Abstract
BACKGROUND
Pre-eclampsia (PE) is a serious hypertensive disorder of pregnancy characterized by excessive production of a soluble form of the vascular endothelial growth factor (VEGF) receptor-1, termed soluble fms-like tyrosine kinase-1 (sFlt-1). This placental-derived factor is believed to be a key contributor to the clinical features of PE. Women with PE are also characterized by the presence of autoantibodies, termed angiotensin type 1 receptor activating autoantibody (AT1-AA), that activate the major angiotensin receptor, AT1. These autoantibodies cause clinical features of PE and elevated sFlt-1 when injected into pregnant mice. The research reported here used this autoantibody-injection model of PE to assess the therapeutic potential of recombinant VEGF121, a relatively stable form of the natural ligand.
METHODS
Immunoglobulin G (IgG) from women with PE was injected into pregnant mice with or without continuous infusion of recombinant VEGF121. Injected mice were monitored for symptoms of PE.
RESULTS
As a result of infusion of recombinant VEGF121 autoantibody-induced hypertension (systolic blood pressure) was reduced from 159 ± 5 to 124 ± 5 mm Hg, proteinuria from 111 ± 16 to 40 ± 5 mg protein/mg creatinine and blood urea nitrogen levels from 31 ± 1 mg/ dl to 18 ± 2 mg/dl, P < 0.05. Histological analysis revealed that autoantibody-induced glomerular damage including the narrowing of Bowman’s space and occlusion of capillary loop spaces was largely prevented by VEGF121 infusion. Finally, impaired placental angiogenesis resulting from AT1-AA injection was significantly improved by VEGF121 infusion.
CONCLUSIONS
The infusion of recombinant VEGF121 significantly attenuated autoantibody-induced features of PE.
Keywords: angiotensin receptor agonistic autoantibody, blood pressure, hypertension, pre-eclampsia, recombinant VEGF121; sFlt-1
Pre-eclampsia (PE) is a serious complication of pregnancy and a leading cause of maternal and neonatal morbidity and mortality.1–3 It is a multisystem disorder generally appearing after the 20th week of gestation and characterized by hypertension, proteinuria, inflammation, and endothelial dysfunction.4–6 Despite intensive research efforts and several large clinical trials, the underlying cause of PE remains a mystery and treatment options continue to be unsatisfactory. It is a commonly held belief that “toxic factors” secreted by the placenta into the maternal circulation are responsible for systemic endothelial dysfunction, hypertension and multi-organ damage.7,8 Prominent among such factors is soluble fms-like tyrosine kinase-1 (sFlt-1), a soluble form of the vascular endothelial growth factor (VEGF) receptor-1. While the data linking enhanced sFlt-1 production in the pathogenesis of PE are compelling, the mechanisms accounting for enhanced production and release of sFlt-1 from placentas of women with PE are not well understood.
We initially showed that angiotensin II stimulates sFlt-1 synthesis and secretion by the placenta during pregnancy.9 Realizing that angiotensin II levels are not increased in women with PE over that occurring during normotensive (NT) pregnancies, we tested the possibility that angiotensin type 1 receptor agonistic autoantibodies (AT1-AAs) harbored by women with PE contribute to increased production of sFlt-1. We found that immunoglobulin G (IgG) from these women, in contrast to IgG from NT pregnant women, stimulates the synthesis and secretion of sFlt-1 via AT1 receptor (AT1R) activation in pregnant mice, human placental explants, and in human trophoblast cells.10 Similar findings have been recently reported by Parrish et al.11 In addition, in women with PE, AT1-AA titers are proportional to serum levels of sFlt-1.12 Overall our published studies suggest that angiotensin II is a significant physiological regulator of sFlt-1 synthesis and secretion during normal pregnancy and that the excessive accumulation of sFlt-1 observed in women with PE is due to the additional activation of AT1Rs mediated by AT1-AAs. Excessive production of sFlt-1 is considered harmful because, as a soluble form of the VEGF receptor-1, it binds to free VEGF and thereby disrupts normal pro-angiogenic signaling. It is this antiangiogenic feature of sFlt-1 that is believed to contribute to the clinical symptoms of PE.13,14 Although PE is a human condition that has not been observed to occur naturally in animals, a number of experimentally-induced animal models, characterized with excessive production of sFlt-1, have been described.15–18 The research reported here used an autoantibody-injection model of PE in pregnant mice to assess the therapeutic potential of VEGF121 infusion to prevent autoantibody-induced clinical features of PE.
Methods
Chemicals
Recombinant mouse VEGF121 (cat. no. CYT-574) was purchased from ProSpec-Tany TechnoGene Ltd. (Rehovot, Israel). Losartan was a generous gift from Merck Research Laboratory (Rahway, NJ). The 7-amino acid (7-aa) peptide (AFHYESQ), an epitope sequence present on the second extracellular loop of the AT1R that is recognized by AT1-AA, was synthesized at the Baylor College of Medicine, Protein Chemistry Core Laboratory (Houston, TX). The specificity of this 7-aa peptide in neutralizing the AT1-AA mediated actions has been verified previously.16,19 Protein G Sepharose 4 Fast Flow, used for IgG isolation, was purchased from Amersham Pharmacia Biotech (Uppsala, Sweden). All other chemicals used in the present study were of high quality grade and were obtained from Sigma Aldrich (St. Louis, MO).
Animals
Eight-week-old timed pregnant C57BL/6 mice (mated with syngeneic males) were obtained from Harlan Laboratories (Indianapolis, IN). The day the copulation plug was detected is designated E0. The mice were housed in the animal care facility of the University of Texas, Houston and had access to food and water ad libitum. Blood was collected from the heart at the time of killing on day E18. All the protocols involving animal studies were reviewed and approved by the Institutional Animal Welfare Committee of the University of Texas Houston Health Science Center.
IgG injection, VEGF121 infusion, losartan and 7-aa peptide administration
We have used our established adoptive transfer animal model of PE as previously described.16 For IgG injections, mice were anesthetized with isofluorane and concentrated IgG (800 μg) purified from 200 μl of patient’s serum (NT or pre-eclamptic) was introduced into embryonic day 13 and 14 pregnant mice by retro orbital injection. Some of the mice (n = 5) injected with the IgG from pre-eclamptic patients were infused with recombinant mouse VEGF121 (180 μg/kg body weight/day on E13) for 5 days using Alzet osmotic minipumps implanted subcutaneously (model 1007D, Alzet, Cupertino, CA). The dose was chosen based on the report by Gilbert et al.20 showing that this dose had pronounced therapeutic benefit in a rat model of PE. Losartan (0.24 mg), an AT1R antagonist, or a 7-aa epitope peptide (1.5 mg) that prevents autoantibody-induced AT1R activation were co-administered with the IgGs. Beginning with the first IgG injection blood pressure was measured daily by the tail cuff method as described below.
Blood pressure measurement
Blood pressure was monitored at the same time daily using a carotid catheter-calibrated eight chamber tail-cuff system (CODA, Kent Scientific, Torrington, CT). Mice were kept warm using a warming pad. After an initial acclimatization of the mice for five cycles, blood pressure was monitored over a period of 20 cycles and averaged for the final blood pressure measurement.
Renal function studies
Urine for creatinine, protein and sodium analysis was collected by placing mice in metabolic cages for 24 h. Total protein in the urine was determined after suitable dilution by the BCA method using a kit (Pierce, Rockford, IL). Creatinine, sodium (urinary and blood) and blood urea nitrogen (BUN) levels were determined at the University of Texas, MD Anderson Cancer Center, Laboratory of Veterinary Medicine.
Hematoxylin and eosin (H&E) staining and quantification in kidneys and placenta
Kidneys and placentas were harvested from the mice on E18, fixed in 4% formaldehyde, dehydrated, and embedded in paraffin. Sections of 4 μm were cut, stained with H&E using standard techniques and analyzed by light microscopy. The extent of renal damage was assessed by quantifying the glomeruli that showed characteristic features of damage in PE: decreased Bowman’s space and occlusion of capillary loop spaces. To examine those features, the glomeruli were counted in 6–9 fields of randomized and blinded slides (×10 magnification), with each field having at least 16–22 glomeruli. The glomeruli in each field were given a score based on the amount of capillary space evident within the Bowman’s capsule. A highest score of 5 was accorded to glomeruli with a normal amount of capillary space within Bowman’s capsule. A score of 1 was assigned to the glomeruli that showed complete loss of capillary space and an intermediate score of 3 was assigned to the glomeruli that displayed reduced, but not completely obliterated, capillary space. The scores for each field were divided by the number of glomeruli to get an average score per glomerulus for each field.
Similarly placental histological quantification was carried by quantifying the number of calcifications/field under ×10 magnification. Placental sections were examined under the microscope and the number of calcifications was counted in each field and then plotted as number of calcifications recorded per field.
CD34 immunostaining in placenta and quantification
Placentas were harvested from the mice on E18, fixed in 4% formaldehyde, dehydrated, and embedded in paraffin. Sections of 4 μm were cut and stained with anti mouse CD34 (cat. no. 553731, BD Pharmingen, San Diego, CA) at a dilution of 1:100 in a humidified chamber at 37 °C for 2 h. Following the primary antibody incubation, anti rat IgG HRP detection kit (cat. no. 551013, BD Pharmingen) was used was used to detect the CD34 staining. The immunohistochemical staining for CD34 positive stains (brown stains) in the labyrinth zone of the placenta was quantified by Image-Pro Plus software (Media Cybernetics, Bethesda, MD). The density of the brown staining (positive for CD34) was measured. The average densities of 6–10 areas per placenta was determined and averaged to get a mean value.
Statistical analysis
Results are expressed as mean ± s.e.m. All the data were subjected to statistical analysis using one-way analysis of variance followed by the Newman Keuls post hoc test or student’s t-test to determine the significance between groups. Statistical programs were run by GraphPad Prism 5, statistical software (GraphPad, San Diego, CA). Statistical significance was set at P < 0.05.
RESULTS
VEGF121 infusion attenuates AT1-AA induced hypertension in pregnant mice
To assess the potential role of VEGF therapy to blunt autoantibody-induced features of PE, we treated autoantibody-injected pregnant mice with recombinant murine VEGF121 administered by an osmotic minipump implanted subcutaneously. We found that blood pressure increased significantly in the mice injected with IgG isolated from women with PE (PE-IgG) relative to that of mice injected with IgG from NT pregnant women (NT-IgG) (Figure 1a). In contrast, the increased blood pressure seen in PE-IgG-injected pregnant mice was completely abolished by co-injection with losartan or a 7-aa epitope peptide (Figure 1b). These findings demonstrate the autoantibody in pre-eclamptic women is capable of increasing blood pressure in pregnant mice via AT1R activation. Additional findings show that the autoantibody-induced hypertension was almost completely blocked by infusion of VEGF121 (159 ± 5 to 124 ± 5 mm Hg, P < 0.05) in pregnant mice (Figure 1a,b).
Figure 1.
Vascular endothelial growth factor (VEGF121) infusion prevents autoantibody-induced hypertension in pregnant mice. A key feature of pre-eclampsia (PE), hypertension, present in the PE-immunoglobulin G (IgG)-injected pregnant mice, was reduced by chronic infusion of VEGF121. In addition, losartan or the 7-amino acid (7-aa) eiptope peptide also reduced autoantibody-induced hypertension. Blood pressure measured (a) on a daily base and (b) on gestation day 18. n = 4–10. * P < 0.05 vs. normotensive (NT)-IgG treatment. **P < 0.05 vs. PE-IgG treatment.
In vivo effect of VEGF121 on renal dysfunction seen in autoantibody-injected pregnant mice
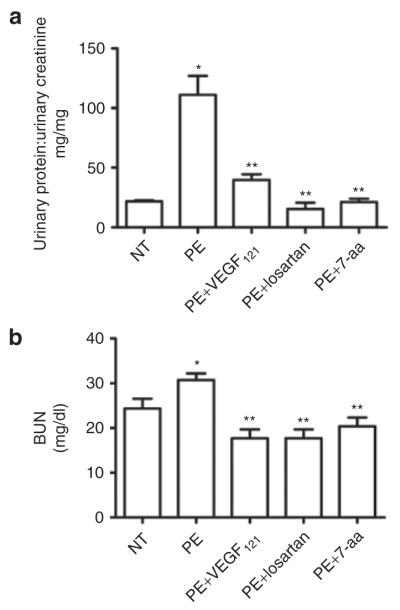
We assessed the effect of VEGF121 on proteinuria, another key maternal feature of PE. The ratio of total urinary protein:creatinine was significantly higher in pregnant mice injected with IgG from women with PE (111 ± 16 mg/ml) compared to mice injected with IgG from NT pregnant women (22 ± 1 mg/ml, P < 0.05) (Figure 2a). Co-injection with losartan or the 7-aa epitope peptide completely blocked autoantibody-induced proteinuria (Figure 2a), indicating the requirement for AT1R activation. VEGF121 infusion significantly decreased urinary protein excretion (40 ± 5 mg/ml, P < 0.05) in mice injected with IgG from women with PE (Figure 2a).
Figure 2.
Vascular endothelial growth factor (VEGF121) infusion protects against antibody-induced renal dysfunction. (a) a key feature of pre-eclampsia (PE), proteinuria, present in the PE-immunoglobulin G (IgG)-injected pregnant mice, was reduced by treatment with VEGF121, losartan or 7-amino acid (7-aa) epitope peptide. (b) increased blood urea nitrogen (BUN) present in the PE-IgG-injected pregnant mice was reduced by the treatment with VEGF121, losartan or the 7-aa epitope peptide. n = 4–6. *P < 0.05 compared to normotensive (NT), **P < 0.05 compared to PE.
An increase in BUN is a physiological response to decreased blood flow in the kidneys and may indicate renal damage. BUN levels were significantly increased in pregnant mice injected with IgG from women with PE (31 ± 1 mg/dl) compared to mice injected with IgG from NT pregnant women (24 ± 2 mg/dl, P < 0.05, Figure 2b). The autoantibody-induced increase in BUN was prevented by co-injection with losartan or the 7-aa epitope peptide (18 ± 2 mg/dl and 20 ± 2 mg/dl respectively, P < 0.05 compared to PE, Figure 2b). Continuous infusion with VEGF121 prevented the increase in BUN that accompanied injection of pregnant mice with IgG from women with PE (18 ± 2 mg/dl, P < 0.05 compared to PE).
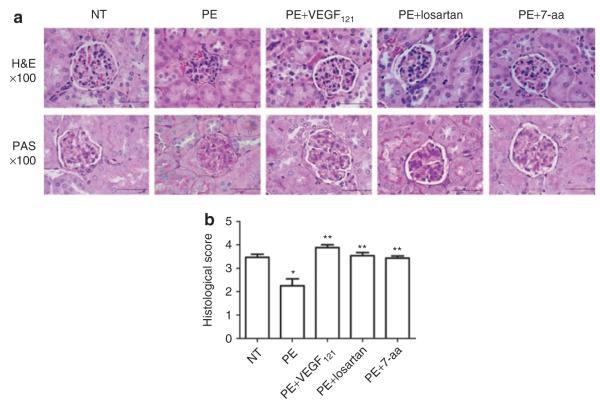
Autoantibody-induced changes in renal histology are prevented by VEGF121 treatment
The impaired renal function associated with PE is also accompanied with characteristic alterations in renal histology, especially that of the glomeruli.5,15,21,22 These renal histological changes are also seen in AT1-AA injected pregnant mice.16 To determine whether VEGF treatment can prevent autoantibody-induced renal histological changes, we injected pregnant mice on days 13 and 14 of gestation with IgG from women with PE in the presence or absence of infused VEGF121. Kidneys were harvested on embryonic day 18 for histological analysis. H&E staining revealed extensive renal damage as evidenced by narrowing or loss of Bowman’s capillary space in the majority of glomeruli (Figure 3a) seen in PE-IgG-injected pregnant mice. The kidneys of pregnant mice injected with IgG from NT pregnant women rarely showed signs of glomerular damage. VEGF121 infusion prevented the renal abnormalities in the mice that resulted from injection with IgG from women with PE (Figure 3a). Co-injection of losartan or the 7-aa epitope peptide also prevented the renal damage seen in the kidneys of the autoantibody-injected mice. The histo-morphometric quantification analysis showed that the extent of glomerular damage was significantly greater in PE-IgG-injected pregnant mice compared to that in NT-IgG-injected pregnant mice and that VEGF121 treatment attenuated the renal damage observed in autoantibody-injected mice (Figure 3b).
Figure 3.
Antibody-induced renal damage in pregnant mice was abrogated by infusion of vascular endothelial growth factor (VEGF121). (a) Hematoxylin and eosin (H&E) staining (top panels) and periodic acidic-Schiff (PAS) (lower panels) staining demonstrate the presence of condensed, hypercellular glomeruli, narrowing of Bowman’s space, and occlusion of capillary lumens in kidneys from pre-eclampsia (PE)-immunoglobulin G (IgG) injected mice. These features were largely prevented when auatoantibody-injected mice were treated with VEGF121, losartan or 7-amino acid (7-aa) epitope peptide (×100, scale bar: 50 μm). No remarkable PAS-positive materials were present in all five groups of the mice. (b) an arbitrary histological quantification among the different groups to assess the renal damage. n = 4–6. *P < 0.05 compared to normotensive (NT), **P < 0.05 compared to PE.
In addition to the H&E staining, we also performed periodic acid–Schiff staining of the kidney sections obtained from the mice from all groups. As with H&E stained sections we noted pathological alterations in glomerular structure, especially loss of Bowman’s space that was prevented by VEGF121, losartan or the 7-aa epitope peptide (Figure 3a, bottom panel). However, no periodic acid–Schiff-positive materials were observed in glomeruli in all of those groups. Overall, histological studies demonstrate typical renal damage in PE-IgG-injected pregnant mice as seen in humans and that these changes were prevented by infusion of VEGF121.
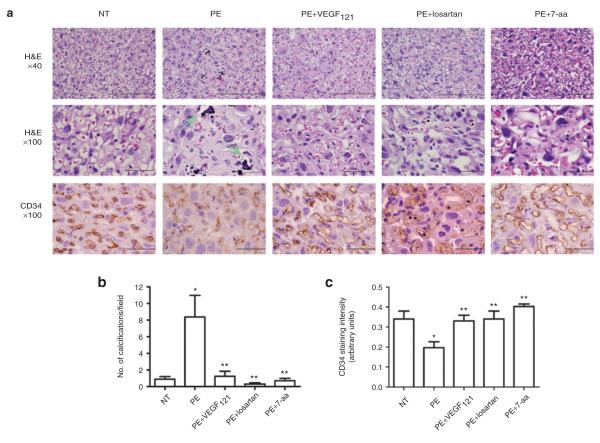
VEGF121 treatment reduces autoantibody-induced placental damage and improves placental endothelial cell content in autoantibody-injected mice
Impaired placental development is often associated with PE and has been observed in AT1-AA-injected pregnant mice.23 In agreement with previous reports23,24 histological analyses of placentas of antibody-injected pregnant mice revealed extensive cellular disorganization of the labyrinth zone and increased intraparenchymal calcifications in pregnant mice injected with IgG from women with PE (Figure 4). These histological changes were not seen in pregnant mice injected with IgG obtained from NT pregnant women. Continuous infusion of VEGF121 prevented the placental pathological changes induced by IgG from women with PE.
Figure 4.
Antibody-induced impairment and decreased angiogensis in mouse placentas can be attenuated by vascular endothelial growth factor (VEGF121) infusion. (a) mouse placentas assessed by hematoxylin and eosin (H&E) staining (top panels, ×40, scale bar: 100 μm and middle panels, ×100, scale bar: 50 μm) indicate that pre-eclampsia (PE)-immunoglobulin G (IgG) injected mice displayed heterogeneity in the labyrinth zone characterized by increased calcifications (arrow). Mouse placental angiogenesis assessed by CD34 immunohistochemical staining (lower panels, scale bar: 50 μm) indicate that PE-IgG injected mice presented decreased angiogenesis. VEGF121, losartan and 7-amino acid (7-aa) epitope peptide remarkably reduced placental calcification (top and middle panels) and improved angiogensis (lower panels). Mice injected with normotensive (NT)-IgG had unremarkable placentas. (b) An arbitrary histological quantification of the number of calcifications obtained per field under ×10 magnification. (c) Quantification of CD34 staining. *P < 0.05 compared to NT, **P < 0.05 compared to PE.
To determine the role of autoantibody-induced placenta-derived antiangiogenic factors in placenta impairment, we analyzed the vasculature of isolated mouse placentas by immunostaining using antibody recognizing CD34, an endothelial cell-specific marker. The results show that CD34 staining was decreased in the labyrinth zone of the placentas of mice injected with IgG from women with PE compared to those injected with IgG from NT pregnant women (Figure 4). Continuous infusion with VEGF121 attenuated autoantibody-induced reduction in angiogenesis as evidenced by improved CD34 staining. Similarly, co-injection of antibody with losartan or the 7-aa epitope peptide also showed increased CD34 staining (Figure 4). Taken together, our findings demonstrate that VEGF121 treatment can prevent AT1-AA-induced placental impairment and decreased endothelial cell content.
DISCUSSION
We report here that continuous infusion of VEGF121 in pregnant mice attenuated AT1-AA-induced hypertension and proteinuria, the hallmark features of PE. In addition, we demonstrate that infusion of VEGF121 blunted other features induced by PE-IgG in the pregnant mice including kidney damage, renal dysfunction featured with increased BUN and placental impairment coupled with decreased endothelial cell content. Overall, our findings reveal that VEGF121 infusion is a promising therapeutic possibility to attenuate autoantibody-induced features of PE.
VEGF is important in renal function where it is highly expressed by glomerular podocytes25 and targets VEGF receptors present on glomerular endothelial cells.26 Initial evidence for the potential importance of VEGF signaling in renal–vascular function came indirectly from clinical trials of anti-VEGF therapy for the treatment of certain cancers, where unexpected side effects included hypertension and proteinuria.27 Maynard et al.5 realized that excessive sFlt-1, because of its anti-VEGF properties, could account for the same features in PE and tested this possibility by introducing recombinant adenoviruses encoding sFlt-1 into rats. Their results showed that sFlt-1 mediated a dose-dependent increase in hypertension, proteinuria and characteristic renal findings termed glomerular endotheliosis when overexpressed in pregnant or nonpregnant rats. Clinical studies have shown that the increase in sFlt-1 in pregnant women precedes the onset of symptoms of PE by several weeks28 and that the levels of circulating sFlt-1 achieved are proportional to disease severity. Other studies have shown that infusion of sFlt-1 via controlled release by osmotic minipumps resulted in hypertension and proteinuria in pregnant rats.29 In a rat model of PE based on experimentally-induced placental ischemia, excessive sFlt-1 production has also been implicated in hypertension and proteinuria.30 We have previously shown that increased sFlt-1 is also associated with the autoantibody-injection model of PE in pregnant mice. It is likely that the beneficial effects of VEGF in these animal models of PE results from neutralizing the antiangiogenic effects of excessive sFlt-1. Thus, the evidence implicating sFlt-1 in the pathogenesis of PE is considerable and compelling and the use of VEGF therapy to neutralize the effects of excessive sFlt-1 is especially attractive.
The placental ischemia model of PE in rats (reduced uteroplacental perfusion pressure (RUPP)) and the autoantibody-injection model in pregnant mice share a number of important features including elevated sFlt-1, tumor necrosis factor-α, interleukin-6, and AT -AA.30–33 1 Additional evidence shows that all of these molecules can induce hypertension and proteinuria when introduced into pregnant animals.5,11,16,31–35 It has also been shown that antagonism of each of these molecules significantly reduces hypertension and proteinuria, indicating that each contributes to disease pathology. Recent evidence supports a role for endothelin-1 as a mediator of sFlt-1-induced hypertension.36 In the latter studies infusion of sFlt-1 resulted in increased production of preproendothelin-1 mRNA in the kidney cortex and hypertension that was prevented by antagonism of the endothelin A receptor, the target of endothelin-1. Thus the hypertensive and proteinuric effects of sFlt-1 are observed in pregnant and nonpregnant animals and are mediated by endothelin-1 signaling pathways. The antihypertensive effects of VEGF therapy may be mediated by stimulating endothelial cells to produce nitric oxide and vasodilatory prostacyclins, resulting in vascular smooth muscle relaxation.37 Because VEGF stimulates the production of vasodilatory substances such as nitric oxide and prostacyclins it is also possible that VEGF121 therapy can function as a general antihypertensive agent to counteract the hypertensive effects of a number of molecules in addition to sFlt-1.
The major focus of the research reported in this study was to evaluate the therapeutic potential of VEGF121 in our autoantibody-injection model of PE in mice. For this reason we examined the potential therapeutic effect of VEGF121 with regard to the defining clinical features of the disease including hypertension, proteinuria, renal abnormalities, and endothelial damage. We have shown here that VEGF121 infusion prevents these autoantibody-induced features of PE. In view of the data presented here showing that VEGF infusion reduced autoantibody-induced placental damage and improves placental endothelial cell content it will be of interest to examine the effects of VEGF121 therapy on fetal and placental weights and development. This issue has been addressed in two experimental models of PE in rat, an adenovirus-induced sFlt-1 over expression model and the RUPP model. No reduction in placental and fetal size was reported for the sFlt-1 over expression model and no effect of VEGF treatment was observed.17 The RUPP model is characterized by reduced fetal weight and no reduction in placental size. The therapeutic dose of VEGF121 did not result in a change in either of these parameters.20 Thus, in the RUPP model of PE the reduction in fetal weight was not corrected by the dose of VEGF121 therapy that was most effective in preventing the maternal features of PE. We have recently reported that the introduction of AT1-AA into pregnant mice resulted in smaller placentas and fetuses.23 It will be interesting to examine the effects of VEGF121 therapy on placental and fetal weights and development in the autoantibody-injection model of PE in pregnant mice.
In conclusion, we provide for the first time in vivo evidence that infusion of VEGF121 significantly attenuates key features of PE induced by AT1-AA in pregnant mice. In addition to the antibody-injection model of PE presented here, the therapeutic benefit of VEGF121 infusion has also been demonstrated in two other animal models of PE. The first was a rat model of PE generated by adenoviral over expression of sFlt-1. In this model, VEGF121 therapy attenuates hypertension and prevents kidney damage.17 More recently the benefit of VEGF121 therapy was shown in an animal model of PE based on experimentally-induced RUPP20 Both of these models, as with the antibody-injection model used in the present report, are characterized by excessive production of sFlt-1. However, the RUPP model and the antibody-injection model are also characterized by increased production of other molecules that have been associated with PE, including tumor necrosis factor-α, interleukin-6, and sEng. Our findings are in agreement with those from the RUPP model and show that VEGF121 prevents hypertension and renal dysfunction in the presence of increased levels of multiple other factors associated with PE. Thus, the potential benefit of VEGF therapy has now been shown in three preclinical models of PE justifying serious consideration of this as a therapeutic approach for use in women with PE.
Acknowledgments
This work was supported by National Institute of Health Grants HL076558, HD34130, American Heart association Grant 10GRNT3760081 and grants from the March of Dimes Foundation and the Texas Higher Education Coordinating Board.
Footnotes
Disclosure: The authors declared no conflict of interest
References
- 1.Roberts JM, Pearson G, Cutler J, Lindheimer M. NHLBI Working Group on Research on Hypertension During Pregnancy. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003;41:437–445. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 2.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 3.Lindheimer MD, Umans JG. Explaining and predicting preeclampsia. N Engl J Med. 2006;355:1056–1058. doi: 10.1056/NEJMe068161. [DOI] [PubMed] [Google Scholar]
- 4.Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173–192. doi: 10.1146/annurev-pathol-121808-102149. [DOI] [PubMed] [Google Scholar]
- 5.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maynard SE, Venkatesha S, Thadhani R, Karumanchi SA. Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr Res. 2005;57:1R–7R. doi: 10.1203/01.PDR.0000159567.85157.B7. [DOI] [PubMed] [Google Scholar]
- 7.Barden A. Pre-eclampsia: contribution of maternal constitutional factors and the consequences for cardiovascular health. Clin Exp Pharmacol Physiol. 2006;33:826–830. doi: 10.1111/j.1440-1681.2006.04448.x. [DOI] [PubMed] [Google Scholar]
- 8.de Groot CJ, Taylor RN. New insights into the etiology of pre-eclampsia. Ann Med. 1993;25:243–249. doi: 10.3109/07853899309147870. [DOI] [PubMed] [Google Scholar]
- 9.Zhou CC, Ahmad S, Mi T, Xia L, Abbasi S, Hewett PW, Sun C, Ahmed A, Kellems RE, Xia Y. Angiotensin II induces soluble fms-Like tyrosine kinase-1 release via calcineurin signaling pathway in pregnancy. Circ Res. 2007;100:88–95. doi: 10.1161/01.RES.0000254703.11154.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou CC, Ahmad S, Mi T, Abbasi S, Xia L, Day MC, Ramin SM, Ahmed A, Kellems RE, Xia Y. Autoantibody from women with preeclampsia induces soluble Fms-like tyrosine kinase-1 production via angiotensin type 1 receptor and calcineurin/nuclear factor of activated T-cells signaling. Hypertension. 2008;51:1010–1019. doi: 10.1161/HYPERTENSIONAHA.107.097790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parrish MR, Murphy SR, Rutland S, Wallace K, Wenzel K, Wallukat G, Keiser S, Ray LF, Dechend R, Martin JN, Granger JP, LaMarca B. The effect of immune factors, tumor necrosis factor-alpha, and agonistic autoantibodies to the angiotensin II type I receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. Am J Hypertens. 2010;23:911–916. doi: 10.1038/ajh.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siddiqui AH, Irani RA, Blackwell SC, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: correlation with disease severity. Hypertension. 2010;55:386–393. doi: 10.1161/HYPERTENSIONAHA.109.140061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu B, Thornton C, Tooher J, Hennessy A. Exogenous soluble VEGF receptor-1 (sFlt-1) regulates Th1/Th2 cytokine production from normal placental explants via intracellular calcium. Hypertens Pregnancy. 2009;28:448–456. doi: 10.3109/10641950902777721. [DOI] [PubMed] [Google Scholar]
- 14.Karumanchi SA, Epstein FH. Placental ischemia and soluble fms-like tyrosine kinase 1: cause or consequence of preeclampsia? Kidney Int. 2007;71:959–961. doi: 10.1038/sj.ki.5002281. [DOI] [PubMed] [Google Scholar]
- 15.Mutter WP, Karumanchi SA. Molecular mechanisms of preeclampsia. Microvasc Res. 2008;75:1–8. doi: 10.1016/j.mvr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14:855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z, Zhang Y, Ma J Ying, Kapoun AM, Shao Q, Kerr I, Lam A, O’Young G, Sannajust F, Stathis P, Schreiner G, Karumanchi SA, Protter AA, Pollitt NS. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension. 2007;50:686–692. doi: 10.1161/HYPERTENSIONAHA.107.092098. [DOI] [PubMed] [Google Scholar]
- 18.Isler CM, Bennett WA, Rinewalt AN, Cockrell KL, Martin JN, Jr, Morrison JC, Granger JP. Evaluation of a rat model of preeclampsia for HELLP syndrome characteristics. J Soc Gynecol Investig. 2003;10:151–153. doi: 10.1016/s1071-5576(03)00009-1. [DOI] [PubMed] [Google Scholar]
- 19.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jüpner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension. 2010;55:380–385. doi: 10.1161/HYPERTENSIONAHA.109.141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henao DE, Saleem MA, Cadavid AP. Glomerular disturbances in preeclampsia: disruption between glomerular endothelium and podocyte symbiosis. Hypertens Pregnancy. 2010;29:10–20. doi: 10.3109/10641950802631036. [DOI] [PubMed] [Google Scholar]
- 22.Strevens H, Wide-Swensson D, Hansen A, Horn T, Ingemarsson I, Larsen S, Willner J, Olsen S. Glomerular endotheliosis in normal pregnancy and pre-eclampsia. BJOG. 2003;110:831–836. [PubMed] [Google Scholar]
- 23.Irani RA, Zhang Y, Blackwell SC, Zhou CC, Ramin SM, Kellems RE, Xia Y. The detrimental role of angiotensin receptor agonistic autoantibodies in intrauterine growth restriction seen in preeclampsia. J Exp Med. 2009;206:2809–2822. doi: 10.1084/jem.20090872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irani RA, Zhang Y, Zhou CC, Blackwell SC, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Autoantibody-mediated angiotensin receptor activation contributes to preeclampsia through tumor necrosis factor-alpha signaling. Hypertension. 2010;55:1246–1253. doi: 10.1161/HYPERTENSIONAHA.110.150540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hakroush S, Moeller MJ, Theilig F, Kaissling B, Sijmonsma TP, Jugold M, Akeson AL, Traykova-Brauch M, Hosser H, Hähnel B, Gröne HJ, Koesters R, Kriz W. Effects of increased renal tubular vascular endothelial growth factor (VEGF) on fibrosis, cyst formation, and glomerular disease. Am J Pathol. 2009;175:1883–1895. doi: 10.2353/ajpath.2009.080792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65:2003–2017. doi: 10.1111/j.1523-1755.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 27.Kuo CJ, Farnebo F, Yu EY, Christofferson R, Swearingen RA, Carter R, von Recum HA, Yuan J, Kamihara J, Flynn E, D’Amato R, Folkman J, Mulligan RC. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci USA. 2001;98:4605–4610. doi: 10.1073/pnas.081615298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 29.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294:H541–H550. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007;50:1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 31.Gilbert JS, Gilbert SA, Arany M, Granger JP. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension. 2009;53:399–403. doi: 10.1161/HYPERTENSIONAHA.108.123513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou CC, Irani RA, Zhang Y, Blackwell SC, Mi T, Wen J, Shelat H, Geng YJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody-mediated tumor necrosis factor-alpha induction contributes to increased soluble endoglin production in preeclampsia. Circulation. 2010;121:436–444. doi: 10.1161/CIRCULATIONAHA.109.902890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension. 2008;52:1168–1172. doi: 10.1161/HYPERTENSIONAHA.108.120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension. 2005;46:1022–1025. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 35.LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, Granger JP. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension. 2008;52:1161–1167. doi: 10.1161/HYPERTENSIONAHA.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy SR, LaMarca BB, Cockrell K, Granger JP. Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension. 2010;55:394–398. doi: 10.1161/HYPERTENSIONAHA.109.141473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibuya M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct. 2001;26:25–35. doi: 10.1247/csf.26.25. [DOI] [PubMed] [Google Scholar]