Summary
Background
Public objection to autopsy has led to a search for minimally invasive alternatives. Imaging has potential, but its accuracy is unknown. We aimed to identify the accuracy of post-mortem CT and MRI compared with full autopsy in a large series of adult deaths.
Methods
This study was undertaken at two UK centres in Manchester and Oxford between April, 2006, and November, 2008. We used whole-body CT and MRI followed by full autopsy to investigate a series of adult deaths that were reported to the coroner. CT and MRI scans were reported independently, each by two radiologists who were masked to the autopsy findings. All four radiologists then produced a consensus report based on both techniques, recorded their confidence in cause of death, and identified whether autopsy was needed.
Findings
We assessed 182 unselected cases. The major discrepancy rate between cause of death identified by radiology and autopsy was 32% (95% CI 26–40) for CT, 43% (36–50) for MRI, and 30% (24–37) for the consensus radiology report; 10% (3–17) lower for CT than for MRI. Radiologists indicated that autopsy was not needed in 62 (34%; 95% CI 28–41) of 182 cases for CT reports, 76 (42%; 35–49) of 182 cases for MRI reports, and 88 (48%; 41–56) of 182 cases for consensus reports. Of these cases, the major discrepancy rate compared with autopsy was 16% (95% CI 9–27), 21% (13–32), and 16% (10–25), respectively, which is significantly lower (p<0·0001) than for cases with no definite cause of death. The most common imaging errors in identification of cause of death were ischaemic heart disease (n=27), pulmonary embolism (11), pneumonia (13), and intra-abdominal lesions (16).
Interpretation
We found that, compared with traditional autopsy, CT was a more accurate imaging technique than MRI for providing a cause of death. The error rate when radiologists provided a confident cause of death was similar to that for clinical death certificates, and could therefore be acceptable for medicolegal purposes. However, common causes of sudden death are frequently missed on CT and MRI, and, unless these weaknesses are addressed, systematic errors in mortality statistics would result if imaging were to replace conventional autopsy.
Funding
Policy Research Programme, Department of Health, UK.
Introduction
Traditional autopsy has changed little in the past century, consisting of external examination and evisceration, dissection of the major organs with identification of macroscopic pathologies and injuries, and histopathology if needed. In the UK, concerns exist about the large number of autopsies done (22% of deaths), and their adequacy. The reductions in consent for hospital autopsies, which might partly indicate clinical disinterest,1 have not been accompanied by a fall in the number of medicolegal autopsies. A review of coroners' services2 questioned the justification of such high numbers of autopsies, and a national audit criticised the number of poor and inadequate autopsy reports.3 Longstanding public objection to dissection of cadavers re-emerged in the UK as a major issue after organ retention scandals in the late 1990s. Some groups—notably Jewish and Muslim communities—have religious objections to autopsy,4 and demand for a minimally-invasive alternative has increased. This demand has been acknowledged in the Coroners and Justice Act 2009.5
A post-mortem MRI service for selected non-suspicious deaths was introduced in Manchester, UK, in the 1990s, in response to demand from the Jewish community and, subsequently, by the much larger Muslim community in the northwest of England. Radiologists provide a cause of death that is accepted by the Coroner with no autopsy in 90% of cases.6 Despite this application of post-mortem MRI, few studies have investigated the accuracy of imaging in the diagnosis of the cause of adult deaths. Findings from a small study7 of ten cases, in which post-mortem MRI was followed by full autopsy, showed important weaknesses of imaging—notably, an inability to detect arterial occlusions and to differentiate between pulmonary oedema and pneumonia. Weustink and colleagues8 reported similar findings in a sample of 30 adult deaths. In 2006, the Department of Health commissioned two post-mortem imaging studies, one in adults and one in neonates and children. We report the findings of the adult study.
We aimed to assess: the accuracy of post-mortem imaging in diagnosis of cause of death in adults; whether radiologists can accurately identify which cases might be diagnosed with post-mortem imaging and therefore would not need full autopsy; the relative accuracy of CT and MRI in detecting post-mortem pathologies and cause of death; interobserver variation in radiological cause of death; and whether accuracy is improved by use of specialist cardiac and neuroradiologists.
Methods
Study design
All deaths were reported to the Oxford or Manchester Coroners between April 4, 2006, and November 26, 2008. We selected for inclusion the first case referred each study day, to ensure no selection bias; timing of case referral to the coroner's office is random. We predetermined study days according to availability of radiology and pathology staff. Exclusion criteria were failure to obtain consent for imaging and severe obesity (patients weighing more than 100 kg). Cases were otherwise unselected, which ensured a representative coronial casemix. The study received ethics approval (Central Office for Research Ethics Committee reference 04/Q1604/56).
Procedures
Radiologists did post-mortem MRI and CT out-of-hours (before 0700 h or after 1800 h) in the departments of radiology at the Churchill Hospital, Oxford, UK, and at Manchester Royal Infirmary. Pathologists did full autopsy after imaging. The workload and casemix at both centres is typical of large city mortuaries in the UK. For cases used for analysis we provided two experienced general cross-sectional radiologists with either CT or MRI scans for each case alternately, together with the clinical history and circumstances of death from the coroner's office. The radiologists, as part of their daily workload, routinely report both body and neuroradiological CT and MRI. The two Manchester-based radiologists had substantial experience of reporting post-mortem MRI, but no experience of post-mortem CT. The two Oxford-based radiologists had no previous experience of post-mortem scanning, which would be typical of a general radiologist at this time. Each radiologist independently completed a reporting proforma. The two radiologists receiving scans from the same imaging modality then produced a consensus report, resulting in independent consensus CT and MRI reports for each case. All four radiologists then reviewed both modalities and reached a consensus about the diagnoses. This report included a formulation of cause of death, an indication of radiological confidence (definite, probable, possible, or unascertained), and whether, in a routine service, autopsy would be needed.
Independent of the general radiology reports, brain imaging was reported by two specialist neuroradiologists who described the pathologies, but did not review the remainder of the imaging to formulate a cause of death. For the first 100 cases, two specialist cardiac radiologists independently reviewed the CT or MRI images for each case alternately (including images of the rest of the body), and then produced a consensus report, including cause of death, based on both techniques. This process resulted in 11 reports for each case: four independent general radiologist reports (two CT and two MRI), consensus MRI and CT reports, a consensus general radiologist report (MRI and CT), three specialist cardiac reports (CT, MRI, and consensus), and one neuroradiology report. Once all reports were complete, we reviewed each case at meetings of the study team, and compared the imaging with pathologists' autopsy findings, which we used as the reference standard. We repeated this process 13 times in batches of ten to 20 cases. Meetings were held between January, 2007, and November, 2010.
Bodies were imaged in sealed body bags in the supine position, with arms adjacent to the body. CT images were acquired on an eight-slice multidetector scanner in Oxford, and on a 16-slice multidetector scanner in Manchester. At both centres, contiguous 3·75 mm axial images were obtained through the brain at 120 kV and variable mAs, with a window level of 40 Hounsfield units (HU) and a width of 80 HU. Volumetric scans were obtained from the vertex to the symphysis pubis at 120 kV with variable mAs, a pitch of 1·675:1, and 0·625 mm collimation. Images were reconstructed with a soft tissue algorithm to provide 5 mm and 1·25 mm slices, and viewed on standard window settings for soft tissue, lung, and bone. See webvideo for an example of a post-mortem CT scan. MRI sequences were sagittal T1-weighted, coronal dual echo and axial fast short tau inversion recovery (STIR) images of the brain, and axial T1-weighted and T2 fast spin echo and coronal T1 and STIR images of the neck, chest, abdomen, and pelvis. After we reviewed the first 30 patients, we replaced the coronal dual echo sequence of the brain with a coronal fluid attenuated inversion recovery (FLAIR) sequence, and obtained a T2-weighted short-axis oblique axial sequence through the heart. Full autopsies were done within 12 h of imaging by six senior consultant pathologists. Specimens were obtained for histology and toxicology assessments as needed.
We present analysis of cause of death data only; the detection of incidental lesions not contributing to death is beyond the functions of the coroner's autopsy. Radiologists and pathologists formulated cause of death according to Office for National Statistics guidance.9 We subclassified radiology and autopsy causes of death according to type of pathology and organ involved. We classified discrepancies between radiology and autopsy cause of death as none, minor, or major, with major indicating involvement of different pathologies or organs. Minor discrepancies were variations within a pathology or organ system that would be of little relevance for the coroner's investigation or for national mortality statistics (eg, ischaemic heart disease vs acute myocardial infarction). Major discrepancies (eg, pulmonary embolism vs myocardial infarction) would affect national mortality statistics, but would not necessarily result in a different verdict by the coroner. We used this definition of major discrepancy rather than one that would change the coroner's verdict because no legal definition exists of natural and unnatural cause of death. The verdict for a specific cause of death is open to interpretation, and there is much variation between different coroners.10
Statistical analysis
We used CIA software (Trevor Bryant version 2.1.2)11 to calculate 95% CIs for paired and unpaired proportions with the Newcombe method,12 and for single proportions with the Wilson method.13 We used Stata (version 11.0) for other statistical methods. We used McNemar's test to calculate p values for paired proportions, and χ2 tests for p values of unpaired proportions. Because no fixed categories are available for reasons of death, calculation of κ values was not appropriate for analysis of interobserver variation between independent radiology reports.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
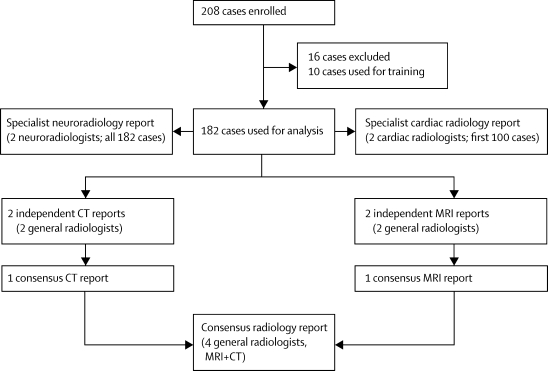
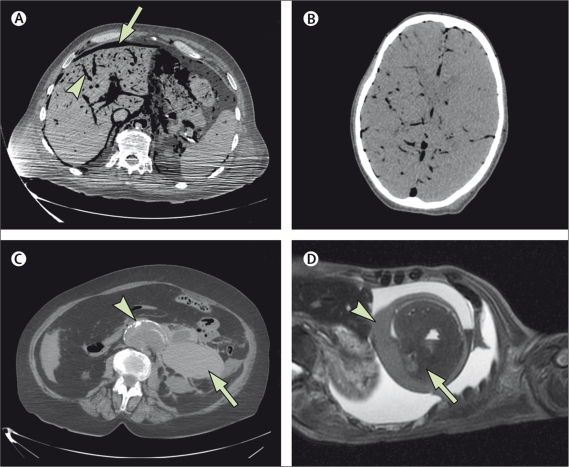
Figure 1 shows the protocol for radiology reporting. We enrolled 208 cases, of which we excluded 15 because of an incomplete imaging procedure (breakdown of MRI or CT scanners, incomplete dataset, or disk failure), and one because the body had been embalmed before examination. We used the first ten cases for training purposes, to familiarise the radiologists with post-mortem changes (figure 2), leaving 182 cases for analysis.
Figure 1.
Protocol for radiology reporting
Figure 2.
Post-mortem changes and pathologies
(A) Axial CT image through the upper abdomen showing extensive intravascular gas (arrowhead), in keeping with decomposition. Free intraperitoneal gas (arrow) is due to decomposition in this patient, but creates difficulty for exclusion of a perforated intra-abdominal viscus. (B) Axial CT image through the brain showing extensive intracranial gas due to decomposition. Differentiation between grey and white matter is poor. (C) Axial CT image showing rupture of an abdominal aortic aneurysm (arrowhead) with extensive retroperitoneal haemorrhage on the left (arrow). (D) Oblique axial (short-axis view) T2-weighted MRI image showing a haemopericardium (arrowhead) due to rupture of a myocardial infarct (arrow).
The most common indication for coronial referral is sudden death of unknown cause (table 1); as such, the most common cause of death was cardiovascular disease (table 2). The major discrepancy rate compared with autopsy was significantly higher (p=0·0046) for MRI than for CT and consensus reports (table 3). We noted 10% (95% CI 3–17) more major discrepancies for MRI than for CT, and 13% (6–19) more for MRI than for consensus reports (table 3). Similarly, in cases with a definite radiological cause of death, the major discrepancy rate compared with autopsy was higher for MRI than for CT and consensus reports (table 3); however, because of the small number of cases, this difference was not significant (MRI vs CT p=0·542; MRI vs consensus reports p=0·481). Analysis of interobserver variation showed that, with exclusion of cases in which one or both radiologist gave the cause of death as unascertained, there was a major discrepancy between the radiologists' cause of death by CT and MRI in about a quarter of cases (table 4).
Table 1.
Circumstances of death and indications for coronial referral
| n (%) | |
|---|---|
| Found dead in community, unknown cause | 99 (54%) |
| Witnessed sudden death in community, unknown cause | 39 (21%) |
| Died in hospital, unknown case | 25 (14%) |
| Postoperative | 11 (6%) |
| Post-trauma | 4 (2%) |
| Suspected industrial disease | 2 (1%) |
| Suspected drug-related death | 2 (1%) |
| Total | 182 |
Table 2.
Autopsy causes of death by type of pathology and organs involved
| n (%) | ||
|---|---|---|
| Type of pathology | ||
| Vascular (eg, thrombosis/infarct/atheroma) | 142 (51%) | |
| Infection | 33 (12%) | |
| Inflammation/fibrosis | 25 (9%) | |
| Anatomical (eg, obstruction/perforation) | 22 (8%) | |
| Biochemical/metabolic | 19 (7%) | |
| Neoplasm | 19 (7%) | |
| Toxic | 7 (3%) | |
| Trauma* | 5 (2%) | |
| Asphyxia | 4 (1%) | |
| Total | 276 | |
| Organ/system involved | ||
| Heart and coronary arteries | 113 (40%) | |
| Respiratory (larynx, airways, lungs) | 58 (20%) | |
| Gastrointestinal | 26 (9%) | |
| CNS | 19 (7%) | |
| Aorta and peripheral arteries | 16 (6%) | |
| Hepatobiliary and pancreatic | 14 (5%) | |
| Endocrine | 11 (4%) | |
| Pulmonary arteries | 10 (4%) | |
| Urinary | 5 (2%) | |
| Multisystem disorder | 4 (1%) | |
| Musculoskeletal | 4 (1%) | |
| Lymphoreticular | 2 (<1%) | |
| Oropharynx | 1 (<1%) | |
| Total | 283 | |
Totals exceed 182 because several pathologies were frequently included in the medical certificate of cause of death.
Three head injuries, one fractured neck of femur, one multiple injuries.
Table 3.
Major discrepancy rate between autopsy and radiology cause of death
| CT | MRI | Consensus CT and MRI | |
|---|---|---|---|
| Major discrepancy rate with autopsy cause of death, all cases (%) | 32% (26–40) | 43% (36–50) | 30% (24–37) |
| Proportion of cases with definite radiological cause of death, no autopsy needed (%) | 34% (28–41) | 42% (35–49) | 48% (41–56) |
| Major discrepancy rate with autopsy when radiologist confidence is definite (%) | 16% (9–27) | 21% (13–32) | 16% (10–25) |
| Major discrepancy rate with autopsy when radiologist confidence is not definite (%) | 41% (33–50) | 59% (49–67) | 44% (34–54) |
Data are % (95% CI) or number (%, 95% CI). Percentages are rounded to nearest whole number.
Table 4.
Comparison of the two independent reports for MRI and CT
| CT | MRI | |
|---|---|---|
| Cases with two complete independent CT and MRI reports | 174/182 (96%) | 172/182 (96%) |
| Cases in which both radiologists gave the cause of death as unascertained (% of total) | 14/174 (8%) | 12/172 (7%) |
| Number in which only one radiologist gave the cause of death as unascertained (% of total) | 29/174 (16%) | 35/172 (20%) |
| Major discrepancy rate in cause of death between the two radiology reports (% of cases in which both radiologists provided a cause of death) | 34/131 (26%) | 27/124 (22%) |
Data are n/N (%).
Levels of confidence for the consensus radiology reports were definite for 88 (48%) of 182 cases, probable for 52 (29%) cases, possible for 29 (16%) cases, and unascertained for 13 (7%) cases. The proportion of cases in which a definite cause of death was provided was 14% (95% CI 6–21) higher for consensus reports than for CT reports (p=0·0005), and 6% (0·5–13) higher for consensus reports than for MRI reports (p=0·05; table 3). The proportion of definite causes of death did not differ significantly between CT and MRI reports (8% difference, 95% CI 1–16, p=0·124; table 3).
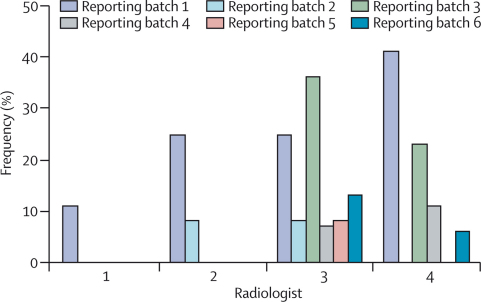
Major discrepancies between radiological and autopsy cause of death were reduced when radiologists indicated that autopsy was not necessary (table 3). When radiologists' confidence was definite, the proportion of cases with a major discrepancy compared with autopsy was 25% less than the non-definite cases for CT (95% CI 11–37; p=0·0006), 37% less for MRI (23–49; p<0·0001), and 28% less for consensus reports (15–44; p<0·0001; table 3). The major discrepancy rate between consensus radiological and autopsy causes of death did not improve with increased experience of comparison between radiology and autopsy (data not shown). All radiologists showed improvement in their formulation of cause of death, although the frequency of major formulation errors, such as sequence errors and unsupported modes of death (figure 3), and rate of improvement (data not shown) differed between radiologists.
Figure 3.
Frequency of formulation errors in the general radiologist causes of death for the first six batches
Major formulation errors are either unsupported modes of death or sequence errors for which no logical causal relation exists between parts Ia, b, and c of the medical certificate of cause of death.
General radiologists requested a neuroradiology report in six of 182 cases. In four of these cases, a CNS disorder was included in the autopsy cause of death. Only one case had a major discrepancy between autopsy and general radiology cause of death; a case of bacterial meningitis missed on imaging in which the neuroradiologists also reported the brain as normal. In a further 15 cases, CNS disorders were included in the cause of death; the most common pathologies were cerebral infarction (four cases), head injury with intracranial haemorrhage (three), and subarachnoid haemorrhage (three). In none would the neuroradiologists' report have altered the general radiologists' cause of death.
We recorded a major discrepancy between consensus cardiac radiology and autopsy causes of death in 30 (31%, 95% CI 23–41) of 97 cases, compared with 31 (32%, 24–42) cases for the general radiologists. Cardiac radiologists provided a definite cause of death in 36 (38%, 30–49) of 97 cases and, in this group, five (14%, 6–29) of 36 showed a major discrepancy with autopsy cause of death.
Imaging was sensitive in the detection of internal haemorrhage, with the consensus radiology reports correctly identifying all ten cases of haemopericardium (six ruptured myocardial infarct, four ruptured aortic aneurysm and dissection), six ruptured aortic aneurysms, and four intracranial haemorrhage (figure 2). In two cases of haemopericardium, the radiologists incorrectly attributed haemorrhage to a ruptured aortic aneurysm or dissection rather than myocardial infarct, and two of three cases of subarachnoid haemorrhage were identified on CT but not MRI. In ten cases, autopsy attributed death to malignancy (three bronchial carcinomas, two pancreatic carcinomas, two colonic carcinoma, one mesothelioma, one carcinoma of gall bladder, one pharyngeal carcinoma). The colonic carcinomas were not identified on imaging. Carcinoma of gall bladder was misidentified as duodenal carcinoma; the pancreatic tumours were also misidentified, one with pulmonary metastases diagnosed as pulmonary malignancy, the other with hepatic metastases as hepatic abscesses. The other malignancies were correctly diagnosed on consensus imaging, although one bronchial carcinoma was correctly reported with MRI but not with CT.
The most common major discrepancies were in the diagnosis of pulmonary embolism, coronary heart disease, pneumonia, and intestinal infarction (table 5). Of 97 cases reported by the cardiac radiologists, death was attributed to pulmonary embolism at autopsy in seven. None was diagnosed by the general radiologists, but three (43%) were correctly identified by the cardiac radiologists. However, death was misattributed to pulmonary embolism by the cardiac radiologists in five cases and by the general radiologists in one case. Radiologists frequently interpreted pneumonic consolidation as pulmonary oedema secondary to cardiac failure (table 5). Other diagnoses missed on imaging were cases of pancreatitis, perforated duodenal ulcer, acute asthma, and overwhelming sepsis with adult respiratory distress syndrome (data not shown). One case of cerebellar infarction reported with imaging was not detected at autopsy. Autopsy attributed death to ileal infarction due to emboli from the heart because of cardiac amyloidosis. Neither disorder was diagnosed with imaging.
Table 5.
Most common sources of major discrepancy between autopsy and consensus radiology cause of death
| Missed on imaging | Overattributed on imaging | |
|---|---|---|
| Coronary heart disease | 12/86 (14%) | 15/95 (16%) |
| Pulmonary embolism | 10/10 (100%) | 1/1 (100%) |
| Bronchopneumonia | 9/28 (32%) | 4/28 (14%) |
| Intestinal infarction | 4/6 (67%) | 1/3 (33%) |
Data are n/N (%). Denominators for the left-hand column are total diagnoses of these disorders in the autopsy causes of death. Denominators in the right-hand column are the total diagnoses of these disorders in the consensus radiology causes of death.
Discussion
Our results show that, in a series of unselected deaths referred to the coroner, a major discrepancy exists between autopsy and imaging causes of death in 30% of cases. Overall, CT was more accurate than MRI in identification of cause of death; the major discrepancy rate compared with autopsy was significantly higher for MRI than for CT and consensus reports.
Published work7,8,14–16 comparing autopsy and imaging comprise mostly anecdotal reports or small case series, and the accuracy of imaging in detection of the wide range of pathological changes seen in coronial practice has not been examined systematically. The most common reason for coronial referral is sudden death of unknown cause. In this respect, our casemix is typical, with coronary heart disease being the most common autopsy diagnosis. In living patients, detection of vascular events by cross-sectional imaging necessitates use of intravenous contrast administration and, therefore, that the most frequent major errors were failure to detect arterial occlusion and infarcts is not surprising. Post-mortem angiographic techniques are being developed and validated17–19 to overcome this weakness. Other deficiencies of conventional cross-sectional imaging shown in this study could be overcome by combination of imaging modalities with minimally-invasive techniques, such as needle biopsy.7,20,21 In a report22 of 20 non-traumatic deaths, investigators recorded a good correlation between CT combined with angiography and needle biopsy and subsequent full autopsy; however, most causes of death provided in that study were modes of death that would not be accepted in the UK legal system.
Post-mortem changes cause specific difficulties for imaging diagnosis. Distinction of intra-abdominal gas due to putrefaction from antemortem perforation of the stomach or bowel can be difficult, which resulted in a missed perforated duodenal ulcer in our study. Similarly, the distinction of post-mortem clot from antemortem thrombus has not proved possible with cross-sectional imaging; a missed diagnosis of pulmonary embolism was one of the most common errors in our study. After use of the first ten cases for training, comparison of the batches in which imaging was reported and reviewed showed an improvement in cause of death formulation with increasing radiologist experience, but no reduction in the major discrepancy rate between radiology and autopsy cause of death.
Panel. Research in context.
Systematic review
We searched Medline from Jan 1, 1980, to Aug 1, 2011, for reviews and primary studies of post-mortem imaging. We excluded studies of fetal and paediatric autopsies and imaging of removed organs. Our search terms “virtopsy”, “virtual autopsy”, “minimally invasive autopsy”, “post mortem angiography”, “post mortem” with “imaging” or “CT” or “MRI”, identified 60 citations, which were mostly forensic case reports. Five small series7,8,14–16 of non-violent adult deaths compared the diagnostic accuracy of post-mortem imaging to traditional autopsy. Only one of these five studies8—the largest one with 30 patients—compared CT with MRI, but full autopsy was done after imaging in only four cases. No study compared accuracy of CT cause of death with that based on MRI.
Interpretation
Our findings show that compared with autopsy, CT is more accurate than MRI in determination of cause of death in adults, and that a major discrepancy exists in the cause of death provided by two radiologists reporting independently in a quarter of cases. When radiologists are confident that the cause of death on imaging is definite, the discrepancy rate between the radiological and autopsy diagnoses is lower and might be acceptable from a medicolegal point of view. The radiologists' ability to accurately identify cases for which their diagnosis is correct is essential for the safe introduction of a minimally invasive autopsy service.
We used full autopsy as the gold standard for diagnosis, but this assumption might not be valid. Imaging could be better than autopsy in detection of some fractures, intracranial pathologies, and pneumothorax. We recorded one case in which a cerebellar infarct evident with imaging was missed at autopsy. Furthermore, although all autopsies were complete, including examination of the cranial cavity, this scenario is not the case in routine coronial practice. The standard of autopsies is highly variable. In a UK-wide audit, one in four autopsy reports were regarded as poor or inadequate.2 The post-mortem report can be audited, but the adequacy of the procedure cannot. Tissue is retained for histology in only a few autopsies, and in even fewer is there a photographic record of the macroscopic pathologies; in most cases, no method is available to review findings. However, a permanent record is available for imaging; therefore, this technique could be a way to audit autopsy practice. The major discrepancy rate in cause of death provided by radiologists reporting the same technique was 22–26%. The destructive nature of traditional autopsy means that reproducibility of diagnosis of pathological changes is difficult to assess. However, autopsy cannot be assumed to be better than imaging; the conclusions of a pathologist doing a second autopsy frequently differ to those of the first.
Which imaging technique should be used? Forensic practices use CT because it provides better spatial resolution than MRI and is effective for showing fractures and haemorrhages. Non-forensic and paediatric practices have used MRI because it provides greater detail of soft tissues than does CT. Our findings show that both techniques have strengths and weaknesses—eg, CT provides visualisation of coronary artery calcification that is not apparent with MRI, whereas acute myocardial infarcts might be seen with MRI but not with CT. Use of both techniques as a routine would have resource implications and evidence to support this use is scarce. We noted only two cases in this study in which a major discrepancy existed with autopsy cause of death for both CT and MRI reports, but not for the consensus radiology report.
CT has important practical advantages, being more widely available, less expensive, and quicker to do than MRI. CT could also be combined with angiography, increasing the accuracy of detection of vascular pathologies. If imaging were to be used as a pre-autopsy screening technique, the overall diagnostic accuracy is less important than radiologists being able to identify those cases in which imaging can correctly diagnose the cause of death. In this study, radiologists indicated that autopsy was unnecessary to confirm the cause of death in almost half of cases. The major discrepancy rate between consensus imaging and autopsy in the group for whom autopsy was not necessary to confirm cause of death could be acceptable for medicolegal purposes because it is similar to the error rate of clinical death certificates on which most registered causes of deaths are based.23 However, because some disorders, such as pulmonary emboli, could not be diagnosed, replacement of autopsy with imaging would result in systematic errors in mortality statistics. If used as a pre-autopsy screen, imaging might avoid unnecessary autopsies (eg, for ruptured aortic aneurysm), identify lesions difficult to diagnose by dissection, and help to guide dissection by identification of pathologies needing further investigation. Therefore, imaging could reduce the number of invasive autopsies at the same time as improving their quality.
The main purpose of a coroner's autopsy is to exclude an unnatural death, which could be achieved without diagnosis of an accurate natural cause. Imaging alone cannot diagnose biochemical and toxicological causes, and is poor in the identification of asphyxial deaths. A minimally invasive autopsy service should include careful external examination of the body by a pathologist to identify superficial signs of injury not detected on imaging. We advocate a flexible multidisciplinary service in which the coroner consults with the pathologist and radiologist to select the most appropriate techniques to investigate each death.
Our findings identify important shortcomings of cross-sectional imaging in the diagnosis of cause of death in adults and provide the evidence needed to refine imaging techniques and enable them to be safely introduced into autopsy services. Practical and clinical governance considerations remain. Where will imaging be done? If clinical facilities are used, providers should ensure that services for living patients are not disrupted. Service providers will need training and assessment in the interpretation of post-mortem imaging. Cost implications are also a concern; MRI in particular is more expensive than is traditional autopsy. Further development of post-mortem imaging is needed and this development must be based on careful consideration of comparisons between radiology and autopsy.
Contributors
ISDR designed the study, assisted in data collection and analysis, and drafted and edited the manuscript. REB, EWB, SHL, JNH, AJ, TP, CP, CR, and ZCT assisted in data collection and edited the manuscript. SM assisted in data analysis and edited the manuscript.
Conflicts of interest
We declare that we have no conflicts of interest.
Acknowledgements
UK Department of Health funded this study. We thank Raymond FT McMahon, Richard Fitzmaurice, Lorna J McWilliam (Manchester Royal Infirmary), and Sanjiv Manek (John Radcliffe Hospital, Oxford) for their assistance undertaking autopsies.
Web Extra Material
Post mortem CT scan (axial and coronal views) of an 83-year-old man who was found collapsed. He had a history of diabetes mellitus and chronic lymphocytic leukaemia. Autopsy showed ischaemic heart disease and head injury consisting of a right temporal fracture with associated traumatic subarachnoid haemorrhage and cerebral contusions. Additionally, widespread lymphadenopathy was present secondary to the leukaemia.
References
- 1.Tsitsikas DA, Brothwell M, Aleong JC, Lister AT. The attitudes of relatives to autopsy: a misconception. J Clin Pathol. 2011;64:412–414. doi: 10.1136/jcp.2010.086645. [DOI] [PubMed] [Google Scholar]
- 2.Luce T, Hodder E, McAuley D. Death certification and investigation in England, Wales and Northern Ireland. The report of a Fundamental Review 2003. June, 2003. http://www.archive2.official-documents.co.uk/document/cm58/5831/5831.pdf (accessed July 1, 2003).
- 3.The Coroner's autopsy: do we deserve better? A report of the National Confidential Enquiry into Patient Outcome and Death. 2006. http://www.ncepod.org.uk/2006Report/Downloads/Coronial Autopsy Report 2006.pdf
- 4.Geller SA. Religious attitudes and the autopsy. Arch Pathol Lab Med. 1984;108:494–496. [PubMed] [Google Scholar]
- 5.Parliament of the UK Coroners and Justice Act 2009. 2009. http://www.legislation.gov.uk/ukpga/2009/25/pdfs/ukpga_20090025_en.pdf (accessed March 26, 2010).
- 6.Bisset RAL, Thomas NB, Turnbull IW, Lee S. Postmortem examinations using magnetic resonance imaging: four year review of a working service. BMJ. 2002;324:1423–1424. doi: 10.1136/bmj.324.7351.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts ISD, Benbow EW, Bisset R. Accuracy of magnetic resonance imaging in determining cause of sudden death in adults: comparison with conventional autopsy. Histopathology. 2003;42:424–430. doi: 10.1046/j.1365-2559.2003.01614.x. [DOI] [PubMed] [Google Scholar]
- 8.Weustink AC, Hunink MGM, van Dijke CF, Renken NS, Krestin GP, Oosterhuis JW. Minimally invasive autopsy: an alternative to conventional autopsy? Radiology. 2009;50:897–904. doi: 10.1148/radiol.2503080421. [DOI] [PubMed] [Google Scholar]
- 9.Office for National Statistics' Death Certification Advisory Group Guidance for doctors certifying cause of death. April, 2005. http://www.nc-hi.com/pdf%20files/certifiers_guidance_v2_tcm69-21289.pdf (accessed Aug 1, 2005).
- 10.Roberts ISD, Gorodkin LM, Benbow EW. What is a natural cause of death? A survey of how Coroners in England and Wales approach borderline cases. J Clin Pathol. 2000;53:367–373. doi: 10.1136/jcp.53.5.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newcombe RG. Improved confidence intervals for the difference between binomial proportions based on paired data. Stat Med. 1998;17:2635–2650. [PubMed] [Google Scholar]
- 12.Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209–212. [Google Scholar]
- 13.Altman DG, Machin D, Bryant TN, Gardner MJ. Statistics with confidence. 2nd edn. BMJ books; Bristol: 2000. [Google Scholar]
- 14.Patriquin L, Kassarjian A, O'Brien M, Andry C, Eustace S. Postmortem whole-body magnetic resonance imaging as an adjunct to the autopsy: preliminary clinical experience. J Magn Reson Imaging. 2001;13:277–287. doi: 10.1002/1522-2586(200102)13:2<277::aid-jmri1040>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Ros PR, Li KC, Vo P, Baer H, Staab EV. Pre-autopsy magnetic resonance imaging: initial experience. Magn Reson Imaging. 1990;8:303–308. doi: 10.1016/0730-725x(90)90103-9. [DOI] [PubMed] [Google Scholar]
- 16.Thali MJ, Yen K, Schweitzer W. Virtopsy, a new imaging horizon in forensic pathology: virtual autopsy by postmortem multislice computed tomography (MSCT) and magnetic resonance imaging (MRI)–a feasibility study. J Forensic Sci. 2003;48:386–403. [PubMed] [Google Scholar]
- 17.Ross S, Spendlove D, Bolliger S. Postmortem whole-body CT angiography: evaluation of two contrast media solutions. AJR. 2008;190:1380–1389. doi: 10.2214/AJR.07.3082. [DOI] [PubMed] [Google Scholar]
- 18.Roberts ISD, Benamore RE, Peebles C, Roobottom C, Traill ZC. Diagnosis of coronary artery disease using minimally invasive autopsy: evaluation of a novel method of post-mortem coronary CT angiography. Clin Radiol. 2011;66:645–650. doi: 10.1016/j.crad.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Saunders SL, Morgan B, Raj V, Robinson CE, Rutty GN. Targeted post-mortem computed tomography cardiac angiography: proof of concept. Int J Legal Med. 2011;125:609–616. doi: 10.1007/s00414-011-0559-4. [DOI] [PubMed] [Google Scholar]
- 20.Huston BM, Malouf NN, Azar HA. Percutaneous needle autopsy sampling. Mod Pathol. 1996;9:1101–1107. [PubMed] [Google Scholar]
- 21.Foroudi F, Cheung K, Duflou J. A comparison of the needle biopsy post mortem with the conventional autopsy. Pathology. 1995;27:79–82. doi: 10.1080/00313029500169532. [DOI] [PubMed] [Google Scholar]
- 22.Bolliger SA, Filograna L, Spendlove D, Thali MJ, Dirnhofer S, Ross S. Postmortem imaging-guided biopsy as an adjuvant to minimally invasive autopsy with CT and postmortem angiography: a feasibility study. Am J Roentgenol. 2010;195:1051–1056. doi: 10.2214/AJR.10.4600. [DOI] [PubMed] [Google Scholar]
- 23.Roulson R, Benbow EW, Hasleton PS. Discrepancies between clinical and autopsy diagnosis and the value of post mortem histology; a meta-analysis and review. Histopathology. 2005;47:551–559. doi: 10.1111/j.1365-2559.2005.02243.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Post mortem CT scan (axial and coronal views) of an 83-year-old man who was found collapsed. He had a history of diabetes mellitus and chronic lymphocytic leukaemia. Autopsy showed ischaemic heart disease and head injury consisting of a right temporal fracture with associated traumatic subarachnoid haemorrhage and cerebral contusions. Additionally, widespread lymphadenopathy was present secondary to the leukaemia.