Abstract
Orai1 proteins have been recently identified as subunits of SOCE (store-operated Ca2+ entry) channels. In primary isolated PACs (pancreatic acinar cells), Orai1 showed remarkable co-localization and co-immunoprecipitation with all three subtypes of IP3Rs (InsP3 receptors). The co-localization between Orai1 and IP3Rs was restricted to the apical part of PACs. Neither co-localization nor co-immunoprecipitation was affected by Ca2+ store depletion. Importantly we also characterized Orai1 in basal and lateral membranes of PACs. The basal and lateral membranes of PACs have been shown previously to accumulate STIM1 (stromal interaction molecule 1) puncta as a result of Ca2+ store depletion. We therefore conclude that these polarized secretory cells contain two pools of Orai1: an apical pool that interacts with IP3Rs and a basolateral pool that interacts with STIM1 following the Ca2+ store depletion. Experiments on IP3R knockout animals demonstrated that the apical Orai1 localization does not require IP3Rs and that IP3Rs are not necessary for the activation of SOCE. However, the InsP3-releasing secretagogue ACh (acetylcholine) produced a negative modulatory effect on SOCE, suggesting that activated IP3Rs could have an inhibitory effect on this Ca2+ entry mechanism.
Keywords: acetylcholine (ACh), Ca2+ signalling, InsP3 receptor (IP3R), Orai1, pancreatic acinar cell (PAC), store-operated Ca2+ entry (SOCE)
Abbreviations: ACh, acetylcholine; [Ca2+]c, cytosolic Ca2+ concentration; ER, endoplasmic reticulum; Fluo-4/AM, Fluo-4 acetoxymethyl ester; fura 2/AM, fura 2 acetoxymethyl ester; IP, immunoprecipitation; IP3R, InsP3 receptor; KO, knockout; PAC, pancreatic acinar cell; PMCA, plasma membrane Ca2+-ATPase; SOCE, store-operated Ca2+ entry; STIM, stromal interaction molecule; TG, thapsigargin; ZO1, zonula occludens 1
INTRODUCTION
PACs (pancreatic acinar cells) are structurally and functionally polarized with secretory granules located in the apical region, whereas the basal and lateral parts contain well-developed rough ER (endoplasmic reticulum). Thin projections of ER are also present in the apical region [1]. Important secretagogues such as ACh (acetylcholine) and CCK (cholecystokinin) utilize InsP3 and Ca2+ signalling cascades to regulate secretion in these cells [2]. The substantial Ca2+ extrusion by the PMCAs (plasma membrane Ca2+-ATPases) in PACs [3] necessitates a well-developed SOCE (store-operated Ca2+ entry) mechanism. IP3R2 (InsP3 receptor 2) and IP3R3 were shown to be the functional IP3Rs in PACs [4]. Local apical Ca2+ transients can be triggered by InsP3 [5,6]. All three types of IP3Rs are found in the apical part of the cell [7–9]. The role of IP3Rs in the activation of SOCE has been the subject of much debate [10]. The original conformational coupling hypothesis suggested that IP3Rs in the store activate Ca2+ entry channels [11,12]. It was later found that STIM (stromal interaction molecule) proteins serve as the Ca2+ sensors in the store; the depletion of ER Ca2+ results in the translocation of STIM to the plasma membrane, where it interacts with and activates Orai channels [13–16]. The notion of conformational coupling was therefore confirmed, however, with STIM rather than IP3R as the primary ER Ca2+ sensor. This does not exclude the possibility that IP3Rs could play some regulatory role in SOCE, particularly considering a recent report describing an interaction between IP3Rs and Orai1 [17]. In PACs, STIM1 was found to form puncta in the basal and lateral subplasmalemmal regions, where it was also shown to co-localize with Orai1 [18]. The basolateral SOCE in PACs is therefore mediated by STIM1 interacting with Orai1. Surprisingly, the highest density of Orai1 was found in the apical region, away from its activator STIM1 [18], but in the area populated with IP3Rs [7–9]. This surprising finding led us to examine the relative positioning of the two proteins, which were found to be closely co-localized. In the second part of the present study, we probed the functional consequences of this co-localization.
MATERIALS AND METHODS
Chemicals
All salts as well as ACh, goat serum, BSA and PBS were obtained from Sigma. Collagenase was from Worthington Biochemicals (Lorne Laboratories). TG (thapsigargin) and caffeine were from Calbiochem. Protease inhibitor cocktail was from Roche Diagnostics. Protein G–Sepharose beads were from GE Healthcare. Clean Blot reagent was from Pierce. Fura 2/AM (fura 2 acetoxymethyl ester) and Fluo-4/AM (Fluo-4 acetoxymethyl ester) were from Invitrogen.
Animals and cell isolation
All animal experiments were conducted in accordance with the Animals (Scientific Procedure) Act of 1986. PACs were isolated from the pancreata of CD1 or BL6 [wild-type or specified KO (knockout)] mice using collagenase digestion as described previously [3].
Immunofluorescence
Freshly isolated PACs were fixed in methanol for 10 min at −20°C. Non-specific antibody binding was blocked for 1 h in 10% goat serum and 1% BSA prior to incubation with primary antibodies for 1 h at room temperature (18–21°C). IP3Rs were visualized by anti-IP3R3 antibodies (BD Transduction Laboratories) or anti-IP3R2 antibodies (rabbit polyclonal, raised against the C-terminal amino acids 2686–2702; a gift from Professor D. Yule, School of Medicine and Dentistry, University of Rochester, Rochester, NY, U.S.A.) or anti-IP3R1 antibodies (rabbit polyclonal, raised against C-terminal amino acids 2735–2749 of mouse IP3R1; a gift from Professor J. Parys (Laboratory of Molecular and Cellular Signalling, Department of Molecular and Cellular Biology, Catholic University of Leuven, Leuven, Belgium). Orai1 channels were stained with an anti-Orai1 antibody (rabbit polyclonal, raised against C-terminal amino acids 278–294, produced by Dr S. Feske) and tight junctions were visualized by anti-occludin antibodies (Zymed Laboratories, Invitrogen) or anti-ZO1 (zonula occludens 1) antibodies (a gift from Dr M. Furuse, Graduate School of Medicine, Kobe University, Kobe, Japan). Appropriate secondary antibodies conjugated to Alexa Fluor® 488, Alexa Fluor® 594 and/or Alexa Fluor® 647 (Invitrogen) were applied for 30 min and coverslips were mounted on to microscope slides with Prolong Gold (Invitrogen). All fluorescent secondary antibodies, used in the present study, were tested on PACs fixed using the same method, but without the application of a primary antibody. None of these secondary antibodies produced any non-specific staining in PACs. Cells were viewed on a Leica TCS SP2 AOBS inverted confocal microscope (Leica Microsystems) equipped with a ×63 oil-immersion objective (numerical aperture=1.4). Optical sections were spaced by 0.5–1 μm. Linear adjustments of contrast and brightness were applied if necessary in Leica Application Suite.
Ca2+ imaging
Freshly isolated PACs were loaded with 2.5 μM fura 2/AM or 2.5 μM Fluo-4/AM for 30 min at room temperature. Fluo-4 labelling was used for experiments involving caffeine. Standard sodium Hepes-based extracellular solution contained 140 mM NaCl, 4.7 mM KCl, 10 mM Hepes, 1 mM MgCl2, 10 mM glucose and 1 mM CaCl2 (pH 7.4). In specific experiments, the Ca2+ concentration in this solution was modified (i.e. reduced to nominally Ca2+ free or increased to 2 mM). For fura 2 imaging, we utilized Till Photonics Imaging System or a RIKEN BSI Olympus Collaboration Center Imaging System. Fura 2 fluorescence was measured with λex at 340 and 380 nm, and λem using a 510 nm high pass filter. Experiments with Fluo-4 loaded cells were conducted on the Till Photonics Imaging System. Fluo-4 labelling was used for experiments involving caffeine because of the strong effect of caffeine on the fluorescence of fura 2. Fluo-4 fluorescence was measured with λex at 470 nm and λem using a 510 nm high pass filter.
Co-immunoprecipitation and Western blotting
PACs were lysed in IP (immunoprecipitation) buffer containing 50 mM Tris/HCl, 150 mM NaCl, 1% Triton X-100, 0.25% sodium deoxycholate, 0.2% SDS, 2 mM EDTA and 2×protease inhibitor cocktail. In each condition, lysate containing 600 μg of protein was added to 2 μg of anti-IP3R antibody (described above) and mixed with 20 μl of Protein G–Sepharose beads in a total volume of 1 ml of IP buffer for 2 h at 4°C. Proteins were eluted, separated on a 4–12% Tris/glycine gradient gel and transferred on to nitrocellulose membranes (VWR). Following blocking in 5% (w/v) non-fat dried skimmed milk powder for 1 h, membranes were probed with anti-Orai1 antibodies (Alomone Labs) and anti-IP3R antibodies or anti-actin antibodies (Sigma). Following staining with Clean Blot, bands were visualized using ECL (enhanced chemiluminescence) Western-blotting substrate in a Bio-Rad gel documentation system. Bands were quantified using the ImageJ gel quantification plug-in.
RESULTS
Orai1 co-localizes with IP3Rs in the apical pole of PACs
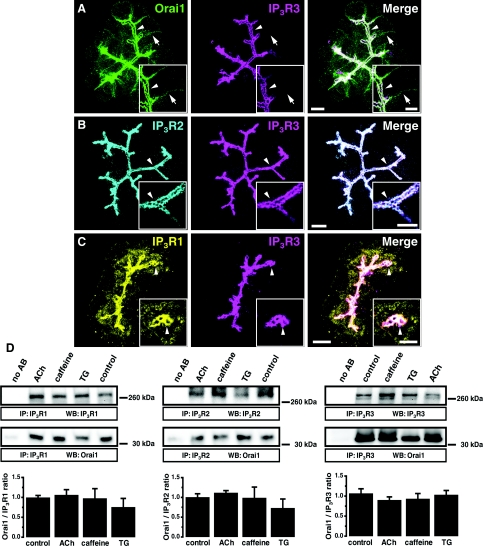
In the apical pole of PACs, we found a striking co-localization of endogenous Orai1 and IP3R3 (Figure 1A, n=7). IP3R2 and IP3R3 are also co-localized in this cellular region (Figure 1B, n=6). The highest density of IP3R1 was observed in the apical region where it co-localized with IP3R3 (Figure 1C, n=6). We can therefore conclude that Orai1 closely co-localizes with all types of IP3Rs in the apical pole of PACs. The apical localization of Orai1 and its co-localization with IP3R3 did not change in conditions when the cells were treated with the InsP3-generating secretagogue ACh (Supplementary Figure S1A available at http://www.BiochemJ.org/bj/436/bj4360231add.htm, n=3), the IP3R inhibitor caffeine (Supplementary Figure S1B, n=3), or the SERCA (sarcoplasmic/endoplasmic reticulum Ca2+-ATPase) pump inhibitor TG (Supplementary Figure S1C, n=3). Orai1 co-immunoprecipitated with all types of IP3Rs (Figure 1D, n=6 for Orai1 and IP3R1; n=7 for Orai1 and IP3R2; n=16 for Orai1 and IP3R3). This co-immunoprecipitation also did not change significantly when the cells were treated with ACh (Figure 1D, n=6 for Orai1 and IP3R1; n=7 for Orai1 and IP3R2; n=14 for Orai1 and IP3R3), caffeine (Figure 1D, n=3 for Orai1 and IP3R1; n=5 for Orai1 and IP3R2; n=4 for Orai1 and IP3R3) or TG (Figure 1D, n=3 for Orai1 and IP3R1; n=5 for Orai1 and IP3R2; n=4 for Orai1 and IP3R3). Importantly actin (which is present at high density in the apical region) was not co-immunoprecipitated with IP3Rs (Supplementary Figure S2 available at http://www.BiochemJ.org/bj/436/bj4360231add.htm, n=4). The observed co-immunopreciptation of Orai1 and IP3Rs does not, of course, guarantee that there is a direct interaction between these proteins, but it is unlikely that the co-immunoprecipitation is due to the interaction of the proteins with actin.
Figure 1. Orai1 co-localization and co-immunoprecipitation with IP3Rs in PACs.
(A) Maximum projection of 20 optical sections spaced 1 μm from each other in a PAC cluster. Orai1 (green) is present in both the basolateral (arrows) and apical (arrowheads) regions. In the apical pole, Orai1 co-localizes with IP3R3 (magenta). Insets: single confocal sections from the same cluster at higher magnification (the arrow and arrowhead points to the same structures as in the main Figure). Here and in (B) and (C) the scale bars correspond to 10 μm in the projections and 5 μm in the insets. (B) Maximum projection of optical sections of a PAC cluster. IP3R2 (cyan) co-localizes with IP3R3 (magenta) in the apical pole of the cells (arrowhead). Insets: single confocal sections from the same cluster at higher magnification (the arrowhead points to the same structures as in the main Figure). (C) Maximum projection of optical sections of a PAC cluster. The highest density of IP3R1 (yellow) is observed in the apical pole of the cells (arrowheads), where it co-localizes with IP3R3 (magenta). Insets: single confocal sections from the same cluster at higher magnification (the arrowhead points to the same structures as in the main Figure). Note that a significant staining for IP3R1 (unlike that for IP3R2 and IP3R3) was also found outside the apical region of the cell. (D) Co-immunoprecipitation of Orai1 with IP3R1 (left panel), IP3R2 (middle panel) or with IP3R3 (right panel) in PAC lysates. The first lane in both panels corresponds to beads that were not bound to anti-IP3R antibodies. Western blots show the IP3Rs and Orai1 eluted from Sepharose beads decorated with the corresponding IP3Rs. Histograms show the quantification of Western blots.
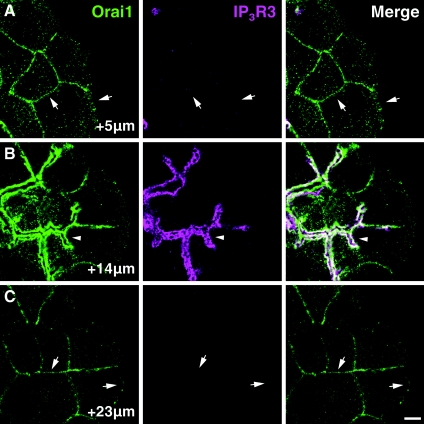
It is essential to note that unlike IP3Rs, Orai1 was observed not only in the apical region, but also on the basal and lateral membranes (shown by arrows in Figure 1A). This was particularly evident in the optical sections that were recorded below (Figure 2A, section at +5 μm) or above (see Figure 2C, section at +23 μm) the apical region of the cells. Orai1 was clearly present outside the apical region, but the intensity of its immunostaining increased substantially in the apical region decorated with IP3Rs (see Figure 2B, section at +14 μm) and in this region Orai1 was always found in the close vicinity of IP3Rs (Figures 1 and 2B, section at +14 μm and Supplementary Figure S1).
Figure 2. Orai1 only co-localizes with IP3Rs in the apical region of the acinar cells: apical and basolateral Orai1.
(A) Confocal section from a cluster of PACs recorded 5 μm from the coverslip. This section is below the apical regions of the cells and Orai1 (green) is clearly visible in the basal and lateral membranes (arrows). (B) Confocal section from the same cluster as in (A), but recorded 14 μm from the top of the coverslip where IP3R3s (magenta) decorate the apical surfaces of the cells. Apical Orai1 present in this section (arrowhead) co-localizes with the IP3R3s. (C) Confocal section of the same acinar cell cluster as in (A) and (B). Confocal section was positioned 23 μm from the coverslip. IP3Rs are no longer visible as this section is above the apical regions of the cells; however, Orai1 is still present in the lateral and basal membranes (arrows). Scale bars correspond to 5 μm.
Orai1 distribution in PACs lacking IP3Rs
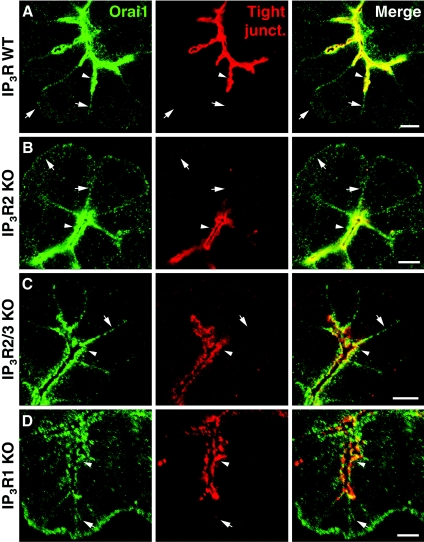
To find out if the IP3Rs are required for the apical positioning of Orai1, we imaged the distribution of Orai1 in PACs from IP3R2 KO mice, from IP3R2/3 double KO mice [4] and from IP3R1 KO mice [19] produced in K. Mikoshiba's laboratory. The apical membrane region of PACs is enriched with occludin and ZO1 (Supplementary Figure S3 available at http://www.BiochemJ.org/bj/436/bj4360231add.htm). As the apical pole cannot be visualized using anti-IP3R antibodies in the cells from the double KO animals, we used tight junction markers, i.e. anti-occludin antibodies and/or anti-ZO1 antibodies, to reveal the apical regions in clusters of acinar cells (both antibodies give very similar staining (Supplementary Figure S3) and were co-localized with IP3Rs in the cells from the wild-type animals (Supplementary Figure S4 available at http://www.BiochemJ.org/bj/436/bj4360231add.htm). In the wild-type animals, Orai1 was mainly found co-localized with these proteins in the apical region, but was also present in the lateral and basal membranes (Figure 3A and Supplementary Movie S1 available at http://www.BiochemJ.org/bj/436/bj4360231add.htm). The distribution of Orai1 in PACs of KO animals was similar to that observed in cells from the wild-type animals (Figure 3). Prominent apical Orai1 staining was present in cells lacking IP3R2 or both IP3R2 and IP3R3 or IP3R1 (Figures 3B–3D; n=5 for both IP3R2 KO mice and for IP3R2/3 double KO mice, n=3 for IP3R1 KO mice). The basolateral presence of Orai1 was also unchanged in the cells from single and double IP3Rs KO animals (Figure 3). These experiments suggest that IP3Rs are not required for the targeting of Orai1 to basolateral or apical membrane regions of PACs.
Figure 3. Orai1 is present in the apical pole of acinar cells lacking IP3Rs.
(A) Confocal section of an acinar cell cluster isolated from wild-type mouse. Orai1 (green) is visible in basolateral membranes (arrows) and in the apical pole (arrowheads) where it co-localizes with tight junctions (red) marking the apical membrane. (B) Confocal section of a cluster of acinar cells isolated from IP3R2 KO mice. Orai1 is apparent in both apical (arrowhead) and basolateral membranes (arrows), its distribution is similar to that in the cells from wild-type animals. (C) Confocal section of an acinar cell cluster isolated from mice lacking both IP3R2 and IP3R3 (IP3R2/3 KO). Orai1 is present in basolateral membranes (arrow) as well as in the apical pole (arrowhead) where it co-localizes with tight junction markers. (D) Confocal section of an acinar cell cluster isolated from IP3R1 KO mice. Orai1 staining is visible in the apical pole (arrowhead) and in basolateral membranes (arrow). Scale bars represent 5 μm.
Effects of the InsP3-generating secretagogue ACh and the IP3R inhibitor caffeine on SOCE in PACs from the wild-type and IP3Rs KO mice
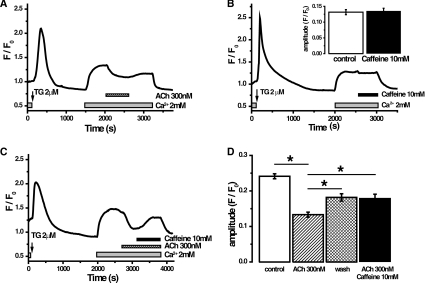
In these experiments, TG was used to deplete Ca2+ stores in cells maintained in nominally Ca2+-free extracellular solution. Addition of Ca2+ to the extracellular solution resulted in SOCE-mediated increase in [Ca2+]c (cytosolic Ca2+ concentration) followed by formation of an elevated [Ca2+]c plateau (Figure 4). Addition of InsP3-producing secretagogue ACh reversibly decreased the amplitude of the plateau (Figure 4A, n=465). Using the Mn quench technique [20] we also observed a small (13±2%) but statistically significant inhibition of the influx by 300 nM ACh (Supplementary Figure S5 available at http://www.BiochemJ.org/bj/436/bj4360231add.htm, n=145 cells in ACh-treated group and n=139 cells in control group). Caffeine (10 mM), which in PACs very efficiently blocks InsP3-induced Ca2+ responses [21] has no effect on its own (Figure 4B, n=169), but it partially reversed the ACh-induced reduction of the plateau (Figure 4C, n=168). These experiments suggest that activation of IP3Rs has a mild inhibitory rather than stimulatory action on SOCE. Caffeine efficiently quenches fura 2, because of this property the experiments described above were conducted using single wavelength indicator fluo-4. We further tested the effects of ACh on TG-induced [Ca2+]c plateau using the ratiometric probe fura 2. The results were similar to that observed using fluo-4, i.e. 300 nM ACh (but not 50 nM ACh) reversibly decreased TG-induced [Ca2+]c plateau (Figures 5A and 5B, n=61 for 50 nM ACh and n=62 for 300 nM ACh). Similar results were found in experiments on PACs from IP3R2 KO mice (Figures 5C and 5D, n=47 for 50 nM ACh and n=33 for 300 nM ACh) and from IP3R2/3 double KO mice (Figures 5E and 5F, n=46 for 50 nM ACh and n=31 for 300 nM ACh); although the recovery phase (on removal of ACh) was less clear in experiments on KO and double KO mice. To assess SOCE more directly and provide an internal control for each experiment we used a two pulse protocol, where cellular Ca2+ stores were depleted using TG in nominally Ca2+-free external solution and then two short pulses of extracellular calcium (2 mM) were applied (Figure 6A). The first pulse (after the TG-induced Ca2+ store depletion) was applied in agonist-free extracellular medium, whereas the second pulse was applied in the presence of ACh (Figure 6A) or in agonist-free extracellular medium (control, results not shown). The changes in the fura 2 340 nm/380 nm ratio were differentiated (the procedure is illustrated in the inset in Figure 6A) and the maximal rate determined. Considering the relatively slow Ca2+ extrusion by PMCA at or near the resting [Ca2+]c [3] and taking into account that the maximal rates of changes were also observed at close to resting [Ca2+]c, we can assume that the maximal derivative reflects the maximal SOCE rate. The maximal SOCE rate during the second pulse was normalized to that recorded during the first pulse, in order to provide an internal control for every cell in each experiment (Figures 6A and 6B). In wild-type cells, a low concentration of ACh (50 nM) slightly reduced the SOCE rate (by 10±3%, n=61; Figure 6B), whereas the treatment with 300 nM ACh resulted in a reduction in the SOCE rate of 27±4% (n=62, Figure 6B). In similar experiments (Supplementary Figure S6 available at http://www.BiochemJ.org/bj/436/bj4360231add.htm), we tested the effect of caffeine on SOCE rate. Caffeine (10 mM) alone did not affect SOCE rate (Supplementary Figure S6, n=48). The ability of ACh to inhibit SOCE was further tested using the two-pulse protocol on the PACs from IP3R2 single KO and IP3R2/3 double KO mice (Figures 6C–6F). These experiments showed that both concentrations of ACh-induced small, but statistically significant, reductions in the SOCE rate in PACs from IP3R2 KO mice (Figures 6C and 6D). Both concentrations reduced SOCE rate by 20±2%. These results were qualitatively similar to those obtained in cells from wild-type animals. The Ca2+ responses in cells from the IP3R2/3 double KO mice were different: the response to TG was drastically reduced in comparison with that in the wild-type and the single KO mice (Figure 6E compared with Figures 6A and 6C). This suggests that IP3Rs amplify the TG response by Ca2+-induced Ca2+ release and that this amplification mechanism is absent in IP3R2/3 double KO mice. The responses to the external Ca2+ pulses in PACs from the double KO mice were, however, surprisingly robust (Figure 6E). In fact the maximal SOCE rate during the first pulse was slightly higher in the cells from the double KO mice (26% higher) than the single KO mice. Using the two-pulse protocol we have not observed changes in the SOCE rate upon the application of 50 or 300 nM ACh in the cells from double KO animals (Figure 6F, n=67 for 50 nM and n=76 for 300 nM). The SOCE rate during the second pulse was however already reduced by approx. 20% in comparison with the first pulse even under control (no ACh) conditions (Figure 6F). It is therefore possible that we do not observe ACh-induced SOCE suppression in these experiments because the ACh simply cannot inhibit the Ca2+ influx any further.
Figure 4. Effect of ACh and caffeine on the cytosolic Ca2+ plateau in PACs.
(A) TG (2 μM)-induced depletion of the ER Ca2+ stores in cells placed in the nominally Ca2+-free extracellular solution. Subsequent addition of 2 mM Ca2+ to the extracellular solution resulted in the increase in [Ca2+]c. ACh application (following the formation of the plateau) caused a decrease in the level of cytosolic Ca2+. Removal of ACh resulted in partial restoration of the amplitude of [Ca2+]c. (B) The application of caffeine (10 mM) did not affect the [Ca2+]c plateau. Inset: quantification of the amplitude of the [Ca2+]c plateau before and after addition of caffeine. (C) Caffeine treatment partially reverses the effect of ACh on [Ca2+]c plateau. (D) Quantification of the results illustrated in (A)–(C). The amplitude of the [Ca2+]c plateau was measured before treatment (white bar), following the addition of ACh (striped bar), following the removal of ACh from the extracellular solution (cross-hatched bar) or following the perfusion with the extracellular solution containing both ACh and caffeine (black bar). *P<0.05.
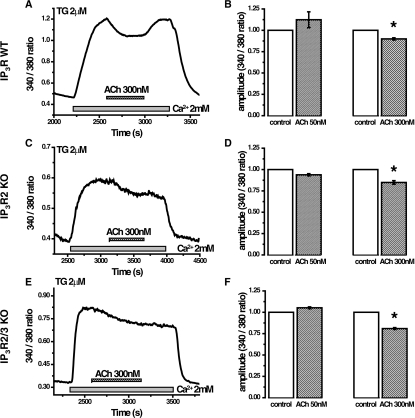
Figure 5. Effect of ACh on the cytosolic Ca2+ plateau in acinar cells isolated from wild-type and IP3R KO mice.
(A) Intracellular Ca2+ stores of fura 2-loaded PACs, isolated from wild-type mice were depleted during a 30 min preincubation in nominally Ca2+-free extracellular solution containing 2 μM TG. The addition of 2 mM Ca2+ to the extracellular solution resulted in the formation of a [Ca2+]c plateau. Subsequent ACh application triggered a small drop of the plateau that could be reversed by removal of ACh. (B) Normalized (to the plateau level before ACh addition) plateau amplitude before (white bars) and following (striped bars) treatment with 50 or 300 nM ACh. Only the application of a large concentration of ACh (300 nM) caused a significant reduction of the plateau amplitude in PACs from wild-type mice (paired Student's t-test, *P<0.05). (C) Intracellular Ca2+ stores of fura 2-loaded PACs, isolated from IP3R2 KO mice, were depleted with 2 μM TG in nominally Ca2+-free extracellular solution and subsequent addition of 2 mM Ca2+ resulted in the formation of a [Ca2+]c plateau. The application of a large concentration of ACh resulted in a small decrease in the plateau. (D) Normalized plateau amplitude, recorded from PACs from IP3R2 KO mice, before (white bars) and following (striped bars) treatment with 50 or 300 nM ACh. Only the application of a 300 nM ACh caused a significant reduction of plateau amplitude in PACs from IP3R2 KO mice (paired Student's t-test, * P<0.05). (E) Intracellular Ca2+ stores of fura 2-loaded PACs, isolated from IP3R2/3 KO mice, were depleted as described above and a cytosolic Ca2+ plateau was formed by introducing 2 mM Ca2+ to the extracellular solution. Subsequent addition of ACh resulted in a decrease in cytosolic Ca2+ levels. (F) Normalized plateau amplitude, recorded from PACs from IP3R2/3 KO mice before (white bars) and following (striped bars) treatment with 50 or 300 nM ACh. Only the application of a 300 nM ACh caused a small but statistically significant reduction of plateau amplitude (paired Student's t-test, *P< 0.05).
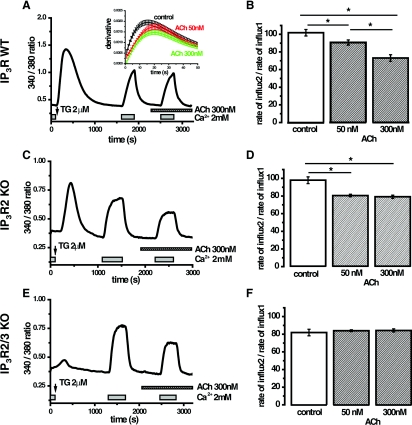
Figure 6. Effects of ACh on the rate of SOCE in PACs isolated from wild-type and IP3R KO mice.
(A) Fura 2 ratio changes upon TG treatment and pulses of external Ca2+ in the absence and presence of 300 nM ACh in PACs from the wild-type animals. To assess the rate of Ca2+ influx the rising phase (induced by a short pulse of 2 mM Ca2+) of the curve was differentiated and the maximum rate of influx was estimated as the maximal value of the derivative (see inset). The same procedure was repeated for the second pulse of external Ca2+ (delivered in the presence or absence of ACh). (B) Summary of the effects of 50 nM ACh and 300 nM ACh on SOCE rate in the cells from wild-type animals. Here, as well as in (D) and (F), the statistical significance was probed by ANOVA, *P<0.05. (C) Fura 2 ratio changes upon TG treatment and pulses of external Ca2+ in the absence and presence of 300 nM ACh in PACs from IP3R2 KO mice. (D) Summary of the effects of 50 and 300 nM ACh on SOCE rate in the cells from IP3R2 KO mice. (E) Fura 2 ratio changes upon TG treatment and pulses of external Ca2+ in the absence and presence of 300 nM ACh in PACs from IP3R2/3 double KO mice. (F) Summary graph showing SOCE rate in control conditions and in the presence of 50 or 300 nM ACh in the cells from IP3R2/3 double KO mice.
DISCUSSION
PACs structurally satisfy the requirements for IP3R and store-operated Ca2+ channel interaction (see Figure 1) suggested as the basis for the original conformational-coupling theory of SOCE [11,12]. We were not able to detect SOCE up-regulation in response to stimulation with the InsP3-producing agonist ACh. The inhibition of IP3Rs with caffeine also had no affect on SOCE. In these respects, the present study yielded important negative results. This finding is in line with conclusions from the study by Woodard et al. [17], which highlighted the importance of the interaction between IP3Rs and Orai1 for different Ca2+ signalling processes, but indicated that the disruption of the interaction between IP3R1 and Orai1 does not prevent TG-induced SOCE and has no significant effect on this process.
The ACh application experiments suggest that IP3Rs could negatively regulate SOCE. This negative modulation could offer some protection against Ca2+ overload induced by InsP3-producing secretagogues. The effect of ACh is, however, moderate and SOCE develops efficiently in the acinar cells lacking both functional types of IP3Rs.
In the present study, the highest density of Orai1 was observed in the apical part of the cell containing IP3Rs, occludin and ZO-1. Importantly, we also found Orai1 along the lateral and basal membranes, far beyond the region containing tight junctions and IP3Rs (Figures 1–3 and Supplementary Movie S1). In this basolateral region, Orai1 was shown to co-localize with STIM1 and form Orai1 and STIM1 puncta following ER store depletion [18]. It is possible that two mechanisms of SOCE operate in PACs; basolateral SOCE mediated by STIM1 and Orai1 proteins will have a reliable Ca2+ source delivered by the interstitial fluid, whereas apical SOCE could help to re-capture Ca2+ transported paracellularly [22], extruded by PMCA (which are active in the apical region [23,24]) and exocytosed with the content of secretory granules. It is conceivable that apical Orai1 could play a role in preventing the build-up of Ca2+ in pancreatic ducts, and consequently, pancreatic stone formation in these structures. The close co-positioning of IP3Rs and Orai1 in the apical region suggests that if activated the apical Orai1 could efficiently re-load strategically important (IP3R containing) Ca2+ stores. The ability of the apical Orai1 to participate in SOCE, the putative mechanism of activation and the physiological function of the apical Orai1 will need to be determined in a separate study. While the present paper was in revision, a study was published by Hong et al. [25] indicating that Orai1 is localized in the apical part of acinar cells where it co-localized with IP3R3. The important difference between this study and our paper is that we observed a substantial presence of Orai1 in the plasma membrane regions outside the apical pole. Indeed our confocal images (Figure 1, Supplementary Figure S1, Figure 3, Supplementary Movie S1 and particularly Figure 2) clearly reveal substantial Orai1 staining outside the plasma membrane regions decorated with IP3Rs, occludin or ZO-1. Also contrary to conclusions from Hong et al. [25] (but see Figure 5A in [25]), in our previous work we have not observed apical localization of STIM1 in cells with depleted ER Ca2+ stores [18]. We therefore consider that the apical function of Orai1 is likely to be STIM1-independent. It is also important to note that the previous electron microscopy investigation of the sub-plasmalemmal ER and the plasma membrane in PACs, revealed ribosome-free rough ER junctions decorated with STIM1 in basal and lateral sub-plasmalemmal regions, but not in the apical pole of the cell [18]. It is therefore difficult to reconcile some of the conclusions (specifically the role of STIM1 in activating apical Orai1 channels) of Hong et al. [25] and our study.
The present study revealed close positioning of IP3Rs and Orai1 channels in the apical pole of the PACs, documented the presence of Orai1 in the apical region of the cells lacking functional IP3Rs and concluded that, in spite of the remarkably close localization of the two proteins, IP3Rs do not activate SOCE in the apical region of PACs.
Online data
AUTHOR CONTRIBUTION
Gyorgy Lur and Mark Sherwood conducted experiments; Gyorgy Lur, Mark Sherwood, Etsuko Ebisui, Lee Haynes, Stefan Feske, Robert Sutton, Robert Burgoyne, Katsuhiko Mikoshiba, Ole Petersen and Alexei Tepikin designed the research project and provided advice on experimental design; and Gyorgy Lur and Alexei Tepikin wrote the paper.
ACKNOWLEDGEMENTS
We acknowledge support from RIKEN BSI-Olympus Collaboration Centre. The authors declare no conflict of interest, except S. F., who is a scientific co-founder of CalciMedica.
FUNDING
This work was supported by the Medical Research Council (U.K.) [grant numbers G0700167, G19/22]; the Wellcome Trust [grant number 080906/Z/06/Z]; and a National Institute for Health Research (U.K.) grant to the Liverpool NIHR Pancreas Biomedical Research Unit. K. M. was supported by a Japan Science and Technology Agency (Japan) grant (Ca2+ oscillations project). S. F. was supported by a National Institutes of Health (U.S.A.) grant [grant number AI066128]. M. S. is a RIKEN Foreign Postdoctoral Research Fellow.
References
- 1.Park M. K., Petersen O. H., Tepikin A. V. The endoplasmic reticulum as one continuous Ca(2+) pool: visualization of rapid Ca(2+) movements and equilibration. EMBO J. 2000;19:5729–5739. doi: 10.1093/emboj/19.21.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen O. H., Tepikin A. V. Polarized calcium signaling in exocrine gland cells. Annu. Rev. Physiol. 2008;70:273–299. doi: 10.1146/annurev.physiol.70.113006.100618. [DOI] [PubMed] [Google Scholar]
- 3.Tepikin A. V., Voronina S. G., Gallacher D. V., Petersen O. H. Pulsatile Ca2+ extrusion from single pancreatic acinar cells during receptor-activated cytosolic Ca2+ spiking. J. Biol. Chem. 1992;267:14073–14076. [PubMed] [Google Scholar]
- 4.Futatsugi A., Nakamura T., Yamada M. K., Ebisui E., Nakamura K., Uchida K., Kitaguchi T., Takahashi-Iwanaga H., Noda T., Aruga J., Mikoshiba K. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science. 2005;309:2232–2234. doi: 10.1126/science.1114110. [DOI] [PubMed] [Google Scholar]
- 5.Ito K., Miyashita Y., Kasai H. Micromolar and submicromolar Ca2+ spikes regulating distinct cellular functions in pancreatic acinar cells. EMBO J. 1997;16:242–251. doi: 10.1093/emboj/16.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorn P., Lawrie A. M., Smith P. M., Gallacher D. V., Petersen O. H. Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell. 1993;74:661–668. doi: 10.1016/0092-8674(93)90513-p. [DOI] [PubMed] [Google Scholar]
- 7.Lee M. G., Xu X., Zeng W., Diaz J., Wojcikiewicz R. J., Kuo T. H., Wuytack F., Racymaekers L., Muallem S. Polarized expression of Ca2+ channels in pancreatic and salivary gland cells. Correlation with initiation and propagation of [Ca2+]i waves. J. Biol. Chem. 1997;272:15765–15770. doi: 10.1074/jbc.272.25.15765. [DOI] [PubMed] [Google Scholar]
- 8.Nathanson M. H., Fallon M. B., Padfield P. J., Maranto A. R. Localization of the type 3 inositol 1,4,5-trisphosphate receptor in the Ca2+ wave trigger zone of pancreatic acinar cells. J. Biol. Chem. 1994;269:4693–4696. [PubMed] [Google Scholar]
- 9.Yule D. I., Ernst S. A., Ohnishi H., Wojcikiewicz R. J. Evidence that zymogen granules are not a physiologically relevant calcium pool. Defining the distribution of inositol 1,4,5-trisphosphate receptors in pancreatic acinar cells. J. Biol. Chem. 1997;272:9093–9098. doi: 10.1074/jbc.272.14.9093. [DOI] [PubMed] [Google Scholar]
- 10.Parekh A. B., Putney J. W., Jr Store-operated calcium channels. Physiol. Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 11.Berridge M. J. Capacitative calcium entry. Biochem. J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irvine R. F. ‘Quantal’ Ca2+ release and the control of Ca2+ entry by inositol phosphates – a possible mechanism. FEBS Lett. 1990;263:5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- 13.Feske S., Gwack Y., Prakriya M., Srikanth S., Puppel S. H., Tanasa B., Hogan P. G., Lewis R. S., Daly M., Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 14.Liou J., Kim M. L., Heo W. D., Jones J. T., Myers J. W., Ferrell J. E., Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luik R. M., Wu M. M., Buchanan J., Lewis R. S. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J. Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roos J., DiGregorio P. J., Yeromin A. V., Ohlsen K., Lioudyno M., Zhang S., Safrina O., Kozak J. A., Wagner S. L., Cahalan M. D., et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodard G. E., Lopez J. J., Jardin I., Salido G. M., Rosado J. A. TRPC3 regulates agonist-stimulated Ca2+ mobilization by mediating the interaction between type I inositol 1,4,5-trisphosphate receptor, RACK1, and Orai1. J. Biol. Chem. 2010;285:8045–8053. doi: 10.1074/jbc.M109.033605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lur G., Haynes L. P., Prior I. A., Gerasimenko O. V., Feske S., Petersen O. H., Burgoyne R. D., Tepikin A. V. Ribosome-free terminals of rough ER allow formation of STIM1 puncta and segregation of STIM1 from IP(3) receptors. Curr. Biol. 2009;19:1648–1653. doi: 10.1016/j.cub.2009.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumoto M., Nakagawa T., Inoue T., Nagata E., Tanaka K., Takano H., Minowa O., Kuno J., Sakakibara S., Yamada M., et al. Ataxia and epileptic seizures in mice lacking type 1 inositol 1,4,5-trisphosphate receptor. Nature. 1996;379:168–171. doi: 10.1038/379168a0. [DOI] [PubMed] [Google Scholar]
- 20.Barrow S. L., Voronina S. G., da S. X., Chvanov M. A., Longbottom R. E., Gerasimenko O. V., Petersen O. H., Rutter G. A., Tepikin A. V. ATP depletion inhibits Ca2+ release, influx and extrusion in pancreatic acinar cells but not pathological Ca2+ responses induced by bile. Pflugers Arch. 2008;455:1025–1039. doi: 10.1007/s00424-007-0360-x. [DOI] [PubMed] [Google Scholar]
- 21.Toescu E. C., O'Neill S. C., Petersen O. H., Eisner D. A. Caffeine inhibits the agonist-evoked cytosolic Ca2+ signal in mouse pancreatic acinar cells by blocking inositol trisphosphate production. J. Biol. Chem. 1992;267:23467–23470. [PubMed] [Google Scholar]
- 22.Jansen J. W., Schreurs V. V., Swarts H. G., Fleuren-Jakobs A. M., de Pont J. J., Bonting S. L. Role of calcium in exocrine pancreatic secreation. VI. Characteristics of the paracellular pathway for divalent cations. Biochim. Biophys. Acta. 1980;599:315–323. doi: 10.1016/0005-2736(80)90077-2. [DOI] [PubMed] [Google Scholar]
- 23.Belan P. V., Gerasimenko O. V., Tepikin A. V., Petersen O. H. Localization of Ca2+ extrusion sites in pancreatic acinar cells. J. Biol. Chem. 1996;271:7615–7619. doi: 10.1074/jbc.271.13.7615. [DOI] [PubMed] [Google Scholar]
- 24.Lee M. G., Xu X., Zeng W., Diaz J., Kuo T. H., Wuytack F., Racymaekers L., Muallem S. Polarized expression of Ca2+ pumps in pancreatic and salivary gland cells. Role in initiation and propagation of [Ca2+]i waves. J. Biol. Chem. 1997;272:15771–15776. doi: 10.1074/jbc.272.25.15771. [DOI] [PubMed] [Google Scholar]
- 25.Hong J. H., Li Q., Kim M. S., Shin D. M., Feske S., Birnbaumer L., Cheng K. T., Ambudkar I. S., Muallem S. Polarized but differential localization and recruitment of STIM1, Orai1 and TRPC channels in secretory cells. Traffic. 2011;12:232–245. doi: 10.1111/j.1600-0854.2010.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.