Abstract
We previously reported that sterol regulatory element-binding protein-1 (SREBP-1) is involved in the transcriptional regulation of androgen receptor (AR) and formation of fatty acid through altered expression of fatty acid synthase (FASN). In this communication, we provide a new finding that SREBP-1 induced oxidative stress in prostate cancer cells through increased production of reactive oxygen species (ROS) and expression of NADPH oxidase 5 (Nox5). We have shown that: 1) Expression of SREBP-1 protein is positively associated with the clinical Gleason grades in human prostate cancer; 2) Genetic overexpression or knockdown of SREBP-1 in prostate cancer cells resulted in corresponding increased or decreased AR, FASN and Nox5 expression, fatty acid and lipid droplet accumulation, and ROS generation; and 3) SREBP-1 induces and promotes the growth, migration, invasion and castration-resistant progression of prostate cancer cells in vitro and in vivo. Our data demonstrate a novel molecular mechanism by which SREBP-1 promotes prostate cancer growth and progression through alterations in the concerted intracellular metabolic and signaling networks involving AR, lipogenesis and ROS in prostate cancer cells.
Keywords: SREBP-1, AR, Nox5, lipogenesis, oxidative stress
Introduction
Cancer progression is the underlying cause of mortality and morbidity in prostate cancer patients. Lethal progression of prostate cancer from androgen-dependent to androgen–refractory (or castration-resistant) status involves multiple mechanisms in which androgens and androgen receptor (AR)-mediated signaling play key roles (1–3). Blockade of androgen action and the AR signaling axis is currently the main treatment for prostate cancer and its progression. Several reports have demonstrated that androgen biosynthesis and AR signaling in prostate cancer cells are intimately affected by lipogenesis (4–6). Lipid raft membrane-related intercellular signaling pathways have been shown to induce AR activity (7–9). High fat diets were shown to promote prostate cancer cell growth and aggressiveness (10, 11) and drugs that interfere with fatty acid and cholesterol metabolism and absorption were demonstrated to reduce prostate cancer growth, angiogenesis and progression (12–14). Identifying the underlying molecular mechanisms linking lipogenesis and AR signaling could facilitate further development of promising therapeutic approaches for human prostate cancer.
Sterol regulatory element-binding protein-1 (SREBP-1) is a critical transcription factor for lipogenesis (15–17). Three major isoforms of SREBP have been identified. SREBP-1a, SREBP-1c and SREBP-2. SREBP proteins (125 kDa) are anchored to the endoplasmic reticulum (ER) membrane. Through proteolytic cleavage (18), the activated amino-terminus (68 kDa) of SREBP translocates into the nucleus to bind SRE (sterol regulatory element) cis-acting elements and trigger gene expression. SRE is found in the promoter regions of genes encoding enzymes for fatty acid, lipid and cholesterol biosynthesis, including fatty acid synthase (FASN), HMG CoA synthase (19, 20) and farnesyl diphosphate synthase (21). SREBP-1 and one of its target genes, FASN, reported to be a metabolic oncogene (22), have been shown to be involved in prostate cancer malignant progression (6, 23). Our recent results demonstrated that SREBP-1 regulated AR promoter activity and expression, and cell viability in prostate cancer cells (5). The data concurred with the observation of up-regulation of SREBP-1 expression in human prostate cancer tissues during castration-resistant progression (23). These experimental and clinical data suggest that SREBP-1 may play an important role in the regulation of prostate cancer cell growth and progression to lethal castration-resistant disease.
In the present study, we revealed a new molecular mechanism by which SREBP-1 promotes prostate cancer growth, survival and lethal progression. We genetically manipulated SREBP-1 using either an expression vector or a sequence-specific shRNA approach to show respectively increased or decreased AR expression, cell proliferation, migration and invasion in prostate cancer cells. Through the induction of FASN expression, SREBP-1 induced fatty acid and lipid droplet formation and accumulation in prostate cancer cells. Furthermore, SREBP-1 increased reactive oxygen species (ROS) levels via increased NADPH oxidase 5 (Nox5) expression in prostate cancer cells. ROS has been shown to induce signal transduction, survival and progression of cancer cells (24, 25). In mouse xenograft models, SREBP-1 promoted human prostate tumor growth and supported the development of a castration-resistant progression phenotype through the induction of AR, FASN and Nox5 expression. SREBP-1 and its downstream AR/lipogenesis/ROS signaling axis therefore provide novel therapeutic targets for prostate cancer treatment.
Materials and Methods
Prostate cancer cell lines, cell culture, reagents and materials
Human prostate cancer cell lines LNCaP and C4-2B (26), were cultured in T-medium (Invitrogen, Carlsbad, CA) supplemented with 5% fetal bovine serum (FBS). SREBP-1 expression vector and shRNA were obtained from OriGene Technologies, Inc. (Rockville, MD) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. Oil Red O and diphenyleneiodonium (DPI) were purchased from Sigma-Aldrich (St. Louis, MO). A human prostate carcinoma tissue microarray was obtained from IMGENEX (San Diego, CA).
Western blot analysis
Cell lysates were prepared from prostate cancer cells as previously described (27). Nuclear extracts were prepared by a NucBuster™ Protein Extraction kit (Novagen, San Diego, CA). Western blot analysis was performed by NOVEX system (Invitrogen). Primary antibodies against human SREBP-1, FASN, AR, Nox5 (Santa Cruz Biotechnology), catalase (Abcam, Cambridge, MA), Akt and phospho-Akt (p-Akt, Ser 473) (Cell Signaling Technology, Danvers, MA) were utilized.
Fatty acid content quantification and Oil Red O staining
The long chain fatty acid contents were determined by a Fatty Acid Quantification kit (MBL International Corporation, Woburn, MA). Lipid droplet formation was assayed by an Oil Red O staining method (28). Oil Red O staining images were examined and recorded by a phase contrast microscope. For quantification, Oil Red O retained in cells were extracted by 100% isopropanol and measured optical absorbance at 500 nm normalized by total cell numbers.
Cell proliferation, in vitro migration and invasion assays
For cell proliferation assay, prostate cancer cells (1 × 105 cells/well) were seeded on 6-well plates for 3-day incubation. Cells were harvested and cell numbers were counted by hemocytometer. The Boyden chamber method was utilized to examine in vitro cell migration and invasion of prostate cancer cells. Briefly, the undersides of the upper Boyden chambers were pre-coated with collagen I (2.5 µg/cm2, for migration assay) or growth factor-depleted Matrigel matrix (1:4 dilution, for invasion assay). Cells (5 × 104 cells) were seeded inside the pre-coated upper chambers. After incubation at 37°C for 12 to 24 h (migration) or 24 to 48 h (invasion), the numbers of migrated or invading cells were measured by the crystal violet staining method (29).
Intracellular ROS determination
Superoxide or hydrogen peroxide were assayed by dihydroethidium (DHE) or 5-(and-6)-chloromethyl-2’,7’-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) (Invitrogen). DHE is oxidized to red fluorescent ethidium by superoxide, and CM-H2DCFDA is oxidized to green fluorescent dichlorofluorescein (DCF) by hydrogen peroxide. Cells were treated with 10 µM DHE or 5 µM CM-H2DCFDA for 30 min respectively at 37 °C. Subsequently, treated prostate cancer cells were washed with PBS and cultured in T-medium for 30 min. The mean fluorescence intensity was determined by flow cytometry FACS Calibur (BD Bioscience, San Jose, CA) as relative ROS (superoxide or hydrogen peroxide) compared to controls.
Mouse xenograft experiments
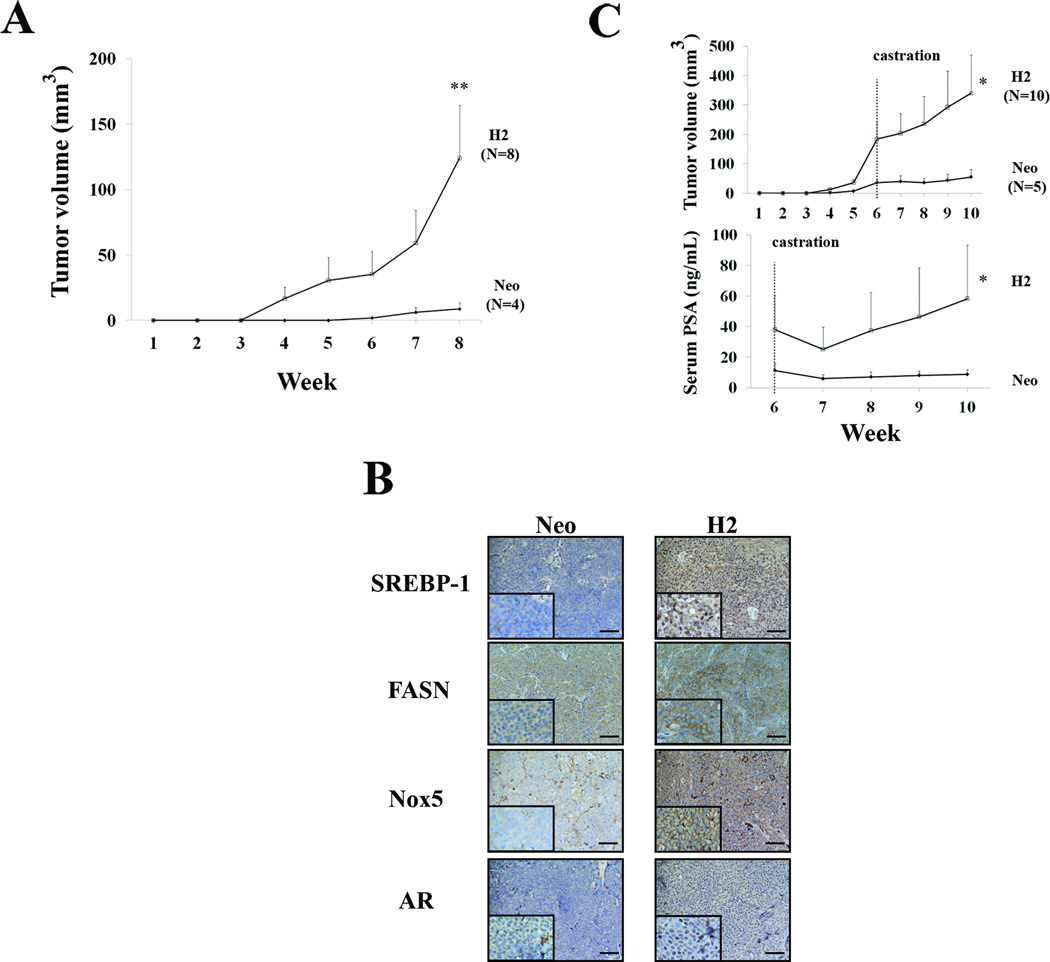
All the mouse experiments were approved and performed in accordance with institutional guidelines. Four-week-old athymic nu/nu male mice (Charles River, Wilmington, MA) were inoculated subcutaneously with control Neo or overexpressing SREBP-1 LNCaP (H2) with 1 × 106 cells per mouse. The tumor burdens were monitored by tumor volume [V = 4/3π × (d/2)2 × D/2, where d is the minor tumor axis and D is the major tumor axis] weekly. To determine the effects of surgical castration on the growth of LNCaP tumors, nu/nu male mice were subcutaneously inoculated with 3 × 106 Neo or H2 cells per mouse. After 6 weeks of tumor growth, mice were either surgically castrated or sham operated. Blood specimens were harvested and serum PSA was determined by AIA-360 Immunoassay Analyzer (Tosoh Bioscience, South San Francisco, CA) weekly. At the end of the animal experiments, mice were euthanized and prostate tumor tissues were harvested, fixed in 10% formalin, dehydrated in ethanol, embedded in paraffin and sectioned for histomorphologic and immunohistochemical (IHC) analyses (5).
Statistical Analysis
Statistical analyses were performed as described previously (30). Student’s t-test and two-tailed distribution were applied in the analysis of statistical significance. Statistical analysis of human prostate carcinoma tissue microarray results was performed using Fisher’s exact test.
Results
Overexpression of SREBP-1 is associated with aggressive pathologic features in human prostate cancer
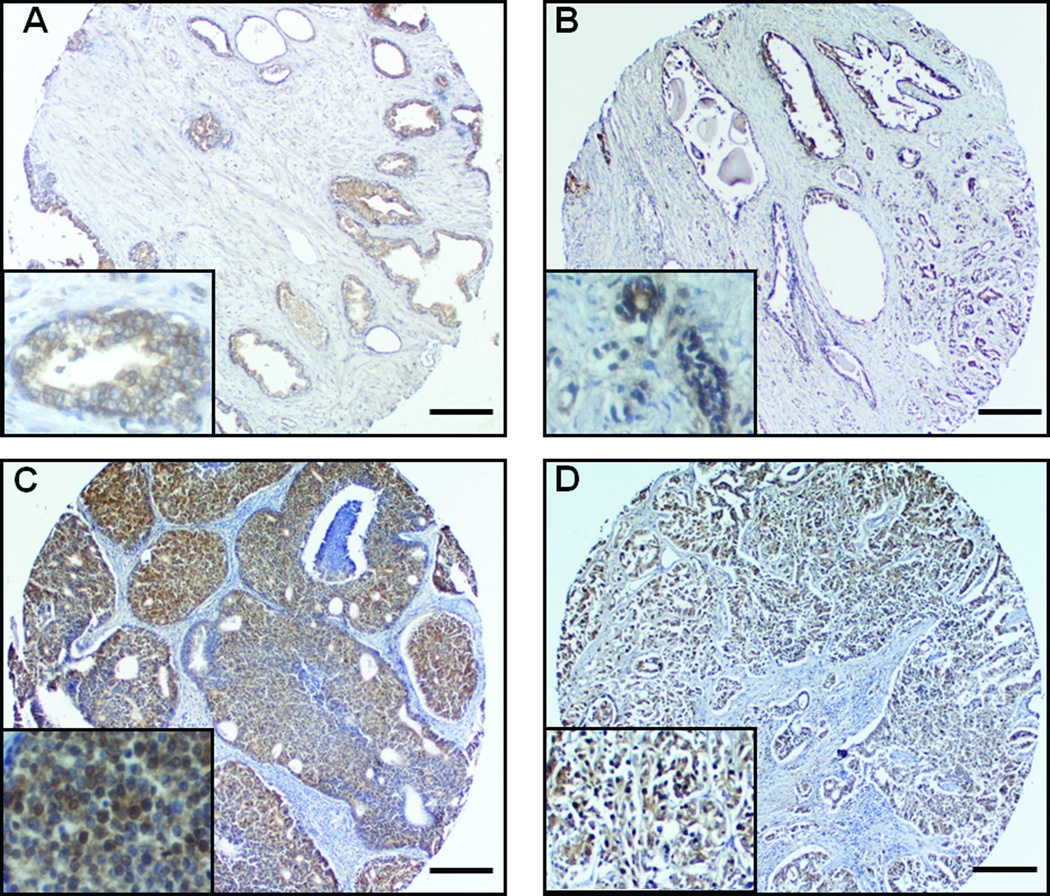
To study the clinical significance of a lipogenic transcription factor, SREBP-1, in prostate cancer progression, we determined the expression of SREBP-1 protein in human prostate carcinoma tissue microarray. We assayed SREBP-1 expression using a clinical prostate cancer progression set representative of tumors at different stages of the disease from normal/benign to localized cancer with different Gleason grades (Fig. 1 and Table 1). SREBP-1 showed only 20% (3/15) positive expression in normal/benign prostate tissues, while expression of SREBP-1 protein increased with higher Gleason grades of disease [from 50% (grade 3) to 71% (grade 5); Table 1]. Interestingly, nuclear SREBP-1 was detected prevalently in grade 4 and 5 prostate cancers (Fig. 1C and D). Statistical analysis revealed that overall SREBP-1 expression levels were strongly correlated with Gleason grades (P = 0.003). These results suggested that expression of SREBP-1 protein is closely linked with the development of aggressive pathologic features in human prostate cancer. SREBP-1 may be a potential prognostic biomarker for human prostate cancer.
Figure 1. Overexpression of SREBP-1 is associated with aggressive pathologic features in human prostate cancer.
A human prostate carcinoma tissue microarray was used to determine expression of SREBP-1. A, Prostate normal/benign tissues; B, Gleason grade 3; C, grade 4; and D, grade 5 of prostate cancer tissues. SREBP-1 protein showed lower expression in human normal/benign tissues, an increasing expression with higher Gleason grade. In particular, SREBP-1 was highly detected in nuclei in grade 4 and 5 prostate cancer. Scale bar = 275 µm.
Table 1.
Expression of SREBP-1 in human prostate carcinoma tissue microarray
| Clinicopathological characteristics |
The numbers of SREBP-1 expression, (%) | |
|---|---|---|
| Positive | Negative | |
| Gleason grade | ||
| Normal/Benign (n#=15) | 3 (20%) | 12 (80%) |
| grade 3 (n=14) | 7 (50%) | 7 (50%) |
| grade 4 (n=27) | 19 (70%) | 8 (30%) |
| grade 5 (n=14) | 10 (71%) | 4 (29%) |
n: the numbers of samples
SREBP-1 induces expression of AR and FASN and increases formation of fatty acid and lipid droplets in prostate cancer cells
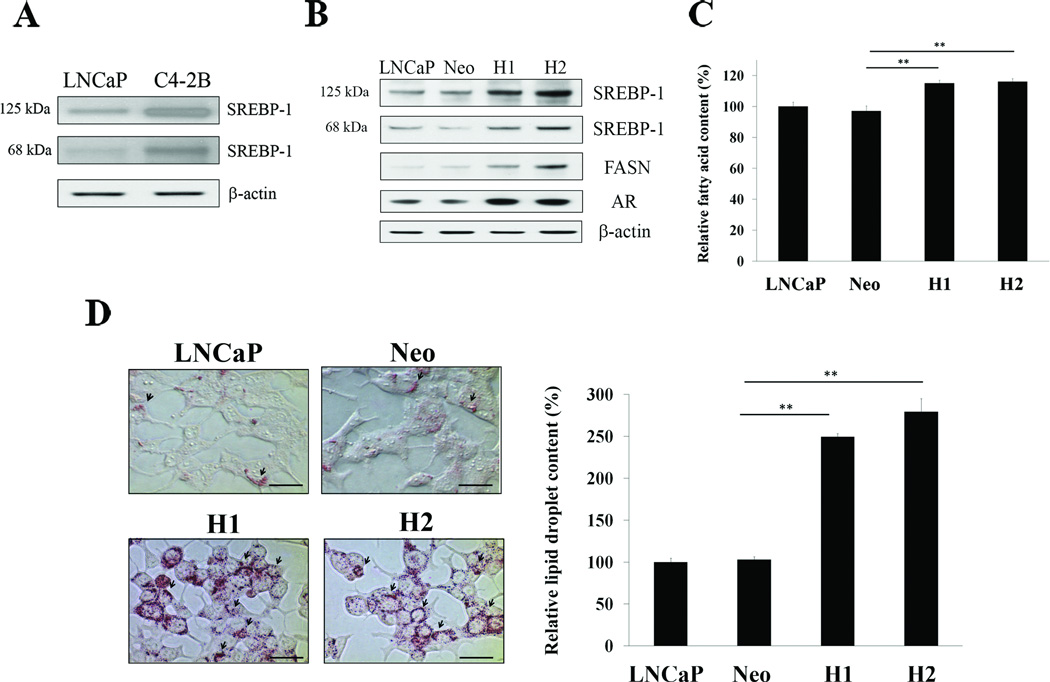
We previously showed that SREBP-1 regulated AR transcriptional expression by binding the 5’-flanking AR promoter region in prostate cancer cells (5). To further investigate the biological functions of SREBP-1 in prostate cancer, we established LNCaP cells stably overexpressing SREBP-1 under the control of a universal CMV promoter (5), since LNCaP cells showed lower intrinsic SREBP-1 [both precursor SREBP-1 (125 kDa) and mature nuclear SREBP-1 (68 kDa)] than aggressive C4-2B cells (Fig. 2A) (26). After antibiotic screening, we selected the two highest stably overexpressing both precursor and nuclear SREBP-1 LNCaP clones, H1 and H2 (Fig. 2B). Consistent with previous observations, SREBP-1 induced expression of AR (5) and FASN (31) in H1 and H2 cells (Fig. 2B). FASN is one of the SREBP-1 target proteins and has been shown as a metabolic oncogene in prostate cancer (32). Because FASN is a key enzyme for de novo synthesis of fatty acid and lipids, we subsequently examined the levels of long chain fatty acid and lipid droplets in cells. As shown in Fig. 2C, fatty acid contents were significantly increased in H1 and H2 compared to untransfected LNCaP and control Neo (empty expression vector-transfected) cells. Also, lipid droplets were highly accumulated in both H1 and H2 compared to control cells (Fig. 2D). The results suggested that SREBP-1 induced expression of AR and FASN and increased accumulation of fatty acid and lipid droplets in prostate cancer cells.
Figure 2. SREBP-1 induces expression of AR and FASN and accumulation of fat in prostate cancer cells.
A, Expression of intrinsic SREBP-1 protein in LNCaP and C4-2B cells was analyzed by Western blot. LNCaP cells showed lower expression of precursor (125 kDa) and mature SREBP-1 (68 kDa) than C4-2B cells. β-actin was used as an internal loading control. B, Expression of FASN and AR was increased in the two highest overexpressing SREBP-1 LNCaP clones, H1 and H2, compared to untransfected LNCaP and control Neo. C, SREBP-1 increased fatty acid contents in H1 and H2. The relative fatty acid content (%) was assigned as 100% in LNCaP. **, P < 0.005, significant differences from Neo. Data represent the mean ± SD of two independent triplicate experiments. D, SREBP-1 greatly increased lipid droplet accumulation in H1 and H2. In the left panel, arrowheads indicate lipid droplets in prostate cancer cells. Scale bars = 40 µm.
SREBP-1 regulates cell proliferation, migration and invasion in prostate cancer cells
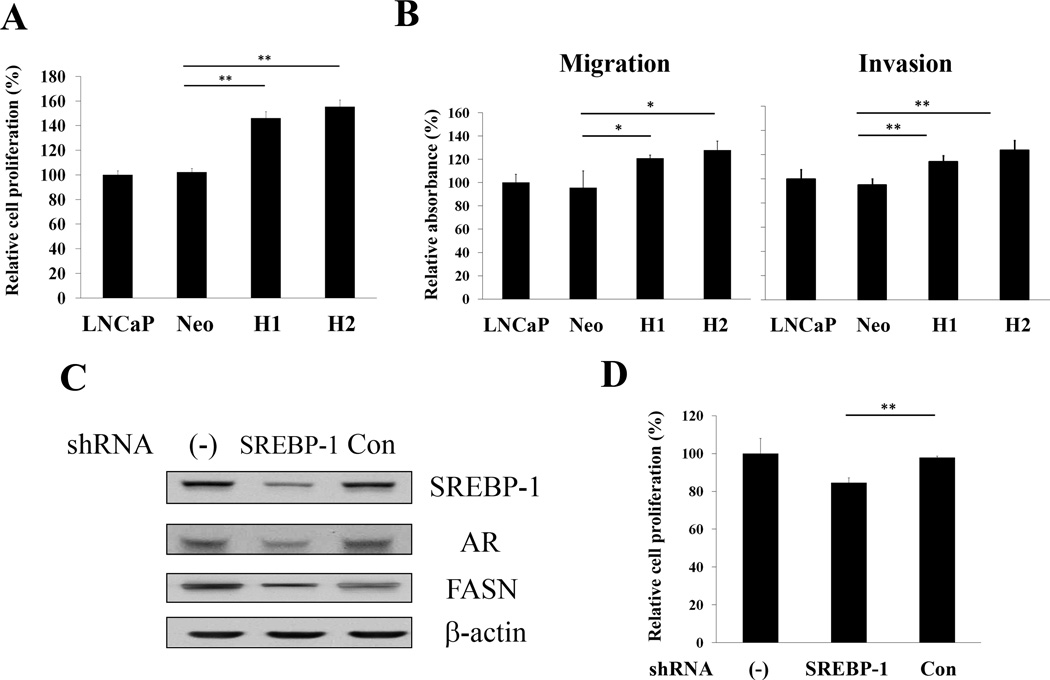
Next, we determined the biological role of SREBP-1 in regulating cell proliferation, migration and invasion in prostate cancer cells. Overexpressing SREBP-1 H1 and H2 cells showed increased cell proliferation compared to untransfected LNCaP and control Neo cells during a three-day incubation (Fig. 3A). One of the hallmarks of progressive and metastatic cells is their ability to invade surrounding tissues and migrate efficiently. The assays of in vitro migration and invasion were conducted by the Boyden chamber method. H1 and H2 showed significantly increased migratory and invasive capabilities compared to the control groups (Fig. 3B). The results supported the conclusion that SREBP-1 promoted cell proliferation, migration and invasion, the hallmarks of progressive cancer cells. Conversely, knock-down of SREBP-1 using a sequence specific shRNA with lentiviral delivery to C4-2B cells as known with high intrinsic SREBP-1 expression (Fig. 2A), showed decreased SREBP-1, AR and FASN expression (Fig. 3C). SREBP-1 shRNA also significantly inhibited cell proliferation (Fig. 3D), in vitro migration (23.8±10.0% inhibition, P = 0.04) and invasion (31.8±10.3% inhibition, P = 0.01) in aggressive C4-2B cells. These data in aggregate revealed that SREBP-1 plays an important role in regulation of cell proliferation, migration and invasion in prostate cancer cells.
Figure 3. SREBP-1 promotes cell proliferation, migration and invasion in prostate cancer cells.
A, H1 and H2 showed increased cell proliferation compared to LNCaP and control Neo during three-day incubation. The relative cell proliferation (%) was assigned as 100% in LNCaP cells. **, P < 0.005, significant differences from Neo. Data represent the mean ± SD of two independent quadruplicate experiments. B, SREBP-1 significantly induced in vitro cell migration and invasion in H1 and H2 compared to control groups. *, P < 0.05 and **, P < 0.005 significant differences from Neo. C, Knocking down SREBP-1 by shRNA with lentiviral delivery in high intrinsic SREBP-1 C4-2B cells showed decreased SREBP-1, AR and FASN expression determined by Western blot. D, Down-regulation of SREBP-1 by shRNA showed decreased cell proliferation of C4-2B. The relative cell proliferation (%) was assigned as 100% in untransfected C4-2B cells (−). **, P < 0.005 significant differences from non-specific control shRNA transfected C4-2B cells (Con).
SREBP-1 induces cell proliferation and progression through increased Nox5 expression and intracellular ROS levels in prostate cancer cells
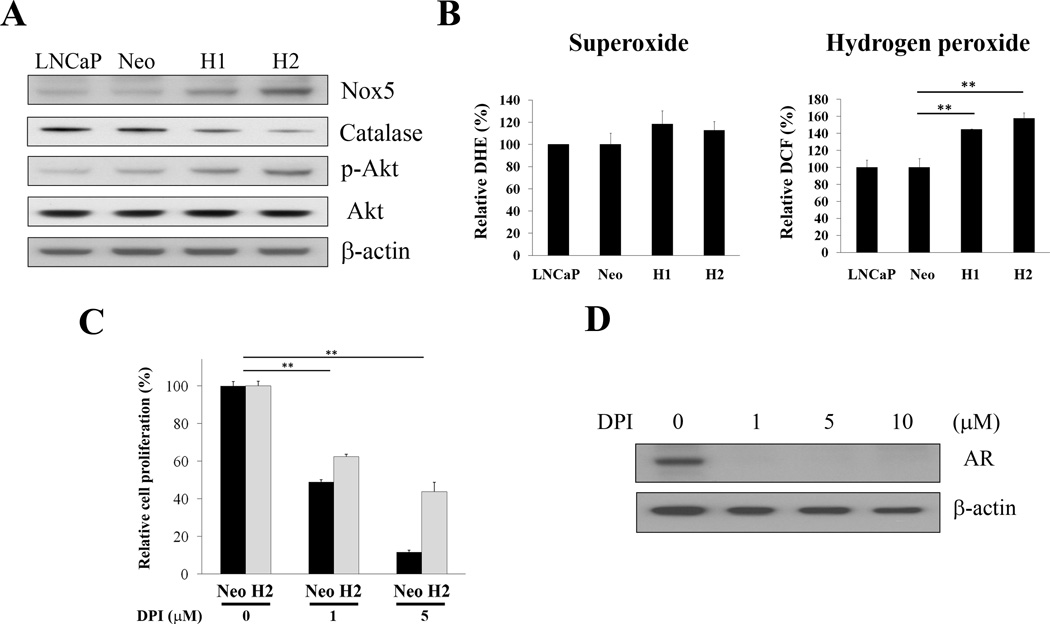
ROS and Nox (a ROS generator), have been reported to regulate cell proliferation, progression, metastasis and radiation resistance of prostate cancer cells (33–35). Our cDNA microarray data revealed that Nox5 was up-regulated in overexpressing SREBP-1 prostate cancer cells compared to control cells (unpublished data). To further determine whether SREBP-1 induces prostate cancer cell proliferation and progression through activation of Nox5 and ROS, we first examined expression of Nox5 in control and SREBP-1stably overexpressing H1 and H2 cells. Consistent with cDNA microarry results, Nox5 protein increased in H1 and H2 compared to Neo and untransfected LNCaP cells (Fig. 4A). We also found that SREBP-1 increased p-Akt expression (Fig. 4A), which is involved in prostate cancer cell proliferation, survival and progression (36). Next, we assessed ROS (superoxide and hydrogen peroxide) levels in cells. The levels of hydrogen peroxide were increased in H1 and H2 cells (Fig. 4B, the right panel); the levels of superoxide, however, were not significantly changed by SREBP-1 (Fig. 4B, the left panel). We also observed that two superoxide degraded enzymes, extracellular superoxide dismutase (SOD3) and mitochondrial SOD (SOD2), were up-regulated in H1 and H2 cells (data not shown). In addition, catalase, an enzyme responsible for hydrogen peroxide degradation, was decreased in H1 and H2 cells (Fig. 4A). To further investigate if SREBP-1 induces prostate cancer cell proliferation through activation of Nox5 and ROS, a Nox inhibitor and ROS scavenger, DPI, was used to treat these prostate cancer cells. As shown in Fig. 4C, cell proliferation in both Neo and H2 cells was affected by DPI in a concentration-dependent inhibition. H2 cells with high Nox5 and ROS levels exhibited a phenotype with increased resistance to DPI-mediated suppression of cell growth (Fig. 4C). It could be due to replenishing intracellular ROS and Nox is easier and faster in H2 than Neo cells. Interestingly, DPI inhibited AR expression in prostate cancer cells (Fig. 4D). Hydrogen peroxide has been demonstrated to affect AR expression in LNCaP cells (37). DPI inhibited AR expression could be through ROS by decreasing hydrogen peroxide in prostate cancer cells. These data collectively indicated that SREBP-1 induced prostate cancer cell proliferation and progression through increased Nox5 expression and intracellular ROS levels.
Figure 4. SREBP-1 induces cell proliferation and progression through alterations of Nox5 and ROS in prostate cancer cells.
A, SREBP-1 induced Nox5 and p-Akt, and inhibited catalase expression in H1 and H2 cells determined by Western blot. B, SREBP-1 increased the levels of hydrogen peroxide in H1 and H2. The levels of superoxide were not significantly changed by SREBP-1. The relative DCF (%) and DHE (%) were assigned as 100% in LNCaP cells. **, P < 0.005, significant differences from Neo. Data represent the mean ± SD of two independent triplicate experiments. C, DPI, a Nox inhibitor and ROS scavenger, inhibited cell proliferation of Neo and H2 in a dose-dependent pattern (0 to 5 µM) during a two-day treatment. H2 cells showed increased resistance to DPI-mediated suppression of cell proliferation. The relative cell proliferation (%) was assigned as 100% for each cell without DPI treatment. **, P < 0.005, significant differences from cells without DPI treatment. Data represent the mean ± SD of two independent quadruplicate experiments. D, DPI also inhibited AR expression in H2 cells.
SREBP-1 promotes prostate tumor growth and castration resistance in subcutaneous xenograft mouse models
Because SREBP-1 expression is increased in advanced forms of human prostate cancer [Fig.1 and (23)], we sought to determine if SREBP-1 confers growth advantages in hormone-naïve mice and resistance to tumor shrinkage in surgically castrated mice. We found that SREBP-1 overexpressing H2 cells inoculated subcutaneously developed a 100% incidence of tumor formation (8/8) in mice. Control Neo cells only exhibited 50% incidence of tumor formation (4/8) during an 8-week observation period. LNCaP classically showed less aggressive and less tumorigenic characteristics in mouse models (26). Furthermore, H2 tumors exhibited a 14-fold increased growth rate over that of the Neo tumors, as assessed by tumor volumes (Neo: 8.8±5.0 mm3 and H2: 124.0±40.0 mm3) after 8 weeks of in vivo growth (Fig. 5A). Consistent with previous Western blot results, IHC data showed that H2 highly expressed SREBP-1 (mostly in nuclei), FASN (cytoplasm), Nox5 (cell membranes) and AR (mostly in nuclei) in comparison to Neo tumors harvested from mouse subcutaneous spaces (Fig. 5B). Next, we sought to determine if SREBP-1 would mediate castration resistance in prostate tumor xenografts grown in mice. We first observed, as expected, that R1881 induced in vitro cell proliferation in control Neo but not SREBP-1 expressing H2 cells (Supplementary Fig. S1). Strikingly, upon castration (week 6), subcutaneous H2 tumor growth continued compared to Neo tumors (Fig. 5C, top panel). Serum PSA levels of both Neo and H2 tumor-bearing mice dropped the first week post-castration (week 7). However, serum PSA levels of H2 mice significantly rebounded four weeks after castration (week 10) compared to Neo mice (Fig. 5C, bottom panel). These results suggested that SREBP-1 regulates prostate tumor occurrence, growth, and even resistance to the effects of castration in mice.
Figure 5. SREBP-1 promotes human prostate tumor growth and castration resistance in mouse subcutaneous xenograft models.
A, Tumor growth was assayed by tumor volume after subcutaneous inoculation of H2 and control Neo cells in mice. SREBP-1 significantly induced the growth of H2 compared to Neo tumors. **, P < 0.005, significant differences from Neo tumors. B, IHC of subcutaneous Neo and H2 tumor specimens. H2 tumors highly expressed SREBP-1 (mostly in nuclei), FASN (cytoplasm), Nox5 (cell membranes) and AR (mostly in nuclei) proteins compared to Neo tumors. Scale bar = 100 µm. C, Mouse castration study. Tumor volumes of subcutaneous H2 tumors continuously increased after mouse castration (week 6) compared to Neo tumors (the top panel). Serum PSA levels of both Neo and H2 tumor-bearing mice dropped the first week post-castration (week 7, bottom panel). However, PSA levels of H2 mice significantly rebounded four weeks after castration (week 10) compared to the Neo group. *, P < 0.05, significant differences from Neo.
Discussion
Aberration of cellular metabolisms has been reported to be strongly linked with cancer. Cancer cells reprogram energy production through aerobic glycolysis followed by lactic acid fermentation in the presence of oxygen (the Warburg effect) (38). Up-regulation of de novo lipogenesis (fatty acid, lipid and cholesterol biosynthesis) in cancer cells is associated with increased needs for membranes and energy storage, and activation of intracellular signaling pathways during uncontrolled cell proliferation and division as well as cancer development and progression (6, 7, 9, 39, 40). Lipogenic activation has also been demonstrated to increase the biosynthesis of androgens in prostate cancer cells (4–6). Androgens and AR signaling are involved in the regulation of prostate cancer development and lethal castration-resistant progression. However, the mechanism of dysregulation of lipogenesis in prostate cancer cells and its contribution to prostate cancer development and progression remain unclear. We previously demonstrated that a key lipogenic transcription factor (15–17), SREBP-1, regulated AR promoter activity and transcriptional expression, and cell viability in prostate cancer cells (5). In the present study, we further revealed a novel molecular mechanism by which SREBP-1 promotes prostate cancer growth and progression through collaborative induction of AR expression, lipogenesis and oxidative stress. The results were confirmed by genetic approaches in which SREBP-1 overexpression or knockdown affected AR, FASN and Nox5 expression, fatty acid and lipid droplet accumulation, and ROS levels in prostate cancer cells. These biochemical alterations were found to be closely associated with cell growth and behavioral changes which can be monitored by prostate cancer cell proliferation, migration and invasion. The data in aggregate suggest that SREBP-1 not only is a crucial mediator for lipogenesis as previously described, but also induces AR, a known survival factor, and increases oxidative stress through induction of Nox5, an important ROS generator, in prostate cancer cells.
ROS and Nox have been closely linked to the initiation and progression of cancer (34, 35, 41, 42). ROS are produced in cells when oxygen is metabolized, including superoxide, hydrogen peroxide and hydroxyl radicals. Excessive ROS accumulation in cells can cause cell injury by damaging vital molecules such as DNA, RNA and proteins. Increased intracellular ROS, however, often leads to the enhanced growth, survival and progression of cancer cells (43–45). Hydrogen peroxide has been shown to induce cell transformation of non-tumorigenic urothelial cells (44) and increase prostate tumorigenicity (42). Significant spheroid growth stimulation occurred when cancer cells were exposed to hydrogen peroxide (46). Our results confirmed that overexpressing SREBP-1 in prostate cancer cells increased ROS (hydrogen peroxide) levels, induced cell proliferation, migration and invasion in vitro, and promoted subcutaneous tumor growth in mice. Furthermore, SREBP-1 also increased p-Akt protein expression (Fig. 4A). The data are consistent with published results where activation of an Akt survival signaling pathway can occur through ROS regulation in several cancer models (25, 47, 48). Together, our results reveal a previously unrecognized role of SREBP-1 promoting prostate cancer growth and survival and increasing prostate cancer cell migration and invasion through augmented Nox5 expression, intracellular ROS and activation of AR and Akt signaling, which ultimately could be responsible for the increased aggressiveness and malignancy of prostate cancer cells commonly associated with castration resistance.
We propose that one of the key mechanisms by which SREBP-1 promotes prostate cancer progression is through the induction of Nox5 gene expression, which enhances ROS levels in cells. We identified a SREBP-1 binding site in the 5′-flanking Nox5 promoter region (data not shown) which suggests that SREBP-1 controls Nox5 expression via promoter transcriptional regulation. Studies using DPI, a Nox activity inhibitor and ROS scavenger, provide additional evidence that SREBP-1 increases ROS levels and promotes prostate cancer cell proliferation through Nox5. Additionally, inhibition of Nox5 expression and decrease of ROS production by Nox5-specific antisense oligonucleotides caused a reduction in the growth of prostate cancer cells (35). This report further supports our findings. Furthermore, DPI has been demonstrated to inhibit cell migration and invasion and decrease MMP-2 and MMP-9 expression and activity in PC3 prostate cancer cells (34). Interestingly, by decreasing Nox activity and ROS levels, DPI greatly inhibited AR expression in LNCaP cells (Fig. 4D). It could be due to DPI decreasing intracellular hydrogen peroxide levels (42), since hydrogen peroxide has been showed to affect AR expression in LNCaP cells (37). These data are consistent with the suggestion that SREBP-1 increases ROS production through transcriptional regulation of Nox5 expression in prostate cancer cells. Targeting Nox, ROS and AR by DPI may be a promising therapeutic approach for the treatment of lethal progression of human prostate cancer.
Very limited information is currently available on the SREBP-1 expression profile of clinical prostate cancer. One early study showed that SREBP-1 was elevated in human primary prostate tumors compared with benign prostatic hypertrophy (23). Additionally, dysregulated SREBP-1 expression may be relevant to prostate cancer castration-resistant progression (23). Our results in a human prostate carcinoma tissue microarray further demonstrated that overexpression of SREBP-1 protein is significantly associated with aggressive pathologic features in human prostate cancer (Fig. 1 and Table 1). Importantly, SREBP-1 was highly expressed in the nuclei of prostate tumor cells with higher Gleason grades (Fig. 1). The precursor of SREBP-1 protein is an ER membrane-bound form. Through a proteolytic process (18), the mature amino-terminal polypeptide is translocated to the nuclei to activate the expression of lipogenesis-related and other genes containing SREBP-1 binding sites in their promoter regions, such as FASN (31), AR (5) and Nox5 (the present study). Furthermore, IHC results of mouse bearing subcutaneous human prostate tumor xenografts demonstrated that SREBP-1 was highly expressed in the nuclei of H2 (i.e. LNCaP-SREBP-1) tumor specimens collected from both intact (Fig. 5B) and castrated (data not shown) mice, which exhibited higher tumor incidences, burdens and serum PSA levels when compared to control Neo tumor specimens. The clinical and animal data collectively indicate that SREBP-1 expression and nuclear translocation play a critical role in the regulation of prostate cancer development and progression to castration resistance. Further investigation of the regulatory mechanism of SREBP-1 nuclear translocation in prostate cancer cells might be of importance.
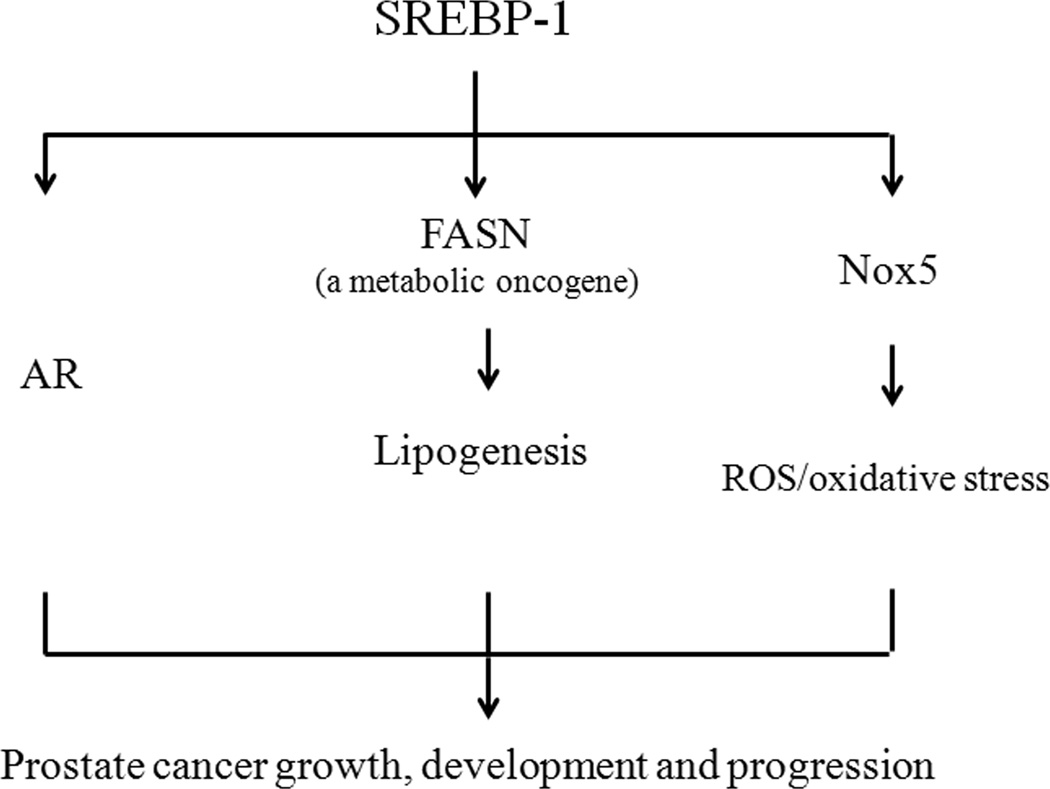
The present studies show for the first time that: 1) Analysis of a human prostate carcinoma tissue microarray with varying grades of diseases revealed that SREBP-1 expression positively correlates with prostate cancer progression. Nuclear translocation of SREBP-1 may also be closely associated with the degree of prostate cancer malignancy. 2) Genetic alterations of SREBP-1 expression led to coordinated regulation of FASN, AR and Nox5 expression in prostate cancer cells. 3) Through the dual induction of FASN and Nox5 expression, SREBP-1 increased fat (fatty acid and lipid droplets) and ROS (hydrogen peroxide) accumulation in prostate cancer cells. 4) SREBP-1 induced prostate cancer cell proliferation, migration and invasion in vitro and promoted prostate tumor growth and castration-resistant progression in vivo. Collectively, the molecular mechanism by which SREBP-1 promotes prostate tumor growth and resistance to castration and androgen responsiveness is through a concerted activation of AR, lipogenesis and ROS signaling networks (Fig. 6). Data presented in this communication are collected from the study of established AR-positive human prostate cancer cell lines and further investigation of this concept in AR-negative prostate cancer cells might be of importance. Also, additional studies may be warranted to define whether the concerted signaling would function in tumor microenvironment, e.g. tumor-stroma interaction. Taken together, we identified SREBP-1, a transcription factor known to regulate fat biosynthesis and homeostasis, promotes and maintains prostate cancer growth and progression by activating and reprogramming the AR/lipogenesis/ROS signaling axis. SREBP-1 and its ancillary regulatory signaling pathways may therefore be novel promising therapeutic targets for the prevention and treatment of lethal progression in human prostate cancer.
Figure 6. Proposed mechanism by which SREBP-1 promotes the growth and progression of prostate cancer by the activation of AR, lipogenesis and ROS/oxidative stress.
SREBP-1 induces AR transcriptional expression and activity in prostate cancer cells. SREBP-1 also activates FASN expression and further induces lipogenesis. By induction of Nox5 expression, SREBP-1 increases ROS levels and oxidative stress in prostate cancer cells. Through a concerted activation of AR, lipogenesis and ROS signaling networks, SREBP-1 promotes cell growth, development and lethal progression of prostate cancer.
Supplementary Material
Acknowledgements
We are grateful to our colleagues for helpful discussions and Mr. Gary Mawyer for editing the manuscript. We would like to thank Drs. Xiaojian Yang and Yueming Wang for the help of mouse study, Mr. Dror Berel (Cedars-Sinai Medical Center) for the assistance of statistical analysis, and Dr. Gene Siegal (Anatomic Pathology, the University of Alabama at Birmingham) for the help of score of human prostate carcinoma tissue microarray. This work was supported by grants from National Institutes of Health Grants 2P01 CA098912 (L.W.K. Chung) and Department of Defense Grants W81XWH-08-1-0321 (W.C. Huang).
References
- 1.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25(2):276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 2.Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst. 2001;93(22):1687–1697. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- 3.Ruizeveld de Winter JA, Janssen PJ, Sleddens HM, et al. Androgen receptor status in localized and locally progressive hormone refractory human prostate cancer. Am J Pathol. 1994;144(4):735–746. [PMC free article] [PubMed] [Google Scholar]
- 4.Leon CG, Locke JA, Adomat HH, et al. Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft model. Prostate. 2010;70(4):390–400. doi: 10.1002/pros.21072. [DOI] [PubMed] [Google Scholar]
- 5.Huang WC, Zhau HE, Chung LW. Androgen receptor survival signaling is blocked by anti-{beta}2-microglobulin monoclonal antibody via a mitogen-activated protein kinase/lipogenic pathway in human prostate cancer cells. J Biol Chem. 2010;285(11):7947–7956. doi: 10.1074/jbc.M109.092759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swinnen JV, Heemers H, van de Sande T, et al. Androgens, lipogenesis and prostate cancer. J Steroid Biochem Mol Biol. 2004;92(4):273–279. doi: 10.1016/j.jsbmb.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Di Vizio D, Solomon KR, Freeman MR. Cholesterol and cholesterol-rich membranes in prostate cancer: an update. Tumori. 2008;94(5):633–639. doi: 10.1177/030089160809400501. [DOI] [PubMed] [Google Scholar]
- 8.Cinar B, Mukhopadhyay NK, Meng G, Freeman MR. Phosphoinositide 3-kinase-independent non-genomic signals transit from the androgen receptor to Akt1 in membrane raft microdomains. J Biol Chem. 2007;282(40):29584–29593. doi: 10.1074/jbc.M703310200. [DOI] [PubMed] [Google Scholar]
- 9.Freeman MR, Cinar B, Lu ML. Membrane rafts as potential sites of nongenomic hormonal signaling in prostate cancer. Trends Endocrinol Metab. 2005;16(6):273–279. doi: 10.1016/j.tem.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Strom SS, Yamamura Y, Forman MR, Pettaway CA, Barrera SL, DiGiovanni J. Saturated fat intake predicts biochemical failure after prostatectomy. Int J Cancer. 2008;122(11):2581–2585. doi: 10.1002/ijc.23414. [DOI] [PubMed] [Google Scholar]
- 11.Kolonel LN, Nomura AM, Cooney RV. Dietary fat and prostate cancer: current status. J Natl Cancer Inst. 1999;91(5):414–428. doi: 10.1093/jnci/91.5.414. [DOI] [PubMed] [Google Scholar]
- 12.Yokomizo A, Shiota M, Kashiwagi E, et al. Statins reduce the androgen sensitivity and cell proliferation by decreasing the androgen receptor protein in prostate cancer cells. Prostate. 2011;71(3):298–304. doi: 10.1002/pros.21243. [DOI] [PubMed] [Google Scholar]
- 13.Solomon KR, Pelton K, Boucher K, et al. Ezetimibe is an inhibitor of tumor angiogenesis. Am J Pathol. 2009;174(3):1017–1026. doi: 10.2353/ajpath.2009.080551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murtola TJ, Visakorpi T, Lahtela J, Syvala H, Tammela T. Statins and prostate cancer prevention: where we are now, future directions. Nat Clin Pract Urol. 2008;5(7):376–387. doi: 10.1038/ncpuro1146. [DOI] [PubMed] [Google Scholar]
- 15.Shimano H. Sterol regulatory element-binding proteins (SREBPs): transcriptional regulators of lipid synthetic genes. Prog Lipid Res. 2001;40(6):439–452. doi: 10.1016/s0163-7827(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 16.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89(3):331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 17.Jackson SM, Ericsson J, Metherall JE, Edwards PA. Role for sterol regulatory element binding protein in the regulation of farnesyl diphosphate synthase and in the control of cellular levels of cholesterol and triglyceride: evidence from sterol regulation-defective cells. J Lipid Res. 1996;37(8):1712–1721. [PubMed] [Google Scholar]
- 18.Wang X, Sato R, Brown MS, Hua X, Goldstein JL. SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell. 1994;77(1):53–62. doi: 10.1016/0092-8674(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Briggs MR, Hua X, Yokoyama C, Goldstein JL, Brown MS. Nuclear protein that binds sterol regulatory element of low density lipoprotein receptor promoter II. Purification and characterization. J Biol Chem. 1993;268(19):14497–14504. [PubMed] [Google Scholar]
- 20.Briggs MR, Yokoyama C, Wang X, Brown MS, Goldstein JL. Nuclear protein that binds sterol regulatory element of low density lipoprotein receptor promoter. I. Identification of the protein and delineation of its target nucleotide sequence. J Biol Chem. 1993;268(19):14490–14496. [PubMed] [Google Scholar]
- 21.Ericsson J, Jackson SM, Lee BC, Edwards PA. Sterol regulatory element binding protein binds to a cis element in the promoter of the farnesyl diphosphate synthase gene. Proc Natl Acad Sci U S A. 1996;93(2):945–550. doi: 10.1073/pnas.93.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menendez JA, Decker JP, Lupu R. In support of fatty acid synthase (FAS) as a metabolic oncogene: extracellular acidosis acts in an epigenetic fashion activating FAS gene expression in cancer cells. J Cell Biochem. 2005;94(1):1–4. doi: 10.1002/jcb.20310. [DOI] [PubMed] [Google Scholar]
- 23.Ettinger SL, Sobel R, Whitmore TG, et al. Dysregulation of sterol response element-binding proteins and downstream effectors in prostate cancer during progression to androgen independence. Cancer Res. 2004;64(6):2212–2221. doi: 10.1158/0008-5472.can-2148-2. [DOI] [PubMed] [Google Scholar]
- 24.Khandrika L, Kumar B, Koul S, Maroni P, Koul HK. Oxidative stress in prostate cancer. Cancer Lett. 2009;282(2):125–136. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clerkin JS, Naughton R, Quiney C, Cotter TG. Mechanisms of ROS modulated cell survival during carcinogenesis. Cancer Lett. 2008;266(1):30–36. doi: 10.1016/j.canlet.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 26.Thalmann GN, Sikes RA, Wu TT, et al. LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. Prostate. 2000;44(2):91–103. doi: 10.1002/1097-0045(20000701)44:2<91::aid-pros1>3.0.co;2-l. Jul 1;44(2) [DOI] [PubMed] [Google Scholar]
- 27.Huang WC, Havel JJ, Zhau HE, et al. {beta}2-Microglobulin Signaling Blockade Inhibited Androgen Receptor Axis and Caused Apoptosis in Human Prostate Cancer Cells. Clin Cancer Res. 2008;14(17):5341–5347. doi: 10.1158/1078-0432.CCR-08-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Xiong W, Chen Y, et al. Evaluation on the phagocytosis of apoptotic spermatogenic cells by Sertoli cells in vitro through detecting lipid droplet formation by Oil Red O staining. Reproduction. 2006;132(3):485–492. doi: 10.1530/rep.1.01213. [DOI] [PubMed] [Google Scholar]
- 29.Nomura T, Huang WC, Zhau HE, et al. {beta}2-Microglobulin Promotes the Growth of Human Renal Cell Carcinoma through the Activation of the Protein Kinase A, Cyclic AMP-Responsive Element-Binding Protein, and Vascular Endothelial Growth Factor Axis. Clin Cancer Res. 2006;12(24):7294–7305. doi: 10.1158/1078-0432.CCR-06-2060. [DOI] [PubMed] [Google Scholar]
- 30.Huang WC, Xie Z, Konaka H, Sodek J, Zhau HE, Chung LW. Human Osteocalcin and Bone Sialoprotein Mediating Osteomimicry of Prostate Cancer Cells: Role of cAMP-Dependent Protein Kinase A Signaling Pathway. Cancer Res. 2005;65(6):2303–2313. doi: 10.1158/0008-5472.CAN-04-3448. [DOI] [PubMed] [Google Scholar]
- 31.Magana MM, Osborne TF. Two tandem binding sites for sterol regulatory element binding proteins are required for sterol regulation of fatty-acid synthase promoter. J Biol Chem. 1996;271(51):32689–32694. doi: 10.1074/jbc.271.51.32689. [DOI] [PubMed] [Google Scholar]
- 32.Baron A, Migita T, Tang D, Loda M. Fatty acid synthase: a metabolic oncogene in prostate cancer? J Cell Biochem. 2004;91(1):47–53. doi: 10.1002/jcb.10708. [DOI] [PubMed] [Google Scholar]
- 33.Lu JP, Monardo L, Bryskin I, et al. Androgens induce oxidative stress and radiation resistance in prostate cancer cells though NADPH oxidase. Prostate Cancer Prostatic Dis. 2010;13(1):39–46. doi: 10.1038/pcan.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68(6):1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 35.Brar SS, Corbin Z, Kennedy TP, et al. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol. 2003;285(2):C353–C369. doi: 10.1152/ajpcell.00525.2002. [DOI] [PubMed] [Google Scholar]
- 36.Li L, Ittmann MM, Ayala G, et al. The emerging role of the PI3-K-Akt pathway in prostate cancer progression. Prostate Cancer Prostatic Dis. 2005;8(2):108–118. doi: 10.1038/sj.pcan.4500776. [DOI] [PubMed] [Google Scholar]
- 37.Shiota M, Yokomizo A, Tada Y, et al. Castration resistance of prostate cancer cells caused by castration-induced oxidative stress through Twist1 and androgen receptor overexpression. Oncogene. 2010;29(2):237–250. doi: 10.1038/onc.2009.322. [DOI] [PubMed] [Google Scholar]
- 38.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 39.Yamashita T, Honda M, Takatori H, et al. Activation of lipogenic pathway correlates with cell proliferation and poor prognosis in hepatocellular carcinoma. J Hepatol. 2009;50(1):100–110. doi: 10.1016/j.jhep.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 40.Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care. 2006;9(4):358–365. doi: 10.1097/01.mco.0000232894.28674.30. [DOI] [PubMed] [Google Scholar]
- 41.Blanchetot C, Boonstra J. The ROS-NOX connection in cancer and angiogenesis. Crit Rev Eukaryot Gene Expr. 2008;18(1):35–45. doi: 10.1615/critreveukargeneexpr.v18.i1.30. [DOI] [PubMed] [Google Scholar]
- 42.Lim SD, Sun C, Lambeth JD, et al. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate. 2005;62(2):200–207. doi: 10.1002/pros.20137. [DOI] [PubMed] [Google Scholar]
- 43.Sikka SC. Role of oxidative stress response elements and antioxidants in prostate cancer pathobiology and chemoprevention--a mechanistic approach. Curr Med Chem. 2003;10(24):2679–2692. doi: 10.2174/0929867033456341. [DOI] [PubMed] [Google Scholar]
- 44.Okamoto M, Kawai K, Reznikoff CA, Oyasu R. Transformation in vitro of a nontumorigenic rat urothelial cell line by hydrogen peroxide. Cancer Res. 1996;56(20):4649–4653. [PubMed] [Google Scholar]
- 45.Dreher D, Junod AF. Role of oxygen free radicals in cancer development. Eur J Cancer. 1996;32A(1):30–38. doi: 10.1016/0959-8049(95)00531-5. [DOI] [PubMed] [Google Scholar]
- 46.Sauer H, Diedershagen H, Hescheler J, Wartenberg M. Calcium-dependence of hydrogen peroxide-induced c-fos expression and growth stimulation of multicellular prostate tumor spheroids. FEBS Lett. 1997;419(2–3):201–205. doi: 10.1016/s0014-5793(97)01456-7. [DOI] [PubMed] [Google Scholar]
- 47.Kwon J, Lee SR, Yang KS, et al. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci U S A. 2004;101(47):16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong-Yun S, Yu-Ru D, Shan-Lin L, Ya-Dong Z, Lian W. Redox stress regulates cell proliferation and apoptosis of human hepatoma through Akt protein phosphorylation. FEBS Lett. 2003;542(1–3):60–64. doi: 10.1016/s0014-5793(03)00338-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.