Abstract
Squamous cell carcinoma of the esophagus (SCCE) carries a poor prognosis due to late diagnosis. Early detection is highly desirable, since surgical and endoscopic resection offers the only possible cure for esophageal cancer. Population screening should be undertaken in high risk areas, and in low or moderate risk areas for people with risk factors (alcoholics, smokers, mate drinkers, history of head and neck cancer, achalasia and lye stricture of the esophagus). Esophageal balloon cytology is an easy and inexpensive sampling technique, but the current methods are insufficient for primary screening due to sampling errors. Conventional endoscopy with biopsy remains the standard procedure for the identification of pre-malignant and early malignant changes in esophageal mucosa and endoscopic detection. It may be enhanced by several techniques such as dye and optic chromoendoscopy, magnifying endoscopy, and optical-based spectroscopic and imaging modalities. Since more than 80% of SCCE deaths occur in developing countries, where expensive techniques such as narrow band imaging (NBI) and autofluorescence imaging are unavailable, the most cost-effective tool for targeting biopsies may be Lugol dye chromoendoscopy, since it is easy, accurate, inexpensive and available worldwide. In ideal conditions, or in developed countries, is it reasonable to think that optimal detection will require a combination of techniques, such as the combination of Lugol’s chromoendoscopy and NBI to identify esophageal areas that require further characterization by a high resolution technique. The efficacy and cost-effectiveness will determine whether these modalities will become part of standard endoscopy practice.
Keywords: Autofluorescence endoscopy, Early diagnosis, Esophageal cancer, Esophageal squamous cell carcinoma, Lugol’s solution, Narrow-band imaging endoscopy
INTRODUCTION
The cancers arising from the esophageal mucosa, including squamous cell carcinoma (SCCE) and adenocarcinoma (ADC), are the eighth most common cancers worldwide, with 482 000 new cases estimated in 2008, and are the sixth most common cause of death from cancer with 4 070 00 deaths in the same year[1]. The majority of new cases occur in developing countries accounting for 83% of cases and 86% of deaths, with incidence ratios varying 16-fold between high incidence regions, such as Southern and Eastern Africa and Eastern Asia, and low incidence regions like Western and Middle Africa and Central America[2]. SCCE is still the most frequent histological type worldwide, even after the 400% increase in the prevalence of ADC in the United States[3,4] and in some countries in Western Europe[5,6] where ADC accounts for more than 80% of new cases[7]. Indeed, the predominance of SCCE is due to its high prevalence in Eastern Asia, as it is observed in some provinces of China, Turkey and Iran, where as much as 120 to 175 new cases are diagnosed per 100 000 inhabitants each year[8]. Intermediate prevalence of SCCE has been observed in France[6], Southern and Eastern Africa[9], and in some countries of South America such as Uruguay, southeast Argentina and southern Brazil, where SCCE still accounts for more than 80% of esophageal cancers[10].
Esophageal cancers carry a high mortality mainly due to its late diagnosis, with a five-year survival of less than 10%[11]. More than 70% of diagnosis are made in patients presenting with dysphagia and weight loss, clinical findings frequently observed in patients in at least stage II disease[12,13]. In developing countries more than 90% of diagnosis of esophageal cancers are at stage II to IV with only 15% to 30% of patients elected for curative surgery[14]. Early diagnosis is uncommon even in developed countries such as France, Japan and the United States, where stage I accounts for just 4% to 25% of new diagnosis[13,15]. This negatively affects the 3- and 5-year survival of patients submitted to multimodality treatments, reaching between 8% to 40% and 5% to 15%, respectively[16]. Diagnosis of early stage lesions is still the best way to improve the chances of cure and survival.
The heterogeneity of risk factors, the differences in geographic distribution and the ethnic groups at risk, make it really difficult to rely on serologic markers for the diagnosis of SCCE. Some attempts have been made, but none of them can be used in clinical practice, due to their low sensitivity[17] or lack of confirmatory values for the diagnosis of early SCCE[18-21]. Since serologic tests are not clinically available yet, more invasive tests are still needed to diagnose SCCE.
Therefore, the aim of the current article is to review some of the most recent efforts that have been made to enhance early diagnosis of SCCE and its precursor lesions.
DEFINITION OF EARLY DIAGNOSIS
Invasive SCCE develops from intraepithelial neoplasia, such as dysplasia and carcinoma in situ, that reaches the lamina propria and extends beyond the submucosa[22]. In a recent Italian study by Ancona and colleagues[23], patients with lesions that were restricted to the esophageal mucosa did not present lymph node metastasis, while lymph node metastasis were observed in 8.3% of patients with tumors restricted to the first third of the submucosa (Sm1). In fact, the diagnosis and treatment of such early stage lesions can improve the survival rates of SCCE, reaching a five-year survival rate of more than 90% after endoscopic or surgical treatment[24].
For the purpose of this review, early esophageal SCCE will be considered high-grade dysplasia, carcinoma in situ, and tumors limited to the upper two thirds of the submucosa. These three types of lesions have a low rate of lymph node metastasis and present higher rates of cure and survival.
RISK FACTORS – WHO SHOULD BE SCREENED?
Early diagnosis of SCCE must not be based on symptoms, since they occur frequently in advanced disease, consequently, screening techniques must be used in asymptomatic individuals exposed to risk factors.
In high risk areas of the “esophageal cancer belt”, such as northern Iran, some provinces of north-central China and north Afghanistan, the main risk factors are poor nutritional and socioeconomic status[25,26], exposure to polycyclic aromatic hydrocarbons (PAH)[27-30], low intake of vegetables and fruits[31], drinking hot beverages[32,33] and there is probably a role for genetic factors[34,35]. These risk factors affect the whole population in these high risk areas, and screening of SCCE in these populations must include the largest number of people that live in these places, with lower costs and less invasive devices.
In moderate and lower risk Western countries, the most important risk factors are the combination of tobacco smoking and excessive alcohol consumption[36-44]. A previous diagnosis of head and neck squamous cell carcinoma has been observed to have a significant impact on the incidence of SCCE[45-47]. Some areas of South America, such as southern Brazil, Uruguay, Paraguay and northwestern Argentina, have a moderate prevalence of SCCE which is influenced by ingestion of a hot beverage called maté. This hot beverage is an infusion of the leaves of Ilex paraguayensis that probably increases the risk of SCCE due to its high temperature[33,38,48-51] and its high content of PAH[52,53]. Some other risk factors that may contribute to SCCE are achalasia, previous radiotherapy for breast cancer, previous caustic injury to the esophagus and thylosis. SCCE screening in moderate and lower risk Western countries must be carried out in subjects exposed to the risk factors described above[54].
CYTOLOGICAL SCREEENING
In the late 1950s a new technique was developed in China to collect cells from the esophageal mucosa utilizing an inflatable balloon covered with a cotton web attached to the tip of a plastic catheter[55]. This device was swallowed and passed down the esophagus to the gastric cardia with the balloon deflated. Once it reached the gastric cardia, the balloon was inflated with air using a 20mL syringe attached to the proximal end of the catheter, and the inflated balloon was gently withdrawn until it reached the upper esophageal sphincter, deflated and then completely withdrawn. The “Chinese Balloon” was designed to be used in multiple patients after simple washing techniques. Other rubber and mechanical disposable balloons and other devices such as sponges have been developed and used in several studies conducted in China which reported that exfoliative cytology may allow early diagnosis of SCCE[55-59].
After collecting squamous cells using this method, slides are stained by the Papanicolaou technique and analyzed. Cytologic findings of atypical squamous cells of undetermined significance (ASCUS), low grade squamous intraepithelial lesions (LSIL), high-grade squamous intraepithelial lesions (HSIL) and carcinoma according to the Bethesda system for squamous cells[60] must undergo upper digestive endoscopy to determine the presence of SCCE.
Many studies in China and one study in Brazil have shown that exfoliative cytology is a good way to collect squamous cells from the esophagus, however, the results of conventional cytology in the diagnosis of SCCE has been discouraging with sensitivities between 39% and 66%[56-59,61-63].
Cytological samples collected using the methods described above are representative of the esophageal mucosa and it is possible that a molecular marker could increase the sensitivity of this inexpensive and simple technique. Immunocytochemical expression of p53 protein was tested recently in southern Brazil, but did not improve the yield of cytological analyses[64]. Two studies have been conducted in China to detect molecular markers that could increase the sensitivity and specificity of balloon cytology[65,66]. One of these studies, published by Adams and colleagues[65], evaluated the presence of methylation in eight genes in esophageal balloon cytology specimens from 147 patients with endoscopic biopsy diagnoses ranging from normal mucosa through to severe squamous dysplasia. This study suggested that evaluation of gene methylation in cytological samples may have utility for the early detection of esophageal squamous dysplasia and early SCCE, however, more sensitive methylation markers will be required for clinical use. The second study by McGruder and colleages[66] tested the telomerase activity measured by real time PCR in esophageal balloon samples in 8 patients from China, and the results seemed to enhance the accuracy of the cytological analysis, however, larger populations and different ethnic groups should be tested before this technique is used in clinical practice.
LUGOL’S DYE CHROMOENDOSCOPY – AN INEXPENSIVE AND SIMPLE SCREENING METHOD
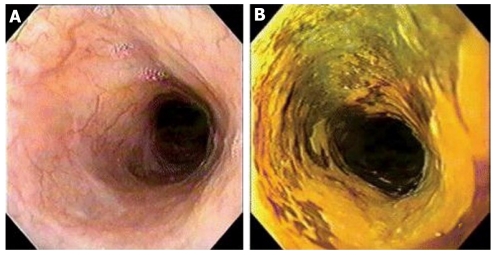
Due to the low sensitivity of conventional endoscopy for the diagnosis of early SCCE[67], new methods were required to evaluate the esophageal mucosa of high risk patients. During the 1990s, multiple reports on Lugol’s dye chromoendoscopy were published and showed how easy, inexpensive and sensitive this method was for detecting early and late squamous cell neoplasia[68-70]. It is based on the lack of absorption of the iodine stain by abnormal squamous tissue, such as inflamed, dysplastic or neoplastic lesions. The esophageal mucosa evaluation occurs during conventional endoscopy, when 10mL to 40 mL of 0.5% to 3% Lugol’s solution is sprayed onto the esophageal mucosa which results in a green-brown, dark-brown, or black discoloration of normal mucosa lasting up to 5 to 8 min (Figure 1). Absence of staining indicates abnormal mucosa that can be biopsied. Lesions with a diameter smaller than 0.5 cm rarely show neoplastic lesions[46].
Figure 1.
Conventional esophagoscopy and Lugol’s chromoendoscopy. A: Conventional esophagoscopy presenting normal appearing mucosa; B: Lugol’s chromoendoscopy disclosed an unstained areaafter multiple biopsies, the diagnosis was high-grade dysplasia.
One of the first reports of Lugol’s dye chromoendoscopy was published by Misumi and colleagues in 1990[71] and demonstrated that this method could reveal esophageal cancer in normal appearing mucosa under conventional endoscopy. Since then, many studies have been published and the use of this method has increased worldwide. Of great interest is a study conducted in China by Dawsey et al[68], where the method showed great sensitivity in revealing early and late SCCE in a high risk population in Linxian province. The same study showed that iodine staining improved the visualization of the lateral margins of the lesions, which was important in guiding endoscopic biopsies and treatment.
Lugol staining of the esophagus has been used in different populations. Studies from Japan and from Brazil which examined alcoholic patients confirmed the significant sensitivity of this chromoendoscopic method when compared with conventional endoscopy[69,72-74]. Patients with previous head and neck cancers were evaluated and similar results were obtained[46,47,75].
The largest series on the use of Lugol’s chromoendoscopy was a multicenter study from France published in 2006 by Dubuc and colleagues[15]. This French study evaluated 1095 patients divided into 4 groups according to exposure to risk factors to SCCE as follows: group 1 –patients with previous diagnosis of head and neck or tracheobronchial squamous cell carcinoma; group 2–patients with alcoholic pancreatitis; group 3 - patients with alcoholic cirrhosis; group 4 - alcohol and tobacco addicts. SCCE and/or dysplasia were observed in 9.9%, 0%, 7.3% and 2.9% in these groups, respectively. Conventional endoscopy detected only 35 esophageal lesions in these patients, while Lugol staining chromoendoscopy detected 67. The difference in diagnostic accuracy was more important for early lesions like low-grade dysplasia, since 77% of these lesions were observed only after spraying of the iodine dye. According to the authors, Lugol’s chromoendoscopy must be used for SCCE screening of patients with previous head and neck or tracheobronchial squamous cell carcinoma.
The pitfalls in its use include increased duration of the procedure, the risk of allergic reactions to the iodine solution and chest pain in some patients[68,70]. The duration of endoscopy is between 5 min and 10 min longer than conventional endoscopy[68,70]. Chest pain and agitation during endoscopy are uncommon, and when they do occur they are easily managed. Indeed, these problems have been surpassed by the advantages of this simple, inexpensive, worldwide available and accurate method for diagnosing early squamous dysplasia and SCCE, when compared to conventional endoscopy[15,46,47,68-72,74-76] and esophageal capsule endoscopy[77].
NARROW-BAND IMAGING – A PROMISING OPTIC-BASED CHROMOENDOSCOPY
Narrow-band imaging (NBI) is a novel, noninvasive optical technique that uses reflected light to visualize the organ surface, and works as an optic-based chromoendoscopic method to detect early lesions[78]. A single-touch of the control knob on the grip of the endoscope allows switching from the standard endoscopy to the NBI filter, emphasizing capillary vessels in the endoscopic images, with image processing in real time[79], and identifying early squamous cell lesions as brownish, well demarcated lesions[80]. NBI when used without magnification has high sensitivity, but a high rate of false-positive lesions, with results similar to Lugol’s chromoendoscopy[81].
Adding magnification to NBI increased the sensitivity and the specificity for the screening of early lesions due to identification of intraepithelial papillary loop (IPCL) patterns such as dilatation, tortuosity, caliber change and variety in shape suggestive of mucosal high-grade neoplasia as shown by Yoshida et al[79]. Ishihara and colleagues[82] identified, in a multivariate analysis, that brownish epithelium and brownish dots were independent factors for the identification of early squamous neoplastic lesions, with an odds ratio of 25.5 [95% confidence interval (CI): 2.4–268] for brownish epithelium and 19.3 (95% CI: 1.8–207.7) for brownish dots. Brownish epithelium and brownish dots had a moderate interobserver agreement in this study. In the same study, IPCL patterns such as dilatation, tortuosity, caliber change and variety in shape were not associated with high-grade dysplasia.
As for Lugol’s chromoendoscopy, the majority of studies on NBI have been conducted in previous head and neck cancer patients. The results are impressive with a sensitivity greater than 90% in this population[80,81,83,84]. However, some pitfalls must be outlined: (1) endoscopes with the NBI system are more expensive than conventional endoscopes and Lugol’s solution; and (2) the NBI technique requires expertise for application. Ishihara and colleagues[85], showed that NBI, when used by less experienced endoscopists, had a sensitivity of 53% in diagnosing high-grade dysplasia.
AUTOFLUORESCENCE IMAGING – AN OPTIC-BASED CHROMOENDOSCOPY FOR MULTIMODALITY APPROACH
Autofluorescence imaging (AFI) is another optic-based chromoendoscopic device designed to detect early lesions. AFI neoplastic areas, that usually involve a thickening of the mucosal layer and increased hemoglobin, emit weaker autofluorescence compared to non-neoplastic areas. In this technique, non-neoplastic areas appear green in color, whereas neoplastic areas are purple or magenta. Some studies have been conducted in the screening of early squamous esophageal lesions and showed that AFI had a higher sensitivity than white-light endoscopy to detect superficial lesions (79% vs 51%, respectively), however, its accuracy was worse than Lugol’s chromoendoscopy or NBI[86,87].
CONCLUSION
Esophageal cancer is a common malignancy with a very poor prognosis. It represents a challenge in medical practice and in the field of public health. It is a devastating disease that continues to have a 5-year survival of less than 10% despite the advances in multimodality therapy. Since surgical and endoscopic resection offer the only possible cure for esophageal cancer, early detection via screening is appealing, particularly in high risk populations. However, so far, there are no guidelines for the screening of SCCE.
Esophageal balloon cytology is a patient-acceptable sampling technique, but the current methods are insufficient for primary screening due to sampling errors. Blind sampling of a large organ misses small lesions and morphologic evaluation of a small percentage of the cell sample misses rare abnormal cells. Molecular markers may be able to help, but the use of biomarkers has to wait for its validation and availability.
Currently there is no single test or testing series that screen for SCCE in a reliable and cost-effective manner, and conventional white light endoscopy with biopsy remains the standard procedure for the identification of pre-malignant and early malignant changes in esophageal mucosa. Endoscopic detection may be enhanced by several techniques such as dye and optic chromoendoscopy, magnifying endoscopy, and optical-based spectroscopic and imaging modalities.
Considering that esophageal cancer is a highly lethal disease, with about 80% of deaths occurring in developing countries, the most efficient and cost-effective tool for targeting biopsies may be Lugol dye chromoendoscopy, since it is an easy, accurate, inexpensive and worldwide available endoscopic technique. In areas of medium and low risk, individual cases should be considered for screening only if the risk and costs to the individual warrant aggressive screening and follow-up evaluation, such as in certain groups of subjects at high risk of the disease such as alcoholics, smokers, mate drinkers, previous head and neck cancer, achalasia and lye stricture of the esophagus.
In ideal conditions, or in developed countries where expensive techniques such as NBI and AFI are available, it is reasonable to think that optimal detection will require a combination of techniques. For example, a suspicious area could be identified initially by NBI or Lugol’s chromoendoscopy, and then further characterized by a high resolution technique, such as confocal endoscopy. The diagnostic performance, availability and cost-effectiveness will determine whether these modalities will become part of standard endoscopy practice.
Footnotes
Peer reviewers: Giampaolo Bresci, MD, UO, Gastroenterrologia, AOUPisana Via a della spina 11, Pisa 56125, Italy; Andreas Sieg, Professor, Dr. med. Andreas Sieg, Practice of Gastroenterology, Bergheimer Str. 56a, D-69117 Heidelberg, Germany
S- Editor Yang XC L- Editor Webster JR E- Editor Yang XC
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2010;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–1187. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook MB, Chow WH, Devesa SS. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977-2005. Br J Cancer. 2009;101:855–859. doi: 10.1038/sj.bjc.6605246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orengo MA, Casella C, Fontana V, Filiberti R, Conio M, Rosso S, Tumino R, Crosignani P, De Lisi V, Falcini F, et al. Trends in incidence rates of oesophagus and gastric cancer in Italy by subsite and histology, 1986-1997. Eur J Gastroenterol Hepatol. 2006;18:739–746. doi: 10.1097/01.meg.0000223905.78116.38. [DOI] [PubMed] [Google Scholar]
- 6.Bosetti C, Levi F, Ferlay J, Garavello W, Lucchini F, Bertuccio P, Negri E, La Vecchia C. Trends in oesophageal cancer incidence and mortality in Europe. Int J Cancer. 2008;122:1118–1129. doi: 10.1002/ijc.23232. [DOI] [PubMed] [Google Scholar]
- 7.Cross AJ, Freedman ND, Ren J, Ward MH, Hollenbeck AR, Schatzkin A, Sinha R, Abnet CC. Meat consumption and risk of esophageal and gastric cancer in a large prospective study. Am J Gastroenterol. 2011;106:432–442. doi: 10.1038/ajg.2010.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gholipour C, Shalchi RA, Abbasi M. A histopathological study of esophageal cancer on the western side of the Caspian littoral from 1994 to 2003. Dis Esophagus. 2008;21:322–327. doi: 10.1111/j.1442-2050.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- 9.Wakhisi J, Patel K, Buziba N, Rotich J. Esophageal cancer in north rift valley of Western Kenya. Afr Health Sci. 2005;5:157–163. [PMC free article] [PubMed] [Google Scholar]
- 10.de Barros SG, Vidal RM, Luz LP, Ghisolfi ES, Barlem GG, Komlós F, Wolff FH, Breyer HP, Pütten AC, Dietz J, et al. [Prevalence of adenocarcinoma of the esophagus and esophagogastric junction in a 10 year period at a cancer referral center in southern Brazil] Arq Gastroenterol. 1999;36:32–36. [PubMed] [Google Scholar]
- 11.CRUK 2009 ‘Cancer survival rates for patients diagnosed 1996-1999’. Survival statistics for the most common cancers. Cited 23/05/2011. Available from: URL: http: //info.cancerresearchuk.org/cancerstats/survival/latestrates/ [Google Scholar]
- 12.Daly JM, Fry WA, Little AG, Winchester DP, McKee RF, Stewart AK, Fremgen AM. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg. 2000;190:562–72; discussion 572-573. doi: 10.1016/s1072-7515(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 13.Schlansky B, Dimarino AJ, Loren D, Infantolino A, Kowalski T, Cohen S. A survey of oesophageal cancer: pathology, stage and clinical presentation. Aliment Pharmacol Ther. 2006;23:587–593. doi: 10.1111/j.1365-2036.2006.02782.x. [DOI] [PubMed] [Google Scholar]
- 14.Abdullah M, Karim AA, Goh KL. Late presentation of esophageal cancer: observations in a multiracial South-East Asian population. J Dig Dis. 2010;11:28–33. doi: 10.1111/j.1751-2980.2009.00410.x. [DOI] [PubMed] [Google Scholar]
- 15.Dubuc J, Legoux JL, Winnock M, Seyrig JA, Barbier JP, Barrioz T, Laugier R, Boulay G, Grasset D, Sautereau D, et al. Endoscopic screening for esophageal squamous-cell carcinoma in high-risk patients: a prospective study conducted in 62 French endoscopy centers. Endoscopy. 2006;38:690–695. doi: 10.1055/s-2006-925255. [DOI] [PubMed] [Google Scholar]
- 16.Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–5067. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, Abnet CC, Wei WQ, Roth MJ, Lu N, Taylor PR, Pan QJ, Luo XM, Dawsey SM, Qiao YL. Serum markers as predictors of esophageal squamous dysplasia and early cancer. Anticancer Res. 2004;24:3245–3249. [PubMed] [Google Scholar]
- 18.Liu WL, Zhang G, Wang JY, Cao JY, Guo XZ, Xu LH, Li MZ, Song LB, Huang WL, Zeng MS. Proteomics-based identification of autoantibody against CDC25B as a novel serum marker in esophageal squamous cell carcinoma. Biochem Biophys Res Commun. 2008;375:440–445. doi: 10.1016/j.bbrc.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 19.Tomita H, Ichikawa D, Ikoma D, Sai S, Tani N, Ikoma H, Fujiwara H, Kikuchi S, Okamoto K, Ochiai T, et al. Quantification of circulating plasma DNA fragments as tumor markers in patients with esophageal cancer. Anticancer Res. 2007;27:2737–2741. [PubMed] [Google Scholar]
- 20.Ikoma D, Ichikawa D, Ueda Y, Tani N, Tomita H, Sai S, Kikuchi S, Fujiwara H, Otsuji E, Yamagishi H. Circulating tumor cells and aberrant methylation as tumor markers in patients with esophageal cancer. Anticancer Res. 2007;27:535–539. [PubMed] [Google Scholar]
- 21.Wu IC, Wu DC, Huang CC, Lin HS, Chen YK, Tsai HJ, Lu CY, Chou SH, Chou YP, Li LH, et al. Plasma decorin predicts the presence of esophageal squamous cell carcinoma. Int J Cancer. 2010;127:2138–2146. doi: 10.1002/ijc.25239. [DOI] [PubMed] [Google Scholar]
- 22.Wang GQ, Abnet CC, Shen Q, Lewin KJ, Sun XD, Roth MJ, Qiao YL, Mark SD, Dong ZW, Taylor PR, et al. Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54:187–192. doi: 10.1136/gut.2004.046631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ancona E, Rampado S, Cassaro M, Battaglia G, Ruol A, Castoro C, Portale G, Cavallin F, Rugge M. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol. 2008;15:3278–3288. doi: 10.1245/s10434-008-0065-1. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu Y, Tsukagoshi H, Fujita M, Hosokawa M, Kato M, Asaka M. Long-term outcome after endoscopic mucosal resection in patients with esophageal squamous cell carcinoma invading the muscularis mucosae or deeper. Gastrointest Endosc. 2002;56:387–390. doi: 10.1016/s0016-5107(02)70043-6. [DOI] [PubMed] [Google Scholar]
- 25.Abnet CC, Kamangar F, Islami F, Nasrollahzadeh D, Brennan P, Aghcheli K, Merat S, Pourshams A, Marjani HA, Ebadati A, et al. Tooth loss and lack of regular oral hygiene are associated with higher risk of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:3062–3068. doi: 10.1158/1055-9965.EPI-08-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Islami F, Kamangar F, Nasrollahzadeh D, Aghcheli K, Sotoudeh M, Abedi-Ardekani B, Merat S, Nasseri-Moghaddam S, Semnani S, Sepehr A, et al. Socio-economic status and oesophageal cancer: results from a population-based case-control study in a high-risk area. Int J Epidemiol. 2009;38:978–988. doi: 10.1093/ije/dyp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roth MJ, Strickland KL, Wang GQ, Rothman N, Greenberg A, Dawsey SM. High levels of carcinogenic polycyclic aromatic hydrocarbons present within food from Linxian, China may contribute to that region’s high incidence of oesophageal cancer. Eur J Cancer. 1998;34:757–758. doi: 10.1016/s0959-8049(97)10071-5. [DOI] [PubMed] [Google Scholar]
- 28.Wornat MJ, Ledesma EB, Sandrowitz AK, Roth MJ, Dawsey SM, Qiao YL, Chen W. Polycyclic aromatic hydrocarbons identified in soot extracts from domestic coal-burning stoves of Henan Province, China. Environ Sci Technol. 2001;35:1943–1952. doi: 10.1021/es001664b. [DOI] [PubMed] [Google Scholar]
- 29.Abedi-Ardekani B, Kamangar F, Hewitt SM, Hainaut P, Sotoudeh M, Abnet CC, Taylor PR, Boffetta P, Malekzadeh R, Dawsey SM. Polycyclic aromatic hydrocarbon exposure in oesophageal tissue and risk of oesophageal squamous cell carcinoma in north-eastern Iran. Gut. 2010;59:1178–1183. doi: 10.1136/gut.2010.210609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamangar F, Strickland PT, Pourshams A, Malekzadeh R, Boffetta P, Roth MJ, Abnet CC, Saadatian-Elahi M, Rakhshani N, Brennan P, et al. High exposure to polycyclic aromatic hydrocarbons may contribute to high risk of esophageal cancer in northeastern Iran. Anticancer Res. 2005;25:425–428. [PubMed] [Google Scholar]
- 31.Zou XN, Taylor PR, Mark SD, Chao A, Wang W, Dawsey SM, Wu YP, Qiao YL, Zheng SF. Seasonal variation of food consumption and selected nutrient intake in Linxian, a high risk area for esophageal cancer in China. Int J Vitam Nutr Res. 2002;72:375–382. doi: 10.1024/0300-9831.72.6.375. [DOI] [PubMed] [Google Scholar]
- 32.Islami F, Pourshams A, Nasrollahzadeh D, Kamangar F, Fahimi S, Shakeri R, Abedi-Ardekani B, Merat S, Vahedi H, Semnani S, et al. Tea drinking habits and oesophageal cancer in a high risk area in northern Iran: population based case-control study. BMJ. 2009;338:b929. doi: 10.1136/bmj.b929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Islami F, Boffetta P, Ren JS, Pedoeim L, Khatib D, Kamangar F. High-temperature beverages and foods and esophageal cancer risk--a systematic review. Int J Cancer. 2009;125:491–524. doi: 10.1002/ijc.24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akbari MR, Malekzadeh R, Nasrollahzadeh D, Amanian D, Sun P, Islami F, Sotoudeh M, Semnani S, Boffeta P, Dawsey SM, et al. Familial risks of esophageal cancer among the Turkmen population of the Caspian littoral of Iran. Int J Cancer. 2006;119:1047–1051. doi: 10.1002/ijc.21906. [DOI] [PubMed] [Google Scholar]
- 35.An JY, Fan ZM, Gao SS, Zhuang ZH, Qin YR, Li JL, He X, Tsao GS, Wang LD. Loss of heterozygosity in multistage carcinogenesis of esophageal carcinoma at high-incidence area in Henan Province, China. World J Gastroenterol. 2005;11:2055–2060. doi: 10.3748/wjg.v11.i14.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown LM, Hoover R, Silverman D, Baris D, Hayes R, Swanson GM, Schoenberg J, Greenberg R, Liff J, Schwartz A, et al. Excess incidence of squamous cell esophageal cancer among US Black men: role of social class and other risk factors. Am J Epidemiol. 2001;153:114–122. doi: 10.1093/aje/153.2.114. [DOI] [PubMed] [Google Scholar]
- 37.Castellsagué X, Muñoz N, De Stefani E, Victora CG, Castelletto R, Rolón PA, Quintana MJ. Independent and joint effects of tobacco smoking and alcohol drinking on the risk of esophageal cancer in men and women. Int J Cancer. 1999;82:657–664. doi: 10.1002/(sici)1097-0215(19990827)82:5<657::aid-ijc7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 38.De Stefani E, Muñoz N, Estève J, Vasallo A, Victora CG, Teuchmann S. Mate drinking, alcohol, tobacco, diet, and esophageal cancer in Uruguay. Cancer Res. 1990;50:426–431. [PubMed] [Google Scholar]
- 39.De Stefani E, Correa P, Oreggia F, Deneo-Pellegrini H, Fernandez G, Zavala D, Carzoglio J, Leiva J, Fontham E, Rivero S. Black tobacco, wine and mate in oropharyngeal cancer. A case-control study from Uruguay. Rev Epidemiol Sante Publique. 1988;36:389–394. [PubMed] [Google Scholar]
- 40.Jiang JM, Zeng XJ, Chen JS, Li JY, Zhang KL, Wu YP, Liu BQ. Smoking and mortality from esophageal cancer in China: a large case-control study of 19,734 male esophageal cancer deaths and 104,846 living spouse controls. Int J Cancer. 2006;119:1427–1432. doi: 10.1002/ijc.21887. [DOI] [PubMed] [Google Scholar]
- 41.Nasrollahzadeh D, Kamangar F, Aghcheli K, Sotoudeh M, Islami F, Abnet CC, Shakeri R, Pourshams A, Marjani HA, Nouraie M, et al. Opium, tobacco, and alcohol use in relation to oesophageal squamous cell carcinoma in a high-risk area of Iran. Br J Cancer. 2008;98:1857–1863. doi: 10.1038/sj.bjc.6604369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandeya N, Williams GM, Sadhegi S, Green AC, Webb PM, Whiteman DC. Associations of duration, intensity, and quantity of smoking with adenocarcinoma and squamous cell carcinoma of the esophagus. Am J Epidemiol. 2008;168:105–114. doi: 10.1093/aje/kwn091. [DOI] [PubMed] [Google Scholar]
- 43.Rossini AR, Hashimoto CL, Iriya K, Zerbini C, Baba ER, Moraes-Filho JP. Dietary habits, ethanol and tobacco consumption as predictive factors in the development of esophageal carcinoma in patients with head and neck neoplasms. Dis Esophagus. 2008;21:316–321. doi: 10.1111/j.1442-2050.2007.00769.x. [DOI] [PubMed] [Google Scholar]
- 44.Wu M, Zhao JK, Hu XS, Wang PH, Qin Y, Lu YC, Yang J, Liu AM, Wu DL, Zhang ZF, et al. Association of smoking, alcohol drinking and dietary factors with esophageal cancer in high- and low-risk areas of Jiangsu Province, China. World J Gastroenterol. 2006;12:1686–1693. doi: 10.3748/wjg.v12.i11.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Do KA, Johnson MM, Doherty DA, Lee JJ, Wu XF, Dong Q, Hong WK, Khuri FR, Fu KK, Spitz MR. Second primary tumors in patients with upper aerodigestive tract cancers: joint effects of smoking and alcohol (United States) Cancer Causes Control. 2003;14:131–138. doi: 10.1023/a:1023060315781. [DOI] [PubMed] [Google Scholar]
- 46.Hashimoto CL, Iriya K, Baba ER, Navarro-Rodriguez T, Zerbini MC, Eisig JN, Barbuti R, Chinzon D, Moraes-Filho JP. Lugol’s dye spray chromoendoscopy establishes early diagnosis of esophageal cancer in patients with primary head and neck cancer. Am J Gastroenterol. 2005;100:275–282. doi: 10.1111/j.1572-0241.2005.30189.x. [DOI] [PubMed] [Google Scholar]
- 47.Muto M, Hironaka S, Nakane M, Boku N, Ohtsu A, Yoshida S. Association of multiple Lugol-voiding lesions with synchronous and metachronous esophageal squamous cell carcinoma in patients with head and neck cancer. Gastrointest Endosc. 2002;56:517–521. doi: 10.1067/mge.2002.128104. [DOI] [PubMed] [Google Scholar]
- 48.Castellsagué X, Muñoz N, De Stefani E, Victora CG, Castelletto R, Rolón PA. Influence of mate drinking, hot beverages and diet on esophageal cancer risk in South America. Int J Cancer. 2000;88:658–664. doi: 10.1002/1097-0215(20001115)88:4<658::aid-ijc22>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 49.de Barros SG, Ghisolfi ES, Luz LP, Barlem GG, Vidal RM, Wolff FH, Magno VA, Breyer HP, Dietz J, Grüber AC, et al. [High temperature “matè” infusion drinking in a population at risk for squamous cell carcinoma of the esophagus] Arq Gastroenterol. 2000;37:25–30. doi: 10.1590/s0004-28032000000100006. [DOI] [PubMed] [Google Scholar]
- 50.Rolón PA, Castellsagué X, Benz M, Muñoz N. Hot and cold mate drinking and esophageal cancer in Paraguay. Cancer Epidemiol Biomarkers Prev. 1995;4:595–605. [PubMed] [Google Scholar]
- 51.Victora CG, Muñoz N, Horta BL, Ramos EO. Patterns of maté drinking in a Brazilian city. Cancer Res. 1990;50:7112–7115. [PubMed] [Google Scholar]
- 52.Fagundes RB, Abnet CC, Strickland PT, Kamangar F, Roth MJ, Taylor PR, Dawsey SM. Higher urine 1-hydroxy pyrene glucuronide (1-OHPG) is associated with tobacco smoke exposure and drinking maté in healthy subjects from Rio Grande do Sul, Brazil. BMC Cancer. 2006;6:139. doi: 10.1186/1471-2407-6-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kamangar F, Schantz MM, Abnet CC, Fagundes RB, Dawsey SM. High levels of carcinogenic polycyclic aromatic hydrocarbons in mate drinks. Cancer Epidemiol Biomarkers Prev. 2008;17:1262–1268. doi: 10.1158/1055-9965.EPI-08-0025. [DOI] [PubMed] [Google Scholar]
- 54.Hirota WK, Zuckerman MJ, Adler DG, Davila RE, Egan J, Leighton JA, Qureshi WA, Rajan E, Fanelli R, Wheeler-Harbaugh J, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570–580. doi: 10.1016/j.gie.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Dawsey SM, Yu Y, Taylor PR, Li JY, Shen Q, Shu YJ, Liu SF, Zhao HZ, Cao SG, Wang GQ. Esophageal cytology and subsequent risk of esophageal cancer. A prospective follow-up study from Linxian, China. Acta Cytol. 1994;38:183–192. [PubMed] [Google Scholar]
- 56.Dawsey SM, Shen Q, Nieberg RK, Liu SF, English SA, Cao J, Zhou B, Wang GQ, Lewin KJ, Liu FS, et al. Studies of esophageal balloon cytology in Linxian, China. Cancer Epidemiol Biomarkers Prev. 1997;6:121–130. [PubMed] [Google Scholar]
- 57.Pan QJ, Roth MJ, Guo HQ, Kochman ML, Wang GQ, Henry M, Wei WQ, Giffen CA, Lu N, Abnet CC, et al. Cytologic detection of esophageal squamous cell carcinoma and its precursor lesions using balloon samplers and liquid-based cytology in asymptomatic adults in Llinxian, China. Acta Cytol. 2008;52:14–23. doi: 10.1159/000325430. [DOI] [PubMed] [Google Scholar]
- 58.Roth MJ, Liu SF, Dawsey SM, Zhou B, Copeland C, Wang GQ, Solomon D, Baker SG, Giffen CA, Taylor PR. Cytologic detection of esophageal squamous cell carcinoma and precursor lesions using balloon and sponge samplers in asymptomatic adults in Linxian, China. Cancer. 1997;80:2047–2059. doi: 10.1002/(sici)1097-0142(19971201)80:11<2047::aid-cncr3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 59.Wang LD, Yang HH, Fan ZM, Lü XD, Wang JK, Liu XL, Sun Z, Jiang YN, He X, Zhou Q. Cytological screening and 15 years’ follow-up (1986-2001) for early esophageal squamous cell carcinoma and precancerous lesions in a high-risk population in Anyang County, Henan Province, Northern China. Cancer Detect Prev. 2005;29:317–322. doi: 10.1016/j.cdp.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 60.Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 61.Yang H, Berner A, Mei Q, Giercksky KE, Warloe T, Yang G, Cui J, Suo Z, Zhang S, Nesland JM. Cytologic screening for esophageal cancer in a high-risk population in Anyang County, China. Acta Cytol. 2002;46:445–452. doi: 10.1159/000326859. [DOI] [PubMed] [Google Scholar]
- 62.Dawsey SM, Lewin KJ, Wang GQ, Liu FS, Nieberg RK, Yu Y, Li JY, Blot WJ, Li B, Taylor PR. Squamous esophageal histology and subsequent risk of squamous cell carcinoma of the esophagus. A prospective follow-up study from Linxian, China. Cancer. 1994;74:1686–1692. doi: 10.1002/1097-0142(19940915)74:6<1686::aid-cncr2820740608>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 63.Lopes AB, Reichert R, Muller LB, Moraes CM, Capra AM, Prolla JC, Diehl AR, Meurer L, Barros SG, Fagundes RB. RS Balloon cytology accuracy for identification of precursor lesions in individuals at risk for squamous cell carcinoma of the esophagus: preliminary results. Gut. 2009;58 [supplement II]:A288–A289. [Google Scholar]
- 64.Lopes AB, Müller LB, Reichert R, Moraes CM, Capra AM, Prolla JC, Diehl AR, Meurer L, de Barros SG, Fagundes RB. Detecting p53 immunoexpression in esophageal mucosa with exfoliative cytology in individuals at risk for squamous cell carcinoma of the esophagus. Acta Cytol. 2010;54:31–38. doi: 10.1159/000324963. [DOI] [PubMed] [Google Scholar]
- 65.Adams L, Roth MJ, Abnet CC, Dawsey SP, Qiao YL, Wang GQ, Wei WQ, Lu N, Dawsey SM, Woodson K. Promoter methylation in cytology specimens as an early detection marker for esophageal squamous dysplasia and early esophageal squamous cell carcinoma. Cancer Prev Res (Phila) 2008;1:357–361. doi: 10.1158/1940-6207.CAPR-08-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McGruder BM, Atha DH, Wang W, Huppi K, Wei WQ, Abnet CC, Qiao YL, Dawsey SM, Taylor PR, Jakupciak JP. Real-time telomerase assay of less-invasively collected esophageal cell samples. Cancer Lett. 2006;244:91–100. doi: 10.1016/j.canlet.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 67.Grüber AC, de Barros SG, Pütten AC, Gigante L, Coelho N, Sekine S, Prolla JC. Esophageal dysplasia and chronic esophagitis: detection at upper gastrointestinal tract endoscopy. Arq Gastroenterol. 1998;35:258–263. [PubMed] [Google Scholar]
- 68.Dawsey SM, Fleischer DE, Wang GQ, Zhou B, Kidwell JA, Lu N, Lewin KJ, Roth MJ, Tio TL, Taylor PR. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer. 1998;83:220–231. [PubMed] [Google Scholar]
- 69.Fagundes RB, de Barros SG, Pütten AC, Mello ES, Wagner M, Bassi LA, Bombassaro MA, Gobbi D, Souto EB. Occult dysplasia is disclosed by Lugol chromoendoscopy in alcoholics at high risk for squamous cell carcinoma of the esophagus. Endoscopy. 1999;31:281–285. doi: 10.1055/s-1999-122. [DOI] [PubMed] [Google Scholar]
- 70.Freitag CP, Barros SG, Kruel CD, Putten AC, Dietz J, Gruber AC, Diehl AS, Meurer L, Breyer HP, Wolff F, et al. Esophageal dysplasias are detected by endoscopy with Lugol in patients at risk for squamous cell carcinoma in southern Brazil. Dis Esophagus. 1999;12:191–195. doi: 10.1046/j.1442-2050.1999.00046.x. [DOI] [PubMed] [Google Scholar]
- 71.Misumi A, Harada K, Murakami A, Arima K, Kondo H, Akagi M, Yagi Y, Ikeda T, Baba K, Kobori Y. Role of Lugol dye endoscopy in the diagnosis of early esophageal cancer. Endoscopy. 1990;22:12–16. doi: 10.1055/s-2007-1012779. [DOI] [PubMed] [Google Scholar]
- 72.Yokoyama A, Ohmori T, Makuuchi H, Maruyama K, Okuyama K, Takahashi H, Yokoyama T, Yoshino K, Hayashida M, Ishii H. Successful screening for early esophageal cancer in alcoholics using endoscopy and mucosa iodine staining. Cancer. 1995;76:928–934. doi: 10.1002/1097-0142(19950915)76:6<928::aid-cncr2820760604>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 73.Yokoyama A, Muramatsu T, Ohmori T, Makuuchi H, Higuchi S, Matsushita S, Yoshino K, Maruyama K, Nakano M, Ishii H. Multiple primary esophageal and concurrent upper aerodigestive tract cancer and the aldehyde dehydrogenase-2 genotype of Japanese alcoholics. Cancer. 1996;77:1986–1990. doi: 10.1002/(SICI)1097-0142(19960515)77:10<1986::AID-CNCR4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 74.Ban S, Toyonaga A, Harada H, Ikejiri N, Tanikawa K. Iodine staining for early endoscopic detection of esophageal cancer in alcoholics. Endoscopy. 1998;30:253–257. doi: 10.1055/s-2007-1001251. [DOI] [PubMed] [Google Scholar]
- 75.Shimizu Y, Tukagoshi H, Fujita M, Hosokawa M, Kato M, Asaka M. Endoscopic screening for early esophageal cancer by iodine staining in patients with other current or prior primary cancers. Gastrointest Endosc. 2001;53:1–5. doi: 10.1067/mge.2001.111387. [DOI] [PubMed] [Google Scholar]
- 76.Bloomfeld RS, Bridgers DI, Pineau BC. Sensitivity of upper endoscopy in diagnosing esophageal cancer. Dysphagia. 2005;20:278–282. doi: 10.1007/s00455-005-0025-x. [DOI] [PubMed] [Google Scholar]
- 77.Heresbach D, Leray E, d’Halluin PN, Cholet F, Lapalus MG, Gaudric M, Ben Soussan E, Gaudin JL, Vahedi K, Quentin V, et al. Diagnostic accuracy of esophageal capsule endoscopy versus conventional upper digestive endoscopy for suspected esophageal squamous cell carcinoma. Endoscopy. 2010;42:93–97. doi: 10.1055/s-0029-1243856. [DOI] [PubMed] [Google Scholar]
- 78.Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, Yoshida S, Hamamoto Y, Endo T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568–577. doi: 10.1117/1.1695563. [DOI] [PubMed] [Google Scholar]
- 79.Yoshida T, Inoue H, Usui S, Satodate H, Fukami N, Kudo SE. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest Endosc. 2004;59:288–295. doi: 10.1016/s0016-5107(03)02532-x. [DOI] [PubMed] [Google Scholar]
- 80.Takenaka R, Kawahara Y, Okada H, Hori K, Inoue M, Kawano S, Tanioka D, Tsuzuki T, Uemura M, Ohara N, et al. Narrow-band imaging provides reliable screening for esophageal malignancy in patients with head and neck cancers. Am J Gastroenterol. 2009;104:2942–2948. doi: 10.1038/ajg.2009.426. [DOI] [PubMed] [Google Scholar]
- 81.Lee YC, Wang CP, Chen CC, Chiu HM, Ko JY, Lou PJ, Yang TL, Huang HY, Wu MS, Lin JT, et al. Transnasal endoscopy with narrow-band imaging and Lugol staining to screen patients with head and neck cancer whose condition limits oral intubation with standard endoscope (with video) Gastrointest Endosc. 2009;69:408–417. doi: 10.1016/j.gie.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 82.Ishihara R, Inoue T, Uedo N, Yamamoto S, Kawada N, Tsujii Y, Kanzaki H, Hanafusa M, Hanaoka N, Takeuchi Y, et al. Significance of each narrow-band imaging finding in diagnosing squamous mucosal high-grade neoplasia of the esophagus. J Gastroenterol Hepatol. 2010;25:1410–1415. doi: 10.1111/j.1440-1746.2010.06378.x. [DOI] [PubMed] [Google Scholar]
- 83.Lee CT, Chang CY, Lee YC, Tai CM, Wang WL, Tseng PH, Hwang JC, Hwang TZ, Wang CC, Lin JT. Narrow-band imaging with magnifying endoscopy for the screening of esophageal cancer in patients with primary head and neck cancers. Endoscopy. 2010;42:613–619. doi: 10.1055/s-0030-1255514. [DOI] [PubMed] [Google Scholar]
- 84.Muto M, Minashi K, Yano T, Saito Y, Oda I, Nonaka S, Omori T, Sugiura H, Goda K, Kaise M, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566–1572. doi: 10.1200/JCO.2009.25.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ishihara R, Takeuchi Y, Chatani R, Kidu T, Inoue T, Hanaoka N, Yamamoto S, Higashino K, Uedo N, Iishi H, et al. Prospective evaluation of narrow-band imaging endoscopy for screening of esophageal squamous mucosal high-grade neoplasia in experienced and less experienced endoscopists. Dis Esophagus. 2010;23:480–486. doi: 10.1111/j.1442-2050.2009.01039.x. [DOI] [PubMed] [Google Scholar]
- 86.Yoshida Y, Goda K, Tajiri H, Urashima M, Yoshimura N, Kato T. Assessment of novel endoscopic techniques for visualizing superficial esophageal squamous cell carcinoma: autofluorescence and narrow-band imaging. Dis Esophagus. 2009;22:439–446. doi: 10.1111/j.1442-2050.2008.00925.x. [DOI] [PubMed] [Google Scholar]
- 87.Suzuki H, Saito Y, Ikehara H, Oda I. Evaluation of visualization of squamous cell carcinoma of esophagus and pharynx using an autofluorescence imaging videoendoscope system. J Gastroenterol Hepatol. 2009;24:1834–1839. doi: 10.1111/j.1440-1746.2009.05941.x. [DOI] [PubMed] [Google Scholar]