Summary
Innocuous touch of the skin is detected by distinct populations of low-threshold mechanoreceptors (LTMRs), which are classified as Aβ-, Aδ- and C-LTMRs. Here, we report genetic labeling of LTMR subtypes and visualization of their relative patterns of axonal endings in hairy skin and the spinal cord. We found that each of the three major hair follicle types of trunk hairy skin; guard, awl/auchene, and zigzag hairs, is innervated by a unique and invariant combination of LTMRs; thus, each hair follicle type is a functionally distinct mechanosensory end organ. Moreover, the central projections of Aβ-, Aδ- and C-LTMRs that innervate the same or adjacent hair follicles form narrow LTMR columns in the dorsal horn. These findings support a model of mechanosensation in which the activities of Aβ-, Aδ- and C-LTMRs are integrated within dorsal horn LTMR columns and processed into outputs that underlie the perception of myriad touch sensations.
Introduction
The first step leading to the perception of touch is activation of low-threshold mechanoreceptors (LTMRs) by mechanical stimuli that include indentation, vibration, or stretch of the skin, and movement or deflection of hair follicles. LTMRs are a diverse group of somatosensory neurons whose cell bodies reside within dorsal root ganglia (DRG) and cranial sensory ganglia. These pseudo-unipolar sensory neurons have one axonal branch that extends to the periphery and associates with a cutaneous mechanosensory end organ, and another branch that penetrates the spinal cord and forms synapses upon second order neurons in the dorsal horn (Rice and Albrecht, 2008). Some LTMRs also have a branch that ascends via the dorsal column to innervate second order neurons of the brainstem dorsal column nuclei (Giuffrida and Rustioni, 1992).
LTMRs are classified as Aβ, Aδ or C based on their action potential conduction velocities (Horch et al., 1977). C-LTMRs are unmyelinated and thus have the slowest conduction velocities, whereas Aδ-LTMRs and Aβ-LTMRs are lightly and heavily myelinated, exhibiting intermediate and rapid conduction velocities, respectively. LTMRs are also classified as slowly-, intermediately-, or rapidly-adapting (SA, IA, and RA-LTMRs) according to their rates of adaptation to sustained mechanical stimuli (Burgess et al., 1968; Johnson and Hsiao, 1992). They are further distinguished by the cutaneous end organs they innervate and their preferred stimuli (Iggo and Andres, 1982). Yet, despite more than 100 years of study, the molecular properties and unique functions of the different populations of LTMRs, the relative patterns of their peripheral and central connections, and thus the logic of LTMR circuit organization underlying the perception of touch remain unclear.
Visualization of LTMR circuits has been hampered by a lack of markers for individual LTMR subtypes, the high degree of complexity of the myriad axonal endings in the skin, and the long distance between LTMR endings in the skin and their connections in the spinal cord and brainstem. Here, we have undertaken a candidate gene approach in combination with an open-ended screen to identify genes that are uniquely expressed in each of the physiologically defined populations of LTMRs. This has allowed us to genetically label Aβ-, Aδ- and C-LTMR populations, both individually and in combination, enabling visualization of the relative patterns of organization of LTMR axonal endings in the skin and spinal cord. We focused our analysis on mouse hairy skin because it covers most of the body and receives rich innervation by several physiologically defined LTMR populations (Koltzenburg et al., 1997). Our findings reveal an exquisite organization of overlapping Aβ-, Aδ- and C-LTMR endings in hairy skin and a principal locus of Aβ-, Aδ- and C-LTMR integration and processing in the spinal cord dorsal horn.
Results
Genetic labeling of C-LTMRs and visualization of their cutaneous axonal endings
To gain an appreciation of the logic of LTMR circuit organization, we sought to identify unique molecular signatures of physiologically distinct LTMR classes and to exploit these features to design molecular-genetic strategies that enable visualization of their respective axonal endings in the skin and spinal cord. We first characterized the C-LTMRs, a large population of neurons implicated in the pleasurable, affective component of touch and injury-induced mechanical hypersensitivity (Olausson et al., 2010; Seal et al., 2009). Though they were identified over 70 years ago (Zotterman, 1939), the molecular properties, peripheral targets, and unique functions of C-LTMRs are unknown. We found that expression of tyrosine hydroxylase (TH), which catalyzes the production of L-DOPA from tyrosine in the catecholamine biosynthesis pathway, is a defining feature of C-LTMRs in adult DRGs. TH is expressed in a large population of small-diameter DRG neurons (Figure 1A-D; Figure S1A) (Brumovsky et al., 2006). These TH+ DRG neurons do not express NFH, a marker for sensory neurons with myelinated axons (Rice and Albrecht, 2008) (Figure 1A), nor do they express CGRP, TrkA or TrpV1, which are markers for peptidergic nociceptors (Molliver et al., 1997) (Figure 1B; Figure S2A and S2B). Rather, nearly all TH+ neurons express the non-peptidergic nociceptor markers cRet and Gfrα2 (Molliver et al., 1997) (Figure 1C; Figure S2I-K). However, surprisingly, the TH+ neurons do not bind the lectin IB4 (Figure 1D), nor do they express MrgprA1, MrgprA3, MrgprA4, MrgprB4, MrgprC11 or MrgprD (Figure S2C-H), all of which are expressed in non-peptidergic nociceptors (Dong et al., 2001; Molliver et al., 1997). Thus, the TH+ DRG neurons are a molecularly unique population of non-peptidergic, small-diameter sensory neurons. We next characterized the physiological properties of these neurons and their sensitivity to cutaneous stimulation using an ex vivo skin-nerve preparation (Woodbury et al., 2001; McIlwrath et al., 2007). Intracellular recordings were performed on 21 cutaneous C-fiber neurons from wild-type animals, followed by immunohistochemical analysis. Five of the cutaneous C-fiber neurons showed classically defined features of C-LTMRs (Bessou et al., 1971; Douglas et al., 1960; Iggo, 1960; Seal et al., 2009): slow conduction velocities (CV = 0.58 ± 0.02 m/sec), trains of spikes in response to application of light mechanical force (1-5 mN); intermediate adaptation to stationary stimuli; robust responses to rapid cooling but not warming of the skin (Figure 1E). All of the identified C-LTMRs stained positively for TH, as well as GFRα2, but they did not bind to IB4 (Figure 1F). The remaining cutaneous C-fiber neurons, which did not exhibit C-LTMR properties, lacked TH immunoreactivity (data not shown). Moreover, more than 80% of TH+ neurons express vGluT3 mRNA, which is expressed in C-LTMRs (Seal et al., 2009) (Figure S2L). Thus, we conclude that adult mouse TH+ DRG neurons are C-LTMRs.
Figure 1. The TH+ adult DRG neurons are C-LTMRs and form longitudinal lanceolate endings associated with zigzag and awl/auchene hair follicles.
(A-D) Double immunostaining of TH and NFH (A) (n=8), TH and CGRP (B) (n=6), TH and cRet (C) (n=4), and TH and IB4 (D) (n=7) on adult thoracic DRG sections. TH+ DRG neurons do not express NFH (<1%) or CGRP (<5%). The majority of TH+ neurons express cRet (>90%) but do not bind to the lectin IB4 (<1%).
(E-F) Intracellular recordings of TH+ DRG neurons were performed using the ex vivo skin nerve preparation. Small-diameter DRG neurons were randomly selected and recorded (n=21). The recorded neurons were then labeled with Neurobiotin, and co-stained with TH and GFRα2 or TH and IB4. Among the recorded neurons, all of those that exhibited classically defined features of C-LTMRs (n=5; See text) (E) are TH+, GFRα2+ and IB4- (F) (arrows).
(G) THCreER; Rosa26IAP mice were treated with 4-HT (1mg per day for three days, from P13 to P15, by oral gavage). Double immunostaining of AP and TH shows that a small number of TH+ DRG neurons (~10%) were labeled with AP (n=2).
(H-K) Whole-mount AP staining of thoracic DRGs and trunk hairy skin shows that DRG cell bodies (H) and their peripheral endings in the skin (I) are sparsely labeled. Individual axonal branches arborize and form longitudinal lanceolate endings (J and K) (n=26).
(L) The longitudinal lanceolate endings of the C-LTMRs are similarly labeled in THCreER;Rosa26LSLtdTomato mice, which enables double labeling of different LTMR endings (n=24). Whole-mount immunostaining of the dsRed signal of hairy skin showed that 80% of C-LTMR lanceolate endings are associated with zigzag hair follicles and 20% with awl/auchene hair follicles, while none were observed to associate with guard hair follicles (n=212 hair follicles examined).
Scale bars: 50 μm (A-D) and (G and H); 100 μm (I and J); 20 μm (F) and (K and L).
To visualize the axonal endings of C-LTMRs, we generated THCreER; Rosa26IAP and THCreER; Rosa26LSL-tdTomato mice (Badea et al., 2009; Madisen et al., 2010; Rotolo et al., 2008). These animals were treated with 4-hydroxytamoxifen (4-HT) daily for three days beginning at P13 to activate Cre-mediated recombination and expression of the placental alkaline phosphatase (AP) or tdTomato reporter genes in a small number of TH+ C-LTMRs (Figure 1G and 1H). These genetic labeling strategies allowed for visualization of C-LTMR peripheral endings in the skin. Surprisingly, the axonal branches of individual C-LTMRs were found to arborize and form longitudinal lanceolate endings that are intimately associated with 18.0 ± 1.7 (330 neurons from 3 animals) hair follicles of back hairy skin (Figure 1I-L). We next assessed the type of hair follicles receiving C-LTMR endings. There are three major types of hair follicles on back hairy skin of mice (Driskell et al., 2009). Guard or tylotrich hairs are the longest and least abundant of the hair follicle types, constituting ~1% of hairs on the skin of the trunk and proximal limbs (Figure S1B, left). Guard hairs have two rows of medulla cells and are typically associated with a pair of sebaceous glands. Awl/auchene hairs are shorter than guard hairs, contain three or four rows of medulla cells, and together constitute ~23% of trunk hair follicles (Figure S1B, middle). The third type, zigzags are the finest, most abundant type of hair follicle, comprising ~76% of trunk hairs. Zigzag hairs have a single row of medulla cells and two or more kinks in the shaft (Figure S1B, right). Interestingly, the longitudinal lanceolate endings of C-LTMRs were observed in association with zigzag (80% of C-LTMR endings) and awl/auchene (20% of endings) hair follicles, but not guard hair follicles (0% of endings) (n = 212 hair follicles examined; Figure 4E). The area of hairy skin innervated by a single C-LTMR, which defines its cutaneous receptive field, is 0.2-0.4 mm2 (Figure 1I and 1J). Longitudinal lanceolate endings have been long thought to belong exclusively to Aβ RA-LTMRs (Takahashi-Iwanaga, 2000), and therefore it was unexpected that the endings of C-LTMRs, which are IA-LTMRs and unmyelinated with slow conduction velocities, would form longitudinal lanceolate endings associated with hair follicles (Figure 1K and 1L). This observation indicates that the peripheral end organ is not the sole determinant of LTMR response or adaptation properties. Moreover, consistent with prior physiological recordings in cats, primates, and humans (Bessou et al., 1971; Kumazawa and Perl, 1977; Vallbo et al., 1999), the peripheral endings of murine C-LTMRs were observed exclusively in hairy skin; C-LTMR projections were absent from glabrous skin of the paws (more than 25 animals were examined; data not shown). Consistent with this, relatively few C-LTMR neurons were found in DRGs that innervate distal limbs as compared to DRGs that innervate trunk and proximal limb hairy skin (Figure S3). Interestingly, cell counts revealed that C-LTMRs are a very large population of neurons. They constitute more than 15% of DRG neurons at non-limb levels and include ~30% of sacral level 1 (S1) DRG neurons (Figure S3B), which provide sensory innervation of the genitalia. Thus, C-LTMRs are an abundant mechanosensory neuronal population that provides rich innervation of trunk and proximal limb hairy skin where they form longitudinal lanceolate endings associated exclusively with zigzag and awl/auchene hair follicles.
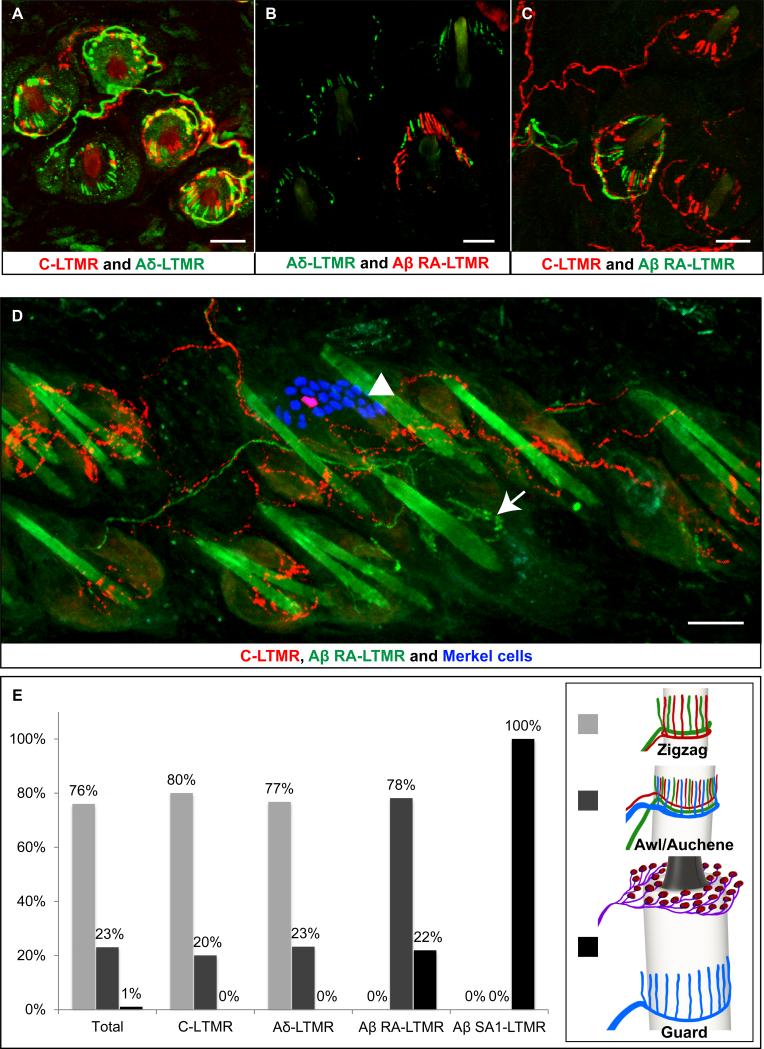
Figure 4. The organization of peripheral axonal endings of Aβ-LTMRs, Aδ-LTMRs, and C-LTMRs in hairy skin.
(A) In hairy skin sections from THCreER ; Rosa26LSL-tdTomato ; TrkBtauEGFP mice, TH+ C-LTMR axonal endings are labeled with tdTomato fluorescence (red) and TrkB+ Aδ-LTMR axonal endings are labeled by GFP immunostaining (green). Virtually every zigzag and awl/auchene hair receives interdigitated C-LTMR and Aδ-LTMR longitudinal lanceolate endings (n=3).
(B) In hairy skin sections from Npy2r-tdTomato; TrkBtauEGFP mice, Aβ RA-LTMR and TrkB+ Aδ-LTMR axonal endings are labeled by dsRed (red) and GFP (green) immunostaining, respectively. Awl/auchene hairs exhibit interdigitated Aδ-LTMR and Aβ RA-LTMR longitudinal lanceolate endings whereas zigzag hairs have Aδ-LTMR endings but not Aβ RA-LTMR endings (n=5).
(C) In hairy skin sections from THCreER ; Rosa26LSL-tdTomato ; Npy2r-GFP mice, TH+ C-LTMR axonal endings are labeled with tdTomato fluorescence (red) and Aβ RA-LTMR axonal endings are labeled by GFP immunostaining (green). Awl/auchene hairs exhibit interdigitated C-LTMR and Aβ RA-LTMR longitudinal lanceolate endings whereas zigzag hairs have C-LTMR endings but not Aβ RA-LTMR endings (n=5).
(D) Whole-mount immunostaining of dsRed, GFP, and Troma1 of hairy skin from THCreER ; Rosa26LSL-tdTomato ; Npy2r-GFP mice reveals the organization of C-LTMR and Aβ RA-LTMR axonal endings around guard hair follicles (n=3). Shown is a representative guard hair follicle associated with a cluster of Troma1+ Merkel cells at the mouth (blue, arrowhead) and innervated by GFP+ Aβ RA-LTMR longitudinal lanceolate endings (green, arrow). Surrounding the guard hair follicle are zigzag and awl/auchene hair follicles, which are innervated by C-LTMRs (red).
(E) Left: quantification of the percentages of zigzag (light grey bars), awl/auchene (dark grey bars) or guard hairs (black bars) among total hairs (n=1493), hairs innervated by C-LTMRs (n=212), hairs innervated by Aδ-LTMRs (n=50), hairs innervated by Aβ RA-LTMRs (n=128), and hairs innervated by Aβ, SA1-LTMRs (n=15). Right: schematic pictures of zigzag, awl/auchene and guard hair follicles showing their unique combinations of LTMR endings.
Scale bars: 20 μm (A-C); 50 μm (D).
Genetic labeling of Aδ-LTMRs and visualization of their cutaneous endings
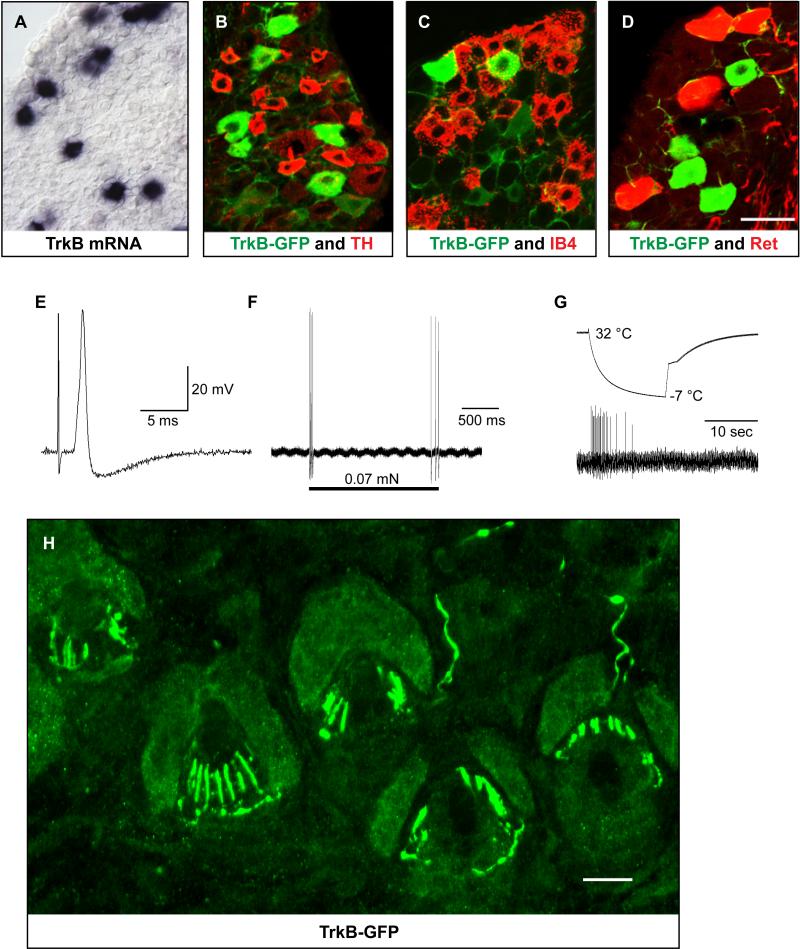
A second major population of LTMRs, the Aδ-LTMRs, or D-hair cells, is maximally excited by gentle touch of hairy skin (Brown and Iggo, 1967; Burgess et al., 1968). Despite being widely studied for decades, their molecular properties, functions in somatosensation, and the morphology of their axonal endings in the skin remain unknown. We tested the idea that Aδ-LTMRs express TrkB, which is a receptor for both brain-derived neurotrophic factor (BDNF) and neurotrophin-4 (NT4) (Klein et al., 1992; Klein et al., 1991). TrkB is expressed at high levels in a subset of medium-diameter DRG neurons (Figure 2A), and prior studies have shown that NT4 is essential for maintenance of adult Aδ-LTMRs (Stucky et al., 1998). To define the physiological and morphological properties of TrkB+ DRG neurons in adult mice, we generated a TrkBtauEGFP knock-in mouse line in which the coding determinants of a tau-enhanced green fluorescent protein (eGFP) fusion protein were introduced via homologous recombination into the first coding exon of the TrkB gene (Figure S4). Using TrkBtauEGFP mice, we found that approximately 7% of adult thoracic DRG neurons express eGFP (Figure 2B-D). These eGFP+ neurons have medium-diameter somata and do not express the C-LTMR marker TH, the non-peptidergic nociceptor marker IB4, or the Aβ RA-LTMR marker cRet (Luo et al., 2009) (Figure 2B-D; Figure S1A). Intracellular recordings using the ex vivo skin-nerve preparation revealed that eGFP+ DRG neurons of adult TrkBtauEGFP mice exhibit all of the characteristic physiological properties of Aδ-LTMRs (Brown and Iggo, 1967; Koltzenburg et al., 1997): exquisite mechanical sensitivity (von Frey threshold < 0.07 mN), rapidly adapting responses to suprathreshold stimuli, intermediate conduction velocities (CV = 5.8 +/- 0.9 m/sec), and narrow uninflected somal spikes (n = 19 neurons from 7 animals; Figure 2E and 2F). Similar to the C-LTMRs, the Aδ-LTMRs responded to rapid cooling of the skin (Figure 2G). We next performed immunohistochemistry using GFP antibodies on sections of skin from adult TrkBtauEGFP mice to visualize the peripheral axonal endings of Aδ-LTMRs. Strikingly, and again much like the C-LTMRs, the Aδ-LTMRs form longitudinal lanceolate endings associated with virtually every zigzag and awl/auchene hair follicle of the trunk (Figure 2H and 4E). Thus, TrkB-expressing neurons of adult DRGs are Aδ-LTMRs, and these neurons form longitudinal lanceolate endings associated with awl/auchene and zigzag hair follicles of trunk hairy skin.
Figure 2. The TrkB+ adult DRG neurons are Aδ-LTMRs and form longitudinal lanceolate endings associated with zigzag and awl/auchene hair follicles.
(A) In situ hybridization for TrkB mRNA using adult thoracic DRG sections shows that TrkB is expressed in a subset of medium-diameter DRG neurons.
(B-D) Double staining of GFP and TH (B) or GFP and IB4 (C) on thoracic DRG sections from TrkBtauEGFP mice shows that TrkB+ DRG neurons are distinct from TH+ C-LTMRs and IB4+ nonpeptidergic nociceptors. Double immunostaining of GFP and dsRed on thoracic DRG sections from RetCreERT2; Rosa26LSL-tdTomato; TrkBtauEGFP mice (D) shows that TrkB+ neurons are distinct from early Ret+ Aβ RA-LTMRs.
(E-G) The ex vivo skin-nerve preparation was used to assess mechanically evoked responses and physiological properties of individual TrkBtauEGFP+ neurons. The TrkBtauEGFP+ neurons exhibit exquisite sensitivity and rapid adaptation to mechanical stimulation (von Frey threshold < 0.07 mN) (E and F) and an average conduction velocity of 5.8 ± 0.9 m/s (n = 19). These neurons also responded to cooling but not warming of the skin (G). The average receptive field size of these neurons is 2 × 2 mm2. Nineteen neurons from 7 different animals were recorded and each displayed physiological properties that are characteristic of D-hair cells, or Aδ-LTMRs. (H) Anti-GFP immunostaining of a section of back hairy skin from TrkBtauEGFP mice shows that the GFP+ neurons form longitudinal lanceolate endings (n=20). 77% of the GFP+ lanceolate endings associate with zigzag hair follicles while the remaining 23% associate with awl/auchene hair follicles (n=50 hair follicles examined).
Scale bars: 20 μm.
Genetic labeling of Aβ-LTMRs and visualization of their cutaneous endings
The most widely studied LTMRs are the Aβ-LTMRs, which are best described for their roles in discriminative touch of glabrous skin (Johnson and Hsiao, 1992). Aβ-LTMRs are classified as either Aβ RA-LTMRs or Aβ SA-LTMRs. They can be further subdivided into RA1- and RA2-, and SA1- and SA2-LTMRs, respectively, although such distinctions in the mouse are somewhat controversial and not entirely clear. In glabrous skin, Aβ SA1-LTMRs form Merkel endings while Aβ RA-LTMRs innervate Meissner corpuscles. In mouse hairy skin, the peripheral endings of Aβ SA1-LTMRs associate with Merkel cells whereas Aβ RA-LTMRs form longitudinal lanceolate endings associated with hair follicles. The majority of Aβ-LTMR central axonal projections terminate in laminae III through V of the spinal cord dorsal horn (Brown, 1981a). Many Aβ-LTMRs also exhibit central branches that ascend via the dorsal columns to the dorsal column nuclei (DCN) of the brainstem. However, in the rodent, the central branches of Aβ-LTMRs located at thoracic levels that innervate trunk and proximal limb hairy skin appear less likely than those located at cervical limb levels to reach the DCN (Giuffrida and Rustioni, 1992).
To establish tools useful for visualizing the axonal projections of specific Aβ-LTMR subtypes, we screened transgenic mouse lines generated by the Gene Expression Nervous System Atlas (GENSAT) project (Gong et al., 2003) for those that express GFP in select populations of Aβ-LTMRs. Utilizing bacterial artificial chromosome (BAC)-based transgenic technology, the GENSAT project created ~1400 mouse lines that express soluble EGFP under the direction of specific nervous system promoters. This technology allows genetically defined cell populations to be tracked either directly via green epifluorescence or through antibody staining. We took advantage of the well-defined patterns of Aβ-LTMR endings in lamina III through V of the dorsal horn and screened the entire GENSAT database for BAC-GFP mouse lines in which GFP is expressed in axons that innervate this region of the spinal cord. One of the mouse lines identified, the Npy2r-GFP line (founder line EJ71), was chosen for further analysis because Npy2r-GFP+ DRG neurons exhibit characteristic features of Aβ RA-LTMRs. Indeed, the central projections of Npy2r-GFP+ DRG neurons are restricted to lamina III through V of the spinal cord dorsal horn (Figure 5C). Moreover, in Npy2r-GFP mice, GFP is expressed in large-diameter DRG neurons that encompass 6% of total thoracic DRG neurons (Figure 3A and 3B; Figure S1A). Double immunostaining showed that these neurons express NFH and have heavily myelinated axons (Figure 3A and 3F). We also generated an Npy2r-tdTomato mouse line (PL27-TOM) using the Npy2r BAC in order to label the same population of DRG neurons with an alternate reporter. Analysis of Npy2r-GFP;Npy2r-tdTomato double transgenic mice showed that the majority of tdTomato+ neurons are large-diameter NFH+ DRG neurons that co-express GFP (Figure S5E-H). Using these transgenic mouse lines, we found that Npy2r-GFP+ neurons are a major subset of the early Ret+ neuronal population (Figure S5A), which includes all Aβ RA-LTMRs (Luo et al., 2009), while they do not express the C-LTMR marker TH, the Aδ-LTMR marker TrkB, the proprioceptor marker parvalbumin, or markers of peptidergic and non-peptidergic nociceptors (Figure 3B and 3C; Figure S5B-D).
Figure 5. The central projections of C-, Aδ-, and Aβ RA-LTMRs terminate in distinct, but partially overlapping laminae of the spinal cord dorsal horn.
(A) In THCreER; Rosa26LSL-tdTomato mice, tdTomato fluorescence shows axonal terminals of individual C-LTMRs located within lamina IIiv of the spinal cord dorsal horn (20-30 μm mediolaterally, 30-40 μm dorsoventrally) (n=24).
(B) In TrkBtauEGFP mice, GFP fluorescence shows central projections of TrkB+ Aδ-LTMRs mainly restricted to lamina III (50-60 μm dorsoventrally) (n=9).
(C) In Npy2r-GFP mice, GFP immunostaining shows central endings of Aβ RA-LTMRs located in lamina III through V (~100 μm dorsoventrally) (n=8).
(D) In spinal cord sections from THCreER; Rosa26LSL-tdTomato ; TrkBtauEGFP mice, the central terminals of C-LTMRs labeled by tdTomato fluorescence (red) are located dorsally, but partially overlapping with the central projections of Aδ-LTMRs, which are visualized by GFP fluorescence (green) (n=3).
(E) In spinal cord sections from THCreER ; Rosa26LSL-tdTomato ; Npy2r-GFP mice, TH+ C-LTMR axonal endings labeled with tdTomato fluorescence (red) are located dorsal to the central projections of Aβ RA-LTMRs labeled by GFP immunostaining (green) (n=5).
(F) In spinal cord sections from RetCreERT2;RosaLSLtdTomato ; TrkBtauEGFP mice, the central projections of Aδ-LTMRs labeled by GFP fluorescence (green) are located dorsally, but partially overlapping with the central projections of Aβ RA-LTMRs, which are labeled by dsRed immunostaining (red) (n=3). Similar findings were made in experiments that used Npy2r-tdTomato; TrkBtauEGFP mice (data not shown).
(G) A schematic of a transverse section of thoracic spinal cord showing the relative positions of the central projections of C-LTMRs, Aδ-LTMRs, and Aβ RA-LTMRs in the dorsal horn.
Scale bars: 50 μm (A-F)
Figure 3. The Aβ RA-LTMRs are labeled with GFP in Npy2r-GFP mice and their GFP+ peripheral axons form longitudinal lanceolate endings associated with guard and awl/auchene hairs.
(A-B) In Npy2r-GFP mice, double immunostaining of GFP and NFH shows that the GFP+ neurons have large-diameter somata and express NFH (n=7). Double staining of GFP and TH shows that Npy2r-GFP+ neurons are distinct from the C-LTMRs (n=5).
(C) In Npy2r-tdTomato; TrkBtauEGFP mice, double immunostaining of dsRed and GFP shows that the Npy2r-GFP+ neurons are distinct from the Aδ-LTMRs (n=5).
(D and E) The ex vivo skin-nerve preparation was used to assess mechanically evoked responses and the physiological properties of individual Npy2r-GFP+ neurons. Intracellular recordings were performed in the same manner as those described for TrkBtauEGFP animals. 18 neurons from 5 different animals were recorded and exhibited low mechanical thresholds (0.07 mN), rapid conduction velocities (13.6 ± 2.7 m/sec) and rapidly adapting responses, which are the hallmark features of Aβ RA-LTMRs.
(F and G) In hairy skin sections from Npy2r-GFP mice, double immunostaining of GFP and MBP (F) (n=2) and GFP and S100 (G) (n=4) shows that Npy2r-GFP+ neurons form longitudinal lanceolate endings associated with hair follicles. These GFP+ endings have MBP+ myelinated parental axons and unmyelinated terminals that are intimately associated with S100+ terminal Schwann cells (G).
(H and I) Whole-mount immunostaining of GFP and the Merkel cell marker Troma1 of hairy skin from Npy2r-GFP mice showed that 22% of the GFP+ lanceolate endings are associated with guard hair follicles, as determined by the presence of Troma1+ Merkel cells at the follicle mouth (H) (n=6); 78% of the GFP+ lanceolate endings are associated with awl/auchene hair follicles, which are smaller in size and do not have Merkel cells (I); zigzag hair follicles are devoid of GFP+ endings (n=128 hair follicles examined).
Scale bars: 20 μm (A-C, F-I).
To directly test the idea that the DRG neurons labeled in Npy2r-GFP mice are Aβ RA-LTMRs, we next performed intracellular recordings from GFP+ neurons of Npy2r-GFP mice using the ex vivo skin-nerve preparation. Npy2r-GFP+ neurons exhibited low threshold mechanical responsiveness (0.07 mN von Frey threshold) with bursts of spikes with fast conduction velocities (13.6 ± 2.7 m/sec) following stimulation of hairy skin. Moreover, all neurons tested exhibited rapidly adapting properties (n = 18 neurons from 5 different mice) (Figure 3D and 3E). Thus, Npy2r-GFP+ neurons are Aβ RA-LTMRs.
We next examined the peripheral cutaneous endings of Aβ RA-LTMRs in relation to Aβ SA-LTMRs and the three major hair follicle types of back hairy skin. A mouse line that selectively labels Aβ SA-LTMRs is not yet available. However, since it is well established that SA1-LTMRs associate exclusively with Merkel cells, which cluster in groups to form “touch domes” in hairy skin ( Iggo and Muir, 1969; Wellnitz et al., 2010), we developed a skin whole-mount immunostaining procedure employing anti-Troma1, which robustly labels Merkel cells (Vielkind et al., 1995), as a surrogate for visualization of Aβ SA1-LTMR endings. Touch domes/Merkel cells were found to associate exclusively with guard hairs (100%, 15/15); none associated with awl/auchene or zigzag hairs (0%, 0/15) (Figure 4E). Conversely, most if not all guard hairs have touch domes. Immunostaining using GFP antibodies on hairy skin of Npy2r-GFP mice showed that all of the peripheral axonal endings of Npy2r-GFP+ DRG neurons form longitudinal lanceolate endings associated with hair follicles (Figure 3F-I). Moreover, double immunostaining of GFP and Troma1 using whole-mount preparations of hairy skin from Npy2r-GFP mice showed that 22% of the GFP+ lanceolate endings in the skin are associated with guard hair follicles (Figure 3H and 4E), while the remaining 78% of the GFP+ lanceolate endings are associated with awl/auchene hair follicles (Figure 3I and 4E). Very few (1-2%) innervated zigzag hair follicles (128 hair follicles examined; Figure 4E). Thus, Aβ RA-LTMRs associate with both guard hairs and awl/auchene hairs, whereas Aβ SA1-LTMRs exclusively associate with guard hairs.
The relative patterns of organization of Aβ-, Aδ-, and C-LTMR endings in hairy skin
Our analysis reveals that the peripheral endings of Aβ-, Aδ-, and C-LTMRs associate with select types of hair follicles. We next addressed the relative organization of cutaneous LTMR endings around individual hair follicles by combining molecular-genetic labeling tools with double or triple immunostaining to simultaneously visualize combinations of LTMR endings in skin preparations. We first generated THCreER ; Rosa26LSL-tdTomato ; TrkBtauEGFP mice to simultaneously visualize C-LTMRs with tdTomato and Aδ-LTMRs with GFP. C-LTMRs and Aδ-LTMRs form morphologically indistinguishable longitudinal lanceolate endings associated with both awl/auchene and zigzag hair follicles with similar percentages: ~20% of both C- and Aδ-LTMRs are associated with awl/auchene hair follicles while ~80% of these neurons associate with zigzag hair follicles (Figure 4E). Strikingly, the C-LTMR and Aδ-LTMR endings around individual zigzag and awl/auchene hair follicles are interdigitated with one another (Figure 4A), suggesting a common mechanism of excitation. This is especially intriguing because both C-LTMRs and Aδ-LTMRs are highly sensitive to skin stimulation, responding to the finest von Frey filament (0.07 mN force application), and also to rapid cooling of the skin (Figure 1E; Figure 2F and 2G).
Similarly, analysis of Npy2r-tdTomato ; TrkBtauEGFPmice, in which Aβ RA-LTMRs are labeled with tdTomato and Aδ-LTMRs with GFP, revealed that individual awl/auchene hair follicles, but not guard or zigzag hair follicles, are innervated by interdigitated Aβ RA-LTMR and Aδ-LTMR endings (Figure 4B). We also generated THCreER ; Rosa26LSL-tdTomato ; Npy2r-GFP mice to simultaneously visualize the endings of C-LTMRs with tdTomato and Aβ RA-LTMRs with GFP (Figure 4C and 4D). Guard hair follicles, which are innervated by Aβ RA-LTMRs, but not C-LTMRs, are surrounded by both awl/auchene hair follicles, which are innervated by interdigitated Aβ RA-LTMRs and C-LTMRs, and zigzag hairs, which are innervated by C-LTMRs but not Aβ RA-LTMRs (Figure 4D). Interestingly, 22% of the Aβ RA-LTMR lanceolate endings and 100% of the Aβ SA1-LTMR/Merkel endings are associated with guard hairs, while 78% of the Aβ RA-LTMR lanceolate endings and none of the Aβ SA1-LTMR/Merkel cells are associated with awl/auchene hairs (Figure 4E). In summary, virtually all zigzag hair follicles are innervated by both C-LTMR and Aδ-LTMR lanceolate endings; Awl/auchene hairs are triply innervated by Aβ RA-LTMR, Aδ-LTMR, and C-LTMR lanceolate endings; Guard hair follicles are innervated by both Aβ RA-LTMR longitudinal lanceolate endings and Merkel cell-associated Aβ SA1-LTMRs (Figure 4E, right). Thus, guard, awl/auchene, and zigzag hair follicle types receive unique and invariant combinations of LTMR endings, indicating that each of the three hair follicle types of mouse hairy skin is a neurophysiologically distinct mechanosensory end organ.
Interestingly, guard, awl/auchene, and zigzag hair follicles are distributed in an approximately 1:23:76 arrangement in mouse trunk hairy skin (Figure 4E; Figure S1B). In fact, two of the three hair types exhibit robust homotypic tiling. Thus, guard and awl/auchene hairs are arranged in an iterative, regularly spaced pattern (distance between guard hairs is 740 ± 24 μm; distance between awl/auchene hairs = 215 ± 5 μm) whereas zigzag hairs densely populate skin areas surrounding the two larger hair follicle types. Moreover, individual Aβ RA-LTMRs labeled with RetCreERT2 form endings that associate with either one or two guard hairs or three or more awl/auchene hairs (Figure S5I and S5J). Likewise, prior physiological recordings and anterograde labeling analyses indicate that the endings of individual Aβ SA1-LTMRs are typically associated with one or two touch domes/Merkel cell complexes (Woodbury and Koerber, 2007) and thus, based on our analysis, one or two guard hairs. Taken together, these findings indicate that hairy skin consists of iterative clusters of three neurophysiologically distinct hair follicles, or “peripheral LTMR units”. Each cluster, or unit, is comprised of: 1) one or two centrally located guard hairs and their associated Aβ RA-LTMR longitudinal lanceolate endings and Aβ SA1-LTMR/Merkel cell complexes; 2) ~20 surrounding, sparsely and evenly distributed awl/auchene hairs with their triply interdigitated Aβ RA-LTMR, Aδ-LTMR and C-LTMR lanceolate endings; and, 3) ~80 interspersed zigzag hairs with their interdigitated C-LTMR and Aδ-LTMR lanceolate endings.
Visualization of LTMR endings in the spinal cord dorsal horn reveals somatotopically-arranged LTMR columns
Since C-LTMRs, Aδ-LTMRs, and most if not all Aβ-LTMRs form synapses onto neurons of the spinal cord dorsal horn, insights into the logic of mechanosensory neuron organization may be gleaned through an appreciation of the relative organization of LTMR central endings in the dorsal horn. Therefore, we next used our LTMR genetic labeling tools to visualize the central endings of Aβ RA-LTMRs, Aδ-LTMRs, and C-LTMRs, both individually and in combination. We observed that each LTMR class exhibits a unique central axon terminal morphology occupying a distinct dorsal horn termination zone (Figure 5A-C). The central endings of individual C-LTMRs sparsely labeled in THCreER ; Rosa26LSL-tdTomato mice or THCreER ; Rosa26IAP mice form dense, flame-shaped terminals within lamina IIiv, overlapping precisely with the PKCγ+ interneurons of the dorsal horn (Figure 5A; Figure S6A and S6B). Moreover, the terminals of each C-LTMR extend rostrocaudally for 200-300 μm (Figure S6C). Terminations of GFP+ Aδ-LTMRs in TrkBtauEGFP mice were found mainly within lamina III, but partially overlapping with the C-LTMR terminals in lamina IIiv (Figure 5B and 5D). Central terminals of GFP+ Aβ RA-LTMRs in Npy2r-GFP mice were found mainly in lamina III through V of the dorsal horn, located below the C-LTMR endings and partially overlapping with the Aδ-LTMR endings in lamina III (Figure 5C, 5E and 5F). Thus, the central projections of Aβ RA-LTMRs, Aδ-LTMRs and C-LTMRs terminate in distinct, but partially overlapping laminae of the spinal cord dorsal horn (Figure 5G).
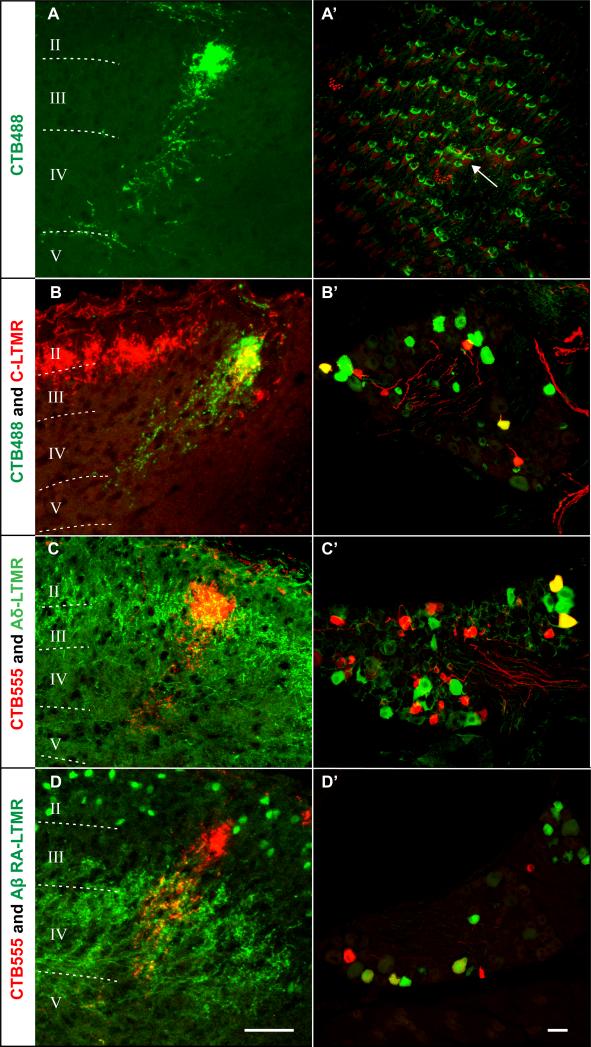
Classic physiological and anatomical studies of cats and primates demonstrated somatotopic organization of wedge-shaped termination zones of primary sensory afferent endings in laminae III-IV of the spinal cord dorsal horn (Brown et al., 1989; Brown and Fuchs, 1975; Koerber and Brown , 1982; Woolf and Fitzgerald, 1986). Here, we observed that the central projections of Aβ RA-LTMRs, Aδ-LTMRs, and C-LTMRs occupy distinct, but partially overlapping laminae of the dorsal horn of the mouse. In addition, we found that Aβ RA-LTMRs, Aβ SA1-LTMRs, Aδ-LTMRs, and C-LTMRs form endings associated with the same or adjacent hair follicles. Thus, it appears likely that a wedge, or column of somatotopically organized primary sensory afferent endings in the dorsal horn represents the alignment of the central projections of Aβ-, Aδ- and C-LTMRs that innervate the same peripheral unit and detect mechanical stimuli acting upon the same small group of hairs follicles. To directly test this idea, we developed a dual labeling strategy that combines somatotopic, retrograde fluorescent labeling of small groups of neurons that innervate a common, small region of hairy skin and simultaneous visualization of the central endings of LTMR subtypes using our LTMR-specific mouse reporter lines. To retrogradely label cutaneous sensory neurons and their central axonal projections, we used fine glass capillaries to subcutaneously inject 0.1-0.3 μl of either CTB488 or CTB555 (2 μg/ul in PBS) into small patches of back hairy skin (0.6-1.8 mm2). These CTB injections labeled small groups of cutaneous sensory neurons innervating skin regions that encompass only 190 ± 23 hair follicles, the approximate size of one or two peripheral LTMR units (Figure 6A’). Consistent with this, the injection sites typically included only one or two guard hairs, as determined by staining the injection sites with anti-Troma1 (Figure 6A’, arrow). We observed that the central endings of CTB-labeled neurons align within a column that is perpendicular to the dorsal border of the ipsilateral dorsal horn, extending from lamina IIiv to V (Figure 6A). These CTB-labeled dorsal horn columns, which are somatotopically organized (Figure S7A and S7A’), extend for several hundred microns along the rostrocaudal axis, with the lengths of extension corresponding to the sizes of the peripheral injection sites (Figure S7B and S7C). We next visualized the LTMR components of the CTB-labeled dorsal horn columns by assessing the extent of overlap between the CTB-labeled afferent endings and the central terminals of genetically labeled C-, Aδ-, and Aβ RA-LTMRs. In THCreER ; Rosa26LSL-tdTomato mice, a single CTB488 injection labeled the somata of several tdTomato+ C-LTMR neurons which were, as expected, randomly distributed in the DRG (Figure 6B’). In the spinal cord, the dorsal most CTB-labeled central terminals overlapped precisely with tdTomato+ C-LTMR terminals in lamina IIiv, exhibiting identical flame-shaped, dense arborization patterns (Figure 6B). Similarly, injections of CTB555 into hairy skin of TrkBtauEGFP mice or Npy2r-GFP mice labeled a small number of GFP+ Aδ-LTMRs or Aβ RA-LTMRs, respectively (Figure 6C’ and 6D’). Importantly, many of the CTB-labeled central endings were co-labeled with GFP+ Aδ-LTMR terminations in lamina III and Aβ RA-LTMR terminations in laminae III through V (Figure 6C and 6D). These findings demonstrate that the dorsal horn LTMR columns consist of the central endings of Aβ-LTMRs, Aδ-LTMRs, and C-LTMRs whose peripheral endings associate in unique patterns with guard, awl/auchene, and zigzag hair follicles within individual peripheral LTMR units (Figure 7). Based on the numbers of guard, awl/auchene and zigzag hairs of the trunk and limbs and the numbers of each LTMR subtype, we estimate that the mouse dorsal horn contains 2,000-4,000 LTMR columns, which corresponds to the approximate number of peripheral LTMR units.
Figure 6. LTMR columns of the spinal cord dorsal horn.
(A-A’) The central (spinal cord dorsal horn) terminations of sensory neurons whose peripheral axonal endings are labeled by microinjection of CTB488 into a small region of back hairy skin (n=80). The size of the dorsal horn axonal termination region for CTB488-labeled neurons is 20-30 μm along the mediolateral axis and ~200 μm along the dorsoventral axis (A). In the example shown (A’), a 1.02 mm2 area of skin containing 123 hair follicles was labeled with CTB488 (green) followed with whole-mount immunostaining of Troma1 (red) (n=9). A single guard hair (arrow) that associates with Troma1+ Merkel cells is located in the middle of the injection area. On average, 190 ± 23 hair follicles were labeled by microinjection of CTB488 into a small region of back hairy skin (n=11).
(B-D) CTB488 or CTB555 were injected into a small region of back hairy skin of mice in which the different populations of LTMRs are genetically labeled. CTB labeled central afferents overlap with C-LTMR central terminals in THCreER; Rosa26LSL-tdTomato mice (B) (n=4), Aδ-LTMR central projections in TrkBtauEGFP mice (C) (n=5) and Aβ RA-LTMR central projections in Npy2r-GFP mice (D) (n=4).
(B’-D’) A small number of cell bodies of each LTMR type are retrogradely labeled by CTB microinjection into the skin. CTB488 (green) and tdTomato fluorescence (red) in a thoracic DRG section from a THCreER; Rosa26LSL-tdTomato mouse (B’) (n=4). CTB555 (red) and GFP fluorescence (green) in a thoracic DRG section of a TrkBtauEGFP mouse (C’) (n=5). CTB555 (red) and GFP fluorescence (green) in a thoracic DRG sections from an Npy2r-GFP mouse (D’) (n=3).
Scale bars: 200 μm (A’), 50 μm (A-D) and (B’-D’).
Figure 7. The organization of LTMR endings in hairy skin and the spinal cord dorsal horn.
The peripheral endings of Aβ-LTMRs, Aδ-LTMRs and C-LTMRs associate with either one or two of the three types of hair follicles of trunk and proximal limb hairy skin. At zigzag hair follicles, C-LTMRs (red) and Aδ-LTMRs (green) form interdigitated longitudinal lanceolate endings; At awl/auchene hair follicles, Aβ RA-LTMRs (blue), Aδ-LTMRs (green), and C-LTMRs (red) form interdigitated longitudinal lanceolate endings; Guard hair follicles are associated with longitudinal lanceolate endings formed by Aβ RA-LTMRs (blue) and clusters of Merkel cells, or touch domes and thus Aβ SA1-LTMRs (purple). The central terminals of LTMRs that innervate the same or adjacent hair follicles within a peripheral LTMR unit are aligned to form a narrow LTMR column in the spinal cord dorsal horn.
Discussion
Decoding the mechanisms by which the individual qualities of a sensory stimulus are extracted from the periphery, and conveyed to, integrated, and processed within the central nervous system requires visualization of the organization of primary sensory neuron projections. Here, we have used a combination of molecular-genetic labeling and somatotopic retrograde tracing approaches to visualize the organization of peripheral and central axonal endings of the physiologically distinct LTMR subtypes that mediate the sense of touch. Our findings support a model in which individual features of a complex tactile stimulus are extracted by three functionally distinct hair follicle types and conveyed via the activities of unique combinations of Aβ-, Aδ- and C-LTMRs to dorsal horn LTMR columns, where these features are represented, integrated, and processed for output to the brain.
Similar to primary sensory neurons of other sensory systems, such as olfactory receptor neurons of the olfactory system and auditory receptors of the auditory system, cutaneous LTMRs of the somatosensory system are poised to extract individual qualities of complex stimuli from the external world. Yet, despite their intense study for decades, the unique functions and the relative organization of LTMR subtype peripheral endings, especially in hairy skin, have remained largely unclear. We found that the peripheral endings of each LTMR subtype associate with either one or two of the three types of trunk and proximal limb hairy skin hair follicles. Furthermore, axons of select LTMR subtypes are intimately associated with one another, having entwined projections and interdigitated lanceolate endings that innervate the same hair follicle. Conversely, each of the three hair follicle types receives a unique and invariant combination of LTMR endings. Indeed, guard hair follicles are uniquely associated with a combination of Aβ RA-LTMR and Aβ SA1-LTMRs; Awl/auchene hairs are triply innervated by Aβ RA-LTMRs, Aδ-LTMRs, and C-LTMRs; and Zigzag hair follicles are innervated by both C-LTMRs and Aδ-LTMRs. These findings are consistent with classic neurophysiological measurements in the cat and rabbit indicating that Aβ RA-LTMRs and Aδ-LTMRs can be differentially activated by deflection of distinct hair follicle types (Brown and Iggo, 1967; Burgess et al., 1968). In addition, because the three hair follicle types exhibit different shapes, sizes, and cellular compositions, they are likely to have distinct deflectional or vibrational tuning properties. Thus, our findings indicate that guard, awl/auchene, and zigzag hairs are physiologically distinct mechanosensory end organs. We suggest that it is the combination of: 1) the relative numbers, unique spatial distributions, and distinct morphological and deflectional properties of the three types of hair follicles, 2) the unique combinations of LTMR subtype endings associated with each of the three hair follicle types, and 3) distinct sensitivities, conduction velocities, spike train patterns, and adaptation properties of the four main classes of hair-follicle-associated LTMRs that enables the hairy skin mechanosensory system to extract and convey to the CNS the complex combinations of qualities that define a touch.
The remarkable organization of peripheral LTMR endings reveals a fundamental feature of the somatosensory system and supports an integrative model of mechanosensation. This integrative model posits that individual mechanical properties of a complex tactile stimulus engage distinct combinations of the three hair follicle types and thus differentially activate the unique combinations of LTMRs with which these follicles associate. We suggest that certain mechanical stimuli, such as the flutter of an insect's wings, raindrops, or light contact with folliage, may preferentially stimulate long guard hairs and thus Aβ RA-LTMRs. On the other hand, stroking of the coat by a nurturing mother may preferentially activate Aδ-LTMRs and C-LTMRs associated with the small, abundant zigzag hair follicles. Indentation of a patch of hairy skin with a blunt object may activate Aβ SA1-LTMRs as well as all other LTMRs associated with hair follicles of the indented and surrounding skin region. Thus, the key feature of this integrative model is that a large number of potential combinations of deflections or vibrations of the three hair follicle types, with or without concomitant skin indentation, endows the somatosensory system with a vast array of potential ensembles of LTMR activities that could encode the properties or qualities that define a particular tactile stimulus.
How are individual properties or qualities of a touch represented and processed within the central nervous system to generate its unique percept? We observed that the central projections of the Aβ-LTMRs, Aδ-LTMRs, and C-LTMRs that innervate a common peripheral LTMR unit align in a columnar manner in the spinal cord dorsal horn. We suggest that dorsal horn LTMR columns are the initial and perhaps principal CNS sites of integration and processing of neural inputs that represent skin indentation and the relative movements of each hair follicle type in a small skin region. It is noteworthy that a distinguishing feature of Aβ-, Aδ- and C-LTMRs is their highly divergent action potential conduction velocities, which in the mouse range from greater than 10 m/sec for Aβ RA- and SA-LTMRs to less than 1 m/sec for C-LTMRs. Thus, a tactile stimulus is likely to be encoded by temporally coordinated ensembles of Aβ RA-, Aβ SA-, Aδ- and C-LTMR activities that converge onto dorsal horn LTMR columns, where these activities are integrated and processed. The outputs of LTMR columns are conveyed via dorsal horn projection neurons that comprise the postsynaptic dorsal column pathway and spinocervical tract to the brain, presumably undergoing further integration with mechanosensory information carried by the direct dorsal column pathway and other sensory inputs (Brown, 1981b; Brown and Franz, 1969; Brown et al., 1987; Cliffer and Giesler, 1989; Giesler and Cliffer, 1985; Giesler et al., 1984). The development of the exquisite organization of peripheral LTMR units and dorsal horn LTMR columns as well as the extent, mechanisms, and functions of integration and processing of Aβ RA-LTMR, Aβ SA-LTMR, Aδ-LTMR, and C-LTMR inputs within LTMR columns are intriguing topics for future studies.
Experimental procedures
Mouse lines
The TrkBtauEGFP mouse line was generated by introducing a tau-enhanced green fluorescent protein (tauEGFP) fusion-Frt-Neomycin-Frt-loxP cassette into the first coding ATG in exon 2 of the TrkB gene (Figure S4A) (See Extended Experimental Procedures for details.) Npy2r-tdTomato transgenic mice were generated as previously described (Gong et al., 2003) using the same Npy2r BAC, RP23-74L7, that was used to generate the Npy2r-GFP mouse line, except that the coding determinants of GFP were replaced by those of tdTomato. Other mouse lines, including the THCreER, RetCreERT2, Npy2r-GFP BAC transgenic, Rosa26 LSL-tdTomato, and Rosa26IAP mouse lines, have been described previously (Badea et al., 2009; Gong et al., 2003; Luo et al., 2009; Madisen et al., 2010; Rotolo et al., 2008).
Electrophysiology
To identify and characterize the TH+ C-LTMRs, the GFP+ Aδ-LTMRs from TrkBtauEGFP mice and the GFP+, Aβ RA-LTMRs from Npy2r-GFP mice, intracellular recordings using an ex vivo skin-nerve preparation of 2 to 3-month old animals were performed, with either dorsal back skin, dorsal cutaneous nerve, thoracic DRGs and spinal cord intact, or dorsal hairy skin, saphenous nerve, lumbar DRGs and spinal cord intact (Woodbury et al., 2001; McIlwrath et al., 2007).
CTB retrograde labeling
P14-P25 mice were anesthetized using isoflurane (Baxter) and the injection sites were shaved. 0.1-0.3 μl of CTB488 or CTB555 (2 μg/μl in PBS) were injected subcutaneously using a fine glass capillary to label sensory afferents that innervate the injection sites. Retrogradely-labeled sensory neurons in DRGs and their central afferents in the dorsal horn were examined 4 to 7 days after injections.
Histochemistry on tissue sections
Immunohistochemistry, in situ hybridization (Luo et al., 2009), and PLAP histochemistry (Liu et al., 2007) were performed using standard methods (See Extended Experimental Procedures for details.)
Whole-mount immunohistochemistry of hairy skin
Back hairy skin from 3-10 weeks old mice was treated with commercial hair remover, wiped clean with tissue paper and tape stripped until glistening. The skin was then dissected, cut into small pieces and fixed in 4% PFA in PBS at 4 °C for 2 hours. The tissue was rinsed in PBS and then washed with PBS containing 0.3% Triton X-100 (0.3% PBST) every 30 minutes for 5-8 hours. Then, the skin was incubated with primary antibodies in 0.3% PBST containing 5% goat/donkey serum and 20% DMSO at room temperature for 3 to 5 days. Tissues were then washed with 0.3% PBST every 30 minutes for 5-8 hours and transferred to secondary antibodies in 0.3% PBST containing 5% goat/donkey serum and 20% DMSO and incubated at room temperature for 2 to 4 days. Tissues were washed with 0.3% PBST every 30 minutes for 5-8 hours. Hairy skin was then dehydrated in 50% methanol for 5 minutes and 100% methanol for 20 minutes, three times, and lastly cleared in BABB (Benzyl Alcohol, sigma 402834; Benzyl Benzoate, sigma B-6630; 1:2) at room temperature for 20 minutes. To identify the types of hair follicles innervated by each LTMR class, similar whole-mount preparations of hairy skin were made without removing the hair. Using a confocal microscope, hair follicles associated with longitudinal lanceolate endings formed by genetically-labeled LTMRs were traced to the corresponding hair shafts. The number of rows of medulla cells in the hair shaft was counted to distinguish zigzag (1 row), awl/auchene (3 or 4 rows) and guard hairs (2 rows) (Figure S1B).
Supplementary Material
Acknowledgements
We thank Michael Caterina, Xinzhong Dong, Ting Guo, Tony Harrington, Steven Hsiao, Alex Kolodkin, Frank Rice, Kevin Wright, and members of the Ginty laboratory for discussions and comments on this study, Dori Reimert for help with generation of the TrkBtauEGFP mouse line, and Jeremy Nathans for providing THCreER and Rosa26IAP animals. This work was supported by NIH grants NS023725 and NS052848 (HRK), NS44094 (CJW), N01 NS-7-2370 (NH), and NS34814 (DDG). NH and DDG are investigators of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badea TC, Hua ZL, Smallwood PM, Williams J, Rotolo T, Ye X, Nathans J. New mouse lines for the analysis of neuronal morphology using CreER(T)/loxP-directed sparse labeling. PloS One. 2009;4:e7859. doi: 10.1371/journal.pone.0007859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessou P, Burgess PR, Perl ER, Taylor CB. Dynamic properties of mechanoreceptors with unmyelinated (C) fibers. J Neurophysiol. 1971;34:116–131. doi: 10.1152/jn.1971.34.1.116. [DOI] [PubMed] [Google Scholar]
- Brown AG. Organization in the spinal cord: the anatomy and physiology of identified neurones. illustrated edn. Springer-Verlag; Berlin ; New York: 1981a. 1981. [Google Scholar]
- Brown AG. The spinocervical tract. Prog Neurobiol. 1981b;17:59–96. doi: 10.1016/0301-0082(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Brown AG, Franz DN. Responses of spinocervical tract neurones to natural stimulation of identified cutaneous receptors. Exp Brain Res. 1969;7:231–249. doi: 10.1007/BF00239031. [DOI] [PubMed] [Google Scholar]
- Brown AG, Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J Physiol. 1967;193:707–733. doi: 10.1113/jphysiol.1967.sp008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AG, Koerber HR, Noble R. An intracellular study of spinocervical tract cell responses to natural stimuli and single hair afferent fibres in cats. J Physiol. 1987;382:331–354. doi: 10.1113/jphysiol.1987.sp016370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PB, Brushart TM, Ritz LA. Somatotopy of digital nerve projections to the dorsal horn in the monkey. Somatosens Mot Res. 1989;6:309–317. doi: 10.3109/08990228909144679. [DOI] [PubMed] [Google Scholar]
- Brown PB, Fuchs JL. Somatotopic representation of hindlimb skin in cat dorsal horn. J Neurophysiol. 1975;38:1–9. doi: 10.1152/jn.1975.38.1.1. [DOI] [PubMed] [Google Scholar]
- Brumovsky P, Hygge-Blakeman K, Villar MJ, Watanabe M, Wiesenfeld-Hallin Z, Hokfelt T. Phenotyping of sensory and sympathetic ganglion neurons of a galanin-overexpressing mouse--possible implications for pain processing. J Chem Neuroanat. 2006;31:243–262. doi: 10.1016/j.jchemneu.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Burgess PR, Petit D, Warren RM. Receptor types in cat hairy skin supplied by myelinated fibers. J Neurophysiol. 1968;31:833–848. doi: 10.1152/jn.1968.31.6.833. [DOI] [PubMed] [Google Scholar]
- Cliffer KD, Giesler GJ., Jr. Postsynaptic dorsal column pathway of the rat. III. Distribution of ascending afferent fibers. J Neurosci. 1989;9:3146–3168. doi: 10.1523/JNEUROSCI.09-09-03146.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- Douglas WW, Ritchie JM, Straub RW. The role of nonmyelinated fibres in signalling cooling of the skin. J Physiol. 1960;150:266–283. doi: 10.1113/jphysiol.1960.sp006386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815–2823. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesler GJ, Jr., Cliffer KD. Postsynaptic dorsal column pathway of the rat. II. Evidence against an important role in nociception. Brain Res. 1985;326:347–356. doi: 10.1016/0006-8993(85)90044-7. [DOI] [PubMed] [Google Scholar]
- Giesler GJ, Jr., Nahin RL, Madsen AM. Postsynaptic dorsal column pathway of the rat. I. Anatomical studies. J Neurophysiol. 1984;51:260–275. doi: 10.1152/jn.1984.51.2.260. [DOI] [PubMed] [Google Scholar]
- Giuffrida R, Rustioni A. Dorsal root ganglion neurons projecting to the dorsal column nuclei of rats. J Comp Neurol. 1992;316:206–220. doi: 10.1002/cne.903160206. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Horch KW, Tuckett RP, Burgess PR. A key to the classification of cutaneous mechanoreceptors. J Invest Dermatol. 1977;69:75–82. doi: 10.1111/1523-1747.ep12497887. [DOI] [PubMed] [Google Scholar]
- Iggo A. Cutaneous mechanoreceptors with afferent C fibres. J Physiol. 1960;152:337–353. doi: 10.1113/jphysiol.1960.sp006491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A, Andres KH. Morphology of cutaneous receptors. Annu Rev Neurosci. 1982;5:1–31. doi: 10.1146/annurev.ne.05.030182.000245. [DOI] [PubMed] [Google Scholar]
- Iggo A, Muir AR. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969;200:763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KO, Hsiao SS. Neural mechanisms of tactual form and texture perception. Annu Rev Neurosci. 1992;15:227–250. doi: 10.1146/annurev.ne.15.030192.001303. [DOI] [PubMed] [Google Scholar]
- Klein R, Lamballe F, Bryant S, Barbacid M. The trkB tyrosine protein kinase is a receptor for neurotrophin-4. Neuron. 1992;8:947–956. doi: 10.1016/0896-6273(92)90209-v. [DOI] [PubMed] [Google Scholar]
- Klein R, Nanduri V, Jing SA, Lamballe F, Tapley P, Bryant S, Cordon-Cardo C, Jones KR, Reichardt LF, Barbacid M. The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell. 1991;66:395–403. doi: 10.1016/0092-8674(91)90628-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerber HR, Brown PB. Somatotopic organization of hindlimb cutaneous nerve projections to cat dorsal horn. J Neurophysiol. 1982;48:481–489. doi: 10.1152/jn.1982.48.2.481. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol. 1997;78:1841–1850. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- Kumazawa T, Perl ER. Primate cutaneous sensory units with unmyelinated (C) afferent fibers. J Neurophysiol. 1977;40:1325–1338. doi: 10.1152/jn.1977.40.6.1325. [DOI] [PubMed] [Google Scholar]
- Liu Q, Vrontou S, Rice FL, Zylka MJ, Dong X, Anderson DJ. Molecular genetic visualization of a rare subset of unmyelinated sensory neurons that may detect gentle touch. Nat Neurosci. 2007;10:946–948. doi: 10.1038/nn1937. [DOI] [PubMed] [Google Scholar]
- Luo W, Enomoto H, Rice FL, Milbrandt J, Ginty DD. Molecular identification of rapidly adapting mechanoreceptors and their developmental dependence on ret signaling. Neuron. 2009;64:841–856. doi: 10.1016/j.neuron.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwrath SL, Lawson JJ, Anderson CE, Albers KM, Koerber HR. Overexpression of neurotrophin-3 enhances the mechanical response properties of slowly adapting type 1 afferents and myelinated nociceptors. Eur J Neurosci. 2007;26:1801–1812. doi: 10.1111/j.1460-9568.2007.05821.x. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- Olausson H, Wessberg J, Morrison I, McGlone F, Vallbo A. The neurophysiology of unmyelinated tactile afferents. Neurosci Biobehav R. 2010;34:185–191. doi: 10.1016/j.neubiorev.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Rice FL, Albrecht PJ. Cutaneous mechanisms of tactile perception: morphological and chemical organization of the innervation to the skin. In: Basbaum AI, Kaneko A, Shepherd GM, Westheimer G, editors. The Senses: A Comprehensive Reference. Academic Press; San Diego: 2008. pp. 1–32. [Google Scholar]
- Rotolo T, Smallwood PM, Williams J, Nathans J. Genetically-directed, cell type-specific sparse labeling for the analysis of neuronal morphology. PloS One. 2008;3:e4099. doi: 10.1371/journal.pone.0004099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucky CL, DeChiara T, Lindsay RM, Yancopoulos GD, Koltzenburg M. Neurotrophin 4 is required for the survival of a subclass of hair follicle receptors. J Neurosci. 1998;18:7040–7046. doi: 10.1523/JNEUROSCI.18-17-07040.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi-Iwanaga H. Three-dimensional microanatomy of longitudinal lanceolate endings in rat vibrissae. J Comp Neurol. 2000;426:259–269. doi: 10.1002/1096-9861(20001016)426:2<259::aid-cne7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Olausson H, Wessberg J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. J Neurophysiol. 1999;81:2753–2763. doi: 10.1152/jn.1999.81.6.2753. [DOI] [PubMed] [Google Scholar]
- Vielkind U, Sebzda MK, Gibson IR, Hardy MH. Dynamics of Merkel cell patterns in developing hair follicles in the dorsal skin of mice, demonstrated by a monoclonal antibody to mouse keratin 8. Acta Anat (Basel) 1995;152:93–109. doi: 10.1159/000147688. [DOI] [PubMed] [Google Scholar]
- Wellnitz SA, Lesniak DR, Gerling GJ, Lumpkin EA. The regularity of sustained firing reveals two populations of slowly adapting touch receptors in mouse hairy skin. J Neurophysiol. 2010;103:3378–3388. doi: 10.1152/jn.00810.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury CJ, Ritter AM, Koerber HR. Central anatomy of individual rapidly adapting low-threshold mechanoreceptors innervating the “hairy” skin of newborn mice: early maturation of hair follicle afferents. The Journal of comparative neurology. 2001;436:304–323. [PubMed] [Google Scholar]
- Woodbury CJ, Koerber HR. Central and peripheral anatomy of slowly adapting type I low-threshold mechanoreceptors innervating trunk skin of neonatal mice. J Comp Neurol. 2007;505:547–561. doi: 10.1002/cne.21517. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Fitzgerald M. Somatotopic organization of cutaneous afferent terminals and dorsal horn neuronal receptive fields in the superficial and deep laminae of the rat lumbar spinal cord. J Comp Neurol. 1986;251:517–531. doi: 10.1002/cne.902510407. [DOI] [PubMed] [Google Scholar]
- Zotterman Y. Touch, pain and tickling: an electro-physiological investigation on cutaneous sensory nerves. J Physiol. 1939;95:1–28. doi: 10.1113/jphysiol.1939.sp003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.