Abstract
The world is currently heavily dependent on oil, especially in the transport sector. However, rising oil prices, concern about environmental impact and supply instability are among the factors that have led to greater interest in renewable fuel and green chemistry alternatives. Lignocellulose is the only foreseeable renewable feedstock for sustainable production of transport fuels. The main technological impediment to more widespread utilization of lignocellulose for production of fuels and chemicals in the past has been the lack of low-cost technologies to overcome the recalcitrance of its structure. Both biological and thermochemical second-generation conversion technologies are currently coming online for the commercial production of cellulosic ethanol concomitantly with heat and electricity production. The latest advances in biological conversion of lignocellulosics to ethanol with a focus on consolidated bioprocessing are highlighted. Furthermore, integration of cellulosic ethanol production into existing bio-based industries also using thermochemical processes to optimize energy balances is discussed. Biofuels have played a pivotal yet suboptimal role in supplementing Africa's energy requirements in the past. Capitalizing on sub-Saharan Africa's total biomass potential and using second-generation technologies merit a fresh look at the potential role of bioethanol production towards developing a sustainable Africa while addressing food security, human needs and local wealth creation.
Keywords: cellulosic ethanol, consolidated bioprocessing, integrating bio-based industries, sustainable biofuels in Africa
1. Introduction
The world is currently heavily dependent (97%) on oil, especially in the transport sector [1]. Rising oil prices, concern about environmental impact and supply instability are among the factors that have led to greater interest in renewable fuel and green chemistry alternatives. Renewable bioenergy, particularly biofuels, has played a pivotal role in Africa in the past and could help address the need for energy expansion in the future [2]. It is estimated that 52 per cent of the developing world and close to 80 per cent of African countries rely on this traditional system to meet their energy needs [3]. Smeets et al. [4] projected that, depending on the level of advancement of agricultural technology, Africa has the largest potential for bioenergy production by 2050 in the world, namely 317 EJ per annum. This could constitute a quarter of the projected total world potential of 1272 EJ per annum.
Biofuels should ideally retain the advantages of fossil fuels with regard to being relatively cheap and rich in energy and should in addition provide a net energy gain, have environmental benefits and be producible in large quantities without impacting on food supplies [5]. Plant biomass is therefore the only foreseeable renewable feedstock for sustainable production of renewable transport fuels. Lignocellulose is globally recognized as the preferred biomass for the production of a variety of fuels and chemicals that may result in the creation of a sustainable chemicals and fuels industry, with significant benefits in agricultural development. Lignocellulose represents the most widespread and abundant source of carbon in nature and is the only source that could provide a sufficient amount of feedstock to satisfy the world's energy and chemicals needs in a renewable manner [6,7]. The main technological impediment to more widespread utilization of lignocellulose for production of fuels and chemicals is the lack of low-cost technologies to overcome the recalcitrance of its structure [8]. Producing biofuels such as ethanol from cellulosic plant material has the potential to meet capacity requirements without impacting directly on food production [9].
Capitalizing on sub-Saharan Africa's biomass potential and bringing back the focus on agriculture merit a fresh look at the bioenergy potential of Africa. For Africa to realize its potential for bioenergy production, advanced agricultural technologies and practices must be employed in a sustainable way to serve the needs of rural and urban communities, foster development of the industrial sector, reduce greenhouse gas (GHG) emissions, develop agricultural infrastructure and lead to land restoration and ecologically healthy landscapes.
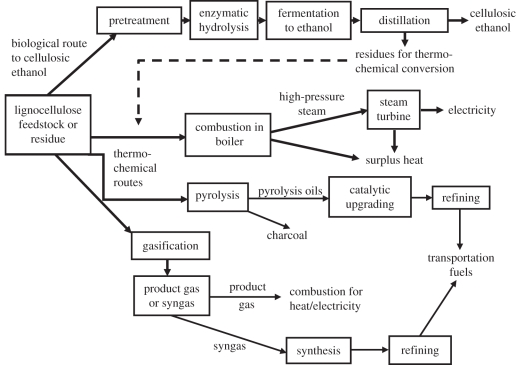
Owing to the wide range of commercial opportunities for second-generation biofuels production, in particular the opportunity for integration of production of such biofuels with existing biomass, fermentation and energy-production industries, both biological and thermochemical conversion processes (summarized in figure 1) should be considered [10,11]. Although biochemical and thermal processes for lignocellulose conversion have comparable efficiencies and economics, the selection of a preferred technology on the basis of the particular industrial scenario for commercialization is still required.
Figure 1.
Alternative process routes for conversion of lignocellulose to bioenergy products. Biological (hydrolysis–fermentation) and thermochemical (combustion, pyrolysis, gasification) routes result in different products, including cellulosic ethanol, electricity, pyrolysis oils, charcoal, surplus heat and other transportation fuels.
This paper will summarize recent developments in second-generation technologies for the production of ethanol from lignocellulose, focusing on consolidated bioprocessing (CBP). Integrating cellulosic ethanol into existing bio-based industries and using thermochemical processes to maximize energy gains and potential electricity production will also be discussed. Lastly, the potential role of bioethanol production towards developing a sustainable Africa while addressing food security, human needs and local wealth creation will be highlighted.
2. Next-generation cellulosic ethanol technologies
Current technologies for biological conversion of biomass commence with a pre-treatment step during which physical and/or chemical processes are used to render the polymeric sugar fractions more accessible to conversion by enzymatic processes [12]. The type of pre-treatment defines the optimal enzyme mixture to be used in subsequent hydrolysis steps and the composition of the hydrolysis products. Four biologically mediated events occur during conversion of pre-treated lignocellulose to ethanol via processes featuring enzymatic hydrolysis: production of depolymerizing enzymes (cellulases and hemicellulases), hydrolysis of the polysaccharide constituents of pre-treated biomass, fermentation of the hexose sugars present and fermentation of pentose sugars present [13]. Improvements in biomass conversion technology generally entail the consolidation of two or more of these steps. Hydrolysis and fermentation steps can be combined in simultaneous saccharification and fermentation (SSF) of hexoses or simultaneous saccharification and co-fermentation (SSCF) of both hexoses and pentoses. The ultimate objective would be a one-step CBP of lignocellulose to bioethanol, in which all four of these steps occur in a single reactor where a single micro-organism or microbial consortium converts pre-treated biomass to a commodity product such as ethanol without added saccharolytic enzymes. CBP would represent a breakthrough for low-cost biomass processing, owing to the economic benefits of process integration [14–17] and avoiding the high costs of enzymes that make the biochemical conversion route unattractive [18,19].
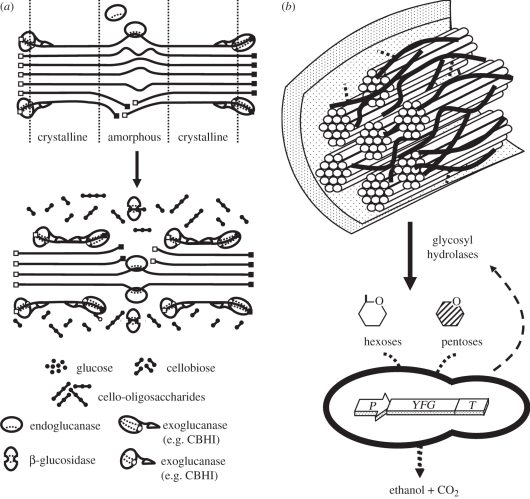
Lignocellulosic plant biomass represents the largest source of renewable carbon and consists of 40–55% cellulose, 25–50% hemicellulose and 10–40% lignin, depending on whether the source is hardwood, softwood or grasses [20]. The main polysaccharide present is water-insoluble cellulose, which represents the major fraction of fermentable sugars. Full enzymatic hydrolysis of crystalline cellulose requires synergistic action of three major types of enzymatic activities: (i) endoglucanases, (ii) exoglucanases, including cellodextrinases and cellobiohydrolases, and (iii) β-glucosidases (figure 2a) [21]. Endoglucanases are active on the non-crystalline or amorphous regions of cellulose and their activities yield cellobiose and cello-oligosaccharides as hydrolysis products. Cellobiohydrolases are processive enzymes that are active on the crystalline regions of cellulose and most yield almost exclusively cellobiose as their main hydrolysis product. In turn, β-glucosidases convert cellobiose and some cello-oligosaccharides to glucose. Hemicellulose refers to a number of heterogeneous structures, such as (arabino)xylan, galacto(gluco)mannan and xyloglucan [20]. These chemically diverse polymers are linked together through covalent and hydrogen bonds, as well as being intertwined, and can be chemically bound to the lignin fraction. Although many pre-treatment protocols remove variable amounts of hemicelluloses, it remains imperative from an economic perspective that sugars contained in the hemicellulose fraction of lignocellulose are also converted to ethanol [22]. The compositions of the major and minor types of hemicelluloses present in lignocellulosic feedstocks and the enzymes required to hydrolyse them are reviewed elsewhere [8,23].
Figure 2.
(a) Schematic of the hydrolysis of amorphous and microcrystalline cellulose by cellulase systems [13]. The filled squares represent reducing ends and the open squares non-reducing ends. Amorphous and crystalline regions are indicated. (b) Lignocellulose conversion to bioethanol in a single bioreactor by a CBP micro-organism is graphically illustrated (adapted from [8]). The enzymatic hydrolysis of the cellulose and hemicellulose fractions to fermentable hexoses and pentoses requires the production of both glycosyl hydrolases (cellulases and hemicellulases), and the subsequent conversion of the hexoses and pentoses to ethanol requires the introduction of pentose-fermenting pathways.
While several micro-organisms can be found in nature with the ability to produce the required enzymes to hydrolyse all the polysaccharides found in lignocellulose, there is no organism with the ability to directly hydrolyse these polysaccharides and ferment the liberated sugar to a desired product such as ethanol at rates and titres required for economic feasibility [24,25]. Strain development is therefore the most important technical obstacle towards the conversion of lignocellulose to commodity products in a CBP configuration [26,27]. Organisms with broad substrate ranges and cellulolytic and/or hemicellulolytic abilities generally suffer from poor growth characteristics or poor product-producing characteristics. These include poor yield, titre and rate or producing mixtures of products where desirable products are produced along with undesirable ones. In comparison, organisms with desirable product-producing qualities often suffer from limited substrate range, including lack of cellulolytic ability, poor fermentation qualities and sensitivity to the inhibitors present in pre-treated lignocellulosic biomass. Two strategies have been followed to develop CBP organisms [25]. The native cellulolytic strategy involves engineering naturally cellulolytic micro-organisms to improve product-related properties. The recombinant cellulolytic strategy involves engineering non-cellulolytic organisms with high product yields so that they express a heterologous cellulase system to enable cellulose utilization (figure 2b).
2.1. Engineering cellulolytic ability into eukaryotic process organisms
The yeast Saccharomyces cerevisiae has long been employed for the industrial production of ethanol from hexose sugars [28–30]. However, this yeast has a number of shortcomings in terms of a CBP-processing organism, such as its inability to hydrolyse cellulose and hemicellulose or use xylose or arabinose. A number of research groups around the world have been working on improving the substrate range of S. cerevisiae to include the monomeric forms of sugars contained in plant biomass [15,22,28,31]. An S. cerevisiae strain that expressed the xylose isomerase gene from the fungus Piromyces sp. E2 was further metabolically engineered to allow anaerobic growth on xylose in synthetic media [32]. Laboratory and industrial S. cerevisiae strains were also engineered to co-ferment the pentose sugars d-xylose and l-arabinose [31].
There have been many reports detailing the expression of one or more cellulase-encoding genes in S. cerevisiae [8]. Strains of S. cerevisiae were created that could grow on and ferment cellobiose, the main product of the action of cellobiohydrolases on cellulosic substrates, at approximately the same rate as on glucose in anaerobic conditions [33]. Recently, the high-affinity cellodextrin transport system of the model cellulolytic fungus Neurospora crassa was reconstituted into S. cerevisiae [34]. This led to the efficient growth of a recombinant strain also producing an intracellular β-glucosidase on cellodextrins up to cellotetraose. Cho et al. [35] showed that, for SSF experiments with a strain producing both a β-glucosidase and enzymes with exo- and endocellulase activity, loadings of externally added cellulase could be reduced. Fujita et al. [36,37] reported co-expression and surface display of cellulases in S. cerevisiae. High-cell-density suspensions of a recombinant strain displaying the Trichoderma reesei endoglucanase II, cellobiohydrolase II and the Aspergillus aculeatus β-glucosidase were able to directly convert 10 g l−1 phosphoric acid swollen cellulose (PASC) to approximately 3 g l−1 ethanol. However, growth of this strain on the cellulosic substrate was not demonstrated. An S. cerevisiae strain co-expressing the T. reesei endoglucanase 1 (cel7B) and the Saccharomycopsis fibuligera β-glucosidase 1 (bgl3A) was able to grow on and convert PASC to ethanol up to 1.0 g l−1 [38]. Jeon et al. [39] constructed a similar strain expressing the S. fibuligera bgl3A and the Clostridium thermocellum cel5E endoglucanase genes that produced significantly more endoglucanase activity than the strain reported by Den Haan et al. [38], and notably improved conversion of PASC to ethanol was achieved. It has been hypothesized that the addition of successful, high-level expression of exocellulases to these strains will enable conversion of crystalline cellulose to ethanol. However, while there have been reports of successful expression of CBH-encoding genes in S. cerevisiae, the titres achieved were generally too low to allow CBP [40].
Several other yeast strains have innate properties that make them attractive as possible CBP organisms [25]. It would be advantageous if the biologically mediated processing steps could occur at an elevated temperature as it would increase enzyme activity, reduce the risk of contamination and decrease the amount of cooling required, thereby decreasing cost. There is subsequently a lot of interest in developing thermophilic or thermotolerant organisms for CBP. Strains of the yeast Kluyveromyces marxianus can grow at temperatures as high as 52°C, and can convert a wide range of substrates, including xylose, to ethanol, and successful SSF with a variety of feedstocks at elevated temperatures has been demonstrated [41–43]. Thermotolerant cellobiohydrolase, endoglucanase and β-glucosidase-encoding genes were expressed in combination in a strain of K. marxianus [44]. The resulting strain was able to grow in synthetic media containing cellobiose or carboxymethylcellulose as the sole carbon source but the hydrolysis of crystalline cellulose was not shown. Recently, a K. marxianus strain was engineered to display T. reesei endoglucanase II and A. aculeatus β-glucosidase on the cell surface [45]. This strain successfully converted 10 g l−1 of a cellulosic β-glucan to 4.24 g l−1 ethanol at 48°C within 12 h.
Some strains of the methylotrophic yeast Hansenula polymorpha have a high capacity for heterologous protein production, are able to grow at elevated temperatures ranging up to 48°C and ferment glucose, cellobiose and xylose to ethanol [46]. A recent report highlighted the promise of H. polymorpha in biomass conversion when strains were constructed that could ferment starch and xylan [47]. Pichia stipitis is one of the best-studied xylose-fermenting yeasts and has a substrate range including all the monomeric sugars present in lignocellulose [48]. Some P. stipitis strains produce low quantities of various cellulases and hemicellulases, among which is a β-glucosidase that allows the yeast to ferment cellobiose; however, P. stipitis cannot use polymeric cellulose as a carbon source [49]. Endoglucanases were successfully produced in H. polymorpha [50] and P. stipitis [51]. As these yeasts are capable of growth on cellobiose the recombinant strains should theoretically have the ability to hydrolyse amorphous cellulose, although this aspect was not tested. The xylanolytic ability of P. stipitis was enhanced by the co-expression of β-xylanase- and β-xylosidase-encoding genes [52]. The resulting strains displayed improved biomass production on medium with birchwood glucuronoxylan as the sole carbohydrate source. Despite the fact that P. stipitis is a relatively poor ethanol producer, it has the ability to remove fermentation inhibition from lignocellulose hydrolysates, by consuming acetic acid and reducing furfural and hydroxymethylfurfural to less harmful substances [53].
2.2. Engineering prokaryotic organisms to hydrolyse polysaccharides
Although Escherichia coli cannot hydrolyse cellulose, nor produce ethanol at appreciable quantities, it has been shown to metabolize all major sugars present in plant biomass, producing a mixture of organic acids and ethanol [54]. Brau & Sahm [55] successfully modified E. coli metabolism by expressing the Zymomonas mobilis pyruvate decarboxylase at high levels. The resulting strain produced ethanol at levels comparable to Z. mobilis. Subsequent work has focused on improving ethanol yields, growth rate, strain stability and ethanol tolerance [56–61]. The Klebsiella oxytoca casAB operon coding for an enzyme IIcellobiose and a phospho-β-glucosidase was expressed in the ethanol-producing strain of E. coli, enabling transformants to efficiently use cellobiose. Several endoglucanases have been expressed in E. coli, allowing it to hydrolyse amorphous and soluble cellulose to shorter cello-oligosaccharides [57,62–66]. Among these are Cel5Z and Cel8Y from Erwinia chrysanthemi. Zhou et al. [66] successfully reconstructed the type II secretion system, the predominant secretion system type in Gram-negative bacteria, encoded by the out genes from E. chrysanthemi, in E. coli. This enabled E. coli to secrete more than 50 per cent of the recombinant Cel5Z it produced.
Klebsiella oxytoca is a hardy, prototrophic bacterium with the ability to transport and metabolize cellobiose, cellotriose, xylobiose, xylotriose, sucrose and all other monomeric sugars present in lignocellulosic biomass [67]. Four fermentation pathways are present in K. oxytoca producing formate, acetate, ethanol, lactic acid, succinate and butanediol [68]. Through metabolic engineering and expression of the Z. mobilis pdc and adhB genes, it was possible for a recombinant K. oxytoca strain to produce ethanol from soluble sugars at 95 per cent of the maximum theoretical yield [69]. Unlike most other ethanol-producing organisms, K. oxytoca has the ability to ferment xylose and glucose at equivalent rates [68]. This significantly shortens the time required to ferment the mixtures of glucose and xylose typically present in lignocellulosic hydrolysates. Zhou & Ingram [67,70] constructed a K. oxytoca strain expressing the E. chrysanthemi cel8Y and cel5Z endoglucanase genes. By also introducing the genes that encode the type II secretion system from E. chrysanthemi, both Cel8Y and Cel5Z were secreted effectively by K. oxytoca. This strain was capable of fermenting amorphous cellulose and producing a small amount of ethanol without the addition of cellulases.
Zymomonas mobilis is a well-known fermenting bacterium that produces ethanol at high rates but cannot ferment or use xylose as the carbon source or hydrolyse polysaccharides [71]. Zhang et al. [71] engineered a Z. mobilis strain capable of fermenting both xylose and arabinose, the major pentose sugars present in plant material. Co-fermentation of 100 g l−1 sugar (glucose : xylose : arabinose—40 : 40 : 20) yielded a final ethanol concentration of 42 g l−1 in 48 h. Brestic-Goachet et al. [72] expressed the E. chrysanthemi cel5Z in Z. mobilis obtaining 1000 IU l−1 activity with 89 per cent of the recombinant endoglucanase secreted to the extracellular medium. Expression of the Ruminococcus albus β-glucosidase enabled Z. mobilis to ferment cellobiose to ethanol very efficiently in 2 days [73].
The thermophilic anaerobic bacterium Thermoanaerobacterium saccharolyticum is also under development for biomass conversion. Thermoanaerobacterium saccharolyticum grows in a temperature range of 45–65°C and a pH range of 4.0–6.5 and is able to ferment a wide range of sugars present in cellulosic biomass, including cellobiose, glucose, xylose, mannose, galactose and arabinose [74]. Unlike most organisms, T. saccharolyticum metabolizes xylose and glucose essentially at the same rate [74,75] but it produces organic acids in addition to ethanol. Knockout mutants were created that produced almost exclusively ethanol from xylose. Furthermore, a strain with hfs and ldh deletions exhibited an increased ethanol yield from consumed carbohydrates [76]. Thermoanaerobacterium saccharolyticum naturally produces both a β-xylanase and a β-xylosidase [77,78], enabling it to ferment xylan directly to ethanol. Furthermore, T. saccharolyticum was able to produce as much ethanol from Avicel with 4 filter paper units (FPU) of externally added enzyme as S. cerevisiae was with 10 FPU in SSF, the result of improved enzyme efficiency at higher temperatures [75]. This shows the potential of this thermophile as a CBP organism if a cellulolytic system can be established.
To date, no ideal organism has been developed for CBP conversion of biomass. Bacteria generally have a high growth rate but lack process robustness. Yeasts are often sufficiently robust, but lack substrate range. Filamentous fungi often have a wide substrate range, but grow relatively slowly and do not produce enough of a desirable product. While the advantages of using the yeasts S. cerevisiae, P. stipitis, K. marxianus and H. polymorpha are well appreciated, the engineered cellulolytic ability of these strains is currently rudimentary. None of the strains are as yet capable of using crystalline cellulose and the high-level production of an exocellulase remains a requirement. New information on secretion pathways, chaperones and metabolic engineering should help alleviate this problem in future. Compared with S. cerevisiae, all of the bacterial species discussed above are relatively sensitive to inhibitors associated with lignocellulosic hydrolysates [27,61,68]. Escherichia coli and K. oxytoca strains capable of breaking down cellulose could also be modified to produce other commodity products such as lactic acid, succinic acid, acetic acid or 2,3-butanediol [79]. It is likely that more than one organism may eventually be used in various biomass conversion processes, and the choice may depend on the sugar composition of the feedstock, the pre-treatment method used and the end product required [80].
3. Process integration to improve economic viability of second-generation biofuels production
The cost disadvantage of second-generation biofuels may be addressed through innovative methods of process integration, in order to minimize the capital investment, maximize energy efficiency and improve overall economics. Various scenarios regarding technical options for process integration, to achieve more attractive financial returns, are presented below.
3.1. Energy integration within lignocellulosic conversion processes
Apart from biochemical conversion of lignocellulose to ethanol, discussed in §2, three thermochemical options are also available: combustion, pyrolysis and gasification. Combustion entails burning of biomass in the presence of air, which generates hot gases at temperatures of around 800–1000°C and energy that can be harvested as heat. Pyrolysis is the conversion of biomass to liquid (bio-oil), solid (char) and gaseous fractions by heating the biomass in the absence of air to about 500°C. Bio-oils can be upgraded to transport fuels, or bio-oils and char can be gasified. Gasification is the conversion of biomass by partial oxidation at temperatures typically in the range of 800–900°C, to generate a combustible gas (called syngas) that can be used for the synthesis of different synthetic fuels (typically using the Fischer–Tropsch process) or burnt for heat production [81].
Several studies have demonstrated the technical, environmental and economic benefits of using process integration within biological and thermochemical processes. Examples of such process integration are the SSF, SSCF and CBP configurations for the production of cellulosic ethanol by the biochemical route (§2). As an example, heat integration within biological [10,19] and thermochemical routes for second-generation biofuels production has the potential to increase overall energy efficiency by as much as 15 per cent [82] and can reduce capital and operational costs substantially [14].
In the biological process for lignocellulose hydrolysis–fermentation, large amounts of energy remain in the non-fermentable lignin-rich residues in the bottom product of the distillation columns. Conversion of these residues through high-efficiency processes, such as a high-pressure boiler coupled with a multi-stage steam turbine [10,83], or advanced biomass integrated gasifier/combined cycle (BIG/CC) systems [84], can provide all the heat and electricity needed for cellulosic ethanol production, together with surplus electricity production for sale [82,84,85]. Energy consumption in the biochemical process can be reduced further by performing enzymatic hydrolysis and/or SSF processes at high substrate loadings, together with recycling of the process streams, both of which have substantial benefits in terms of process energy efficiency and economics [86,87]. Hot vapour generated by evaporation steps for sugar concentration and/or water recovery can provide heat for distillation [84], one example of how the energy needs of ethanol distillation can be minimized through heat integration and pinch analysis [83]. Anaerobic digestion for waste-water treatment can be used to produce methane-rich biogas that can be captured and used to generate electricity and/or process heating [88]. Similarly, the integrated production of synthetic biofuels and electricity from lignocellulose in the gasification–synthesis process route will provide higher energy efficiencies than production of synfuels alone [11,82].
3.2. Energy integration between lignocellulosic conversion processes and adjacent industrial processes
In addition to performing process and energy integration within a particular second-generation biofuels production process, integration of second-generation biofuels production with adjacent industrial processes can address both energy efficiency and production costs for the lignocellulose conversion process. Process integration with adjacent industrial processes can be broadly classified as integration (i) with electricity production from biomass or fossil fuels, (ii) with biomass processing for pulp or sugar production, (iii) of first- and second-generation biofuels production by the biological route, (iv) of second-generation biofuels production by the thermochemical route with petrochemical processing, and (v) integration of biological and thermochemical processing of lignocellulose to second-generation biofuels. Examples of such process integrations, and the associated economic benefits, are presented below.
3.2.1. Integration with electricity production from biomass or fossil fuels
Integration of second-generation biofuels production with dedicated electricity production from coal, natural gas or biomass can provide benefits in economies of scale (financial) and process efficiency. Both the biological (hydrolysis–fermentation) and thermochemical (gasification–synthesis) processes require the use of advanced, high-efficiency equipment for electricity production, to satisfy process requirements and provide surplus electricity for sale [89,90]. However, advanced equipment such as BIG/CC systems [84,91,92] have high capital costs per unit electricity [19,93].
Economies of scale achieved through integration of electricity production in second-generation biofuel processes with electricity production in adjacent industrial facilities can reduce capital investment per unit of electricity substantially [24,90,92,94–96]. Integration and scale-up of electricity and steam production can be achieved by combining feedstocks for electricity generation, such as lignin-rich residues from biological processing or residual syngas from gasification synthesis, and using heat recovery/integration in both biofuel and electricity generation for steam production and drying/evaporation [90,94,95]. The resulting maximization of electricity production will increase revenue to second-generation biofuels production, since it minimizes domestic/industrial heat production, for which limited markets are available [97]. Increasing the overall energy efficiency of the combined biofuel–electricity production processes will have substantial benefits in reducing the GHG emissions of processes [97]. Opportunities for sharing of feedstock supply and handling infrastructure and logistics will also exist when integrating second-generation biofuels production with electricity production from biomass. These opportunities are similar to benefits in feedstock supply through integration considered in §3.2.2.
3.2.2. Integration with biomass processing for pulp or sugar production
Integration of biofuels production from lignocellulose with existing biomass processes such as pulp-and-paper industries or sugar production can provide efficiency and economic benefits owing to potential for feedstock supply and/or energy integration [24,98,99]. The combined costs of raw material (lignocellulose) supply and onsite handling thereof contribute substantially to the total capital and operational cost of second-generation biofuels, even though lignocellulose is often considered to be inexpensive [10,18,19,24,83,100].
Feedstock costs can be reduced by co-locating and integration of feedstock supply for biofuels production into existing industrial processes, owing to economies of scale benefits, the potential availability of biomass (e.g. residues) and lower transportation costs [98,101]. For both pulp mills and sugar production mills, the potential availability of residues, not suitable for or useful in primary biomass processing, presents an attractive opportunity for feedstock supply. Furthermore, sharing of pre-existing logistics, supply chain and infrastructure for feedstock supply with an existing biomass processing operation can reduce capital and operational cost substantially, especially when considering the significant contribution to production costs from these costs [85,96,99]. The Brazilian sugar industry, an example for Africa, is prioritizing the co-location of lignocellulose conversion with existing sugarcane-processing plants for cost minimization [102], which may include the combination of surplus bagasse from a number of nearby mills for economies of scale, and/or using sugarcane agricultural residues (SCARs) to provide energy to primary sugarcane milling, thus liberating additional bagasse for conversion [99,102].
Although bagasse liberated from sugar milling can be considered free of transportation costs [99], the feedstock does have economic value, while supply is often limited. Although highly efficient sugar mills can liberate up to 50 per cent of the bagasse present in cane supply as surplus [103], the availability of bagasse at present-day mills is highly variable and often limiting. Many conventional sugar mills in Africa are designed to dispose of bagasse residues by inefficient burning, resulting in energy-inefficient operations compared with international state-of-the-art mills [102], and limited availability of surplus bagasse. The economic cost of sugarcane bagasse is therefore coupled to the cost of capital investments required to improve the energy efficiency, and/or the harvesting/transportation cost of replacing bagasse with another source of biomass for energy production, e.g. SCAR. Design and construction of a sugar mill with co-located second-generation biofuels as an integrated, greenfields project are considered beneficial, and likely to provide bagasse at low cost.
Co-location of second-generation biofuels with pulp or sugar production will also provide economic benefits in terms of energy supply (steam, electricity) to second-generation biofuels. Co-generation of electricity from sugar and pulp mills is widely considered to be an attractive option for a sustainable energy future. The integration of such co-generation between primary biomass processing and second-generation biofuels production can provide substantial economies of scale, making the capital costs of highly efficient electricity generation affordable, and can reduce the cost of second-generation biofuels production by 20 per cent in, for example, Sweden [24,104].
3.2.3. Integration of biological first- and second-generation biofuels production
Integration of second-generation biofuels production by the hydrolysis–fermentation of lignocellulose with production of the same biofuels from sugar/starch with first-generation technology will provide technical, environmental and economic benefits in addition to those considered above [96], reduce capital costs and investor risk and increase economic attractiveness [100]. Particular aspects of process integration would be feedstock supply, fermentation, water and nutrient recycle, distillation and additional opportunities for utilities/energy integration to provide process demand [94,101]. Sugar-rich crops for first-generation ethanol production, such as sugarcane, sweet sorghum and sugarbeet, are particularly attractive for integrated first- and second-generation processes, being able to supply feedstock to both processes and sharing the cost of feedstock production and logistics with associated environmental benefits [96,100]. These crops also allow construction of a flexible manufacturing process, capable of making both crystallized sugar and ethanol from the extracted plant juices. This allows switching between products according to market conditions, which is practised in some Brazilian sugar mills, and has also been suggested for sweet sorghum processing in Northern China [100]. Potential benefits of combined fermentation–distillation processes for ethanol production from lignocellulose may include (i) replacing exogenous nutrient supplements with sugar juice and/or molasses, which are rich in nutrients [88], (ii) mixing of sugars from juice and lignocellulose to increase ethanol concentrations at the end of the cellulose fermentation, and (iii) scale-up of ethanol purification/distillation to achieve economies of scale and improve energy efficiency [99]. Similar integration possibilities also exist in grain (small grains, corn, etc.) fermentation, where ethanol production from starch may be supplemented with sugars from bran (starch fibre) and polysaccharide-rich waste streams such as thin stillage [85,105].
3.2.4. Integration of thermochemical second processes with fossil fuel processing
Conversion of lignocellulose into biofuels by thermochemical processes such as gasification and pyrolysis may be integrated with existing fossil fuel processing. Examples of co-gasification of biomass with coal and development of pyrolysis oil as feedstock to oil refineries are considered here.
The gasification–synthesis route for production of biofuels from lignocellulose is based on a similar process for fuels and chemical production from coal, which has been in commercial operation at Sasol (South Africa) for more than 35 years [18]. The synthesis and downstream-processing parts of this process route are well established, and the key to biomass conversion is therefore the production of syngas of acceptable quality [96,106]. One attractive means to integrate biomass processing into existing coal gasification–synthesis processes is through co-gasification of biomass with coal, which may provide substantial synergies in terms of both gas and liquid yields [107]. Biomass co-gasification with coal in existing gasifiers, with associated capital cost benefits, may be complemented with syngas production from biomass in stand-alone gasifiers, both using existing synthesis–purification equipment to produce a ‘blended’ synthetic fuel.
Pyrolysis of biomass, in particular fast pyrolysis for the production of pyrolysis oils, is rapidly developing in terms of its potential to produce low-cost transportation fuels from lignocellulose [18]. Whereas the production of bio-oil is a well-established, low-cost process [82], the upgrading of bio-oil to transportation fuels through catalytic hydrogenation and/or decarboxylation is not as well developed [18,108]. Refining of upgraded bio-oils may be integrated with existing oil refineries, to further reduce the costs of transportation fuel production. Pyrolysis products may also be gasified for syngas production, either in stand-alone units or by co-gasification with biomass.
3.2.5. Integration of biological and thermochemical processing of lignocellulose to second-generation biofuels
Whereas biological and thermochemical processing of lignocellulose are often considered as competing technologies, integration of these processes may achieve improved energy efficiency and economic returns for second-generation biofuels production. For example, conversion of carbohydrates in lignocellulose to ethanol by the biological route may be combined with thermochemical (gasification–synthesis) processing of the non-fermentable lignin residues, for which overall energy efficiencies as high as 70 per cent have been demonstrated for future mature technology scenarios [92,106]. Scenarios that integrate biological and thermochemical processing enable waste heat from the thermochemical process to power the biological process, resulting in higher overall process efficiencies [106]. Alternative scenarios for process integration to improve economies of second-generation biofuels production in Africa should be considered through rigorous process modelling coupled with economic analysis [18,82], taking location-specific factors into account.
4. Sustainable bioenergy production in africa
Africa still remains a large consumer of traditional sources of energy, mainly fuel wood, and a greater proportion of its population faces energy insecurity [109]. The availability and accessibility of socially and environmentally acceptable sources of energy are still very low and disproportionate between rural and urban areas. With the exception of fuel wood, other energy sources (coal, crude oil and more recently biofuels) have been the major sources of power driving the transport and industry sectors.
Several conversion paths are being studied for total conversion of biomass into biofuels, in particular for production of bioethanol from cellulosic feedstocks. The benefits of the total conversion of cellulosic feedstock to bioethanol are different in different geographical regions. In developed countries, the main thrust for ethanol production is the replacement of fossil fuels in the transportation sector. The situation is different for developing countries, such as those in the Southern African Development Community (SADC) region, because of their unique socio-economic needs, especially the chronic food and energy insecurity, extreme poverty, high unemployment rate and degradation of the natural environment. Therefore, biofuels in Africa have increasingly received attention for their potential not only to reduce GHG emissions and increase energy supply but also to open new markets for agricultural surplus (thus additional revenue for farmers) and provide employment opportunities and local economic development opportunities in rural areas, just to mention a few [110].
4.1. Biofuels production potential in Africa
The biofuels industry in Africa is being developed gradually in most African countries with assistance from international agencies [111]. Some of the major biofuels and technologies that have been reported in Africa include: biogas, thermal gasification, biodiesel, bioethanol and most recently, albeit at the research and/or developmental level, the second-generation biofuels devoted to total biomass conversion.
4.2. Ethanol production
In SADC, sugarcane production, an important feedstock for bioethanol production, is growing steadily (2.5% per annum) [111]. Most of this potential in biofuels for the region is in domestic markets, especially in the transport sector. This has been attributed to the contribution from rehabilitation programmes in post-conflict countries (Angola and Mozambique). The region has great potential to produce and meet the growing demand for lead phase-out programmes in fuel for transport. Current figures for cultivated land (6%) are very low and suggest that availability of land may not be a constraint to increasing production of biomass for fuel production [112]. For bioethanol, most production plants in Africa are in SADC and active participants include South Africa, Malawi, Swaziland, Mauritius and Zimbabwe. There are also substantial amounts of sugarcane and a large potential for doubling current production in the region [111].
Bioethanol production requires biomass with significant starch or sugars, which is fermented through enzymatic biological processes to generate liquid biofuel [3]. The current major feedstock in the production of biofuels in the world is starchy biomass, which accounts for nearly 53 per cent of all bioethanol production. Maize, wheat, sorghum and other starchy materials are the main starchy feedstocks used in bioethanol production. The second method uses sugarcane and sugarbeet biomass, the feedstock that is already in sugar form, and the rest of the processes are the same as in starchy biomass; the last method uses biomass from cellulosic materials such as bagasse, straw and wood biomass [81].
While the technology associated with the first two feedstocks (starchy biomass and sugarcane) is available and can be replicated, maize and other starchy biomass feedstocks have a very important role in food security in sub-Saharan Africa. To some extent, the use of these feedstocks (maize included) in the promotion of biofuel production makes it less attractive for most parts of Africa [110]. On the other hand, secondary products from, for example, processing of sugar from sugarcane generate co-products like bagasse, molasses and fibre, which can be used to generate electricity and provide additional revenue if exported [113]. Countries such as Mauritius have successfully used this technology and supplied electricity to the national grid, contributing up to 40 per cent of all domestic power consumption [114]. Molasses, another form of waste from crystalline sugar production, can also be used as feedstock in bioethanol production. This pathway has a very high unexploited potential in Africa. For example, in Tanzania, only 30 per cent of the molasses produced from sugar production is exported and used as animal feed while 70 per cent goes to waste [112]. Hence, in light of current debates on the potential negative impact of increasing biofuel production on food security, sugarcane molasses offers a viable option.
4.3. Energy crops
Several potential energy crops have been highlighted for biofuels production in sub-Saharan Africa [113]. Ethanol is the most promising biofuel product that can be produced from different raw materials in many African countries (table 1), with most of the ethanol coming from molasses. On the other hand, Jatropha and oil seeds are the main feedstocks for producing biodiesel, which is used to run stationary generators for electricity generation and as a diesel substitute for transportation.
Table 1.
Biofuels potential in selected African countries in megalitres (Ml).
| country | raw material | biodiesel (Ml) | ethanol (Ml) |
|---|---|---|---|
| Benin | cassava | — | 20 |
| Burkina Faso | sugarcane | — | 20 |
| Ivory Coast | molasses | — | 20 |
| Ghana | Jatropha | 50 | — |
| Guinea Bissau | cashew | — | 10 |
| Mali | molasses | — | 20 |
| Malawi | molasses | — | 146 |
| Kenya | molasses | — | 413 |
| Ethiopia | molasses | — | 80 |
| Niger | Jatropha | 10 | — |
| Nigeria | sugarcane | — | 70 |
| Sudan | molasses | — | 408 |
| Swaziland | molasses | — | 480 |
| Senegal | molasses | — | 15 |
| Tanzania | molasses | — | 254 |
| Togo | Jatropha | 10 | — |
| Uganda | molasses | — | 119 |
Source: [113].
4.4. Biofuels production in Africa is hampered by economic and technical factors
Examples of first-generation biofuels production plants with attractive economic returns can be found in Africa, in particular in least developed countries with preferred access to developed world markets. However, the commercial production of second-generation biofuels is constrained by economic and technical concerns [96]. Owing to the recalcitrance of lignocellulose to biological degradation, the cost of production of a second-generation biofuel is dominated by investment costs; for example, second-generation ethanol by the biological route requires substantially higher capital investment than first-generation ethanol [100]. Such economic concerns place second-generation technologies at an even greater disadvantage than first-generation technologies, despite their potential for improved environmental and socio-economic benefits, since they are not deemed economically viable without government subsidies. In addition, while methods for lignocellulose pre-treatment/fractionation are available, these have not been optimized for local substrates and novel African bioenergy crops.
4.5. Markets opportunities for bioenergy in Africa
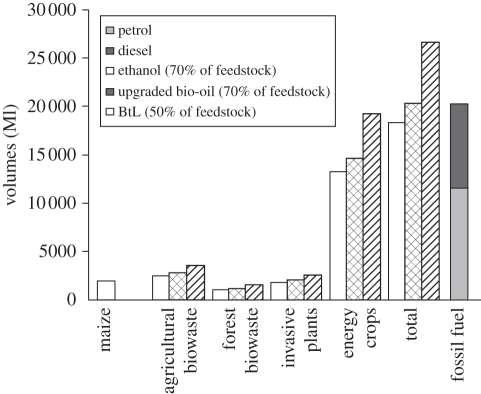
The outlook of market potential for biofuels in Africa is varied with sub-Saharan Africa having the most potential and North Africa having the least potential. The potential value of biofuels for sub-Saharan Africa by 2010–2013, as estimated by global growth consultancy Frost and Sullivan in the Africa Review of Business Technology, March 2008, was between US$1.54 and US$1.83 bn. However, if the next-generation technologies unlock the potential of converting all cellulosic biomass, the potential value could be significantly higher. Figure 3 compares the potential biofuels production from agricultural and forestry residues, invasive plants and energy crops in South Africa, in relation to the current fossil fuel and the industrial biofuels strategy's target for 400 Ml per annum. In this case, when considering the use of only 50–70% of this plant biomass with second-generation biochemical and thermochemical technologies, South Africa could very well exchange the bulk of its current liquid fossil fuel usage (currently 21.2 billion litres per annum) with renewable biofuels. One of the factors stimulating domestic demand on the continent is the implementation of the lead phase-out programmes in petrol. In West Africa, a market opportunity study for biofuels indicated that locally produced anhydrous ethanol favourably competed with petrol [118]. Ethanol programmes that produce a blend of ethanol and petrol (gasohol) for use in existing fleets of motor vehicles have been implemented in Malawi, Zimbabwe and Kenya [2].
Figure 3.
Potential biofuels production from lignocellulosic biomass available in South Africa [6] (assuming only 50–70% was used) when advanced second-generation biochemical and thermochemical technologies are available. Optimal biofuels yields estimated when the appropriate technologies are available, including (i) biochemical processing of maize-to-ethanol = 460 l ton−1 [115] or lignocellulosic-to-ethanol = 280 l ton−1 (only polysaccharide fraction) [116], and (ii) thermochemical upgrade of bio-oils from fast pyrolysis = 310 l ton−1 [117] and thermochemical biomass-to-liquid (BtL) = 570 l ton−1 [82].
Notwithstanding, sugarcane-producing countries have great potentials in the ethanol gel fuel industry [111], which can be directed for local consumption at the household level. The ethanol gel and specially adapted ethanol cooking stoves such as SuperBlu stoves [17,119] can lessen the burden of women and girls, who spend most of their time fetching fuelwood for their households. Such technologies provide alternatives to paraffin or open fire cooking and heating that are associated with fire hazards, indoor pollution and inefficient conversion (in the case of fuelwood). Johnson & Matsika [120] estimated a market of 10 billion litres is needed to substitute for 30 per cent of all cooking fuels in sub-Saharan Africa. Despite offering more socio-economic and environmental benefits than traditional cooking and heating energy, the uptake of clean fuel cooking and heating technologies in rural areas of Africa has been very low, mainly because of lack of distribution infrastructure, high costs and lack of awareness [121]. However, several pilot projects run by non-governmental organizations and private companies such as Millennium Gelfuel Initiative, which began in 2000 as a public–private partnership, have demonstrated some level of acceptance of the gelfuel technology by households in Malawi, South Africa and Zimbabwe [122].
4.6. Bioenergy production and the environment
According to the Intergovernmental Panel on Climate Change, agricultural production and access to food in many regions may be severely compromised by climate variability and change [123]. The area suitable for agriculture, the length of growing seasons and the yield potential of some crops mainly in arid areas are expected to decrease. The adverse impacts of mitigation measures being taken under the Kyoto Protocol such as carbon sinks and the expansion of monocrop plantations for biofuels (e.g. palm oil, soya, sugar cane, Jatropha) have been associated with undermining small-scale traditional livelihoods of indigenous people (e.g. rotational agriculture, pastoralism, hunting and gathering), which usually have higher biodiversity as opposed to the monocrops. However, on whether biofuels decrease or increase the emissions, it will be important to appraise the entire energy chain when comparing options, and it is equally important to analyse the production and emissions based on best practices, including innovative ways to manage crops and soils, such as zero-tillage approaches; and also examine forestry management that includes judicious forest use without burning and other activities that generate high emissions.
In dry areas, the production of fast-growing biofuel crops will naturally be associated with the competition for water between food and fuel crops and may become the overriding issue in the fuels versus food debate. Improvement in crop productivity as well as the shift from high water-use biofuel crops (such as sugarcane) to drought-tolerant crops (such as sweet sorghum and Jatropha) can be used as options to address the issue of water scarcity. Despite what is often said about production of biofuel crops on dry and marginal lands, irrigation in low-rainfall ecologies is required for optimal yields. This may have the undesirable water salinity problem in many regions [123].
With biofuels and the carbon markets, it has been suggested that ways need to be found to link small-scale farmers to the global carbon market. This should occur without creating bureaucracies or additional burdens for them while also establishing clear indicators for accounting for carbon and providing payments to poor farmers for such environmental services. Additionally, options for financing are much broader and are emerging rapidly. The growing market for carbon projects and activities, through both the Clean Development Mechanism (CDM) and voluntary markets, demonstrates that the sequestration of carbon could offer opportunities for smallholder agriculturalists to gain from the mitigation potential of the agriculture sector. However, in the global carbon market, the participation of developing countries, particularly the poorest communities within them, has been extremely challenging, because of the costs, modalities and procedures for CDM verification.
4.7. Policy and institutional framework for bioenergy industry development
Political commitment and support for the development of the necessary regulatory instruments for advancement of bioenergy in Africa are very important. A number of governments in Africa have made some progress in coming up with definitive policy strategies that have unveiled the economic benefits of renewable energy. For instance, operating bioethanol programmes that blend ethanol and petrol (gasohol) for motor vehicles exist in Southern African countries such as Malawi, Zimbabwe and South Africa [2]. However, most of the policy instruments are embedded within the energy policies for the countries, which vary from country to country. Consequently, policies in particular specific for second-generation biofuels are generally lacking. While most of the biofuels policies and regulatory frameworks have centred on the first generation of commercially viable biofuels, owing to the advancement in research and development and the sector's associated expansion and growth, future policies are likely to extend to other second-generation biofuels [118].
However, the implementation structures for bioenergy policies are another source of confusion, with some pieces of the mandates scattered in different departments and falling under different ministries [2]. The cooperation and interaction of all relevant stakeholders, including civil society and the private sector, can lead to the development of a conducive policy instrument that can foster significant growth in biofuels development. An example of such multistakeholder partnerships is the Competence Platform on Energy Crop and Agroforestry Systems for Arid and Semi-arid Ecosystems (COMPETE) that aims at developing cross-sectoral work packages for evaluating Africa's potential for sustainable provision of bioenergy and develops innovative tools for financing national and local and strategic policy mechanisms for developing bioenergy systems [124]. Furthermore, one of the suggestions that has been proposed as fundamental in the ‘bioenergy revolution’ has been the organization of smallholder farmers and producers in order to facilitate their access to markets and enable them to commercially interact with large private entities engaged in the energy markets [123].
From a rural development perspective, at the microeconomic level, biofuel policy development must aim at contributing to the larger developmental goals, but not at the expense of more pertinent issues such as food and social security. According to Sulle & Nelson [125], most of the biofuels production systems in Africa are characterized by large-scale and concentrated ownership operations, which in the African context would affect the land tenure system and rural development policies. Thus, biofuel development could, without appropriate policy guidelines, increase pressure on land to the disadvantage of poor rural people and deprive the locals of their customary land rights. Notwithstanding, secure access to land tenure is a much broader issue in most developing countries that generally affects agricultural production and so biofuels are not its main driver. Foreign investors that involve the locals in the biofuels value chains, such as the locals participating as outgrowers, formation of village land trust and establishment of equity-based ventures, represent the positive models for improving local livelihoods and the environment [125].
5. Concluding remarks
Great strides have been made in the development of second-generation technologies for cellulose conversion to bioethanol and other biofuels. New opportunities using agricultural residues, energy crops or invasive plant species for biofuel production potentially allow for the sustainable production of biofuels in significant quantities, while at the same time stabilizing food production by providing alternative markets to farmers, as well as addressing human development specifically in rural communities. First-generation biofuels production has been modest in Africa (table 1), compared with the huge potential of Africa to produce biomass [4].
Although the geographical potential of Africa to produce biofuels is at least as large as any other continent, recent developments in second-generation technologies have not had much impact in Africa. Partly, this is because Africa faces pressing human challenges associated with an interconnected set of issues involving poverty, food security, economic development, gender issues, health and energy security. The limited resources of many African economies, together with fragmented trade and economic policies, apparently limit the abilities of governments to provide financial incentives for second-generation biofuels production.
However, the positive considerations of second-generation biofuels production, such as GHG emission reductions, renewability, absence of competition with food/feed, negligible land-use impacts and sustainability, should translate into a greater willingness by governments and consumers to accept higher prices for second-generation biofuels, compared with those produced by first-generation technologies [100]. Policies to enable such price differentials in the market place have not been implemented in Africa. Fossil fuels remain generally cheap, and the development of a sufficient policy environment, which can provide attractive economic returns for second-generation biofuels through incentives, subsidies and carbon taxes, mandated blending, carbon emissions legislation and the financial benefits of carbon trading, is lacking in the African context.
To actively take a step in the right direction, actions need to be considered to ensure that Africa benefits along the full value chain of biofuel production and utilization. Some worthy actions could include:
— proper analysis, understanding and consensus on the potential of bioenergy, including biofuels production with second-generation technologies to realize a sustainable Africa;
— African scalable demonstration projects using second-generation technologies for learning perspectives, e.g. training to strengthen local manpower; this could also show best practices in energy efficiency and resource protection in transport, electricity supply, cooking and other household needs; and
— alignment of international, regional and local policies on trade, aid, land tenure and development, needed to facilitate integrated value chains of agriculture and forestry for food and bioenergy in Africa.
Biofuels present one of the most cost-effective solutions for a global sustainable low-carbon-energy future. This future demands sustainable agriculture and forestry in Africa to supply food and bioenergy in support of Africa and the world. A sustainable globe requires a sustainable Africa, and it is for Africa to step up to this challenge.
Footnotes
One contribution of 9 to a Theme Issue ‘Biorenewables, the bio-based economy and sustainability’.
References
- 1.IEA 2008. World Energy Outlook 2008. Paris, France: International Energy Agency, OECD [Google Scholar]
- 2.Amigun B., Sigamoney R., von Blottmitz H. 2008. Commercialization of biofuels industry in Africa. A review article. Renew. Sust. Energ. Rev. 12, 690–711 10.1016/j.rser.2006.10.019 (doi:10.1016/j.rser.2006.10.019) [DOI] [Google Scholar]
- 3.Cotula L., Dyer N., Vermeulen S. 2008. Fuelling exclusion? The biofuels boom and poor people's access to land. London, UK: International Institute for Environment and Development [Google Scholar]
- 4.Smeets E. M. W., Faaij A. P. C., Lewandowski I. M., Turkenburg W. C. 2007. A bottom-up assessment and review of global bio-energy potentials to 2050. Prog. Energ. Combust. Sci. 33, 56–106 10.1016/j.pecs.2006.08.001 (doi:10.1016/j.pecs.2006.08.001) [DOI] [Google Scholar]
- 5.Hill J., Nelson E., Tilman D., Polasky S., Tiffany D. 2006. Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc. Natl Acad. Sci. USA 103, 11 206–11 210 10.1073/pnas.0604600103 (doi:10.1073/pnas.0604600103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynd L. R., von Blottnitz H., Tait B., de Boer J., Pretorius I. S., Rumbold K., Van Zyl W. H. 2003. Converting plant biomass to fuels and commodity chemicals in South Africa: a third chapter? S. Afr. J. Sci. 99, 499–507 [Google Scholar]
- 7.Marrison C. I., Larson E. D. 1996. A preliminary analysis of the biomass energy production potential in Africa in 2025 considering projected land needs for food production. Biomass Bioenerg. 10, 337–351 10.1016/0961-9534(95)00122-0 (doi:10.1016/0961-9534(95)00122-0) [DOI] [Google Scholar]
- 8.Van Zyl W. H., Lynd L. R., Den Haan R., McBride J. E. 2007. Consolidated bioprocessing for bioethanol production using Saccharomyces cerevisiae. Adv. Biochem. Eng. Biotechnol. 108, 205–235 10.1007/10_2007_061 (doi:10.1007/10_2007_061) [DOI] [PubMed] [Google Scholar]
- 9.Dale B. E., Bals B. D., Kim S., Eranki P. 2010. Biofuels done right: land efficient animal feeds enable large environmental and energy benefits. Environ. Sci. Technol. 44, 8385–8389 10.1021/es101864b (doi:10.1021/es101864b) [DOI] [PubMed] [Google Scholar]
- 10.Aden A., Foust T. 2009. Technoeconomic analysis of the dilute sulfuric acid and enzymatic hydrolysis process for the conversion of corn stover to ethanol. Cellulose 16, 535–545 10.1007/s10570-009-9327-8 (doi:10.1007/s10570-009-9327-8) [DOI] [Google Scholar]
- 11.Swanson R. M., Platon A., Satrio J. A., Brown R. C. 2010. Techno-economic analysis of biomass-to-liquids production based on gasification. Fuel 89, S11–S19 10.1016/j.fuel.2010.07.027 (doi:10.1016/j.fuel.2010.07.027) [DOI] [Google Scholar]
- 12.Stephanopoulos G. 2007. Challenges in engineering microbes for biofuels production. Science 315, 801–804 10.1126/science.1139612 (doi:10.1126/science.1139612) [DOI] [PubMed] [Google Scholar]
- 13.Lynd L. R., Weimer P. J., Van Zyl W. H., Pretorius I. S. 2002. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 66, 506–577 10.1128/MMBR.66.3.506-577.2002 (doi:10.1128/MMBR.66.3.506-577.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galbe M., Liden G., Zacchi G. 2005. Production of ethanol from biomass—research in Sweden. J. Sci. Ind. Res. 64, 905–919 [Google Scholar]
- 15.Hahn-Hägerdal B., Karhumaa K., Fonseca C., Spencer-Martins I., Gorwa-Grauslund M. F. 2007. Towards industrial pentose-fermenting yeast strains. Appl. Microbiol. Biotechnol. 74, 937–953 10.1007/s00253-006-0827-2 (doi:10.1007/s00253-006-0827-2) [DOI] [PubMed] [Google Scholar]
- 16.Hamelinck C. N., van Hooijdonk G., Faaij A. P. C. 2005. Ethanol from lignocellulosic biomass: techno-economic performance in short-, middle- and long-term. Biomass Bioenerg. 28, 384–410 10.1016/j.biombioe.2004.09.002 (doi:10.1016/j.biombioe.2004.09.002) [DOI] [Google Scholar]
- 17.Robinson J. 2006. Bio-ethanol as a household cooking fuel: a mini pilot study of the SuperBlu stove in peri-urban Malawi. PhD dissertation, Loughborough University, UK [Google Scholar]
- 18.Anex R. P., et al. 2010. Techno-economic comparison of biomass-to-transportation fuels via pyrolysis, gasification, and biochemical pathways. Fuel 89, S29–S35 10.1016/j.fuel.2010.07.015 (doi:10.1016/j.fuel.2010.07.015) [DOI] [Google Scholar]
- 19.Kazi F. K., Fortman J. A., Anex R. P., Hsu D. D., Aden A., Dutta A., Kothandaraman G. 2010. Techno-economic comparison of process technologies for biochemical ethanol production from corn stover. Fuel 89, S20–S28 10.1016/j.fuel.2010.01.001 (doi:10.1016/j.fuel.2010.01.001) [DOI] [Google Scholar]
- 20.Sun Y., Cheng J. 2002. Hydrolysis of lignocellulosic material for ethanol production: a review. Bioresour. Technol. 83, 1–11 10.1016/S0960-8524(01)00212-7 (doi:10.1016/S0960-8524(01)00212-7) [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y. H., Lynd L. R. 2004. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: noncomplexed cellulase systems. Biotechnol. Bioeng. 88, 797–824 10.1002/bit.20282 (doi:10.1002/bit.20282) [DOI] [PubMed] [Google Scholar]
- 22.Hahn-Hägerdal B., Wahlbom C. F., Gardonyi M., Van Zyl W. H., Cordero O. R., Jonsson L. J. 2001. Metabolic engineering of Saccharomyces cerevisiae for xylose utilization. Adv. Biochem. Eng. Biotechnol. 73, 53–84 [DOI] [PubMed] [Google Scholar]
- 23.Girio F. M., Fonseca C., Carvalheiro F., Duarte L. C., Marques S., Bogel-Lukasik R. 2010. Hemicelluloses for fuel ethanol: a review. Bioresour. Technol. 101, 4775–4800 10.1016/j.biortech.2010.01.088 (doi:10.1016/j.biortech.2010.01.088) [DOI] [PubMed] [Google Scholar]
- 24.Hahn-Hägerdal B., Galbe M., Gorwa-Grauslund M. F., Lidén G., Zacchi G. 2006. Bio-ethanol—the fuel of tomorrow from the residues of today. Trends Biotechnol. 24, 549–556 10.1016/j.tibtech.2006.10.004 (doi:10.1016/j.tibtech.2006.10.004) [DOI] [PubMed] [Google Scholar]
- 25.Lynd L. R., Van Zyl W. H., McBride J. E., Laser M. 2005. Consolidated bioprocessing of cellulosic biomass: an update. Curr. Opin. Biotechnol. 16, 577–583 10.1016/j.copbio.2005.08.009 (doi:10.1016/j.copbio.2005.08.009) [DOI] [PubMed] [Google Scholar]
- 26.Alfenore S., Molina-Jouve C., Guillouet S. E., Uribelarrea J.-L., Goma G., Benbadis L. 2002. Improving ethanol production and viability of Saccharomyces cerevisiae by a vitamin feeding strategy during fed-batch process. Appl. Microbiol. Biotechnol. 60, 67–72 10.1007/s00253-002-1092-7 (doi:10.1007/s00253-002-1092-7) [DOI] [PubMed] [Google Scholar]
- 27.Bothast R. J., Nichols N. N., Dien B. S. 1999. Fermentations with new recombinant organisms. Biotechnol. Prog. 15, 867–875 10.1021/bp990087w (doi:10.1021/bp990087w) [DOI] [PubMed] [Google Scholar]
- 28.Kuyper M., Hartog M. M., Toirkens M. J., Almering M. J., Winkler A. A., Van Dijken J. P., Pronk J. T. 2005. Metabolic engineering of a xylose-isomerase-expressing Saccharomyces cerevisiae strain for rapid anaerobic xylose fermentation. FEMS Yeast Res. 5, 399–409 10.1016/j.femsyr.2004.09.010 (doi:10.1016/j.femsyr.2004.09.010) [DOI] [PubMed] [Google Scholar]
- 29.Nissen T. L., Kielland-Brandt M. C., Nielsen J., Villadsen J. 2000. Optimization of ethanol production in Saccharomyces cerevisiae by metabolic engineering of the ammonium assimilation. Metab. Eng. 2, 69–77 10.1006/mben.1999.0140 (doi:10.1006/mben.1999.0140) [DOI] [PubMed] [Google Scholar]
- 30.Van Dijken J. P., et al. 2000. An interlaboratory comparison of physiological and genetic properties of four Saccharomyces cerevisiae strains. Enzyme Microb. Technol. 26, 706–714 10.1016/S0141-0229(00)00162-9 (doi:10.1016/S0141-0229(00)00162-9) [DOI] [PubMed] [Google Scholar]
- 31.Karhumaa K., Wiedemann B., Hahn-Hagerdal B., Boles E., Gorwa-Grauslund M. F. 2006. Co-utilization of l-arabinose and d-xylose by laboratory and industrial Saccharomyces cerevisiae strains. Microb. Cell Fact. 5, 18–28 10.1186/1475-2859-5-18 (doi:10.1186/1475-2859-5-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuyper M., Winkler A. A., Van Dijken J. P., Pronk J. T. 2004. Minimal metabolic engineering of Saccharomyces cerevisiae for efficient anaerobic xylose fermentation: a proof of principle. FEMS Yeast Res. 4, 655–664 10.1016/j.femsyr.2004.01.003 (doi:10.1016/j.femsyr.2004.01.003) [DOI] [PubMed] [Google Scholar]
- 33.Van Rooyen R., Hahn-Hagerdal B., La Grange D. C., Van Zyl W. H. 2005. Construction of cellobiose-growing and fermenting Saccharomyces cerevisiae strains. J. Biotechnol. 120, 284–295 10.1016/j.jbiotec.2005.06.013 (doi:10.1016/j.jbiotec.2005.06.013) [DOI] [PubMed] [Google Scholar]
- 34.Galazka J. M., Tian C., Beeson W. T., Martinez B., Glass N. L., Cate J. H. 2010. Cellodextrin transport in yeast for improved biofuel production. Science 330, 84–86 10.1126/science.1192838 (doi:10.1126/science.1192838) [DOI] [PubMed] [Google Scholar]
- 35.Cho K. M., Yoo Y. J., Kang H. S. 1999. δ-Integration of endo/exo-glucanase and β-glucosidase genes into the yeast chromosomes for direct conversion of cellulose to ethanol. Enzyme Microb. Technol. 25, 23–30 10.1016/S0141-0229(99)00011-3 (doi:10.1016/S0141-0229(99)00011-3) [DOI] [Google Scholar]
- 36.Fujita Y., Ito J., Ueda M., Fukuda H., Kondo A. 2004. Synergistic saccharification, and direct fermentation to ethanol, of amorphous cellulose by use of an engineered yeast strain codisplaying three types of cellulolytic enzyme. Appl. Environ. Microbiol. 70, 1207–1212 10.1128/AEM.70.2.1207-1212.2004 (doi:10.1128/AEM.70.2.1207-1212.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujita Y., et al. 2002. Direct and efficient production of ethanol from cellulosic material with a yeast strain displaying cellulolytic enzymes. Appl. Environ. Microbiol. 68, 5136–5141 10.1128/AEM.68.10.5136-5141.2002 (doi:10.1128/AEM.68.10.5136-5141.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Den Haan R., Rose S. H., Lynd L. R., Van Zyl W. H. 2007. Hydrolysis and fermentation of amorphous cellulose by recombinant Saccharomyces cerevisiae. Metab. Eng. 9, 87–94 10.1016/j.ymben.2006.08.005 (doi:10.1016/j.ymben.2006.08.005) [DOI] [PubMed] [Google Scholar]
- 39.Jeon E., Hyeon J. E., Suh D. J., Suh Y. W., Kim S. W., Song K. H., Han S. O. 2009. Production of cellulosic ethanol in Saccharomyces cerevisiae heterologous expressing Clostridium thermocellum endoglucanase and Saccharomycopsis fibuligera beta-glucosidase genes. Mol. Cells 28, 369–373 10.1007/s10059-009-0131-y (doi:10.1007/s10059-009-0131-y) [DOI] [PubMed] [Google Scholar]
- 40.Den Haan R., McBride J. E., La Grange D. C., Lynd L. R., Van Zyl W. H. 2007. Functional expression of cellobiohydrolases in Saccharomyces cerevisiae towards one-step conversion of cellulose to ethanol. Enzyme Microb. Technol. 40, 1291–1299 10.1016/j.enzmictec.2006.09.022 (doi:10.1016/j.enzmictec.2006.09.022) [DOI] [Google Scholar]
- 41.Fonseca G. G., Bombert A. K., Heinzle E., Wittmann C. 2007. Physiology of the yeast Kluyveromyces marxianus during batch and chemostat cultures with glucose as the sole carbon source. FEMS Yeast Res. 7, 422–435 10.1111/j.1567-1364.2006.00192.x (doi:10.1111/j.1567-1364.2006.00192.x) [DOI] [PubMed] [Google Scholar]
- 42.Fonseca G. G., Heinzle E., Wittmann C., Gombert A. K. 2008. The yeast Kluyveromyces marxianus and its biotechnological potential. Appl. Microbiol. Biotechnol. 79, 339–354 10.1007/s00253-008-1458-6 (doi:10.1007/s00253-008-1458-6) [DOI] [PubMed] [Google Scholar]
- 43.Rajoka M. I., Khan S., Shahid R. 2003. Kinetics and regulation studies of the production of β-galactosidase from Kluyveromyces marxianus grown on different substrates. Food Technol. Biotechnol. 41, 315–320 [Google Scholar]
- 44.Hong J., Wang Y., Kumagai H., Tamaki H. 2007. Construction of thermotolerant yeast expressing thermostable cellulase genes. J. Biotechnol. 130, 114–123 10.1016/j.jbiotec.2007.03.008 (doi:10.1016/j.jbiotec.2007.03.008) [DOI] [PubMed] [Google Scholar]
- 45.Yanase S., Hasunuma T., Yamada R., Tanaka T., Ogino C., Fukuda H., Kondo A. 2010. Direct ethanol production from cellulosic materials at high temperature using the thermotolerant yeast Kluyveromyces marxianus displaying cellulolytic enzymes. Appl. Microbiol. Biotechnol. 88, 381–388 10.1007/s00253-010-2784-z (doi:10.1007/s00253-010-2784-z) [DOI] [PubMed] [Google Scholar]
- 46.Ryabova O. B., Chmil O. M., Sibirny A. A. 2003. Xylose and cellobiose fermentation to ethanol by the thermotolerant methylotrophic yeast Hansenula polymorpha. FEMS Yeast Res. 4, 157–164 10.1016/S1567-1356(03)00146-6 (doi:10.1016/S1567-1356(03)00146-6) [DOI] [PubMed] [Google Scholar]
- 47.Voronovsky A. Y., Rohulya O. V., Abbas C. A., Sibirny A. A. 2009. Development of strains of the thermotolerant yeast Hansenula polymorpha capable of alcoholic fermentation of starch and xylan. Metab. Eng. 11, 234–242 10.1016/j.ymben.2009.04.001 (doi:10.1016/j.ymben.2009.04.001) [DOI] [PubMed] [Google Scholar]
- 48.Jeffries T., Shi N. Q. 1999. Genetic engineering for improved xylose fermentation by yeasts. Adv. Biochem. Eng. Biotechnol. 65, 117–161 [DOI] [PubMed] [Google Scholar]
- 49.Jeffries T. W., et al. 2007. Genome sequence of the lignocellulose-bioconverting and xylose-fermenting yeast Pichia stipitis. Nat. Biotechnol. 25, 319–326 10.1038/nbt1290 (doi:10.1038/nbt1290) [DOI] [PubMed] [Google Scholar]
- 50.Papendieck A., Dahlems U., Gellissen G. 2002. Technical enzyme production and whole-cell biocatalysis: application of Hansenula polymorpha. In Hansenula polymorpha: biology and applications (ed. Gellissen G.), pp. 255–271 Weinham, Germany: Wiley-VCH [Google Scholar]
- 51.Piotek M., Hagedorn J., Hollenberg C. P., Gellissen G., Srasser A. W. M. 1998. Two novel gene expression systems based on the yeasts Schwanniomyces occidentalis and Pichia stipitis. Appl. Microbiol. Biotechnol. 50, 331–338 10.1007/s002530051300 (doi:10.1007/s002530051300) [DOI] [PubMed] [Google Scholar]
- 52.Den Haan R., Van Zyl W. H. 2003. Enhanced xylan degradation and utilisation by Pichia stipitis overproducing fungal xylanolytic enzymes. Enzyme Microb. Technol. 33, 620–628 10.1016/S0141-0229(03)00183-2 (doi:10.1016/S0141-0229(03)00183-2) [DOI] [Google Scholar]
- 53.Agbogbo F. K., Coward-Kelly G. 2008. Cellulosic ethanol production using the naturally occurring xylose-fermenting yeast, Pichia stipitis. Biotechnol. Lett. 30, 1515–1524 10.1007/s10529-008-9728-z (doi:10.1007/s10529-008-9728-z) [DOI] [PubMed] [Google Scholar]
- 54.Alterthum F., Ingram L. O. 1989. Efficient ethanol production from glucose, lactose, and xylose by recombinant Escherichia coli. Appl. Environ. Microbiol. 55, 1943–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brau B., Sahm H. 1986. Cloning and expression of the structural gene for pyruvate decarboxylase of Zymomonas mobilis in Escherichia coli. Arch. Microbiol. 144, 296–301 10.1007/BF00410966 (doi:10.1007/BF00410966) [DOI] [Google Scholar]
- 56.Chen J., Zhang W., Tan L., Wang Y., He G. 2009. Optimization of metabolic pathways for bioconversion of lignocellulose to ethanol through genetic engineering. Biotechnol. Adv. 27, 593–598 10.1016/j.biotechadv.2009.04.021 (doi:10.1016/j.biotechadv.2009.04.021) [DOI] [PubMed] [Google Scholar]
- 57.Da Silva G. P., De Araujo E. F., Silva D., Guimaraes W. V. 2005. Ethanolic fermentation of sucrose, sugarcane juice and molasses by Escherichia coli strain KO11 and Klebsiella oxytoca strain P2. Braz. J. Microbiol. 36, 395–404 [Google Scholar]
- 58.Ingram L. O., Conway T., Alterthum F. 1991. Ethanol production by Escherichia coli strains co-expressing Zymomonas PDC and ADH genes. United States of America, Patent 5000000 [Google Scholar]
- 59.Ingram L. O., Conway T., Clark D. P., Sewell G. W., Preston J. F. 1987. Genetic engineering of ethanol production in Escherichia coli. Appl. Environ. Microbiol. 53, 2420–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohta K., Beall D. S., Mejia J. P., Shanmugam K. T., Ingram L. O. 1991. Genetic improvement of Escherichia coli for ethanol production: chromosomal integration of Zymomonas mobilis genes encoding pyruvate decarboxylase and alcohol dehydrogenase. Appl. Environ. Microbiol. 57, 893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamano L. P., York S. W., Ingram L. O. 1998. Isolation and characterization of ethanol-tolerant mutants of Escherichia coli KO11 for fuel ethanol production. J. Ind. Microbiol. Biotechnol. 20, 132–138 10.1038/sj.jim.2900496 (doi:10.1038/sj.jim.2900496) [DOI] [PubMed] [Google Scholar]
- 62.Seon P. J., Russell J. B., Wilson D. B. 2007. Characterization of a family 45 glycosyl hydrolase from Fibrobacter succinogenes S85. Anaerobe 13, 83–88 10.1016/j.anaerobe.2006.12.003 (doi:10.1016/j.anaerobe.2006.12.003) [DOI] [PubMed] [Google Scholar]
- 63.Srivastava R., Kumar G. P., Srivastava K. K. 1995. Construction of a recombinant cellulolytic Escherichia coli. Gene 164, 185–186 10.1016/0378-1119(95)00437-B (doi:10.1016/0378-1119(95)00437-B) [DOI] [PubMed] [Google Scholar]
- 64.Wood B. E., Beall D. S., Ingram L. O. 1997. Production of recombinant bacterial endoglucanase as a co-product with ethanol during fermentation using derivatives of Escherichia coli KO11. Biotech. Bioeng. 55, 547–555 (doi:10.1002/(SICI)1097-0290(19970805)55:3<547::AID-BIT12>3.0.CO;2-D) [DOI] [PubMed] [Google Scholar]
- 65.Yoo J. S., Jung Y. J., Chung S. Y., Lee Y. C., Choi Y. L. 2004. Molecular cloning and characterization of CMCase gene (celC) from Salmonella typhimurium UR. J. Microbiol. 42, 205–210 [PubMed] [Google Scholar]
- 66.Zhou S., Davis F. C., Ingram L. O. 2001. Gene integration and expression and extracellular secretion of Erwinia chrysanthemi endoglucanase CelY (celY) and CelZ (celZ) in ethanologenic Klebsiella oxytoca P2. Appl. Environ. Microbiol. 67, 6–14 10.1128/AEM.67.1.6-14.2001 (doi:10.1128/AEM.67.1.6-14.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou S., Ingram L. 1999. Engineering endoglucanase-secreting strains of ethanologenic Klebsiella oxytoca P2. J. Ind. Microbiol. Biotechnol. 22, 600–607 10.1038/sj.jim.2900666 (doi:10.1038/sj.jim.2900666) [DOI] [PubMed] [Google Scholar]
- 68.Ohta K., Mejia J. P., Shanmugam K. T., Ingram L. O. 1991. Metabolic engineering of Klebsiella oxytoca M5A1 for ethanol production from xylose and glucose. Appl. Environ. Microbiol. 57, 2810–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wood B. E., Ingram L. O. 1992. Ethanol production from cellobiose, amorphous cellulose, and crystalline cellulose by recombinant Klebsiella oxytoca containing chromosomally integrated Zymomonas mobilis genes for ethanol production and plasmids expressing thermostable cellulase genes from Clostridium thermocellum. Appl. Environ. Microbiol. 58, 2103–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou S., Ingram L. O. 2001. Simultaneous saccharification and fermentation of amorphous cellulose to ethanol by recombinant Klebsiella oxytoca SZ21 without supplemental cellulase. Biotechnol. Lett. 23, 1455–1462 10.1023/A:1011623509335 (doi:10.1023/A:1011623509335) [DOI] [Google Scholar]
- 71.Zhang M., Chou Y. C., Picataggio S. K., Finkelstein M. 1998. Single Zymomonas mobilis strain for xylose and arabinose fermentation. United States Patent 5843760. Filed 1 December 1998 [Google Scholar]
- 72.Brestic-Goachet N., Gunasekaran P., Cami B., Baratti J. C. 1989. Transfer and expression of an Erwinia chrysanthemi cellulase gene in Zymomonas mobilis. J. Gen. Microbiol. 135, 893–902 [Google Scholar]
- 73.Yanase H., Nozaki K., Okamoto K. 2005. Ethanol production from cellulosic materials by genetically engineered Zymomonas mobilis. Biotechnol. Lett. 27, 259–263 10.1007/s10529-004-8295-1 (doi:10.1007/s10529-004-8295-1) [DOI] [PubMed] [Google Scholar]
- 74.Shaw A. J., Jenney F. E., Adams M. W. W., Lynd L. R. 2008. End-product pathways in the xylose fermenting bacterium, Thermoanaerobacterium saccharolyticum. Enzyme Microb. Technol. 42, 453–458 10.1016/j.enzmictec.2008.01.005 (doi:10.1016/j.enzmictec.2008.01.005) [DOI] [Google Scholar]
- 75.Shaw A. J., Podkaminer K. K., Desai S. G., Bardsley J. S., Rogers S. R., Thorne P. G., Hogsett D. A., Lynd L. R. 2008. Metabolic engineering of a thermophilic bacterium to produce ethanol at high yield. Proc. Natl Acad. Sci. USA 105, 13 769–13 774 10.1073/pnas.0801266105 (doi:10.1073/pnas.0801266105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shaw A. J., Hogsett D. A., Lynd L. R. 2009. Identification of the [FeFe]-hydrogenase responsible for hydrogen generation in Thermoanaerobacterium saccharolyticum and demonstration of increased ethanol yield via hydrogenase knockout. J. Bacteriol. 191, 6457–6464 10.1128/JB.00497-09 (doi:10.1128/JB.00497-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee Y. E., Lowe S. E., Henrissat B., Zeikus J. G. 1993. Characterization of the active site and thermostability regions of endoxylanse from Thermoanaerobacterium saccharolyticum B6A-RI. J. Bacteriol. 175, 5890–5898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee Y. E., Lowe S. E., Zeikus G. J. 1993. Regulation and characterization of xylanolytic enzymes of Thermoanaerobacterium saccharolyticum B6A-RI. Appl. Environ. Microbiol. 59, 763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ji X. J., Huang H., Zhu J. G., Ren L. J., Nie Z. K., Du J., Li S. 2009. Engineering Klebsiella oxytoca for efficient 2,3-butanediol production through insertional inactivation of acetaldehyde dehydrogenase gene. Appl. Microbiol. Biotechnol. 85, 1751–1758 10.1007/s00253-009-2222-2 (doi:10.1007/s00253-009-2222-2) [DOI] [PubMed] [Google Scholar]
- 80.La Grange D. C., Den Haan R., Van Zyl W. H. 2010. Engineering cellulolytic ability into bioprocessing organisms. Appl. Microbiol. Biotechnol. 87, 1195–1208 10.1007/s00253-010-2660-x (doi:10.1007/s00253-010-2660-x) [DOI] [PubMed] [Google Scholar]
- 81.McKendry P. 2002. Energy production from biomass (part 2): conversion technologies. Bioresour. Technol. 83, 47–54 10.1016/S0960-8524(01)00119-5 (doi:10.1016/S0960-8524(01)00119-5) [DOI] [PubMed] [Google Scholar]
- 82.Leibbrandt N. 2010. Techno-economic study for sugarcane bagasse to liquid biofuels in South Africa: a comparison between biological and thermochemical process routes. PhD dissertation, Stellenbosch University, South Africa [Google Scholar]
- 83.Piccolo C., Bezzo F. 2009. A techno-economic comparison between two technologies for bioethanol production from lignocellulose. Biomass Bioenerg. 33, 478–491 10.1016/j.biombioe.2008.08.008 (doi:10.1016/j.biombioe.2008.08.008) [DOI] [Google Scholar]
- 84.Reith J. H., den Uil H., de Laat W. T. A. M., Niessen J. J., de Jong E., Elbersen H. W., Weusthuis R., Van Dijken J. P., Raamsdonk L. 2002. Co-production of bio-ethanol, electricity and heat from biomass residues. Report ECN-RX-02-030, Energy Research Centre, The Netherlands [Google Scholar]
- 85.Cardona C. A., Sanchez O. J. 2007. Fuel ethanol production: process design trends and integration opportunities. Bioresour. Technol. 98, 2415–2457 10.1016/j.biortech.2007.01.002 (doi:10.1016/j.biortech.2007.01.002) [DOI] [PubMed] [Google Scholar]
- 86.Martin M., Ahmetovic E., Grossmann I. E. In press Optimization of water consumption in second generation bioethanol plants. Ind. Eng. Chem. Res. 10.1021/ie101175p (doi:10.1021/ie101175p) [DOI] [Google Scholar]
- 87.Wingren A., Galbe M., Zacchi G. 2003. Techno-economic evaluation of producing ethanol from softwood: comparison of SSF and SHF and identification of bottlenecks. Biotechnol. Prog. 19, 1109–1117 10.1021/bp0340180 (doi:10.1021/bp0340180) [DOI] [PubMed] [Google Scholar]
- 88.Banerjee S., Mudliar S., Sen R., Giri B. 2009. Commercializing lignocellulosic bioethanol: technology bottlenecks and possible remedies. Biofuel. Bioprod. Bioref. 4, 77–93 10.1002/bbb.188 (doi:10.1002/bbb.188) [DOI] [Google Scholar]
- 89.Larson E. D., Jin H., Celik F. E. 2009. Large-scale gasification-based coproduction of fuels and electricity from switchgrass. Biofuel. Bioprod. Bioref. 3, 174–194 10.1002/bbb.137 (doi:10.1002/bbb.137) [DOI] [Google Scholar]
- 90.Laser M., Jin H., Jayawardhana K., Dale B. E., Lynd L. R. 2009. Coproduction of ethanol and power from switchgrass. Biofuel. Bioprod. Bioref. 3, 195–218 10.1002/bbb.133 (doi:10.1002/bbb.133) [DOI] [Google Scholar]
- 91.Jin H., Larson E. D., Celik F. E. 2009. Performance and cost analysis of future, commercially mature gasification-based electric power generation from switchgrass. Biofuel. Bioprod. Bioref. 3, 142–173 10.1002/bbb.138 (doi:10.1002/bbb.138) [DOI] [Google Scholar]
- 92.Laser M., Jin H., Jayawardhana K., Dale B. E., Lynd L. R. 2009. Projected mature technology scenarios for conversion of cellulosic biomass to ethanol with coproduction thermochemical fuels, power, and/or animal feed protein. Biofuel. Bioprod. Bioref. 3, 231–246 10.1002/bbb.131 (doi:10.1002/bbb.131) [DOI] [Google Scholar]
- 93.Larsson E. D., Williams R. H., Real M. R. 2010. A review of biomass integrated-gasifier/gas turbine combined cycle technology and its application in sugarcane industries, with an analysis for Cuba. Energ. Sust. Dev. 5, 54–76 10.1016/S0973-0826(09)60021-1 (doi:10.1016/S0973-0826(09)60021-1) [DOI] [Google Scholar]
- 94.Easterly J. 2002. AES Greenidge Bioethanol Co-Location Assessment: Final Report. Report NREL/SR-510-33001, National Renewable Energy Laboratory, Golden, CO.
- 95.Sassner P., Galbe M., Zacchi G. 2008. Techno-economic evaluation of bioethanol production from three different lignocellulosic materials. Biomass Bioenerg. 32, 422–430 10.1016/j.biombioe.2007.10.014 (doi:10.1016/j.biombioe.2007.10.014) [DOI] [Google Scholar]
- 96.Sims R., Taylor M., Saddler J., Mabee W. 2008. From 1st- to 2nd-generation biofuel technologies—an overview of current industry and RD&D activities. See www.iea.org/papers/2008/2nd_Biofuel_Gen_Exec_Sum.pdf [DOI] [PubMed]
- 97.Eriksson G., Kjellström B. 2010. Assessment of combined heat and power (CHP) integrated with wood-based ethanol production. Appl. Energ. 87, 3632–3641 10.1016/j.apenergy.2010.06.012 (doi:10.1016/j.apenergy.2010.06.012) [DOI] [Google Scholar]
- 98.Goh C. S., Tan K. T., Lee K. T., Bhatia S. 2010. Bio-ethanol from lignocellulose: status, perspectives and challenges in Malaysia. Bioresour. Technol. 101, 4834–4841 10.1016/j.biortech.2009.08.080 (doi:10.1016/j.biortech.2009.08.080) [DOI] [PubMed] [Google Scholar]
- 99.Soccol C. R., et al. 2010. Bioethanol from lignocelluloses: status and perspectives in Brazil. Bioresour. Technol. 101, 4820–4825 10.1016/j.biortech.2009.11.067 (doi:10.1016/j.biortech.2009.11.067) [DOI] [PubMed] [Google Scholar]
- 100.Gnansounou E., Dauriat A. 2010. Techno-economic analysis of lignocellulosic ethanol: a review. Bioresour. Technol. 101, 4980–4991 10.1016/j.biortech.2010.02.009 (doi:10.1016/j.biortech.2010.02.009) [DOI] [PubMed] [Google Scholar]
- 101.Galbe M., Sassner P., Wingren A., Zacchi G. 2007. Process engineering economics of bioethanol production. Adv. Biochem. Eng. Biotechnol. 108, 303–327 10.1007/10_2007_063 (doi:10.1007/10_2007_063) [DOI] [PubMed] [Google Scholar]
- 102.Seabra J. E. A., Tao L., Chuma H. L., Macedo I. C. 2010. A techno-economic evaluation of the effects of centralized cellulosic ethanol and co-products refinery options with sugarcane mill clustering. Biomass Bioenerg. 34, 1065–1078 10.1016/j.biombioe.2010.01.042 (doi:10.1016/j.biombioe.2010.01.042) [DOI] [Google Scholar]
- 103.Botha T., von Blottnitz H. 2006. A comparison of the environmental benefits of bagasse-derived electricity and fuel ethanol on a life-cycle basis. Energ. Policy 34, 2654–2661 10.1016/j.enpol.2004.12.017 (doi:10.1016/j.enpol.2004.12.017) [DOI] [Google Scholar]
- 104.von Sivers M., Zacchi G. 1995. A techno-economical comparison of three processes for the production of ethanol from pine. Bioresour. Technol. 51, 43–52 10.1016/0960-8524(94)00094-H (doi:10.1016/0960-8524(94)00094-H) [DOI] [Google Scholar]
- 105.Linde M., Galbe M., Zacchi G. 2010. Bioethanol production from non-starch carbohydrate residues in process streams from a dry-mill ethanol plant. Bioresour. Technol. 99, 6505–6511 10.1016/j.biortech.2007.11.032 (doi:10.1016/j.biortech.2007.11.032) [DOI] [PubMed] [Google Scholar]
- 106.Laser M., Larson E., Dale B., Wang M., Greene N., Lynd L. R. 2009. Comparative analysis of efficiency, environmental impact, and process economics for mature biomass refining scenarios. Biofuel. Bioprod. Bioref. 3, 247–270 10.1002/bbb.136 (doi:10.1002/bbb.136) [DOI] [Google Scholar]
- 107.Sonobe T., Worasuwannarak N., Pipatmanomai S. 2008. Synergies in co-pyrolysis of Thai lignite and corncob. Fuel Process. Technol. 89, 1371–1378 10.1016/j.fuproc.2008.06.006 (doi:10.1016/j.fuproc.2008.06.006) [DOI] [Google Scholar]
- 108.Wright M. M., Daugaard D. E., Satrio J. A., Brown R. C. 2010. Techno-economic analysis of biomass fast pyrolysis to transportation fuels. Fuel 89, S10. 10.1016/j.fuel.2010.07.029 (doi:10.1016/j.fuel.2010.07.029) [DOI] [Google Scholar]
- 109.ICSU 2007. Sustainable energy in sub-Saharan Africa. Science Plan, Pretoria, South Africa: ICSU Regional Office for Africa [Google Scholar]
- 110.Meyer F., Strauss P. G., Funke T. 2008. Modelling the impacts of macro-economic variables on the South African biofuels industry. Agrekon 47, 327–345 10.1080/03031853.2008.9523803 (doi:10.1080/03031853.2008.9523803) [DOI] [Google Scholar]
- 111.Darkwah L., Hammond A. B., Ramde E., Kemausuor F., Addo A. 2007. Biofuels industry development in Africa. Background paper prepared for AU/Brazil/UNIDO High Level Seminar on Biofuels in Africa (30 July–1 August 2007). Addis Ababa, Ethiopia: African Union Commission [Google Scholar]
- 112.Gnansounou E., Panichelli L., Villegas J. D. 2007. Sustainable liquid biofuels for transport: the context of the Southern Africa development community (SADC). Working paper, Ecole Polytechnic Federal de Lausanne, Switzerland [Google Scholar]
- 113.Jumbe C. B. L., Msiska F. B. M., Madjera M. 2009. Biofuels development in sub-Saharan Africa: are the policies conducive? Energ. Policy 37, 4980–4986 10.1016/j.enpol.2009.06.064 (doi:10.1016/j.enpol.2009.06.064) [DOI] [Google Scholar]
- 114.Deepchand K. 2005. Sugar cane bagasse energy cogeneration—lessons from Mauritius. In Proc. Parliamentarian Forum on Energy Legislation and Sustainable Development, Cape Town, South Africa, 5–7 October 2005 [Google Scholar]
- 115.Overend R. P. 2007. Biomass conversion technologies. Golden, CO: National Renewable Energy Laboratory [Google Scholar]
- 116.Gibbs D. 1998. Global warming and the need for liquid fuels from biomass. BioEnergy '98. In Proc. of the 8th National Bioenergy Conf., Madison, WI, 4–8 October 1998, pp. 1344–1353 [Google Scholar]
- 117.Huber G. W., Iborra S., Corma A. 2006. Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem. Rev. 106, 4044–4098 10.1021/cr068360d (doi:10.1021/cr068360d) [DOI] [PubMed] [Google Scholar]
- 118.UEMOA. 2008 Sustainable bioenergy development in UEMOA member countries The West African Economic and Monetary Union and The Hub for Rural Development in West and Central Africa. See http://www.globalproblems-globalsolutions-files.org/gpgs_files/pdf/UNF_Bioenergy/UNF_Bioenergy_full_report.pdf
- 119.Lambe F. 2008. Cooking on ethanol. Environment and Poverty Times, Arendal, Norway: UNEP/GRID-Arendal [Google Scholar]
- 120.Johnson F. X., Matsika E. 2008. Bioenergy trade and regional development: the case of bioethanol in Southern Africa. Energ. Sust. Dev. 10, 42–53 10.1016/S0973-0826(08)60506-2 (doi:10.1016/S0973-0826(08)60506-2) [DOI] [Google Scholar]
- 121.Schlag N., Zuzarte F. 2008. Market barriers to clean cooking fuels in sub-Saharan Africa: a review of literature. An SEI Working Paper. See http://www.sei-international.org/mediamanager/documents/Publications/Climate/market_barriers_clean_cooking_fuels_21april.pdf
- 122.Utria B. E. 2004. Ethanol and gelfuel: clean renewable cooking fuels for poverty alleviation in Africa. Energ. Sust. Dev. 8, 107–114 10.1016/S0973-0826(08)60472-X (doi:10.1016/S0973-0826(08)60472-X) [DOI] [Google Scholar]
- 123.IFAD 2008. Challenges and opportunities for smallholder farmers in the context of climate change and new demands on agriculture in conjunction with the Thirty-first Session of IFAD's Governing Council. See www.ifad.org/events/gc/31/roundtable/proceedings.pdf
- 124.Janssen R., Rutz D., Helm P., Woods J., Diaz-Chavez R. 2009. Bioenergy for sustainable development in Africa—environmental and social aspects. See www.compete-bioafrica.net/sustainability/OD
- 125.Sulle E., Nelson F. 2009. Biofuels, land access and rural livelihoods in Tanzania. London, UK: IIED [Google Scholar]