Abstract
Little data are available regarding the safety and immunologic response to pandemic H1N1 influenza vaccine in recipients of allogeneic hematopoietic stem cell transplantation (HSCT). We measured serum antibody titers against A/California/7/2009 H1N1 using a hemagglutination inhibition assay in 82 allogeneic HSCT recipients who received the 2009 H1N1 vaccine between November 2009 and January 2010 after it became available at our institution. The median time between HSCT and vaccination was 19 months (range, 2.5–94 months), and the median time from vaccination to specimen collection was 56 days (range, 14–140 days). Seroprotective antibody titers (hemagglutination inhibition titer ≥1:40) against 2009 H1N1 influenza A virus were detected in 51% of patients. The presence of chronic graft-versus-host disease and type of conditioning regimen did not affect the rate of detection of seroprotective titers after vaccination. Patients were more likely to have a seroprotective titer the farther away from HSCT they were (adjusted odds ratio, 1.79 per year; 95% confidence interval, 1.12–2.85). Rituximab administration in the year before vaccination was associated with a lack of seroprotective titer (adjusted odds ratio, 0.11; 95% confidence interval, 0.01–0.97). The vaccine was safe and well tolerated. Strategies are needed to improve the influenza vaccine response in this population, especially those receiving immunotherapy.
Keywords: Immune response, Swine Influenza, rituximab, bone marrow transplantation
INTRODUCTION
Influenza A is an important pathogen in allogeneic hematopoietic stem cell transplantation (HSCT) recipients, with reported rates of pneumonia of 29%–80% and case fatality rates of 10%–25% [1–7]. In April 2009, a novel H1N1 influenza A virus of swine origin was identified in Mexico and spread rapidly throughout the world, reaching pandemic levels. Given that the 2009 H1N1 virus continues to circulate globally, and the fact that the 2009 H1N1 antigen will be a component of the standard influenza vaccination moving forward, we investigated the safety and serologic titers to the monovalent 2009 H1N1 vaccination in allogeneic HSCT recipients at our institution, and examined factors associated with the serologic response in this population.
MATERIALS AND METHODS
Outpatient allogeneic HSCT recipients at our institution who received unadjuvanted 2009 H1N1 vaccine (15 μg of hemagglutinin antigen, lot nos. 102046P1, 102130P1, 102131P1, and 102138P1; Novartis Vaccines, Cambridge, MA) between November 2009 and January 2010 were studied. Only serum samples collected at subsequent clinic visits between December 2009 and April 2010 were analyzed. No baseline (ie, prevaccination) serum samples were available. This study was approved by the Office for Human Research Studies at Dana-Farber/Harvard Cancer Center.
Pertinent clinical data, including patient demographic information, underlying diseases, graft donor source and date, conditioning regimen, graft-versus-host disease (GVHD), immunosuppressant use, previous influenza vaccinations, influenza-like illnesses, use of oseltamivir, results of any diagnostic testing for influenza infection between April 2009 and blood collection time, and any adverse events potentially associated with H1N1 vaccination, were recorded. Anti-influenza antibody titers were measured by Focus Diagnostics (Cypress, CA) using a standard hemagglutination inhibition (HAI) assay [8]. All specimens were tested in triplicate against H1N1: A3/California/7/2009, H1N1: A1/Brisbane/59/2007, H3N2: A2/Brisbane/10/2007, and B strain: B/Brisbane/60/2008. The primary immunogenicity endpoint was the proportion of subjects with a geometric mean antibody titer of ≥1:40, which is the typical minimum titer associated with protection as defined by the Centers for Disease Control and Prevention and the Food and Drug Administration [8]. Logistic regression was used for univariate and multivariate analyses. All statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
Serum samples from 82 allogeneic HSCT recipients who returned to clinic following receipt of the 2009 H1N1 vaccination during the study period were available for analysis. Clinical characteristics are summarized in Table 1. Two additional patients had documented H1N1 influenza before vaccination and were excluded from the analysis; both had high HAI titers. Patients received the vaccination and returned for follow-up at the discretion of the treating physician. The median patient age at the time of vaccination was 55 years (range, 20–71 years). The median time between HSCT to vaccination was 578 days (interquartile range [IQR], 358–879 days; range, 77–2819 days), and the median time between vaccination and specimen collection was 56 days (range, 14–140 days). The median donor age was 40 years (IQR, 30–47 years; range, 23–65 years). Forty-one patients had chronic GVHD at time of vaccination, and two patients had acute GVHD. Sixty-one patients were receiving an immunosuppressive medication at time of vaccination. Thirty-two patients had received the 2009 seasonal influenza vaccine.
Table 1.
Patient Characteristics and Serologic Response Rates to the 2009 H1N1 Vaccination
| n | % | Seroprotective Titers (HAI Titer ≥1:40) against 2009 H1N1 Influenza A Virus | P Value | |
|---|---|---|---|---|
| Number of patients | 82 | 100% | 51.2% | |
| Median age, years (IQR, range) | 55 (47–61, 20–71) | |||
| <60 | 56 | 68.3% | 51.8% | 1.00 |
| ≥60 | 26 | 31.7% | 50.0% | |
| Sex | ||||
| Male | 40 | 48.8% | 45.0% | .38 |
| Female | 42 | 51.2% | 57.1% | |
| Race/ethnicity | .71 | |||
| White | 76 | 92.7% | 52.6% | |
| Nonwhite | 6 | 7.3% | 33.3% | |
| Black | 4 | 4.9% | ||
| Asian | 2 | 2.4% | ||
| Donor age, years (IQR, range) | 40 (30–47, 23–65) | |||
| Underlying disease | ||||
| Acute myelogenous leukemia | 26 | 31.7% | 57.7% | |
| Chronic lymphoblastic leukemia | 12 | 14.6% | 41.7% | |
| Myelodysplastic syndrome | 12 | 14.6% | 41.7% | |
| Non-Hodgkin lymphoma | 10 | 12.2% | 40.0% | |
| Aacute lymphoblastic leukemia | 10 | 12.2% | 30.0% | |
| Chronic myelogenous leukemia | 4 | 4.9% | 75.0% | |
| Multiple myeloma | 4 | 4.9% | 75.0% | |
| Other | 4 | 4.9% | 100.0% | |
| Type of transplantation | .19 | |||
| Matched related donor | 35 | 42.7% | 60.0%* | |
| Unrelated donor | 47 | 57.3% | 44.7%* | |
| Matched unrelated donor | 35 | 42.7% | 45.7% | |
| Mismatched unrelated donor | 9 | 11.0% | 44.4% | |
| Cord blood transplant | 3 | 3.7% | 33.3% | |
| Conditioning regimen | ||||
| Myeloablative | 38 | 46.3% | 47.4% | .66 |
| Nonmyeloablative | 44 | 53.7% | 54.5% | |
| GVHD at vaccination | ||||
| No GVHD | 39 | 47.6% | 59.0% | .19 |
| Any GVHD | 43 | 52.4% | 44.2% | |
| Acute | 2 | 2.4% | ||
| Chronic | 41 | 50.0% | ||
| Immunosuppression at time of vaccination | 61 | 74.4% | 47.5% | .32 |
| None | 21 | 25.6% | 61.9% | |
| Tacrolimus | 46 | 56.1% | 50.0% | .83 |
| No tacrolimus | 36 | 43.9% | 52.8% | |
| Sirolimus | 28 | 34.1% | 53.6% | .82 |
| No sirolimus | 54 | 65.9% | 50.0% | |
| MMF | 18 | 21.9% | 33.3% | .11 |
| No MMF | 64 | 78.1% | 56.2% | |
| Prednisone | 45 | 54.9% | 44.4% | .19 |
| No prednisone | 37 | 45.1% | 59.5% | |
| Past season influenza vaccination | ||||
| 2009 | 32 | 39.0% | 43.7% | .36 |
| No 2009 | 50 | 61.0% | 56.0% | |
| 2008 | 31 | 37.8% | 58.1% | .37 |
| No 2008 | 51 | 62.2% | 47.1% | |
| 2007 | 17 | 20.7% | 70.6% | .10 |
| No 2007 | 65 | 79.3% | 46.1% | |
| 2006 | 11 | 13.4% | 63.6% | .52 |
| No 2006 | 71 | 86.6% | 49.3% | |
| Median time from HSCT to vaccination, days | 578 | |||
| IQR, 358–879 | ||||
| Range, 77–2819 | ||||
| Time from HSCT to vaccination | .09 | |||
| <6 months | 8 | 9.8% | 37.5% | |
| 6 months-1 year | 14 | 17.0% | 50.0% | |
| 1–2 years | 31 | 37.8% | 38.7% | |
| > 2 years | 29 | 35.4% | 69.0% | |
| Median time from vaccination to specimen collection, days | 56 | |||
| IQR, 39–80 | ||||
| Range, 14–140 | ||||
| Intravenous immunoglobulin between vaccination date and sampling date | 6 | 7.3% | 33.3% | |
| No intravenous immunoglobulin | 76 | 92.7% | 52.6% | .43 |
| Rituximab within 1 year before vaccination | 11 | 13.4% | 9.1% | .003 |
| No rituximab | 71 | 86.6% | 57.7% | |
| Alemtuzumab | 1 | 1.2% | 0% | .49 |
| No alemtuzumab | 81 | 98.8% | 51.8% | |
| Influenza-like illness | 16 | 19.5% | 43.7% | .58 |
| No influenza-like illness | 66 | 80.5% | 53.0% | |
| Causes of influenza-type illness | ||||
| Respiratory syncytial virus | 1 | 1.2% | ||
| Parainfluenza | 2 | 2.4% | ||
| Unknown | 13 | 15.8% | ||
| History of exposure | 1 | 1.2% | ||
| Oseltamivir treatment | 8 | 9.8% | .48 | |
| Influenza testing | ||||
| Negative | 14 | 17.0% | ||
| Not performed | 68 | 83.0% | ||
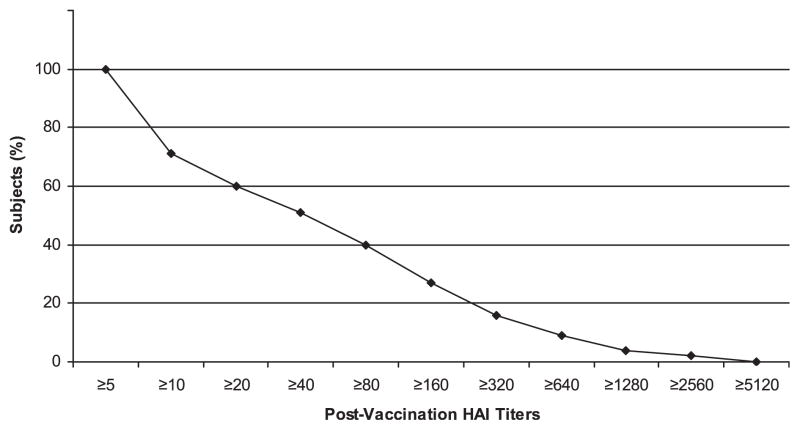
Seroprotective titers (HAI titer ≥1:40) against 2009 H1N1 influenza A virus were detected in 51.2% of the patients (Table 1). Figure 1 presents the HAI titer distribution in the study group. HAI titers ≥1:40 to the seasonal influenza strains were variable: 50% to 2007 H1N1, 57.3% to 2007 H3N2, and 20.7% to 2008 B.
Figure 1.
Reverse cumulative distribution curve of HAI titers.
Age ≥60 years, sex, race/ethnicity, HSCT type (donor source), conditioning regimen, presence or absence of GVHD, type of immunosuppression, previous seasonal influenza vaccination, history of influenza-like illness, or history of oseltamivir treatment did not influence the rate of achieving a seroprotective titer against 2009 H1N1 on univariate or multivariate analysis. The relevant factors affecting seroprotection against 2009 H1N1 are summarized in Table 2. Of note, no patient studied underwent T cell–depleted transplantation. In the year before vaccination, rituximab was administered to 7 patients as part of a clinical trial for chronic GVHD prophylaxis and to 4 patients who received rituximab to treat chronic GVHD. Rituximab therapy (375 mg/m2) in the year preceding vaccination was associated with a significantly diminished rate of seroprotective titers to the 2009 H1N1 vaccination (9.1%; adjusted odds ratio [OR], 0.11; 95% confidence interval [CI], 0.01–0.97; P = .04). Patients who received mycophenolate mofetil (MMF) therapy had a lower rate of seroprotective titers compared with those who did not receive MMF (33.3% vs 56.2%); however, the difference was not statistically significant (P = .11). Seroprotective titer rates were higher in patients with longer intervals between HSCT and vaccination (adjusted OR, 1.79 per year after HSCT; 95% CI, 1.12–2.85; P = .01). The rate of seroprotection was 37% in patients who received the 2009 H1N1 vaccine less than 6 months after HSCT, 50.0% in those who did so between 6 months and 1 year after HSCT, 38.7% in those who did so between 1 and 2 years after HSCT, 69% in those who did so more than 2 years after HSCT. The 2009 H1N1 vaccine was safe and well tolerated; no significant vaccine associated adverse events were noted. No patient had confirmed H1N1 influenza infection from the time of vaccination to the time of specimen collection.
Table 2.
Seroprotective Titers against 2009 H1N1 Influenza A Virus and the Influence of Relevant Variables
| Variable | n | 2009 H1N1 Titer ≥1:40 | Univariate OR (95% CI) | Multivariate OR (95% CI) | P Value |
|---|---|---|---|---|---|
| Age ≥60 years | 26 | 50.0% | 0.93 (0.37–2.36) | 0.93 (0.31–2.78) | .89 |
| GVHD | 43 | 44.2% | 0.55 (0.23–1.32) | 0.59 (0.14–2.43) | .47 |
| Rituximab use within 1 year of vaccination | 11 | 9.1% | 0.07 (0.009–0.60) | 0.11 (0.01–0.97) | .04 |
| MMF use | 18 | 33.3% | 0.39 (0.13–1.16) | 0.41 (0.09–1.80) | .24 |
| Prednisone use | 45 | 44.4% | 0.54 (0.23–1.32) | 0.80 (0.22–2.98) | .74 |
| Time to vaccination, per year after HSCT | — | — | 1.58 (1.09–2.30) | 1.79 (1.12–2.85) | .01 |
DISCUSSION
The serologic response rate to the seasonal influenza vaccine and the clinical efficacy of vaccination are reportedly influenced by various factors, including age, timing of vaccination after HSCT, GVHD, and exposure to immunosuppressant medications (eg, steroids, rituximab, alemtuzumab) [9–14]. A poor serologic response to vaccine antigens has been documented in HSCT recipients receiving seasonal influenza vaccination within the first 6 months after HSCT [11]. A comparison of humoral and cellular immune responses to influenza vaccination in HSCT recipients and healthy volunteers revealed that overall, the HSCT recipients had a lower protective humoral response to the vaccination, with a particularly diminished response seen in those vaccinated within the first 6 months after transplantation; however, the vaccine still elicited a strong T cell–mediated immune response in both groups [10,15]. We found a 37% rate of seroprotection in the patients who received 2009 H1N1 vaccination less than 6 months after HSCT; our sample was small, however. The variable rate of seasonal trivalent influenza vaccination in our cohort (39% in 2009, 37.8% in 2008, 20.7% in 2007, and 13.4% in 2006) makes it difficult to discern the overall vaccine immune response to these antigens versus possible previous seasonal influenza infection. Overall, seroprotection against all of the influenza viruses observed was modest, even in patients who received the trivalent vaccine, with particularly low titers to influenza B, consistent with previous reports [16,17].
In our study cohort, the overall rate of seroprotection after 2009 H1N1 vaccination was modest (51.2%) compared with the rate of 96.7% (95% CI, 91.7%–98.7%) observed in the general population [8]. Historically, the response rate to seasonal influenza vaccination in HSCT recipients has been found to be blunted compared with healthy individuals (29%–34% vs 46%–62%) [12]. Rituximab therapy initiated within 1 year of vaccination was significantly associated with a decreased rate of seroprotective titers. This finding might be attributed to the prolonged depletion of B lymphocytes after rituximab administration [18], or might be related to loss of antigen presentation or B cells’ function. A poor response to seasonal influenza vaccination has been reported in patients who received rituximab or alemtuzumab therapy for lymphoma [9]. As reported previously with seasonal influenza vaccination [11], a longer interval between HSCT and influenza vaccination is correlated with better serologic response to the 2009 H1N1 influenza vaccination, with an increasing OR of 1.79 (95% CI, 1.12–2.85) per year after HSCT for achieving a seroprotective level. Importantly, the presence of chronic GVHD, concurrent immunosuppressive therapy (including prednisone), and the type of conditioning regimen did not influence the rate of seroprotection against 2009 H1N1 influenza virus in our cohort. Previous studies found lower titers in patients with GVHD and patients who received more intensive conditioning regimens [11,14]; although these observations are plausible, the small number of patients makes the data less reliable.
Although our analysis lacks baseline 2009 H1N1 antibody titers because it was carried out in an epidemic setting, with vaccinations administered as they became available, it is highly unlikely that our findings were affected by preexisting immunity to H1N1 from infection. Such an effect would lead to an overestimation of the seroprotective titer rate. Preexisting antibody titers against 2009 H1N1 influenza A virus in up to 34% of individuals born before 1950 have been reported [19]; however, our data show no difference in the seroprotective titer rate between patients or donors older and younger than age 60. We also found no difference in the seroprotective titer rate between patients with and without an influenza-like illness, patients with and without oseltamivir therapy, and patients with and without seasonal influenza vaccination. In 2 recent studies, vaccination with seasonal nonadjuvanted or adjuvanted influenza vaccines induced little or no cross-reactive antibody response to 2009 H1N1 in any age group [19,20]. The 2009 H1N1 vaccine proved safe and well tolerated, with no significant vaccine-associated adverse events noted, in agreement with another study [21].
In summary, we found a modest rate of seroprotective titers against 2009 H1N1 influenza virus in our cohort of allogeneic HSCT recipients. Based on these data, administration of influenza vaccination relatively early after transplantation should be considered in all HSCT recipients. Given the modest response rate, strategies to improve influenza vaccine response are needed, and patients should continue to use protective measures to prevent viral respiratory infections. These data further demonstrate the impact of rituximab on the response to the influenza vaccine and may have implications for the response to other vaccines as well.
Acknowledgments
We thank David W. Kubiak, PharmD, BCPS, Anne McDonnell, PharmD, Emily Mui, PharmD, and Juliette Kim, PharmD for their help in retrieving pharmacy data on vaccine administration oseltamivir, intravenous immunoglobulin, rituximab, and alemtuzumab use. This study was supported in part by National Cancer Institute Grant CA142106 and the Jock and Bunny Adams Education and Research Fund.
Footnotes
Financial disclosure: The authors have no conflicts of interest to disclose.
References
- 1.Ljungman P. Respiratory virus infections in bone marrow transplant recipients: the European perspective. Am J Med. 1997;102:44–47. doi: 10.1016/s0002-9343(97)00010-7. [DOI] [PubMed] [Google Scholar]
- 2.Ljungman P. Respiratory virus infections in stem cell transplant patients: the European experience. Biol Blood Marrow Transplant. 2001;7(Suppl):5S–7S. doi: 10.1053/bbmt.2001.v7.pm11777102. [DOI] [PubMed] [Google Scholar]
- 3.Ljungman P, Ward KN, Crooks BN, et al. Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2001;28:479–484. doi: 10.1038/sj.bmt.1703139. [DOI] [PubMed] [Google Scholar]
- 4.Nichols WG, Guthrie KA, Corey L, et al. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004;39:1300–1306. doi: 10.1086/425004. [DOI] [PubMed] [Google Scholar]
- 5.Whimbey E, Elting LS, Couch RB, et al. Influenza A virus infections among hospitalized adult bone marrow transplant recipients. Bone Marrow Transplant. 1994;13:437–440. [PubMed] [Google Scholar]
- 6.Bowden RA. Respiratory virus infections after marrow transplant: the Fred Hutchinson Cancer Research Center experience. Am J Med. 1997;102:27–30. doi: 10.1016/s0002-9343(97)00007-7. [DOI] [PubMed] [Google Scholar]
- 7.Champlin RE, Whimbey E. Community respiratory virus infections in bone marrow transplant recipients: the M.D. Anderson Cancer Center experience. Biol Blood Marrow Transplant. 2001;7(Suppl):8S–10S. doi: 10.1053/bbmt.2001.v7.pm11777103. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg ME, Lai MH, Hartel GF, et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med. 2009;361:2405–2413. doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- 9.Ljungman P, Nahi H, Linde A. Vaccination of patients with haematological malignancies with one or two doses of influenza vaccine: a randomised study. Br J Haematol. 2005;130:96–98. doi: 10.1111/j.1365-2141.2005.05582.x. [DOI] [PubMed] [Google Scholar]
- 10.Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant. 2008;42:637–641. doi: 10.1038/bmt.2008.264. [DOI] [PubMed] [Google Scholar]
- 11.Engelhard D, Nagler A, Hardan I, et al. Antibody response to a two-dose regimen of influenza vaccine in allogeneic T cell-depleted and autologous BMT recipients. Bone Marrow Transplant. 1993;11:1–5. [PubMed] [Google Scholar]
- 12.Pauksen K, Linde A, Hammarstrom V, et al. Granulocyte macrophage colony-stimulating factor as immunomodulating factor together with influenza vaccination in stem cell transplant patients. Clin Infect Dis. 2000;30:342–348. doi: 10.1086/313663. [DOI] [PubMed] [Google Scholar]
- 13.Fiore AE, Shay DK, Broder K, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57(RR-7):1–60. [PubMed] [Google Scholar]
- 14.Machado CM, Cardoso MR, da Rocha IF, et al. The benefit of influenza vaccination after bone marrow transplantation. Bone Marrow Transplant. 2005;36:897–900. doi: 10.1038/sj.bmt.1705159. [DOI] [PubMed] [Google Scholar]
- 15.Avetisyan G, Aschan J, Hassan M, et al. Evaluation of immune responses to seasonal influenza vaccination in healthy volunteers and in patients after stem cell transplantation. Transplantation. 2008;86:257–263. doi: 10.1097/TP.0b013e3181772a75. [DOI] [PubMed] [Google Scholar]
- 16.Yalcin SS, Kondolot M, Albayrak N, et al. Serological response to influenza vaccine after hematopoetic stem cell transplantation. Ann Hematol. 2010 Sep;89(9):913–918. doi: 10.1007/s00277-009-0897-1. [DOI] [PubMed] [Google Scholar]
- 17.Egoz N, Morag B, Klingberg W, et al. Influenza immunization: serologic and clinical responses in military units. Infection. 1977;5:71–75. doi: 10.1007/BF01642083. [DOI] [PubMed] [Google Scholar]
- 18.Cerny T, Borisch B, Introna M, et al. Mechanism of action of rituximab. Anticancer Drugs. 2002;13(Suppl 2):S3–S10. doi: 10.1097/00001813-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 19.Hancock K, Veguilla V, Lu X, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–1952. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 20.Echevarria-Zuno S, Mejia-Arangure JM, Mar-Obeso AJ, et al. Infection and death from influenza A H1N1 virus in Mexico: a retrospective analysis. Lancet. 2009;374:2072–2079. doi: 10.1016/S0140-6736(09)61638-X. [DOI] [PubMed] [Google Scholar]
- 21.Redelman-Sidi G, Sepkowitz KA, Huang CK, et al. 2009 H1N1 influenza infection in cancer patients and hematopoietic stem cell transplant recipients. J Infect. 2010 Apr;60(4):257–263. doi: 10.1016/j.jinf.2010.01.009. [DOI] [PubMed] [Google Scholar]