Abstract
Endoscopic submucosal dissection (ESD), an endoscopic procedure for the treatment of gastric epithelial neoplasia without lymph node metastases, spread rapidly, primarily in Japan, starting in the late 1990s. ESD enables en bloc resection of lesions that are difficult to resect using conventional endoscopic mucosal resection (EMR). However, in comparison to EMR, ESD requires a high level of endoscopic competence and a longer resection time. Thus, ESD is associated with a higher risk of adverse events, including intraoperative and postoperative bleeding and gastrointestinal perforation. In particular, because of a higher incidence of intraoperative bleeding with mucosal incision and submucosal dissection, which are distinctive endoscopic procedures in ESD, a strategy for endoscopic hemostasis, mainly by thermo-coagulation hemostasis using hemostatic forceps, is important. In addition, because of iatrogenic artificial ulcers that always form after ESD, endoscopic hemostasis and appropriate pharmacotherapy during the healing process are essential.
Keywords: Artificial ulcer, Endoscopic hemostasis, Endoscopic submucosal dissection, Gastric epithelial neoplasia, Hemostatic forceps
INTRODUCTION
Endoscopic submucosal dissection (ESD) is a novel en-doscopic procedure developed in the 1990s[1,2], and is characterized by the use of electrosurgical knives for mucosal incision and submucosal dissection[3-15]. In ESD, the resected size and shape of tumors can be controlled, and even lesions difficult to resect by endoscopic mucosal resection (EMR) can be resected en bloc by ESD. As this technique permits en bloc resection of tumors, ESD has the advantages of enabling accurate pathological assessment and reducing the risk of local recurrence[2,16-19].
However, ESD requires a higher level of endoscopic competence than EMR. In addition, as a result of ESD being used to treat larger lesions and lesions with ulcerative findings, operation time is longer, with a higher risk of adverse events such as bleeding and gastrointestinal perforation[20-29]. The incidence of procedure-related bleeding is higher with ESD than with EMR, and to permit safe completion of ESD, control of bleeding is very important. In this article, we discuss the characteristics of ESD-related bleeding (intraoperative and postoperative bleeding) and endoscopic hemostasis. Furthermore, to prevent postoperative bleeding, we also discuss the pharmacotherapy of artificial ulcers after ESD.
ENDOSCOPIC HEMOSTASIS USING HEMOSTATIC FORCEPS
Endoscopic hemostatic methods for peptic ulcers include various techniques, such as local injection of hypertonic saline-epinephrine (HSE) and ethanol, mechanical hemostasis using endoscopic hemoclips, and thermo-coagulation hemostasis[30,31]. Local injection of HSE alone is inferior to combination therapy with other hemostatic methods, but the clear superiority of any one method has not been definitively established[32]. Thermo-coagulation devices include contact thermal devices such as heater probes and hemostatic forceps, and non-contact thermal devices such as an argon plasma coagulator[33,34].
For hemostasis of ESD intraoperative bleeding, Enomoto et al[35] reported the usefulness of a method of thermo-coagulation hemostasis using monopolar hemostatic forceps in combination with an endoscope equipped with a water-jet system. Hemostatic technique in ESD, which differs from hemostasis for usual gastrointestinal bleeding, is often characterized by the need for repeated hemostasis during both mucosal incision and submucosal dissection. In addition, precise hemostatic maneuvers are required, in order not to interfere with the subsequent procedure after hemostatic treatment[36,37]. Therefore, hemostatic forceps, which enable reliable hemostasis when, with re-holding of the ruptured vessels permissible several times before coagulation, bleeding points can be accurately grasped, are useful for hemostasis in ESD-related bleeding[38,39] (Figure 1).
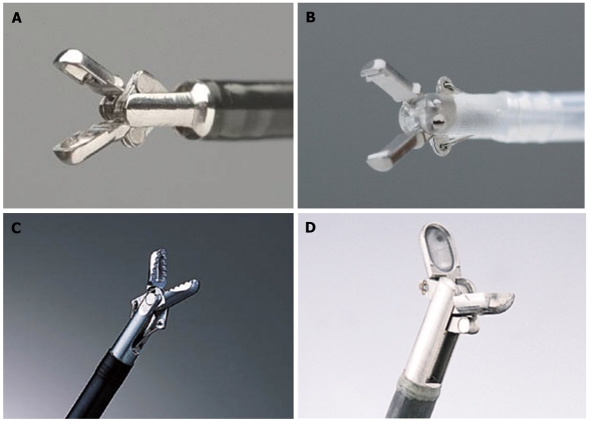
Figure 1.
Hemostatic forceps tips. A: Monopolar hemostatic forceps (HDB2422W; Pentax, Tokyo, Japan); B: Bipolar hemostaticforceps (H-S2518; Pentax, Tokyo, Japan); C: Hemostatic forceps (Coagrasper: FD-410LR; Olympus, Tokyo, Japan); D: Hot biopsy forceps (FD-1L-1; Olympus, Tokyo, Japan).
With wider use of ESD, hemostasis using hemostatic forceps has become routine at medical centers, and its usefulness for bleeding from exposed vessels at the base of peptic ulcers has also been reported[40,41]. Moreover, the usefulness not only of monopolar, but also of bipolar hemostatic forceps, has been reported[42].
MANAGEMENT OF BLEEDING DURING AND AFTER ESD
ESD-related bleeding includes intraoperative bleeding associated with procedures such as mucosal incision and submucosal dissection, and delayed bleeding, which occurs postoperatively from exposed vessels at ulcer bases. Appropriate management of each type of bleeding is required.
Endoscopic hemostasis for intraoperative bleeding
In ESD, the incidence of intraoperative bleeding, which is to some degree unavoidable given the nature of techniques such as incision and dissection, is as high as 22.6%[16]. In particular, with ESD for lesions in the upper third of the stomach, because of abundant vessels in the submucosa, the incidence of intraoperative bleeding is relatively high[43]. To predict intraoperative bleeding, identification of the submucosal vascular structure by preoperative endoscopic ultrasonography can be useful[44].
Of the series of techniques in ESD, bleeding is inevitable with submucosal local injection and mucosal incision because they are blind procedures in the vascular-rich submucosal tissue. To produce higher hemostatic ability, a small amount of epinephrine to a concentration of 0.0005% is added to the submucosal cushion (glyceol, Chugai Pharmaceutical Co., Tokyo Japan). On the other hand, during submucosal dissection, bleeding can be avoided at all sites by making every effort to visually identify vessels and not perform dissection blindly. Oyama et al[45] noted that identification of vessels prior to submucosal dissection and prophylactic thermo-coagulation are most important in preventing ESD intraoperative bleeding. Toyonaga et al[13,46] stated that knowing the correct layer of the submucosa containing fewer vessels and existing fibrous tissue, is important in reducing ESD intraoperative bleeding.
When bleeding occurs during ESD, by washing out the blood with the water-jet system and using a transparent attachment hood, a clear visual field can be maintained, and bleeding points can be rapidly identified[35]. For bleeding from vessels smaller than the electrosurgical knife tip or arm, hemostasis by thermo-coagulation with the knife is usually possible. For bleeding from vessels larger than the electrosurgical knife tip or arm, or bleeding for which hemostasis with the knife is difficult, hemostatic forceps are used (Figure 2). Fujishiro et al[47] reported that hemostatic forceps for vessels smaller than 2 mm in diameter, and hot biopsy forceps for vessels larger than 2 mm in diameter, are useful. When hemostasis by thermo-coagulation cannot be achieved, hemostasis using endoscopic hemoclips is necessary, so that subsequent procedures are not hindered.
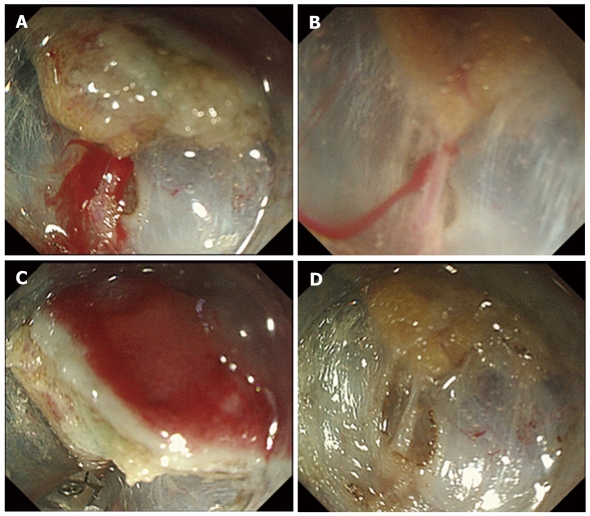
Figure 2.
Hemostatic procedure for endoscopic submucosal dissectionintraoperative bleeding using hemostatic forceps. A: Pulsatile bleeding is observed during submucosal dissection; B: By filling the tip attachment with water, the bleeding point can be pinpointed and identified; C: After identifying the bleeding point, the vessel is securely grasped by hemostatic forceps, and thermo-coagulation is performed; D: Complete hemostasis is achieved, without excessive coagulation.
Hemostasis for delayed bleeding
Delayed bleeding after ESD occurs in 0%-9% of cases[6,16,18,28,48-54] (Table 1). For resected lesions located in the middle and lower third of the stomach, the incidence is higher. Bleeding occurs when vessels at ulcer bases rupture due to physical stimulation by peristalsis or due to chemical stimulation, for example, by bile reflux[48]. Delayed bleeding often occurs within 24 h postoperatively and is related to lesion location, size, and ulcerfindings[48,55]. For delayed bleeding, in almost all cases, hemostasis is achieved with urgent endoscopic hemostasis[56]. However, cases requiring vascular embolization because endoscopic hemostasis could not be achieved[57], and cases complicated by disseminated intravascular coagulation the day after delayed bleeding[58] have been reported, so caution is necessary.
Table 1.
Delayed bleeding rate of endoscopic submucosal dissection for gastric epithelial neoplasia
| Author | Year | Total cases | Delayed bleeding (%) | En bloc resection rate (%) |
| Oda et al[48] | 2005 | 945 | 6 | 93 |
| Kakushima et al[49] | 2006 | 383 | 3.4 | 91 |
| Imagawa et al[18] | 2006 | 196 | 0 | 93 |
| Onozato et al[50] | 2006 | 171 | 7.6 | 94 |
| Oka et al[16] | 2006 | 195 | 6.2 | 83 |
| Hirasaki et al[51] | 2007 | 112 | 7.1 | 96 |
| Ono et al[6] | 2008 | 161 | 8.7 | 99 |
| Hoteya et al[52] | 2009 | 572 | 4.9 | 95 |
| Isomoto et al[53] | 2009 | 510 | 1.8 | 95 |
| Tsuji et al[54] | 2010 | 398 | 5.8 | NA |
| Akasaka et al[28] | 2011 | 1188 | 3.1 | 95 |
NA: Not analyzed.
To prevent delayed bleeding, prophylactic coagulation of exposed vessels at the bases of artificial ulcers that occur after ESD lesion resection is very useful. According to Takizawa et al[59], the cause of delayed bleeding is due more to insufficient prophylactic thermo-coagulation than insufficient primary hemostasis during ESD[60], because the site of delayed bleeding is not the site of endoscopic hemostasis during surgery. In addition, a study has been conducted on the prevention of delayed bleeding by evaluation of blood flow at ulcer bases using endoscopic Doppler ultrasound (US). Uedo et al[61], based on blood flow detected using Doppler US, reported that, by coagulation of vessels seen at artificial ulcer bases after ESD lesion resection, delayed bleeding is reduced, and unnecessary thermo-coagulation of vessels without blood flow can be avoided. On the other hand, Choi et al[62] reported that prophylactic closure of gastric EMR-induced ulcers with metal hemoclips prevent delayed bleeding.
In 2008, a survey of treatment methods for peptic and artificial ulcer bleeding was conducted at nine departments of high-volume center hospitals in Japan[63]. For endoscopic hemostasis of peptic ulcer bleeding, the number one method used was clipping (32.9%), followed by coagulation forceps (23.5%). In contrast, for artificial ulcer bleeding, coagulation forceps (77.8%) were used significantly more. In addition, the proportion of patients who underwent second-look endoscopy, compared to peptic ulcers, was significantly lower for artificial ulcers (86% and 71%, respectively).
The effectiveness of second-look endoscopy after hemostasis of peptic ulcer bleeding has previously been shown[64,65]. However, according to Goto et al[66], for artificial ulcers, no significant difference in the incidence of delayed bleeding before and after second-look endoscopy was found. This suggests that delayed bleeding after ESD, irrespective of whether second-look endoscopy is performed, may develop. However, for artificial ulcers located in the lower third of the stomach, compared to ulcers located in the upper and middle third of the stomach, because delayed bleeding occurs earlier, careful follow-up observation or early second-look endoscopy may be useful[54,66].
MANAGEMENT OF ARTIFICAL GASTRIC ULCERS AFTER ESD
Pharmacotherapy of artificial ulcers that develop after ESD lesion resection is also important to prevent delayed bleeding. However, management must take into account the differences in etiology between peptic ulcers and artificial ulcers after ESD.
Comparison of peptic ulcers and artificial ulcers
Currently, proton pump inhibitors (PPIs) are the drugs of first choice for treatment of peptic ulcers, and when a PPI cannot be used, an H2-receptor antagonist (H2RA) is selected. Treatment is generally for 8 wk. A meta-analysis of ulcer healing rates reported significantly higher ulcer healing rates with PPIs than with H2RAs[67,68]. In addition, in a meta-analysis of the efficacy of preventing recurrence of bleeding gastric ulcers, no differences in rebleeding rates, surgical intervention rates, or mortality rates between the two classes of drugs were reported[69].
The etiology of artificial ulcers after gastric ESD and peptic ulcers also differs greatly[70]. First, peptic ulcers develop, at least in part, due to hyperacidity, whereas artificial ulcers form in a hypoacidic environment in which there is severe mucosal atrophy. Second, peptic ulcers develop at sites where there is breakdown of gastric mucosal defense mechanisms, whereas artificial ulcers occur iatrogenically at sites where mucosal defense mechanisms are intact. Third, peptic ulcers include ulcers deeper than the submucosa, and inflammation spreads in the ulcer periphery, whereas artificial ulcers, because they basically occur due to submucosal dissection, are relatively shallow ulcers down to the submucosa, and the inflammation is localized. Despite these differences, treatment of an artificial ulcer after gastric ESD, based on treatment for a peptic ulcer, is empiric, with an anti-acid drug for 8 wk[63] (Table 2).
Table 2.
Healing process of gastric artificial ulcers after endoscopic submucosal dissection
| Author | Year | Total cases | Drugs administration | Weeks |
Ulcer healing rate (%) |
Average ulcer size |
||
| 4 wk | 8 wk | Maximal diameter (mm) | Resected area (mm2) | |||||
| Kakushima et al[71] | 2004 | 70 | PPI + sucralfate | 8 | NA | 100 | 34.7 | NA |
| Lee et al[76] | 2004 | 26 | OPZ 20 mg | 1 | 12 | NA | NA | 503 |
| 34 | OPZ 20 mg | 4 | 15 | NA | NA | 575 | ||
| Yamaguchi et al[78] | 2005 | 29 | OPZ 20 mg | 8 | NA | NA | 27.8 | NA |
| 28 | Famotidine 40 mg | 8 | NA | NA | 22.4 | NA | ||
| Uedo et al[79] | 2007 | 73 | RPZ 20 mg | 8 | NA | 83 | 41 | NA |
| 70 | Cimetidinde 800 mg | 8 | NA | 89 | 40.5 | NA | ||
| Asakuma et al[80] | 2009 | 28 | RPZ 20 mg + ES 3.0 g | 8 | 40.7 | 96.3 | NA | 1306 |
| 28 | RPZ 20 mg | 8 | 11.5 | 76.9 | NA | 1274 | ||
| Kato et al[81] | 2010 | 31 | RPZ 10 mg + rebamipide 300 mg | 4 | 68 | NA | 35 | NA |
| 31 | RPZ 10 mg | 4 | 35 | NA | 31 | NA | ||
| Fujiwara et al[82] | 2011 | 30 | RPZ 20 mg + rebamipide 300 mg | 8 | NA | 86.7 | 41 | 1453 |
| 31 | RPZ 20 mg | 8 | NA | 54.8 | 42.8 | 1521 | ||
| Niimi et al[77] | 2011 | 55 | RPZ 10 mg | 2 | NA | 80.0 | 32.7 | NA |
NA: Not analyzed; PPI: Proton pump inhibitor; OPZ: Omeprazole; RPZ: Rabeprazole; ES: Ecabet sodium.
Anti-acid drugs for artificial ulcers
For artificial ulcers that develop after ESD for gastric mucosal lesions without preoperative ulcer findings, Kakushima et al[71] reported that healing occurred within 8 wk with PPI administration for 8 wk, irrespective of ulcer size or location. In addition, factors that influence artificial ulcer healing such as artificial ulcer size, location, Helicobacter pylori infection status, and extent of gastric mucosal atrophy had no effect. However, with fibrosis deeper than the submucosa of lesions prior to ESD, healing may be delayed[72,73]. According to Huang et al[74], although the recurrence rate of ESD artificial ulcers is lower than that of peptic ulcers, Helicobacterpylori infection and lesion ulcer findings are risk factors for recurrence. In contrast, Oh et al[75] reported that, because the extent of healing of artificial ulcers 4 wk after ESD is determined by the size of the ulcer initially formed, the duration of PPI treatment should be decided based on this parameter.
For artificial ulcers after EMR, Lee et al[76] compared PPIs in 1-wk and 4-wk treatment groups. They found that, after 4 wk, ulcer size, stage, subjective symptoms, and use of other mucosal-protective antiulcer drugs did not significantly differ between the groups. Niimi et al[77] reported that administration of PPI for 2-wk for artificial ulcers after ESD may be sufficient to help them heal. These results suggest that, for artificial ulcers, unlike peptic ulcers, the importance of acid secretion inhibition in the ulcer healing process may be low.
Yamaguchi et al[78] compared PPI-treatment and H2RA-treatment groups in patients with artificial ulcers after EMR. They reported no differences in the incidence of delayed bleeding or ulcer size at 30 d and 60 d postoperatively. They did state that artificial ulcers healed more easily than peptic ulcers, and they concluded that, for artificial ulcers with severe bleeding within 24 h after surgery, treatment with H2RA drugs, whose onset of inhibition of gastric acid secretion is more rapid than that with PPIs, is appropriate.
Uedo et al[79] compared PPI-treatment and H2RA-treatment groups in patients with artificial ulcers after ESD. There were no differences in the incidence of delayed bleeding or ulcer healing rates between the groups. However, the cumulative non-bleeding rate using the Kaplan-Meier method was significantly higher in the PPI group. Moreover, on multivariate analysis, PPI treatment was an independent factor in reducing the rate of delayed bleeding. Their results suggested that PPIs are more effective than H2RAs for preventing ESD delayed bleeding.
For post-EMR ulcers and post-ESD ulcers, in terms of formation by endoscopic resection, with the exception of size, the pathophysiology is the same. However, in studies to date, with regard to ulcer healing and prevention of delayed bleeding when artificial ulcers are treated with acid secretion inhibitors, there is no agreement in the results. Regarding the need for and duration of treatment with acid secretion inhibitors for artificial ulcers, there is still room for debate.
Mucosal-protective antiulcer drugs in artificial ulcers
In the treatment of peptic ulcers, there is no evidence that combined therapy with a PPI and a mucosal-protective antiulcer drug is superior to a PPI alone. However, in artificial ulcers, an additive effect of mucosal-protective antiulcer drugs has been reported (Table 2). Asakuma et al[80] compared combined therapy with a PPI (rabeprazole 20 mg/d) and ecabet sodium (3.0 g/d) vs the PPI alone for artificial ulcers after ESD. At 4 wk and 8 wk, ulcer healing rates were significantly higher in the combined treatment group. In addition, Kato et al[81] compared combined therapy with a PPI (rabeprazole 10 mg/d) and rebamipide (300 mg/d) vs the PPI alone for artificial ulcers after ESD. At 4 wk, the ulcer scarring rate was significantly higher in the combined treatment group. Similarly, Fujiwara et al[82] compared combined therapy with a PPI (rabeprazole 20 mg/d) and rebamipide (300 mg/d) vs the PPI alone for artificial ulcers after ESD. At 8 wk, the ulcer scarring rate was significantly higher in the combined treatment group.
Thus, among the mucosal-protective antiulcer drugs, there are drugs that accelerate ulcer healing. This may be attributable to differences in the etiology between artificial ulcers and peptic ulcers, as previously mentioned, but further evidence must be accumulated.
CONCLUSION
With the increasing use of ESD for gastric epithelial neoplasia, management of ESD-related bleeding and artificial ulcers after lesion resection has become an important issue not only in Japan, but throughout the world. Therefore, more effective endoscopic hemostatic methods and appropriate pharmacotherapy of artificial ulcers, taking into account their etiology, are becoming increasingly important. Moreover, safer and more reliable ESD techniques must be developed.
ACKNOWLEDGMENTS
The authors would like to express their deepest thanks to Ms. Kazu Konishi for her excellent secretarial assistance.
Footnotes
Supported by A Grant-in-Aid for Cancer Research from the Ministry of Health, Labor and Welfare of Japan, in part
Peer reviewer: David Friedel, MD, Gastroenterology, Winthrop University Hospital, 222 Station Plaza North, Suite 428, Mineola, NY 11501, United States
S- Editor Yang XC L- Editor Webster JR E- Editor Li JY
References
- 1.Fujishiro M. Endoscopic submucosal dissection for stomach neoplasms. World J Gastroenterol. 2006;12:5108–5112. doi: 10.3748/wjg.v12.i32.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kakushima N, Fujishiro M. Endoscopic submucosal dissection for gastrointestinal neoplasms. World J Gastroenterol. 2008;14:2962–2967. doi: 10.3748/wjg.14.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225–229. doi: 10.1136/gut.48.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, Yoshida S. New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy. 2001;33:221–226. doi: 10.1055/s-2001-12805. [DOI] [PubMed] [Google Scholar]
- 5.Gotoda T. A large endoscopic resection by endoscopic submucosal dissection procedure for early gastric cancer. Clin Gastroenterol Hepatol. 2005;3:S71–S73. doi: 10.1016/s1542-3565(05)00251-x. [DOI] [PubMed] [Google Scholar]
- 6.Ono H, Hasuike N, Inui T, Takizawa K, Ikehara H, Yamaguchi Y, Otake Y, Matsubayashi H. Usefulness of a novel electrosurgical knife, the insulation-tipped diathermic knife-2, for endoscopic submucosal dissection of early gastric cancer. Gastric Cancer. 2008;11:47–52. doi: 10.1007/s10120-008-0452-0. [DOI] [PubMed] [Google Scholar]
- 7.Oyama T, Kikuchi Y. Aggressive endoscopic mucosal resection in the upper GI tract-hook knife EMR method. MInim Invasive Ther Allied Technol. 2002;11:291–295. doi: 10.1080/13645706.2003.11873728. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto H, Kawata H, Sunada K, Sasaki A, Nakazawa K, Miyata T, Sekine Y, Yano T, Satoh K, Ido K, et al. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy. 2003;35:690–694. doi: 10.1055/s-2003-41516. [DOI] [PubMed] [Google Scholar]
- 9.Inoue H, Kudo S. A novel procedure of en block EMR using triangle-tipped knife (abstract) Gastrointest Endosc. 2003;57:AB86. [Google Scholar]
- 10.Yahagi N, Fujishiro M, Kakushima N, Kobayashi K, Hashimoto T, Oka M, Iguchi M, Enomoto S, ichinose M, Niwa H, et al. Endoscopic submucosal dissection for early gastric cancer using the tip of an electrosurgical snare (thin type) Dig Endosc. 2004;16:34–38. [Google Scholar]
- 11.Fujishiro M, Yahagi N, Nakamura M, Kakushima N, Kodashima S, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A, et al. Endoscopic submucosal dissection for rectal epithelial neoplasia. Endoscopy. 2006;38:493–497. doi: 10.1055/s-2006-925398. [DOI] [PubMed] [Google Scholar]
- 12.Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Shimizu Y, Oka M, et al. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol. 2006;4:688–694. doi: 10.1016/j.cgh.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Toyonaga T, Nishino E, Dozaiku T, Ueda C, Hirooka T. Management to prevent bleeding during endoscopic submucosal dissection using the flush knife for gastric tumors. Dig Endosc. 2007;19 Suppl 1:S14–18. [Google Scholar]
- 14.Fujishiro M, Kodashima S, Goto O, Ono S, Muraki Y, Kakushima N, Omata M. Successful en bloc resection of superficial esophageal cancer treated by endoscopic submucosal dissection with a splash needle. Endoscopy. 2008;40 Suppl 2:E81–E82. doi: 10.1055/s-2007-995538. [DOI] [PubMed] [Google Scholar]
- 15.Akahoshi K, Akahane H. A new breakthrough: ESD using a newly developed grasping type scissor forceps for early gastrointestinal tract neoplasms. World J Gastrointest Endosc. 2010;2:90–96. doi: 10.4253/wjge.v2.i3.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877–883. doi: 10.1016/j.gie.2006.03.932. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe K, Ogata S, Kawazoe S, Watanabe K, Koyama T, Kajiwara T, Shimoda Y, Takase Y, Irie K, Mizuguchi M, et al. Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc. 2006;63:776–782. doi: 10.1016/j.gie.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 18.Imagawa A, Okada H, Kawahara Y, Takenaka R, Kato J, Kawamoto H, Fujiki S, Takata R, Yoshino T, Shiratori Y. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy. 2006;38:987–990. doi: 10.1055/s-2006-944716. [DOI] [PubMed] [Google Scholar]
- 19.Isomoto H, Yamaguchi N. Endoscopic submucosal dissection in the era of proton pump inhibitors. J Clin Biochem Nutr. 2009;44:205–211. doi: 10.3164/jcbn.SR09-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A, et al. Successful nonsurgical management of perforation complicating endoscopic submucosal dissection of gastrointestinal epithelial neoplasms. Endoscopy. 2006;38:1001–1006. doi: 10.1055/s-2006-944775. [DOI] [PubMed] [Google Scholar]
- 21.Fujishiro M. Perspective on the practical indications of endoscopic submucosal dissection of gastrointestinal neoplasms. World J Gastroenterol. 2008;14:4289–4295. doi: 10.3748/wjg.14.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujishiro M. Endoscopic submucosal dissection for gastric cancer. Curr Treat Options Gastroenterol. 2008;11:119–124. doi: 10.1007/s11938-008-0024-8. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka M, Ono H, Hasuike N, Takizawa K. Endoscopic submucosal dissection of early gastric cancer. Digestion. 2008;77 Suppl 1:23–28. doi: 10.1159/000111484. [DOI] [PubMed] [Google Scholar]
- 24.Cao Y, Liao C, Tan A, Gao Y, Mo Z, Gao F. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy. 2009;41:751–757. doi: 10.1055/s-0029-1215053. [DOI] [PubMed] [Google Scholar]
- 25.Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228–1235. doi: 10.1016/j.gie.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 26.Conlin A, Kaltenbach T, Kusano C, Matsuda T, Oda I, Gotoda T. Endoscopic resection of gastrointestinal lesions: advancement in the application of endoscopic submucosal dissection. J Gastroenterol Hepatol. 2010;25:1348–1357. doi: 10.1111/j.1440-1746.2010.06402.x. [DOI] [PubMed] [Google Scholar]
- 27.Onogi F, Araki H, Ibuka T, Manabe Y, Yamazaki K, Nishiwaki S, Moriwaki H. “Transmural air leak”: a computed tomographic finding following endoscopic submucosal dissection of gastric tumors. Endoscopy. 2010;42:441–447. doi: 10.1055/s-0029-1244013. [DOI] [PubMed] [Google Scholar]
- 28.Akasaka T, Nishida T, Tsutsui S, Michida T, Yamada T, Ogiyama H, Kitamura S, Ichiba M, Komori M, Nishiyama O, et al. Short-term outcomes of endoscopic submucosal dissection (ESD) for early gastric neoplasm: multicenter survey by osaka university ESD study group. Dig Endosc. 2011;23:73–77. doi: 10.1111/j.1443-1661.2010.01062.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim YJ, Park DK. Management of complications following endoscopic submucosal dissection for gastric cancer. World J Gastrointest Endosc. 2011;3:67–70. doi: 10.4253/wjge.v3.i4.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conway JD, Adler DG, Diehl DL, Farraye FA, Kantsevoy SV, Kaul V, Kethu SR, Kwon RS, Mamula P, Rodriguez SA, et al. Endoscopic hemostatic devices. Gastrointest Endosc. 2009;69:987–996. doi: 10.1016/j.gie.2008.12.251. [DOI] [PubMed] [Google Scholar]
- 31.Anjiki H, Kamisawa T, Sanaka M, Ishii T, Kuyama Y. Endoscopic hemostasis techniques for upper gastrointestinal hemorrhage: A review. World J Gastrointest Endosc. 2010;2:54–60. doi: 10.4253/wjge.v2.i2.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adler DG, Leighton JA, Davila RE, Hirota WK, Jacobson BC, Qureshi WA, Rajan E, Zuckerman MJ, Fanelli RD, Hambrick RD, et al. ASGE guideline: The role of endoscopy in acute non-variceal upper-GI hemorrhage. Gastrointest Endosc. 2004;60:497–504. doi: 10.1016/s0016-5107(04)01568-8. [DOI] [PubMed] [Google Scholar]
- 33.Watson JP, Bennett MK, Griffin SM, Matthewson K. The tissue effect of argon plasma coagulation on esophageal and gastric mucosa. Gastrointest Endosc. 2000;52:342–345. doi: 10.1067/mge.2000.108412. [DOI] [PubMed] [Google Scholar]
- 34.Fujishiro M, Yahagi N, Nakamura M, Kakushima N, Kodashima S, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A, et al. Safety of argon plasma coagulation for hemostasis during endoscopic mucosal resection. Surg Laparosc Endosc Percutan Tech. 2006;16:137–140. doi: 10.1097/00129689-200606000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Enomoto S, Yahagi N, Fujishiro M, Oka M, Kakushima N, Iguchi M, Yanaoka K, Arii K, Tamai H, Shimizu Y, et al. Novel endoscopic hemostasis technique for use during endoscopic submucosal dissection. Endoscopy. 2007;39 Suppl 1:E156. doi: 10.1055/s-2006-925254. [DOI] [PubMed] [Google Scholar]
- 36.Enomoto S, Yahagi N, Fujishiro M, Iguchi M, Ichinose M. Endoscopic hemostasis using high-frequency hemostatic forceps for hemorrhagic gastric ulcer. Nihon Rinsho. 2004;62:513–518. [PubMed] [Google Scholar]
- 37.Enomoto S, Yahagi N, Fujishiro M, Oka M, Muraki Y, Deguchi H, Ueda K, Inoue I, Maekita T, Magari H, et al. Assessment of intraoperative bleeding during endoscopic submucosal dissection and endoscopic hemostasis using high-frequency hemostatic forceps. J Wakayama Med. 2009;60:124–129. [Google Scholar]
- 38.Fujishiro M, Abe N, Endo M, Kawahara Y, Shimoda R, Nagata S, Homma K, Morita Y, Uedo N. Retrospective multicenter study concerning electrocautery forceps with soft coagulation for nonmalignant gastroduodenal ulcer bleeding in Japan. Dig Endosc. 2010;22 Suppl 1:S15–S18. doi: 10.1111/j.1443-1661.2010.00962.x. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida N, Naito Y, Kugai M, Inoue K, Wakabayashi N, Yagi N, Yanagisawa A, Yoshikawa T. Efficient hemostatic method for endoscopic submucosal dissection of colorectal tumors. World J Gastroenterol. 2010;16:4180–4186. doi: 10.3748/wjg.v16.i33.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagata S, Kimura S, Ogoshi H, Hidaka T. Endoscopic hemostasis of gastric ulcer bleeding by hemostatic forceps coagulation. Dig Endosc. 2010;22 Suppl 1:S22–S25. doi: 10.1111/j.1443-1661.2010.00973.x. [DOI] [PubMed] [Google Scholar]
- 41.Coumaros D, Tsesmeli N. Active gastrointestinal bleeding: use of hemostatic forceps beyond endoscopic submucosal dissection. World J Gastroenterol. 2010;16:2061–2064. doi: 10.3748/wjg.v16.i16.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kataoka M, Kawai T, Yagi K, Tachibana C, Tachibana H, Sugimoto H, Hayama Y, Yamamoto K, Nonaka M, Aoki T, et al. Clinical evaluation of emergency endoscopic hemostasis with bipolar forceps in non-variceal upper gastrointestinal bleeding. Dig Endosc. 2010;22:151–155. doi: 10.1111/j.1443-1661.2010.00949.x. [DOI] [PubMed] [Google Scholar]
- 43.Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264–269. doi: 10.1016/s0016-5107(88)71327-9. [DOI] [PubMed] [Google Scholar]
- 44.Kikuchi D, Iizuka T, Hoteya S, Yamashita S, Nakamura M, Kuroki Y, Mitani T, Fujimoto A, Matsui A, Nishida N, et al. Usefulness of endoscopic ultrasound for the prediction of intraoperative bleeding of endoscopic submucosal dissection for gastric neoplasms. J Gastroenterol Hepatol. 2011;26:68–72. doi: 10.1111/j.1440-1746.2010.06412.x. [DOI] [PubMed] [Google Scholar]
- 45.Oyama T, Tomori A, Hotta K, Miyata Y. Hemostasis with hook knife during endoscopic submucosal dissection. Dig Endosc. 2006;18 Suppl 1:S128–130. [Google Scholar]
- 46.Toyonaga T, Nishino E, Hirooka T, Ueda C, Noda K. Intraoperative bleeding in endoscopic submucosal dissection in the stomach and strategy for prevention and treatment. Dig Endosc. 2006;18 Suppl 1:S123–127. [Google Scholar]
- 47.Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Tateishi A, Omata M. Management of bleeding concerning endoscopic submucosal dissection with the flex knife for stomach neoplasm. Dig Endosc. 2006;18 Suppl 1:S119–122. [Google Scholar]
- 48.Oda I, Gotoda T, Hamanaka H, Eguchi T, Saito Y, Matsuda T, Bhandari P, Emura F, Saito D, Ono H. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc. 2005;17:54–58. [Google Scholar]
- 49.Kakushima N, Fujishiro M, Kodashima S, Muraki Y, Tateishi A, Omata M. A learning curve for endoscopic submucosal dissection of gastric epithelial neoplasms. Endoscopy. 2006;38:991–995. doi: 10.1055/s-2006-944808. [DOI] [PubMed] [Google Scholar]
- 50.Onozato Y, Ishihara H, Iizuka H, Sohara N, Kakizaki S, Okamura S, Mori M. Endoscopic submucosal dissection for early gastric cancers and large flat adenomas. Endoscopy. 2006;38:980–986. doi: 10.1055/s-2006-944809. [DOI] [PubMed] [Google Scholar]
- 51.Hirasaki S, Kanzaki H, Matsubara M, Fujita K, Ikeda F, Taniguchi H, Yumoto E, Suzuki S. Treatment of over 20 mm gastric cancer by endoscopic submucosal dissection using an insulation-tipped diathermic knife. World J Gastroenterol. 2007;13:3981–3984. doi: 10.3748/wjg.v13.i29.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoteya S, Iizuka T, Kikuchi D, Yahagi N. Benefits of endoscopic submucosal dissection according to size and location of gastric neoplasm, compared with conventional mucosal resection. J Gastroenterol Hepatol. 2009;24:1102–1106. doi: 10.1111/j.1440-1746.2009.05811.x. [DOI] [PubMed] [Google Scholar]
- 53.Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331–336. doi: 10.1136/gut.2008.165381. [DOI] [PubMed] [Google Scholar]
- 54.Tsuji Y, Ohata K, Ito T, Chiba H, Ohya T, Gunji T, Matsuhashi N. Risk factors for bleeding after endoscopic submucosal dissection for gastric lesions. World J Gastroenterol. 2010;16:2913–2917. doi: 10.3748/wjg.v16.i23.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929–942. doi: 10.1007/s00535-006-1954-3. [DOI] [PubMed] [Google Scholar]
- 56.Messmann H, Probst A. Management of endoscopic submucosal dissection complications. Endoscopy. 2009;41:712–714. doi: 10.1055/s-0029-1214992. [DOI] [PubMed] [Google Scholar]
- 57.Lee CK, Park JY, Lee TH, Lee SH, Chung IK, Park SH, Kim HS, Kim SJ. Superselective microcoil embolization for endoscopically uncontrollable bleeding after endoscopic submucosal dissection. Endoscopy. 2009;41 Suppl 2:E109–E110. doi: 10.1055/s-0029-1214627. [DOI] [PubMed] [Google Scholar]
- 58.Kang SH, Kim JI, Kim EM, Moon HS, Kim SH, Lee BS, Sung JK, Jeong HY. A rare case of disseminated intravascular coagulation after endoscopic submucosal dissection for early gastric cancer. Endoscopy. 2010;42 Suppl 2:E33–E34. doi: 10.1055/s-0029-1214438. [DOI] [PubMed] [Google Scholar]
- 59.Takizawa K, Oda I, Gotoda T, Yokoi C, Matsuda T, Saito Y, Saito D, Ono H. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection--an analysis of risk factors. Endoscopy. 2008;40:179–183. doi: 10.1055/s-2007-995530. [DOI] [PubMed] [Google Scholar]
- 60.Okano A, Hajiro K, Takakuwa H, Nishio A, Matsushita M. Predictors of bleeding after endoscopic mucosal resection of gastric tumors. Gastrointest Endosc. 2003;57:687–690. doi: 10.1067/mge.2003.192. [DOI] [PubMed] [Google Scholar]
- 61.Uedo N, Takeuchi Y, Ishihara R, Hanaoka N, Inoue T, Kizu T, Higashino K, Iishi H, Tatsuta M, Chak A, et al. Endoscopic Doppler US for the prevention of ulcer bleeding after endoscopic submucosal dissection for early gastric cancer: a preliminary study (with video) Gastrointest Endosc. 2010;72:444–448. doi: 10.1016/j.gie.2010.03.1128. [DOI] [PubMed] [Google Scholar]
- 62.Choi KD, Jung HY, Lee GH, Oh TH, Jo JY, Song HJ, Hong SS, Kim JH. Application of metal hemoclips for closure of endoscopic mucosal resection-induced ulcers of the stomach to prevent delayed bleeding. Surg Endosc. 2008;22:1882–1886. doi: 10.1007/s00464-008-9743-0. [DOI] [PubMed] [Google Scholar]
- 63.Fujishiro M, Abe N, Endo M, Kawahara Y, Shimoda R, Nagata S, Homma K, Morita Y, Uedo N. Current managements and outcomes of peptic and artificial ulcer bleeding in Japan. Dig Endosc. 2010;22 Suppl 1:S9–14. doi: 10.1111/j.1443-1661.2010.00961.x. [DOI] [PubMed] [Google Scholar]
- 64.Villanueva C, Balanzó J, Torras X, Soriano G, Sáinz S, Vilardell F. Value of second-look endoscopy after injection therapy for bleeding peptic ulcer: a prospective and randomized trial. Gastrointest Endosc. 1994;40:34–39. doi: 10.1016/s0016-5107(94)70006-0. [DOI] [PubMed] [Google Scholar]
- 65.Chiu PW, Lam CY, Lee SW, Kwong KH, Lam SH, Lee DT, Kwok SP. Effect of scheduled second therapeutic endoscopy on peptic ulcer rebleeding: a prospective randomised trial. Gut. 2003;52:1403–1407. doi: 10.1136/gut.52.10.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goto O, Fujishiro M, Kodashima S, Ono S, Niimi K, Hirano K, Yamamichi N, Koike K. A second-look endoscopy after endoscopic submucosal dissection for gastric epithelial neoplasm may be unnecessary: a retrospective analysis of postendoscopic submucosal dissection bleeding. Gastrointest Endosc. 2010;71:241–248. doi: 10.1016/j.gie.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 67.Di Mario F, Battaglia G, Leandro G, Grasso G, Vianello F, Vigneri S. Short-term treatment of gastric ulcer. A meta-analytical evaluation of blind trials. Dig Dis Sci. 1996;41:1108–1131. doi: 10.1007/BF02088227. [DOI] [PubMed] [Google Scholar]
- 68.Tunis SR, Sheinhait IA, Schmid CH, Bishop DJ, Ross SD. Lansoprazole compared with histamine2-receptor antagonists in healing gastric ulcers: a meta-analysis. Clin Ther. 1997;19:743–757. doi: 10.1016/s0149-2918(97)80098-7. [DOI] [PubMed] [Google Scholar]
- 69.Gisbert JP, González L, Calvet X, Roqué M, Gabriel R, Pajares JM. Proton pump inhibitors versus H2-antagonists: a meta-analysis of their efficacy in treating bleeding peptic ulcer. Aliment Pharmacol Ther. 2001;15:917–926. doi: 10.1046/j.1365-2036.2001.01012.x. [DOI] [PubMed] [Google Scholar]
- 70.Goto O, Fujishiro M, Kodashima S, Minatsuki C, Niimi K, Ono S, Yamamichi N, Koike K. Short-term healing process of artificial ulcers after gastric endoscopic submucosal dissection. Gut Liver. 2011;5:293–297. doi: 10.5009/gnl.2011.5.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kakushima N, Yahagi N, Fujishiro M, Iguchi M, Oka M, Kobayashi K, Hashimoto T, Omata M. The healing process of gastric artificial ulcers after endoscopic submucosal dissection. Dig Endosc. 2004;16:327–331. [Google Scholar]
- 72.Kakushima N, Fujishiro M, Yahagi N, Kodashima S, Nakamura M, Omata M. Helicobacter pylori status and the extent of gastric atrophy do not affect ulcer healing after endoscopic submucosal dissection. J Gastroenterol Hepatol. 2006;21:1586–1589. doi: 10.1111/j.1440-1746.2006.04321.x. [DOI] [PubMed] [Google Scholar]
- 73.Kakushima N, Fujishiro M, Kodashima S, Kobayashi K, Tateishi A, Iguchi M, Imagawa A, Motoi T, Yahagi N, Omata M. Histopathologic characteristics of gastric ulcers created by endoscopic submucosal dissection. Endoscopy. 2006;38:412–415. doi: 10.1055/s-2006-925166. [DOI] [PubMed] [Google Scholar]
- 74.Huang Y, Kakushima N, Takizawa K, Tanaka M, Ikehara H, Yamaguchi Y, Matsubayashi H, Ono H, Oishi T, Nakajima T. Risk factors for recurrence of artificial gastric ulcers after endoscopic submucosal dissection. Endoscopy. 2011;43:236–239. doi: 10.1055/s-0030-1255927. [DOI] [PubMed] [Google Scholar]
- 75.Oh TH, Jung HY, Choi KD, Lee GH, Song HJ, Choi KS, Chung JW, Byeon JS, Myung SJ, Yang SK, et al. Degree of healing and healing-associated factors of endoscopic submucosal dissection-induced ulcers after pantoprazole therapy for 4 weeks. Dig Dis Sci. 2009;54:1494–1499. doi: 10.1007/s10620-008-0506-5. [DOI] [PubMed] [Google Scholar]
- 76.Lee SY, Kim JJ, Lee JH, Kim YH, Rhee PL, Paik SW, Rhee JC. Healing rate of EMR-induced ulcer in relation to the duration of treatment with omeprazole. Gastrointest Endosc. 2004;60:213–217. doi: 10.1016/s0016-5107(04)01683-9. [DOI] [PubMed] [Google Scholar]
- 77.Niimi K, Fujishiro M, Goto O, Kodashima S, Minatsuki C, Hirayama I, Mochizuki S, Ono S, Yamamichi N, Kakushima N, et al. Prospective single-arm trial of two-week rabeprazole treatment for ulcer healing after gastric endoscopic submucosal dissection. Dig Endosc. 2011:In press. doi: 10.1111/j.1443-1661.2011.01178.x. [DOI] [PubMed] [Google Scholar]
- 78.Yamaguchi Y, Katsumi N, Tauchi M, Toki M, Nakamura K, Aoki K, Morita Y, Miura M, Morozumi K, Ishida H, et al. A prospective randomized trial of either famotidine or omeprazole for the prevention of bleeding after endoscopic mucosal resection and the healing of endoscopic mucosal resection-induced ulceration. Aliment Pharmacol Ther. 2005;21 Suppl 2:111–115. doi: 10.1111/j.1365-2036.2005.02484.x. [DOI] [PubMed] [Google Scholar]
- 79.Uedo N, Takeuchi Y, Yamada T, Ishihara R, Ogiyama H, Yamamoto S, Kato M, Tatsumi K, Masuda E, Tamai C, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol. 2007;102:1610–1616. doi: 10.1111/j.1572-0241.2007.01197.x. [DOI] [PubMed] [Google Scholar]
- 80.Asakuma Y, Kudo M, Matsui S, Okada M, Kawasaki M, Umehara Y, Ichikawa T, Kitai S. Comparison of an ecabet sodium and proton pump inhibitor (PPI) combination therapy with PPI alone in the treatment of endoscopic submucosal dissection (ESD)--induced ulcers in early gastric cancer: prospective randomized study. Hepatogastroenterology. 2009;56:1270–1273. [PubMed] [Google Scholar]
- 81.Kato T, Araki H, Onogi F, Ibuka T, Sugiyama A, Tomita E, Nagaki M, Moriwaki H. Clinical trial: rebamipide promotes gastric ulcer healing by proton pump inhibitor after endoscopic submucosal dissection--a randomized controlled study. J Gastroenterol. 2010;45:285–290. doi: 10.1007/s00535-009-0157-0. [DOI] [PubMed] [Google Scholar]
- 82.Fujiwara S, Morita Y, Toyonaga T, Kawakami F, Itoh T, Yoshida M, Kutsumi H, Azuma T. A randomized controlled trial of rebamipide plus rabeprazole for the healing of artificial ulcers after endoscopic submucosal dissection. J Gastroenterol. 2011;46:595–602. doi: 10.1007/s00535-011-0372-3. [DOI] [PubMed] [Google Scholar]