Summary
Background
Indications for chemotherapy in gestational trophoblastic disease include raised human chorionic gonadotropin (hCG) concentrations 6 months after uterine evacuation of hydatidiform mole, even when values are falling. We aimed to establish whether chemotherapy is always necessary in these patients.
Methods
We retrospectively identified women registered between January, 1993, and May, 2008, at Charing Cross Hospital, London, UK, who had persistently high hCG concentrations 6 months after evacuation of hydatidiform mole. Rates of hCG normalisation, relapse, and death were assessed in patients continued under surveillance and those who received chemotherapy after 6 months. We postulated that a surveillance policy would be clinically acceptable if hCG values returned to normal in 75% of patients or more.
Findings
76 (<1%) of 13 960 patients with hydatidiform moles had persistently high hCG concentrations of more than 5 IU/L 6 months after evacuation. 66 (87%) patients continued under surveillance and hCG values spontaneously returned to normal without chemotherapy in 65 (98%) of these patients. Values in one patient did not become normal because of chronic renal failure, but she remains healthy. Ten patients received chemotherapy, and hCG concentrations returned to normal in eight (80%) of these individuals (surveillance vs chemotherapy groups p=0·044) and remained slightly high (6–11 IU/L) in two without any associated clinical problems off treatment. We noted no significant differences between individuals in the surveillance and chemotherapy groups, apart from lower median hCG concentrations 6 months after evacuation in those under surveillance than in those given chemotherapy (13 IU/L, range 5–887, vs 157 IU/L, range 6–6438; p=0·004). Overall, there were no deaths in this series.
Interpretation
A surveillance policy seems to be clinically acceptable in patients with low and declining concentrations of hCG 6 months after evacuation of hydatidiform mole.
Funding
National Commissioning Group, Imperial Experimental Cancer Medicine Centre, Imperial Biomedical Research Centre, and Cancer Research UK.
Introduction
Genetically, partial and complete hydatidiform moles overexpress paternal genes. Complete moles are diploid and androgenetic in origin,1,2 whereas partial moles are triploid and have one maternal set and two paternal sets of chromosomes.3,4 Hydatidiform moles occur more frequently in east Asia than in most western populations, in which the incidence seems to be fairly uniform.5 In the UK, about one to three per 1000 pregnancies are either complete or partial moles.6 Hydatidiform moles can affect women of any reproductive age, but relative risks are higher in those younger than 16 years or older than 45 years than in other age groups.7,8
Most women with molar pregnancy present with vaginal bleeding in the first trimester and all have a raised serum or urine human chorionic gonadotropin (hCG) concentration (5 IU/L or more in serum on the Charing Cross hCG assay). Subsequent pelvic ultrasonography might show a failing normal, abnormal, or possible molar pregnancy, but cannot enable diagnosis of hydatidiform mole, so a dilatation and curettage is necessary.9 This procedure allows histological examination of the products of conception and an appropriate diagnosis can be made.10
Residual tissue spontaneously regresses in most patients, with a corresponding normalisation of hCG concentrations in about 92% of patients.6 However, malignant transformation to gestational trophoblastic neoplasia after complete hydatidiform mole occurs in roughly 15% of cases and after partial hydatidiform moles in 0·5–1%.11–13 This malignant transformation requires prompt recognition so that lifesaving chemotherapy can be instituted. About 8% of UK patients with hydatidiform moles undergo chemotherapy, usually as single drug treatment with methotrexate or dactinomycin.6
To ensure rapid detection of post-mole gestational trophoblastic neoplasia, all patients with hydatidiform moles in the UK are registered with one of three centralised hCG monitoring and surveillance programmes (Ninewells Hospital, Dundee; Weston Park Hospital, Sheffield; and Charing Cross Hospital, London).9 The most common reason for diagnosis of post-mole gestational trophoblastic neoplasia is a plateau or rise in hCG values. Another internationally agreed reason for diagnosis of gestational trophoblastic neoplasia and initiation of chemotherapy is a persistently raised hCG concentration at 6 months, even if the value is falling (panel 1).6,14,15 This criterion is used because increasing tumour age is thought to entail a worse prognosis or high risk of resistance to single agent chemotherapy, or both, presumably because of further acquired genetic mutations with time.16 Increased time intervals between antecedent pregnancy and start of chemotherapy are associated with higher mortality rates.16–19
Panel 1. Charing Cross Hospital indications for chemotherapy and FIGO 2000 criteria for diagnosis of gestational trophoblastic neoplasia.
Charing Cross Hospital indications for chemotherapy in gestational trophoblastic disease
-
•
Histological evidence of choriocarcinoma
-
•
Evidence of metastases in brain, liver, or gastrointestinal tract, or radiological opacities larger than 2 cm on chest radiography
-
•
Pulmonary, vulval, or vaginal metastases unless human chorionic gonadotropin concentrations are falling
-
•
Heavy vaginal bleeding, or evidence of gastrointestinal or intraperitoneal haemorrhage
-
•
Rising hCG concentrations in two consecutive samples or steady concentrations in three consecutive samples after evacuation of molar pregnancy
-
•
Serum hCG greater than 20 000 IU/L more than 4 weeks after evacuation, because of the risk of uterine perforation
-
•
Raised hCG concentrations 6 months after evacuation, even if decreasing
FIGO 2000 criteria for the diagnosis of gestational trophoblastic neoplasia after hydatidiform mole
-
•
Consistently high hCG concentrations last for four measurements for 3 weeks or longer (days 1, 7, 14, and 21)
-
•
Rise of hCG on three consecutive measurements done every week, during 2 weeks or longer (days 1, 7, and 14)
-
•
Histological diagnosis of choriocarcinoma
-
•
hCG concentration high for 6 months or more
hCG=human chorionic gonadotropin.
However, evidence from twin and singleton molar-pregnancy studies20,21 showing no increased frequency of gestational trophoblastic neoplasia or disease resistant to single-agent therapy in moles that were evacuated late as opposed to early in pregnancy suggests that the malignant potential of moles is established very early in the first trimester. Moreover, other data suggest that such acquired adverse mutations probably occur much later than 6 months after evacuation in the natural course of the disease. Thus, the crucial time for an adverse outcome for most patients with gestational trophoblastic neoplasia seems to be 2·8 years from time of causal pregnancy,17 or for placental site trophoblastic tumour after 4 years.18 Additionally, chemotherapy given at or after 6 months subsequent to evacuation was effective in 28 women,22 suggesting that, if surveillance were undertaken and failed, effective salvage treatment could still be given. Collectively, these clinical results indicate that chemotherapy might not be necessary in women who have an increased but falling hCG concentration 6 months after uterine evacuation of a hydatidiform mole.
Here, we postulate that a policy of continued surveillance after 6 months would be clinically acceptable if hCG concentrations spontaneously returned to normal in more than 75% of patients within 12 months of evacuation, with less than 25% developing gestational trophoblastic neoplasia and requiring chemotherapy. Therefore, we retrospectively analysed the outcome of patients with persistently raised but falling hCG concentrations 6 months after evacuation of hydatidiform moles.
Methods
Patients and study design
We retrospectively identified patients with persistently raised concentrations of serum hCG at 6 months after evacuation of molar pregnancy between January, 1993, and May, 2008, from the electronic database of gestational trophoblastic disease at Charing Cross Hospital. At this hospital, hCG detection and monitoring is done with a non-commercial, one-site, in-house, competitive hCG radioimmunoassay that uses a rabbit polyclonal antibody. Serum hCG concentrations of less than 5 IU/L are classed as normal (in urine, less than 25 IU/L).23 Once registered, women with molar pregnancies have serum and urinary hCG concentrations measured every 2 weeks after evacuation. For patients whose hCG concentrations spontaneously return to normal within 8 weeks of follow-up, monitoring is continued for as long as 6 months from time of uterine evacuation. When the time taken for the hCG concentration to normalise is longer than 8 weeks, hCG monitoring is continued for a further 6 months of normal values.24
Clinical records were reviewed and data such as patients' age at registration, date of antecedent pregnancy and uterine evacuation, histological subtype, treatment, and outcome were noted. All histological changes were centrally reviewed as previously described.7,12 Patients with non-molar pregnancies, pregnancy within 6 months of follow up, and false-positive hCG values were excluded.
All identified patients were reviewed and discussed in multidisciplinary meetings every week. Criteria for review in clinic 6 months after evacuation were based on steady or rising hCG concentrations, symptoms such as vaginal bleeding, or hCG concentration of more than 100 IU/L. Patients who were reviewed in clinic underwent a chest radiograph and doppler ultrasound scan of the pelvis. Decision to treat with chemotherapy or continue surveillance was jointly made by the consultant and patient after discussion. No formal randomisation process was used.
hCG concentrations of women who continued under surveillance were monitored according to a surveillance programme, which entailed measurements of blood and urine samples every 2 weeks until normal, and then monthly urine samples for a further 6 months.24 The women who started chemotherapy sent hCG serum samples twice a week until normal, and then once a week until 6 weeks after completion of chemotherapy. At this point, patients given chemotherapy were reviewed in clinic with a repeat doppler ultrasound of the pelvis before post-treatment monitoring of hCG in serum and urine began. Monitoring was done with decreasing frequency until eventually urine samples were necessary only every 6 months.25
The primary outcome assessed in patients with persistently raised hCG 6 months after evacuation was rate of spontaneous hCG normalisation under continued surveillance. We also investigated normalisation rates in patients who received chemotherapy. Additionally, frequency of antecedent complete and partial hydatidiform moles in the two groups, hCG concentrations at registration, peak hCG between registration and 6 months, hCG at 6 months, time to normalisation, and rates of resistance to chemotherapy, relapse, and death were also established. Because use of oral contraceptives while hCG is still high might increase risk of gestational trophoblastic neoplasia,26 use of these hormones was documented.
Local institutional review board approval was obtained for this study. The study was done with anonymised patient records and hence informed consent was not needed.
Statistical analyses
Enrolment of 66 patients under surveillance allowed exclusion of hCG normalisation rates of less than 75% with 100% power, with a two-sided α of 0·05 for a 95% observed rate of hCG normalisation. Fisher's exact test was used to compare rates of spontaneous hCG normalisation and frequency of complete and partial hydatidiform moles between chemotherapy and surveillance groups. Mann-Whitney U test was used to test for differences in registration, peak, and 6 month hCG concentrations between chemotherapy and surveillance groups, and 6 month hCG concentrations between responding and non-responding patients who received chemotherapy. We assessed the correlation between hCG concentration at 6 months and time to remission with Spearman's rank correlation coefficient. We did statistical analyses with SPSS (version 17·0).
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Between Jan 1, 1993, and May 1, 2008, 13 960 patients with hydatidiform mole were registered. 974 (7%) required chemotherapy, and hCG normalised spontaneously in 12 910 (92%) within 6 months of evacuation. The remaining 76 (<1%) patients had persistently high hCG values 6 months after evacuation and were included in our study (figure 1). The median age of the included patients was 30 years (range 15–51). Histologically, 46 (61%) of these patients had complete moles, 25 (33%) had partial moles, and five (7%) were unclassified. Median plasma hCG concentration at registration was 1343·5 IU/L (range 7–1 809 437), peak hCG was 1386 IU/L (range 6–1 809 437), hCG at 6 months was 13·5 IU/L (range 3–6438), and time to hCG normalisation was 7·5 months (range 6·1–32·2; webappendix p 1). Overall hCG concentration at 6 months was modestly correlated with time to hCG normalisation (table).
Figure 1.
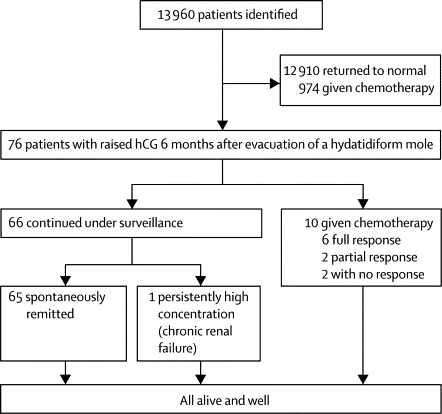
Management and outcome of patients with high hCG concentrations 6 months after evacuation of hydatidiform mole
hCG=human chorionic gonadotropin.
Table.
Correlation of hCG concentrations at 6 months with time from initial uterine evacuation to remission
| Number of patients (n=76) | Median hCG concentration at 6 months (IU/L) | Correlation between hCG concentration at 6 months and time to remission | |
|---|---|---|---|
| <8 months | 46 (61%) | 12·5 (3–117) | r=0·233; p=0·049 |
| 8–12 months | 20 (26%) | 17 (6–887) | .. |
| >12 months | 7 (9%) | 12 (7–798) | .. |
| No return to normal | 3 (4%) | 7 (6–18) | .. |
Data are n (%) or median (range). hCG=human chorionic gonadotropin.
66 (87%) patients continued under surveillance and did not have chemotherapy (figure 1). 38 (58%) of these individuals had complete hydatidiform mole, 24 (36%) had partial mole, and four (6%) were unclassified (webappendix p 1). hCG concentrations returned to normal in 65 of these patients (98%; 95% CI 95·5–100). Values became normal within 8 months of evacuation in 44 (67%) of patients under surveillance, in the next 4 months in 15 (23%), and times were longer than 1 year for only six (9%). Five of the patients whose times to normalisation were longer than 1 year had normal hCG concentrations within 18 months of evacuation. The sixth patient was offered treatment after three consecutive rises in hCG concentrations, but she declined chemotherapy and was lost to follow-up because she emigrated out of the UK. On return, she had a successful pregnancy and her hCG subsequently normalised without chemotherapy. Therefore, only one patient in the surveillance group continued to have slightly raised hCG concentrations (range 8–11). This individual was on haemodialysis for end-stage renal failure and persistent hCG concentrations were therefore attributed to impaired renal clearance. Thus, no patients in the surveillance group developed gestational trophoblastic neoplasia that needed chemotherapy, developed drug resistance, or had a relapse. The observed rate of hCG normalisation in the surveillance group, suggests that the true hCG normalisation rate is at least 85% with 99% confidence, and is greater than 90% with 67% power in the surveillance group.
Of the ten patients who started on low-risk chemotherapy with methotrexate and folinic acid25 6 months after evacuation of their hydatidiform mole (figure 1), eight (80%) had complete and two (20%) had partial molar pregnancies (webappendix pp 1–2). All these women had stage I disease with low FIGO 2000 risk factor scores27 of between 1 and 3. Six (60%) patients had a complete response to treatment and two (20%) individuals showed only a partial response but their hCG concentrations returned to normal after cessation of chemotherapy (one within 1 week and one in 8 months), and so were classified as having a partial response (webappendix p 2). The remission rate was 80·0% (95% CI 55·2–100).
The treatment of the other two (20%) patients given chemotherapy was subsequently stopped. They remained well, with slightly increased hCG concentrations (range 6–11 IU/L) for 3 and 15 years, and have since had successful pregnancies. Patients with partial and full responses to chemotherapy had higher hCG concentrations at 6 months than did those who had no response (median hCG concentrations of 345 IU/L vs 7 IU/L; p=0·037).
Comparison of patient characteristics in surveillance and chemotherapy groups identified no significant differences in histology (complete or partial mole), age at diagnosis, year of diagnosis, hCG concentration at registration, and peak hCG concentrations (webappendix p 1). However, patients under surveillance had lower median hCG concentrations 6 months after evacuation than did those who received chemotherapy (figure 2). Importantly, hCG concentrations returned to normal in more patients under surveillance than in those on chemotherapy (p=0·044), with no significant differences in time to normalisation between the groups (webappendix pp 1–3). No deaths occurred in either the surveillance or treatment groups, with an overall median follow up of 2·1 years (range 0·6–14·7). No patients included took oral contraceptives while their hCG concentrations were increased.
Figure 2.
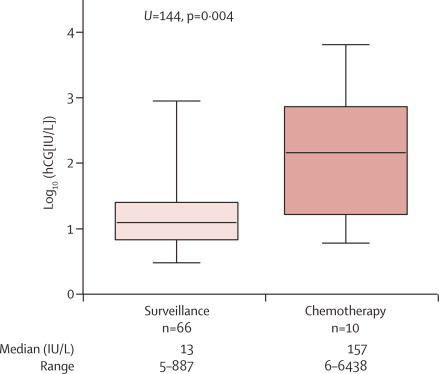
hCG concentrations at 6 months of patients who received chemotherapy and those continued under surveillance
hCG=human chorionic gonadotropin.
Discussion
We have shown that when hCG concentrations are still falling 6 months after evacuation of a hydatidiform mole, spontaneous normalisation will occur in almost all cases without chemotherapy. Moreover, chemotherapy seems to be unhelpful in 20–40% of patients given this treatment. Therefore, when available, a stringent surveillance protocol could be preferable to chemotherapy (panel 2).
Panel 2. Research in context.
Systematic review
We searched PubMed for reports from January, 1980, to December, 2010, that contained the terms “GTD”, “molar pregnancy”, “hydatidiform mole”, “trophoblastic disease”, “complete hydatidiform mole”, or “partial hydatidiform mole”, and combined these terms with “surveillance”, “follow-up”, “monitoring”, “treatment criteria”, “low risk”, or “duration from evacuation” to identify any other studies in this area of research. Previous reports showed that one standard indication for chemotherapy in women who have had a molar pregnancy is a persistently high hCG concentration 6 months after uterine evacuation of the mole, even if it is still falling.6,14,15 We identified no studies that specifically looked at whether continued hCG surveillance 6 months after evacuation of molar disease from the uterus is clinically acceptable. However, one study showed that chemotherapy could help patients with an increased hCG concentration beyond 6 months to recover safely.22
Interpretation
As far as we are aware, our study is the first to investigate whether continued hCG surveillance is a clinically acceptable approach as opposed to chemotherapy. Our findings directly challenge the present clinical dogma,6,14,15 and provide data showing that continued surveillance for women with high but falling hCG concentrations 6 months after uterine evacuation of a molar pregnancy is clinically acceptable because nearly all patients will spontaneously remit. The results are important because they will change international practice and spare women unnecessary exposure to chemotherapy and its toxic effects.
Chemotherapy has several disadvantages. For example, although low-risk single-agent chemotherapy regimens are generally well tolerated,25,28 they might still be associated with some short-term side-effects. Additionally, if patients are assumed to not be responding to this regimen and are then switched to multi-agent chemotherapy regimens, toxic effects greatly increase in the short term,19,29,30 and unlike single-agent treatment also in the long term.31 Furthermore, patients in the UK are advised not to become pregnant for 12 months after chemotherapy. By contrast, individuals under surveillance would have to wait only 6 months after their first normal hCG measurement before they could attempt a new pregnancy.24
We do not know if any patients successfully given chemotherapy might have spontaneously remitted had they been in the surveillance group. So how should a decision be made about which patients are suitable for observation and which should be given chemotherapy? This question is clearly difficult to answer. One possibility might be the use of a cutoff hCG concentration for requirement of a clinic review with further investigation. Our findings suggest that hCG concentrations can spontaneously return to normal in patients with 6 month hCG measurements of lower than 887 IU/L, which could indicate an upper limit for cutoff of a surveillance policy. Alternatively, on the basis of median hCG value of patients who responded to chemotherapy, a cutoff hCG of 345 IU/L could be suggested.
Some patients with a high hCG concentration might not need treatment. Two (3%) patients in the surveillance group had hCG values higher than 345 IU/L and still spontaneously remitted. However, four of six patients who responded fully to chemotherapy had hCG concentrations greater than 345 IU/L (data not shown). On the basis of the few data available, a cutoff of 345 IU/L on the Charing Cross radioimmunoassay for hCG would be reasonable until more information is generated.
Other commercial hCG assays can variably detect the many hCG fragments or isoforms produced in patients with cancer,23 and so the cutoff value suggested might vary depending on the hCG assay used. One solution to this difficulty might be provided through use of hyperglycosylated hCG (hCG-H) measurements.32 Preliminary work suggests that an increased ratio of hCG-H to total hCG might identify patients with highly invasive gestational trophoblastic neoplasia likely to benefit from chemotherapy.32,33 However, this approach will not be validated until commercial hCG-H assays are more readily available.
So does surveillance of this group of women increase the risk that high-risk, drug-resistant disease or placental site trophoblastic tumour will develop and potentially reduce survival? Encouragingly, no patients died in our cohort, or in a group of 28 patients given chemotherapy beyond 6 months at another centre (all had low-risk disease and were cured).22 Additionally, previous twin and singleton studies have shown that delayed evacuation does not increase the risk of development of gestational trophoblastic neoplasia.20,21
We cannot exclude the possibility that some of these hydatidiform moles could have developed into placental site trophoblastic tumours,34 which are associated with slightly raised hCG concentrations.18 Reassuringly, this development has not yet happened in our cohort. Thus, our findings suggest that the practice of close surveillance could be adopted in the knowledge that these women are not being exposed to a significantly increased risk of life-threatening gestational trophoblastic neoplasia including placental site trophoblastic tumour.
This study is limited by retrospective data and small patient numbers. A further prospective study in a multicentre setting would be useful. However, in the absence of these data, we recommend that women with persistently high hCG concentrations 6 months after evacuation should continue regular hCG monitoring rather than begin chemotherapy. Chemotherapy should only be considered in patients whose hCG concentrations are greater than 345 IU/L, and when radiological evidence of disease is present or when hCG levels are consistently plateaued or rising.
Acknowledgments
Acknowledgments
MJS thanks the National Commissioning Group and Department of Health for continuing support of the GTD service. MJS is supported by the Imperial College Experimental Cancer Medicine Centre grant from Cancer Research UK, the Department of Health, and the Imperial College Biomedical Research Centre. RA is supported by a Clinician Scientist Fellowship from Cancer Research UK.
Contributors
MJS conceived and designed the study. ST, DS, and MJS participated in data collection. RH did hCG assays. PMS, RA, and MJS treated the patients. ST, RA, and MJS contributed to data analysis and interpretation. ST, RA, and MJS wrote the report. All authors have seen and approved the final version of the report.
Conflicts of interest
We declare that we have no conflicts of interest.
Web Extra Material
References
- 1.Kajii T, Ohama K. Androgenetic origin of hydatidiform mole. Nature. 1977;268:633–634. doi: 10.1038/268633a0. [DOI] [PubMed] [Google Scholar]
- 2.Fisher RA, Newlands ES. Gestational trophoblastic disease: molecular and genetic studies. J Reprod Med. 1998;43:81–97. [PubMed] [Google Scholar]
- 3.Fisher RA, Lawler SD, Ormerod MG, Imrie PR, Povey S. Flow cytometry used to distinguish between complete and partial hydatidiform moles. Placenta. 1987;8:249–256. doi: 10.1016/0143-4004(87)90048-8. [DOI] [PubMed] [Google Scholar]
- 4.Lage JM, Mark SD, Roberts DJ, Goldstein DP, Bernstein MR, Berkowitz RS. A flow cytometric study of 137 fresh hydropic placentas: correlation between types of hydatidiform moles and nuclear DNA ploidy. Obstet Gynecol. 1992;79:403–410. doi: 10.1097/00006250-199203000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Palmer JR. Advances in the epidemiology of gestational trophoblastic disease. J Reprod Med. 1994;39:155–162. [PubMed] [Google Scholar]
- 6.Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. Lancet. 2010;376:717–729. doi: 10.1016/S0140-6736(10)60280-2. [DOI] [PubMed] [Google Scholar]
- 7.Sebire NJ, Foskett M, Fisher RA, Rees H, Seckl M, Newlands E. Risk of partial and complete hydatidiform molar pregnancy in relation to maternal age. BJOG. 2002;109:99–102. doi: 10.1111/j.1471-0528.2002.t01-1-01037.x. [DOI] [PubMed] [Google Scholar]
- 8.Savage P, Williams J, Wong SL. The demographics of molar pregnancies in England and Wales from 2000–2009. J Reprod Med. 2010;55:341–345. [PubMed] [Google Scholar]
- 9.Sebire NJ, Seckl MJ. Gestational trophoblastic disease: current management of hydatidiform mole. BMJ. 2008;337:a1193. doi: 10.1136/bmj.a1193. [DOI] [PubMed] [Google Scholar]
- 10.Fowler DJ, Lindsay I, Seckl MJ, Sebire NJ. Routine pre-evacuation ultrasound diagnosis of hydatidiform mole: experience of more than 1000 cases from a regional referral center. Ultrasound Obstet Gynecol. 2006;27:56–60. doi: 10.1002/uog.2592. [DOI] [PubMed] [Google Scholar]
- 11.Bagshawe KD, Dent J, Webb J. Hydatidiform mole in England and Wales 1973–83. Lancet. 1986;328:673. doi: 10.1016/s0140-6736(86)90179-0. [DOI] [PubMed] [Google Scholar]
- 12.Seckl MJ, Fisher RA, Salerno GA. Choriocarcinoma and partial hydatidiform moles. Lancet. 2000;356:36–39. doi: 10.1016/S0140-6736(00)02432-6. [DOI] [PubMed] [Google Scholar]
- 13.Hancock BW, Nazir K, Everard JE. Persistent gestational trophoblastic neoplasia after partial hydatidiform mole incidence and outcome. J Reprod Med. 2006;51:764–766. [PubMed] [Google Scholar]
- 14.Kohorn EI. The new FIGO 2000 staging and risk factor scoring system for gestational trophoblastic disease: description and critical assessment. Int J Gynecol Cancer. 2001;11:73–77. doi: 10.1046/j.1525-1438.2001.011001073.x. [DOI] [PubMed] [Google Scholar]
- 15.Ngan HY, Bender H, Benedet JL, Jones H, Montruccoli GC, Pecorelli S. Gestational trophoblastic neoplasia, FIGO 2000 staging and classification. Int J Gynaecol Obstet. 2003;83(suppl 1):175–177. doi: 10.1016/s0020-7292(03)90120-2. [DOI] [PubMed] [Google Scholar]
- 16.Bagshawe KD. Risk and prognostic factors in trophoblastic neoplasia. Cancer. 1976;38:1373–1385. doi: 10.1002/1097-0142(197609)38:3<1373::aid-cncr2820380342>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.Powles T, Young A, Sammit A. The significance of the time interval between antecedent pregnancy and diagnosis of high-risk gestational trophoblastic tumours. Br J Cancer. 2006;95:1145–1147. doi: 10.1038/sj.bjc.6603416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmid P, Nagai Y, Agarwal R. Prognostic markers and long-term outcome of placental-site trophoblastic tumours: a retrospective observational study. Lancet. 2009;374:48–55. doi: 10.1016/S0140-6736(09)60618-8. [DOI] [PubMed] [Google Scholar]
- 19.Kim SJ, Bae SN, Kim JH. Effects of multiagent chemotherapy and independent risk factors in the treatment of high-risk GTT—25 years experiences of KRI-TRD. Int J Gynaecol Obstet. 1998;60(suppl 1):S85–S96. doi: 10.1016/S0020-7292(98)80010-6. [DOI] [PubMed] [Google Scholar]
- 20.Sebire NJ, Foskett M, Paradinas FJ. Outcome of twin pregnancies with complete hydatidiform mole and healthy co-twin. Lancet. 2002;359:2165–2166. doi: 10.1016/S0140-6736(02)09085-2. [DOI] [PubMed] [Google Scholar]
- 21.Seckl MJ, Dhillon T, Dancey G. Increased gestational age at evacuation of a complete hydatidiform mole: does it correlate with increased risk of requiring chemotherapy? J Reprod Med. 2004;49:527–530. [PubMed] [Google Scholar]
- 22.Gillespie AM, Kumar S, Hancock BW. Treatment of persistent trophoblastic disease later than 6 months after diagnosis of molar pregnancy. Br J Cancer. 2000;82:1393–1395. doi: 10.1054/bjoc.1999.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey RA, Mitchell HD, Stenman UH. Differences in total human chorionic gonadotropin immunoassay analytical specificity and ability to measure human chorionic gonadotropin in gestational trophoblastic disease and germ cell tumors. J Reprod Med. 2010;55:285–295. [PubMed] [Google Scholar]
- 24.Sebire NJ, Foskett M, Short D. Shortened duration of human chorionic gonadotropin surveillance following complete or partial hydatidiform mole: evidence for revised protocol of a UK regional trophoblastic disease unit. BJOG. 2007;114:760–762. doi: 10.1111/j.1471-0528.2007.01320.x. [DOI] [PubMed] [Google Scholar]
- 25.McNeish IA, Strickland S, Holden L. Low risk persistent gestational trophoblastic disease: outcome following initial treatment with low-dose methotrexate and folinic acid, 1992–2000. J Clin Oncol. 2002;20:1838–1844. doi: 10.1200/JCO.2002.07.166. [DOI] [PubMed] [Google Scholar]
- 26.Stone M, Dent J, Kardana A, Bagshawe KD. Relationship of oral contraceptive to development of trophoblastic tumour after evacuation of hydatidiform mole. BJOG. 1976;86:913–916. doi: 10.1111/j.1471-0528.1976.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 27.FIGO Oncology Committee FIGO staging for gestational trophoblastic neoplasia 2000. Int J Gynaecol Obstet. 2002;77:285–287. doi: 10.1016/s0020-7292(02)00063-2. [DOI] [PubMed] [Google Scholar]
- 28.Alazzam M, Tidy J, Hancock BW, Osborne R. First line chemotherapy in low risk gestational trophoblastic neoplasia. Cochrane Database Syst Rev. 2009;1:CD007102. doi: 10.1002/14651858.CD007102.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Bower M, Newlands ES, Holden L. EMA/CO for high-risk gestational trophoblastic tumours: results from a cohort of 272 patients. J Clin Oncol. 1997;15:2636–2643. doi: 10.1200/JCO.1997.15.7.2636. [DOI] [PubMed] [Google Scholar]
- 30.Lurain JR, Nejad B. Secondary chemotherapy for high-risk gestational trophoblastic neoplasia. Gynecol Oncol. 2005;97:618–623. doi: 10.1016/j.ygyno.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Rustin GJS, Newlands ES, Lutz J-M. Combination but not single agent methotrexate chemotherapy for gestational trophoblastic tumours (GTT) increases the incidence of second tumours. J Clin Oncol. 1996;14:2769–2773. doi: 10.1200/JCO.1996.14.10.2769. [DOI] [PubMed] [Google Scholar]
- 32.Cole LA, Butler SA, Khanlian SA. Gestational trophoblastic diseases: 2. Hyperglycosylated hCG as a reliable marker of active neoplasia. Gynecol Oncol. 2006;102:151–159. doi: 10.1016/j.ygyno.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 33.Palmieri C, Dhillon T, Fisher RA. Management and outcome of healthy women with a persistently elevated beta-hCG. Gynecol Oncol. 2007;106:35–43. doi: 10.1016/j.ygyno.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 34.Palmieri C, Fisher RA, Sebire NJ. Placental site trophoblastic tumour arising from a partial hydatidiform mole. Lancet. 2005;366:688. doi: 10.1016/S0140-6736(05)67143-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


