Abstract
Cardiovascular disease is a leading cause of death in the Western world. Therefore, detection and quantification of atherosclerotic disease is of paramount importance to monitor treatment and possible prevention of acute events. Vascular ultrasound is an excellent technique to assess the geometry of vessel walls and plaques. The high temporal as well as spatial resolution allows quantification of luminal area and plaque size and volume. While carotid arteries can be imaged non-invasively, scanning of coronary arteries requires invasive intravascular catheters. Both techniques have already demonstrated their clinical applicability. Using linear array technology, detection of disease as well as monitoring of pharmaceutical treatment in carotid arteries are feasible. Data acquired with intravascular ultrasound catheters have proved to be especially beneficial in understanding the development of atherosclerotic disease in coronary arteries. With the introduction of vascular elastography not only the geometry of plaques but also the risk for rupture of plaques might be identified. These so-called vulnerable plaques are frequently not flow-limiting and rupture of these plaques is responsible for the majority of cerebral and cardiac ischaemic events. Intravascular ultrasound elastography studies have demonstrated a high correlation between high strain and vulnerable plaque features, both ex vivo and in vivo. Additionally, pharmaceutical intervention could be monitored using this technique. Non-invasive vascular elastography has recently been developed for carotid applications by using compound scanning. Validation and initial clinical evaluation is currently being performed. Since abundance of vasa vasorum (VV) is correlated with vulnerable plaque development, quantification of VV might be a unique tool to even prevent this from happening. Using ultrasound contrast agents, it has been demonstrated that VV can be identified and quantified. Although far from routine clinical application, non-invasive and intravascular ultrasound VV imaging might pave the road to prevent atherosclerotic disease in an early phase. This paper reviews the conventional vascular ultrasound techniques as well as vascular ultrasound strain and vascular ultrasound VV imaging.
Keywords: ultrasound imaging, vascular, elastography, vulnerable plaque, contrast-enhanced ultrasound
1. Introduction
The vascular system is the crucial transportation mechanism of the body: the arterial system delivers oxygen and nutrients to the organs and the venous system is responsible for the removal of waste products. Owing to genetic factors and Western lifestyle, the function of the vascular system is seriously threatened. A substantial number of people develop atherosclerotic disease when they age. It has been observed that the global stiffness of the vascular system increases with age. An increasing stiffness leads to chronic pressure overload of the left ventricle and may eventually result in heart failure. Consequently, assessment of the global stiffness of the arterial system is of diagnostic value. Furthermore, not only the global stiffness is affected but also local disease may occur. Initially, fatty streaks may develop that in time may grow into atherosclerotic plaques. The size and composition of these plaques is a major determinant of the risk for the patient. A small fibrous plaque is most of the time not flow-limiting owing to the fact that the artery remodels: the luminal area is kept similar by compensatory expansion of the vessel [1]. But with increasing plaque size the vessel wall is unable to compensate further and narrowing of the lumen occurs. This narrowing results in complaints especially when the need for oxygen and nutrients is increased, for example, during physical effort. A life-threatening situation occurs when the main constituents of the plaque are not fibrous and/or calcified tissue but when it is composed of a large lipid core that is shielded from the blood by a thin fibrous cap. This so-called vulnerable plaque or thin cap fibro atheroma has a high risk for rupture [2]. The risk for rupture is further increased by the presence of macrophages in the fibrous cap [3]. After rupturing, the contact between the core and blood will result in a thrombogenic reaction. Rupture of a vulnerable plaque is responsible for 60–80% of all myocardial infarctions and strokes [4–6]. Therefore, early detection and recognition of such plaques before rupture is important to prevent the occurrence of stroke and myocardial infarction.
In past years, ultrasound research primarily focused on improving the quantification of the stiffness of the arterial wall. By assessment of the luminal area or diameter over the pressure cycle, an indication of the local wall stiffness was obtained (distensibility). The global stiffness can be determined from the velocity with which the arterial pulse wave travels over the arterial wall. The pulse wave velocity can be measured between two sites a known distance apart using a specific feature of the pressure waveform to calculate the transit time. Furthermore, Doppler techniques are used to estimate the blood velocity and flow. In this way, a haemodynamic quantification of the grade of stenosis can be obtained which is of crucial importance for planning surgical or interventional therapy. Echography is also the most used technique to quantify the geometry and size of the plaque. Geometry and size of the plaque are usually quantified by measuring the thickness of the two innermost layers of the vessel (the intima media thickness; IMT) or the luminal area reduction. Recently, more emphasis has been put on characterization of the composition of the plaque. It is known that standard echography has a poor sensitivity and specificity to identify the composition of plaques. However, by using more sophisticated techniques like spectral analysis [7] and elastography [8,9], the composition can be determined. In spectral analysis techniques, not only the amplitude of the reflected ultrasound signal is used, but also the frequency content of the reflected signal and the attenuation of the ultrasound signal as a function of echo depth are determined. In elastography, the acoustic amplitude or a parameter related to that is not visualized, but ultrasound is used to quantify the deformation of tissue. Initially, this technique was developed for tumour detection since these are predominantly much stiffer than the surrounding tissue. For this application, an ultrasound dataset is acquired and subsequently the tissue is deformed and a second dataset is captured. The local displacement and the local strain of the tissue are derived from comparison of the two datasets. For vascular applications, the pulsatile intraluminal pressure is used as deformation force and therefore application of elastography in arteries is feasible without the need for applying an external force.
Prevention of cardiovascular disease is more effective than treating it. Hunting for vulnerable atherosclerotic plaques is an approach, but detecting the processes that are crucial in the pathogenesis of vulnerable plaques might be even more effective. When the vessel wall thickens, it will have more problems getting all the nutrition into its cells. Caused by hypoxia, a network of very small vessels (approx. 20–100 µm in diameter) will grow into the vessel wall and into the atherosclerotic plaque [10]. This network of vessels is referred to as vasa vasorum (VV). VV delivers oxygen, but is primarily a cleaning mechanism to get low-density lipoprotein out of the plaques back into the blood [11]. The main problem though is that these vessels are immature. Their endothelial cells are not well aligned and there are holes between them. As a result they can leak red blood cells. These red blood cells cause inflammation leading to vulnerable plaques [12].
In this paper, conventional vascular and intravascular ultrasound techniques are described. Furthermore, elastography and VV imaging using invasive as well as non-invasive echography will be discussed and their potential to identify the development and presence of vulnerable plaques.
2. Ultrasound imaging
Ultrasound scanning is always a compromise between resolution and penetration. With increasing frequency, the resolution is improved: a resolution of 500 µm is obtained for an ultrasound system using a 5 MHz pulse whereas this resolution improves to 125 µm for a 20 MHz pulse. Nowadays, non-invasive imaging of superficial arteries and veins is performed with linear array transducers operating at frequencies between 5 and 18 MHz. However, since the penetration depth of the ultrasound pulse decreases with increasing frequency arteries located deeper in the body like coronary arteries cannot be imaged using these high frequencies. The disadvantage of using a 5 MHz system conventionally used for cardiac imaging is that it is not capable of adequately imaging coronary arteries because of the poor resolution. Especially, when intimal thickening and early plaque development is studied, another approach is required. Therefore, invasive intraluminal echocatheters operating at 20–40 MHz are used for imaging coronary arteries.
3. Vascular ultrasound scanning
Linear array transducers are composed of 64 up to 256 and more elements to generate a high resolution image in both the axial direction (determined by the frequency and bandwidth) and lateral direction (determined by the frequency, number of elements and distance between the elements). When 256 ultrasound lines per frame are scanned, typically 50 f s–1 can be obtained. This not only allows assessment of the geometry, but also visualization of the dynamics of the arterial wall and plaque.
3.1. Non-invasive vascular ultrasound scanning
For carotid applications, the common carotid artery as well as the internal and external carotid artery can be imaged. During a routine evaluation of the supraortic vessels, the array transducer is positioned in the longitudinal direction of the artery. The carotid wall as well as the eventually present intimal thickening or plaque can be quantified. Since the acoustic impedance of blood and the intimal layer is different, this transition is characterized by a bright reflection. Additionally, the transition between the medial and adventitial layer is characterized by a bright reflection. Furthermore, the echogenicity of the intima-media layer is characterized by a low echogenicity whereas the adventitial layer has an increased echogenicity. The relatively high resolution with respect to magnetic resonance imaging (MRI) and the ability to provide a detailed image of the arterial wall and plaque instead of the lumen only as provided by the gold standard computed tomography (CT) make ultrasound imaging an excellent technique for studying the presence and progression of plaque. Multiple serial measurements can be made to accurately monitor changes in atherosclerosis. Furthermore, no X-ray radiation exposure is present and the procedure is relatively simple and inexpensive.
There is a wealth of data indicating that the carotid IMT as non-invasively measured with a linear array transducer is an excellent surrogate marker for cardiovascular disease. Since development of atherosclerosis is a systemic disease, carotid plaques are also indicative for the risk for coronary events [13] and the severity of coronary artery disease [14,15]. Observational studies have demonstrated that carotid IMT is a strong predictor of future stroke and myocardial infarctions [16], for future myocardial infarctions [17] and increased risk for clinical coronary events [18].
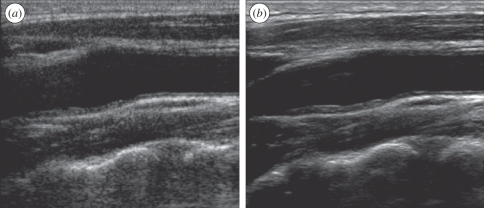
The image quality as provided by linear array scanning has improved considerably over the last decade. Owing to an increasing bandwidth of the transducers, the resolution in especially the axial direction has increased. Furthermore, since the sensitivity of the used transducer materials has been improved, higher frequencies can be employed also resulting in better resolution. The image quality has also been improved by reducing the speckle. Since the speckle pattern can be characterized as spatial noise, a different pattern is observed when scanning the same region from another position. By using electronic beam steering, one region can be imaged from multiple angles without moving the transducer itself. The combination of the various angular acquisitions is called spatial compounding. It results in images that preserve the specular reflection of the arterial wall and other tissue boundaries by averaging the speckle pattern. In this way, smoother images are obtained without deterioration of the tissue features (figure 1).
Figure 1.
Echogram of the longitudinal view of a common carotid artery using (a) conventional scanning and (b) scanning using spatial compounding. The speckle pattern is diminished while the specular reflections are preserved.
4. Intravascular ultrasound scanning
Intravascular ultrasound (IVUS) is a technology that was invented in 1972 [19]. An ultrasound unit is mounted on the tip of a catheter. This unit can consist of a single element piezoelectric material with a frequency range between 30 and 45 MHz or of an array of 64 elements with a centre frequency of 20 MHz [20]. For the single element catheters, the beam will be steered by mechanically rotating the element and for the array the beam will be steered electronically. These catheters can be advanced through the groin, through the aorta all the way into the coronary arteries. In this way, a tomographic image of the vessel, vessel wall and atherosclerosis can be created.
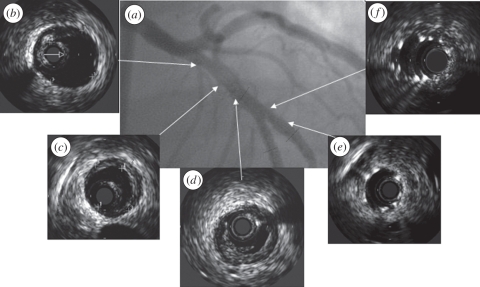
Ab initio it was used for a more accurate diagnosis of atherosclerosis. The gold standard still is X-ray angiography, which can display a shadow image of the free lumen. The disadvantage of X-ray imaging is that the lumen is imaged and not the diseased artery wall. Dissections are easily missed by angiography and can be easily found by IVUS [21]. IVUS has played an important role in the development of standard of care in treatment by balloon angioplasty and by stents. The exact size of balloon and stent needed can be carefully determined and stent malapposition can be easily assessed [22] (figure 2). In this way, IVUS has taught us to which pressure the balloons should be inflated to get optimal stent deployment [23]. With the advent of the drug-eluting stent, this became even more important since the dosage of the drugs is dependent on the contact between the stent struts and the vessel wall [24]. IVUS plays a major role in clinical trials. Plaque progression and regression can be accurately measured using IVUS and in such manner the efficacy of new cardiovascular drugs can be assessed [25]. For the assessment of the risk for acute cardiovascular events, plaque composition is more important than plaque volume [3]. Plaque composition cannot be directly assessed from ultrasound, but additional data processing as described below provides tools to determine relevant plaque components.
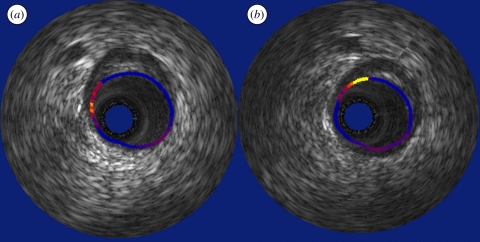
Figure 2.
(a) Angiogram with corresponding IVUS echograms (b–f) of a coronary artery demonstrating that the luminal area as provided by the echogram does not provide information on the presence of plaque (b,c). Furthermore, the presence of (d) a dissection and (e) the adequate positioning and (f) malapposition of a stent can be visualized by IVUS.
5. Vascular strain imaging
With ultrasound strain imaging, an indication of the mechanical properties of the vessel wall and plaque can be obtained. The technique is based on the principle that soft tissue deforms more than hard tissue when an external force is applied. To measure strain, an image of the tissue in an initial condition is made and a second image is made after applying a force. Comparison of the pre- and post-deformation registration produces the local tissue displacements. These displacements can be converted into tissue strains by spatial derivation.
Ophir et al. [26] were first to describe the concept of strain imaging using ultrasound data. Ultrasound data are very suitable for strain imaging, since the periodicity of ultrasound allows very accurate estimation of the displacement. Ophir et al. determined the local tissue displacements by cross-correlating one-dimensional windows of pre- and post-deformation radiofrequency (RF) data. The location of the peak of the normalized cross-correlation corresponds to the time shift between the two RF signals caused by the displacement of the tissue. Since time corresponds to depth in ultrasound, the time shift can be translated into a local tissue displacement for the tissue in the window of interest. By repeating this cross-correlation procedure for multiple depths the displacement profile along the ultrasound beam can be estimated. In the next step, spatial derivation can be applied to obtain strains. Instead of direct point-to-point spatial derivation often a least squares strain estimator (LSQSE) is applied [27]. An LSQSE calculates a least squares linear fit through the displacement values. The slope of this fit corresponds to the strain. This is done to reduce the error in displacement estimates caused by high frequency noise. RF-based techniques have optimal performance when the strain is measured along the ultrasound beam. Perpendicular to this direction, the displacement estimate is primarily based on the envelope information and consequently less accurate [28].
Based on speckle-tracking techniques as developed for cardiac strain imaging, these techniques have recently also been evaluated for vascular strain imaging. The advantage of these optical flow-based techniques is the limited computational load and consequently the possibility for real-time implementation. However, since these techniques are based on using the envelope of the ultrasound signal only, the accuracy will be worse than for RF-based techniques [28].
5.1. Non-invasive vascular strain imaging
Linear array transducers are used to non-invasively obtain ultrasound images of superficial arteries, like the carotid and femoral arteries. The images can be acquired both for longitudinal and transverse cross sections of the arteries at frame-rates of 50–100 Hz. When imaging in the longitudinal direction, the ultrasound beams are aligned with the radial deformations of the vessel. Non-invasive strain imaging in the longitudinal direction was described by Bonnefous et al. [29] using cross-correlation analysis and later by Hasegawa [30] and Kanai et al. [31] using the phase tracking method. The method of Bonnefous et al. [29] was tested in vitro in cadaverous human arterial samples. A pulsating blood flow was generated through the arterial segments and ultrasound data were obtained in a longitudinal plane. The obtained radial strain images were compared with histology data. Low strains were found in hard lesions and high strains were observed in soft lesions. The phase shift approach of Hasegawa & Kanai [32] was experimentally validated using a pressurized homogeneous vessel-mimicking phantom. The technique was also applied to in vitro recordings of a femoral artery and to in vivo recordings of carotid arteries [33,34]. For the femoral artery, again low strains were observed for the calcified, stiff regions and higher strains were observed in regions that contained mainly smooth muscle cells and collagen. For the in vivo carotid recordings, systolic and diastolic pressures were combined with the strain estimates to derive elastic moduli. These ‘elasticity’ images revealed lower elastic moduli for lipid rich regions compared with regions that mainly contained collagen and smooth muscle cells [35]. This method was recently extended and now also allows estimation of the lateral strain component (along the vessel axis) [36].
Since longitudinal scanning does not provide information on the whole plaque, transverse scanning is required. In this case, the ultrasound beams and the radial strain are not aligned anymore. Consequently, the radial strain can only be determined by using two-dimensional strain estimation (along the ultrasound beam as well as perpendicular to the beam). Different methods are available to estimate strains in two dimensions. Ribbers et al. [37] applied a two-dimensional multi-step algorithm to derive axial and lateral strains and composed radial and circumferential strain images out of the axial and lateral strain estimates. Based on the strain values a soft and a hard plaque were identified in phantoms. The initial clinical applicability was demonstrated using in vivo recordings in longitudinal and transverse cross sections of 12 carotid arteries. This technique was further extended by Hansen et al. Instead of cross-correlating the data once for fixed window sizes, it is repeated iteratively with decreasing window sizes [28,38]. In the first iteration, global motion and small strains are detected. In the following iterations this initial guess is used and strains are estimated more locally. The use of shorter windows is needed to be able to detect larger strains. Large strains cause the signals to decorrelate fast and therefore window sizes must be restricted [39].
A different method for strain imaging is the Lagrangian speckle motion estimator (LSME) as introduced by Maurice et al.: one ultrasound frame is deformed in multiple iterations until it matches the next frame best. The translations and deformations of the pre-deformation image that are required to find the optimal match correspond to the two-dimensional displacements and strains that occurred between pre- and post-deformation situation. The LSME approach allows estimation of the radial and circumferential strains in transverse imaging planes, although in this case isotropy and incompressibility are assumed. The method was validated using several vessel-mimicking phantoms with varying stiffness [40]. The results demonstrated the potential of the technique to differentiate between hard and soft tissue. In Schmitt et al. [41], the technique was applied in vivo: four subjects were imaged during five to seven heart cycles in a longitudinal plane. Two of the subjects were young and healthy, the other two subjects were 75-year-old asymptomatic patients with severe carotid stenosis. It was illustrated that the hard calcified stenotic tissue strained less than the wall tissue. Furthermore, it was found that the strain pattern in the stenotic region was more heterogeneous than the strain pattern for the vessel walls of the healthy subjects.
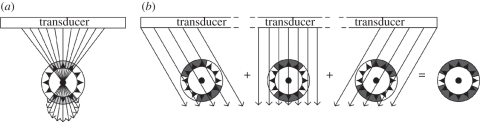
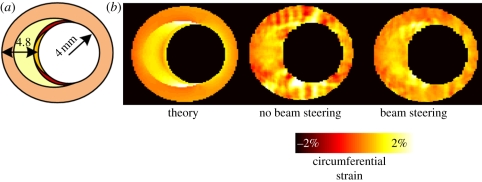
There are several ways to circumvent the use of the lateral strain component when using RF-based strain estimation for strain imaging in transverse imaging planes. Nakagawa et al. [42] changed the time delays of adjacent transducer elements to electronically steer the ultrasound beam through the centre of the lumen to obtain radial strains for a larger segment of the cross section (figure 3a). The method was applied to a pressurized rubber tube and in vivo ultrasound registrations of a carotid artery. The disadvantage of this method is that only a part of the arterial cross section can be imaged. Hansen et al. [38,43] used beam steering of entire image planes to acquire axial strain data for various regions of the cross section. These methods for radial and circumferential strain imaging in transverse planes show significantly improved radial and circumferential strain estimates compared with strain imaging without beam steering. By correcting for the angle between the axial and radial strain, radial strains are acquired for various circle segments of the cross sections which were combined afterwards (figure 3b). More recently, Hansen et al. [44] proposed a new method in which radial and circumferential strains were obtained by projection of axial strain data only from two or more acquisitions at multiple steering angles. Phantom experiments were performed to validate the technique and revealed an even better performance when compared with the initial beam steering technique. In figure 4, the improvement in performance when using beam steering is illustrated for a vulnerable plaque simulation. In vivo recordings of a healthy human carotid artery demonstrated that the usage of two angles to reconstruct radial and circumferential strain is possible in a pulsating environment [45].
Figure 3.
Beam steering methods for vascular strain imaging in transverse planes. (a) Method by Nakagawa et al. and (b) method by Hansen et al.
Figure 4.
Circumferential strain reconstructions for a vulnerable plaque model. (a) The theoretical strain image is shown which was derived by finite element modelling. Ivory, lipids (25 kPa); orange, vessel wall (1000 kPa); yellow, centre of fibrous cap (1250 kPa); red, shoulders of cap (1500 kPa). (b) Strain images derived from simulated ultrasound data are shown. The left image was derived using conventional single angle data. The right image was estimated using beam steering at angles of −30°, 0° and 30°.
5.2. Intravascular strain imaging
For vascular applications, the pulsatile intraluminal pressure is used as source for deformation. Since the strains in the radial and circumferential direction are the principal directions, there is a great benefit of using intravascular catheters: the ultrasound beams are more or less in the radial direction whereas the circumferential strain can be determined from displacements perpendicular to the ultrasound beams. For acquiring an intravascular elastogram, an IVUS catheter was inserted in a coronary artery and RF data were recorded for the full circumference for a low and a high intraluminal pressure. Next, radial displacements were calculated by applying the aforementioned cross-correlation technique to each separate ultrasound line. From the displacements, the strain is calculated and plotted next to the IVUS echograms. A different way of visualization is by plotting only the inner ring of strain values (the eventually rupture prone region) in the B-mode data at the lumen vessel wall boundary [46]. Such an image is referred to as a palpogram (figure 5).
Figure 5.
IVUS palpogram of a non-culprit lesion. The patient had a myocardial infarction caused by an occlusion of the left anterior descending coronary artery. Recordings were made at the same location in the right coronary artery, (a) directly after infarct and (b) at three months follow up. The eccentric lesion was not occluding, but characterized by increased strain, which was worse after three months.
The first reports on IVUS strain imaging came from three different research groups [47–49]. The performance of the technique was first tested on homogeneous vessel-mimicking phantoms and vessel-mimicking phantoms with layers of soft or hard plaques. The strain in the homogeneous vessel was in accordance with theory. Furthermore, the harder and softer plaques were correctly identified [47]. Next, the technique was tested in vitro on excised coronary and femoral arteries. The obtained radial strain maps were locally compared with corresponding histology. It was shown that IVUS strain imaging enabled differentiation between fibrous, fatty and fibro-fatty plaques based on their strain values [50]. Then, the step towards in vivo imaging was taken. IVUS strain imaging also proved to be successful for differentiating between fibrous and fatty materials using data from atherosclerotic iliac and femoral arteries of a Yucatan minipig [51]. In a later study, its ability to correctly classify vulnerable and stable plaques was investigated. IVUS strain data from 54 cross-sectional recordings of excised coronary arteries were compared with vulnerable plaque classification based on histology. It was shown that the technique identified vulnerable plaques in vitro with a sensitivity of 88 per cent and specificity of 89 per cent [52]. Next, the correlation between three-dimensional IVUS strain imaging and clinical symptoms was investigated [53]. Three-dimensional IVUS palpograms were estimated from RF data obtained during catheter pullback for 55 patients. A high correlation was found between the number of high strain spots and the clinical symptoms of the patients. Patients suffering from unstable angina or from an acute myocardial infarction had significantly more high strain spots than patients with stable angina.
The number of studies that confirm the usefulness of intravascular ultrasound strain imaging of the coronary arteries for vulnerable plaque detection is still increasing. In the Integrated Biomarker and Imaging Study (IBIS), it was shown that the density of spots with strain values above 0.9 per cent decreased significantly in acute coronary syndrome patients using standard of care treatment [54]. In IBIS-2, it was shown that patients with vulnerable plaques that were treated with Darapladib, an inhibitor of an enzyme associated with inflammation activity, showed a significant decrease in strain values in 12 months time [55]. Patients who were treated using standard of care also showed a small decrease in strain values, resulting in non-significant treatment effect.
However, a major drawback of IVUS elastography will always remain its invasive nature, which implies that the technique can only be applied to patients who are already in the catheterization laboratory for catheterization. In other words, the technique can only be applied to people who already have clinical symptoms. As explained before, most people who are at risk of having a myocardial infarction or a stroke are asymptomatic [56]. Since ultrasound is relatively cheap and harmless compared with other imaging modalities like MRI and CT, a non-invasive variant for vascular ultrasound strain imaging would be ideal for preventive screening of these populations as well. Since atherosclerosis is a systemic disease the local findings in the carotid artery might also serve as a surrogate marker for overall disease in a certain patient [57].
6. Vasa vasorum imaging
Since the presence and size of the VV are related to plaque vulnerability it is currently believed that early identification and treatment of abundance of VV will prevent many cardio- and neurovascular events. Vascular ultrasound has potential to provide this information.
6.1. Non-invasive vasa vasorum imaging
Based on knowledge obtained from myocardial perfusion measurements, echographic methods have been developed to identify the perfusion of atherosclerosis using vascular ultrasound in combination with ultrasound contrast agents. As initially reported by Feinstein et al. in 2004, ultrasound contrast agents revealed real-time visualization of the carotid artery adventitial VV network in patients undergoing routine carotid ultrasonography [58,59]. Several independent reports using similar techniques confirmed the initial discoveries including the observations that adventitial VV and intra-plaque angiogenesis can be visualized [60–69].
6.2. Intravascular ultrasound vasa vasorum imaging
With the recent interest in VV of the carotid arteries using ultrasound contrast agents, there is accompanying interest in developing an IVUS application for measurement of coronary VV. However, since IVUS systems generally operate at acoustic frequencies in the range of 20–50 MHz, these systems will provide high spatial resolution (approx. 125 to 50 µm, respectively) with cross-sectional images of the lumen and walls of larger blood vessels, but these high frequencies may not provide optimization of the harmonic contrast effect resulting in visualization of the coronary artery VV. Ultrasound contrast agents have primarily been designed to be used at low frequencies, from 1 to 5 MHz. As identified above, commercial contrast agents have been used clinically for carotid VV identification with ultrasound systems that operate at frequencies ranging from 7 to 12 MHz. Despite conventional belief, Goertz et al. [70] have shown that it is possible to identify contrast agent signals at the higher frequencies used specifically for IVUS. Harmonic IVUS seems a fruitful approach for this [71]. Conventional IVUS elements have a bandwidth that is too limited for optimal harmonic imaging though. A new generation single crystal technology or composite transducers based on dual resonance layers (capacitive micromachined ultrasound transducers or CMUTs) are under development [72–74]. The harmonic IVUS system that approaches current IVUS practice employs filtered transmission and reception of signals. Granada & Feinstein [75] showed enhanced adventitial signal in coronary arteries in a swine model for atherosclerosis using this system. A problem associated with filtering is the inhibition of resolution. Methods that require less steep filtering provide preserved resolution using multi-pulse sequences, similar to pulse inversion and power modulation and these processes occur prior to filtering. In the near future, inherent tissue motion can be compensated for by using novel sequence design [76]. Goertz et al. reported detailed detection and localization of VV in an atherosclerotic rabbit aorta model using a dual frequency IVUS element and pulse inversion followed by filtering out of the harmonic [77] or subharmonic signals [78]. Figure 6 shows an example of VV detection in this model.
Figure 6.
In vivo results in an atherosclerotic rabbit aorta using an ultrasound contrast agent. (a) Fundamental mode at 20 MHz, 10 s after injection where changes in adventitial enhancement are not evident. (b) At 10 s after injection, the harmonic mode (transmit at 20 MHz, receive at 40 MHz) shows significant adventitial enhancement, consistent with the detection of adventitial microvessels. The white dots are contrast agents in the vasa vasorum and the bright ring contrast agents attached to the luminal border. The dynamic range of the fundamental and harmonic images is 40 and 25 dB, respectively.
7. Discussion and conclusion
Vascular ultrasound is an excellent technique to frequently scan arteries. Owing to the use of non-ionizing radiation, its excellent temporal and spatial resolution, the short acquisition time with real-time visualization and its relatively low costs, vascular ultrasound is the imaging modality for screening a population. However, the clinical use will strongly depend on the sensitivity and specificity to identify the rupture proneness of atherosclerotic plaques. IMT as measured using ultrasound has proved to be associated with the risk for a cerebral event. However, this association is found in large-scale studies and, at present, it seems that the published evidence to quantitatively support the use of a carotid IMT measurement to help in risk stratification on top of a risk function is limited [79]. Presently, risk prediction using three-dimensional plaque volume as assessed by vascular ultrasound in combination with CT and MRI is investigated in a large cohort of normal individuals [80]. Although conventional echo features like echolucent regions in carotid plaques are associated with the risk for future strokes in symptomatic patients, the prediction of future events in asymptomatic patients with echolucent regions remains low [81].
With the introduction of vascular elastography, information has become available potentially capable of identifying plaque vulnerability. Ex vivo validation studies using intravascular ultrasound elastography [52] have demonstrated that an increased strain value at the lumen vessel wall boundary has a high sensitivity and specificity to identify plaques with typical vulnerable plaque features [2]. Furthermore, in patients it was demonstrated that the number and area of high strain regions were associated with clinical symptoms and blood serum markers [53]. With the implementation of elastography using non-invasive ultrasound, screening of an asymptomatic population becomes feasible. The recently started studies in patients are required to demonstrate the effectiveness of vascular elastography to identify vulnerable plaques and the possibility to intervene for prevention of cardiovascular events.
The current limitation of vascular elastography is the huge computational load to calculate the elastograms. Especially, since frame-rates of the order of 50 per second are required for RF-based elastographic techniques, the current processing speed of ultrasound machines is not sufficient. However, more and more dedicated hardware is integrated in ultrasound machines to address this problem. Using graphical processing unit boards normally used in the gaming industry, strain calculation speed can be improved orders of magnitude. This principle is already used for shear wave imaging (Aixplorer ultrasound system, Supersonic Imagine, Aix-en-Provence, France).
Quantification of the VV remains a major issue confronting the future development and implementation of ultrasound contrast agent detection methods as a clinically useful imaging technique. For good quantification, it is crucial to understand the artefacts that are present in the images [82]. Different pulse sequences will produce different artefacts. Designing pulse sequences with minimal artefacts and taking the remaining artefacts into account will be an important step forward. Additionally, three-/four-dimensional volumetric imaging will be necessary for mapping and quantification of VV within vascular systems. Currently, all the observational studies which use ultrasound contrast agents for VV detection and quantification employ two-dimensional ultrasound imaging and semi-quantitative approaches [61,62,65,67,68]. IVUS VV detection is not ready for clinical use yet. Quantification and a thoughtful strategy for three-dimensional reconstruction of the VV remain to be developed [83]. And once developed, IVUS catheters and scanning methods require regulatory approvals. Yet, there is promise for the development of a significant extension of the functionality of IVUS imaging. In the near future, IVUS imaging of the coronary VV might serve as an additional biomarker of plaque vulnerability.
In conclusion, ultrasound is an elegant and relatively cheap technology for vascular scanning. It reveals the geometry of the artery as well as the presence of plaques. Since the carotid artery can be scanned using non-invasive techniques, its application is much more widespread than coronary artery scanning. The latter application requires intravascular catheters and consequently the clinical applicability is limited to the catheterization laboratory and in patients undergoing an interventional procedure. With the introduction of vascular strain imaging and VV imaging, the presence and development of plaque vulnerability might be identified paving the road to prevention of cardiovascular events.
References
- 1.Pasterkamp G., Schoneveld A. H., van der Wal A. C., Haudenschild C. C., Clarijs R. J., Becker A. E., Hillen B., Borst C. 1998. Relation of arterial geometry to luminal narrowing and histologic markers for plaque vulnerability: the remodeling paradox. J. Am. Coll. Cardiol. 32, 655–662 10.1016/S0735-1097(98)00304-0 (doi:10.1016/S0735-1097(98)00304-0) [DOI] [PubMed] [Google Scholar]
- 2.Schaar J. A., et al. 2004. Terminology for high-risk and vulnerable coronary artery plaques. Eur. Heart J. 25, 1077–1082 10.1016/j.ehj.2004.01.002 (doi:10.1016/j.ehj.2004.01.002) [DOI] [PubMed] [Google Scholar]
- 3.Davies M. J. 1996. Stability and instability: two faces of coronary atherosclerosis. Circulation 94, 2013–2020 [DOI] [PubMed] [Google Scholar]
- 4.Carr S., Farb A., Pearce W. H., Virmani R., Yao J. S. 1996. Atherosclerotic plaque rupture in symptomatic carotid artery stenosis. J. Vasc. Surg. 23, 755–765 10.1016/S0741-5214(96)70237-9 (doi:10.1016/S0741-5214(96)70237-9) [DOI] [PubMed] [Google Scholar]
- 5.Davies M. J., Thomas A. C. 1985. Plaque fissuring—the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br. Heart J. 53, 363–373 10.1136/hrt.53.4.363 (doi:10.1136/hrt.53.4.363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falk E., Shah P., Fuster V. 1995. Coronary plaque disruption. Circulation 92, 657–671 [DOI] [PubMed] [Google Scholar]
- 7.Nair A., Kuban B. D., Tuzcu E. M., Schoenhagen P., Nissen S. E., Vince D. G. 2002. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation 106, 2200–2206 10.1161/01.CIR.0000035654.18341.5E (doi:10.1161/01.CIR.0000035654.18341.5E) [DOI] [PubMed] [Google Scholar]
- 8.de Korte C. L., van der Steen A. F. W., Céspedes E. I., Pasterkamp G., Carlier S. G., Mastik F., Schooneveld A. H., Serruys P. W., Bom N. 2000. Characterization of plaque components and vulnerability with intravascular ultrasound elastography. Phys. Med. Biol. 45, 1465–1475 10.1088/0031-9155/45/6/305 (doi:10.1088/0031-9155/45/6/305) [DOI] [PubMed] [Google Scholar]
- 9.Schaar J. A., de Korte C. L., Mastik F., Baldewsing R., Regar E., Feyter P., Slager C. J., van der Steen A. F., Serruys P. W. 2003. Intravascular palpography for high-risk vulnerable plaque assessment. Herz 28, 488–495 10.1007/s00059-003-2488-6 (doi:10.1007/s00059-003-2488-6) [DOI] [PubMed] [Google Scholar]
- 10.Sluimer J. C., et al. 2008. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J. Am. Coll. Cardiol. 51, 1258–1265 10.1016/j.jacc.2007.12.025 (doi:10.1016/j.jacc.2007.12.025) [DOI] [PubMed] [Google Scholar]
- 11.Moreno P. R., Purushothaman K. R., Sirol M., Levy A. P., Fuster V. 2006. Neovascularization in human atherosclerosis. Circulation 113, 2245–2252 10.1161/CIRCULATIONAHA.105.578955 (doi:10.1161/CIRCULATIONAHA.105.578955) [DOI] [PubMed] [Google Scholar]
- 12.Sluimer J. C., et al. 2009. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions: relevance of compromised structural integrity for intraplaque microvascular leakage. J. Am. Coll. Cardiol. 53, 1517–1527 10.1016/j.jacc.2008.12.056 (doi:10.1016/j.jacc.2008.12.056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodis H. N., Mack W. J., LaBree L., Selzer R. H., Liu C. R., Liu C. H., Azen S. P. 1998. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann. Intern. Med. 128, 262–269 [DOI] [PubMed] [Google Scholar]
- 14.Steinvil A., Sadeh B., Arbel Y., Justo D., Belei A., Borenstein N., Banai S., Halkin A. 2011. Prevalence and predictors of concomitant carotid and coronary artery atherosclerotic disease. J. Am. Coll. Cardiol. 57, 779–783 10.1016/j.jacc.2010.09.047 (doi:10.1016/j.jacc.2010.09.047) [DOI] [PubMed] [Google Scholar]
- 15.Bots M. L. 2006. Carotid intima-media thickness as a surrogate marker for cardiovascular disease in intervention studies. Curr. Med. Res. Opin. 22, 2181–2190 10.1185/030079906X148472 (doi:10.1185/030079906X148472) [DOI] [PubMed] [Google Scholar]
- 16.Bots M. L., Witteman J. C., Grobbee D. E. 1993. Carotid intima-media wall thickness in elderly women with and without atherosclerosis of the abdominal aorta. Atherosclerosis 102, 99–105 10.1016/0021-9150(93)90088-C (doi:10.1016/0021-9150(93)90088-C) [DOI] [PubMed] [Google Scholar]
- 17.Salonen J. T., Salonen R. 1991. Ultrasonographically assessed carotid morphology and the risk of coronary heart disease. Arterioscler. Thromb. 11, 1245–1249 [DOI] [PubMed] [Google Scholar]
- 18.Chambless L. E., Heiss G., Folsom A. R., Rosamond W., Szklo M., Sharrett A. R., Clegg L. X. 1997. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study 1987–1993. Am. J. Epidemiol. 146, 483–494 [DOI] [PubMed] [Google Scholar]
- 19.Bom N., Lancee C. T. 1972. Apparatus for ultrasonically examining a hollow organ. UK Patent no. 1402192 [Google Scholar]
- 20.Bom N., van der Steen A. F. W., Lancee C. T. 2003. The technical potential of IVUS: history and principles. In Vascular ultrasound (eds Saijo Y., van der Steen A. F. W.), pp. 51–65 Tokyo, Japan: Springer [Google Scholar]
- 21.Metz J. A., Yock P. G., Fitzgerald P. J. 1997. Intravascular ultrasound: basic interpretation. Cardiol. Clin. 15, 1–15 10.1016/S0733-8651(05)70314-3 (doi:10.1016/S0733-8651(05)70314-3) [DOI] [PubMed] [Google Scholar]
- 22.von Birgelen C., et al. 1997. ECG-gated three-dimensional intravascular ultrasound: feasibility and reproducibility of the automated analysis of coronary lumen and atherosclerotic plaque dimensions in humans. Circulation 96, 2944–2952 [DOI] [PubMed] [Google Scholar]
- 23.Nissen S. E., Yock P. 2001. Intravascular ultrasound: novel pathophysiological insights and current clinical applications. Circulation 103, 604–616 [DOI] [PubMed] [Google Scholar]
- 24.Degertekin M., Serruys P. W., Tanabe K., Lee C. H., Sousa J. E., Colombo A., Morice M. C., Ligthart J. M., de Feyter P. J. 2003. Long-term follow-up of incomplete stent apposition in patients who received sirolimus-eluting stent for de novo coronary lesions: an intravascular ultrasound analysis. Circulation 108, 2747–2750 10.1161/01.CIR.0000103666.25660.77 (doi:10.1161/01.CIR.0000103666.25660.77) [DOI] [PubMed] [Google Scholar]
- 25.Nissen S. E., et al. 2006. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 295, 1556–1565 10.1001/jama.295.13.jpc60002 (doi:10.1001/jama.295.13.jpc60002) [DOI] [PubMed] [Google Scholar]
- 26.Ophir J., Céspedes E. I., Ponnekanti H., Yazdi Y., Li X. 1991. Elastography: a method for imaging the elasticity in biological tissues. Ultrason. Imaging 13, 111–134 10.1016/0161-7346(91)90079-W (doi:10.1016/0161-7346(91)90079-W) [DOI] [PubMed] [Google Scholar]
- 27.Kallel F., Ophir J. 1997. A least-squares strain estimator for elastography. Ultrason. Imaging 19, 195–208 [DOI] [PubMed] [Google Scholar]
- 28.Lopata R. G. P., Nillesen M. M., Hansen H. H., Gerrits I. H., Thijssen J. M., de Korte C. L. 2009. Performance evaluation of methods for two-dimensional displacement and strain estimation using ultrasound radio frequency data. Ultrasound Med. Biol. 35, 796–812 10.1016/j.ultrasmedbio.2008.11.002 (doi:10.1016/j.ultrasmedbio.2008.11.002) [DOI] [PubMed] [Google Scholar]
- 29.Bonnefous O., Brevannes L., Denis E., Sananes J. C., Montaudon M., Laurent F. H., Drouillard J. 1996. New noninvasive echographic technique for arterial wall characterization. Radiology 201, 1129 [Google Scholar]
- 30.Hasegawa H., Kanai H., Chubachi N., Koiwa Y. 1997. Non-invasive evaluation of Poisson's ratio of arterial wall using ultrasound. Electron. Lett. 33, 340–342 10.1049/el:19970219 (doi:10.1049/el:19970219) [DOI] [Google Scholar]
- 31.Kanai H., Sato M., Koiwa Y., Chubachi N. 1996. Transcutaneous measurement and spectrum analysis of heart wall vibrations. IEEE Trans. Ultrason. Ferroelectr. Freq. Contr. 43, 791–810 10.1109/58.535480 (doi:10.1109/58.535480) [DOI] [Google Scholar]
- 32.Hasegawa H., Kanai H. 2008. Reduction of influence of variation in center frequencies of RF echoes on estimation of artery-wall strain. IEEE Trans. Ultrason. Ferroelectr. Freq. Contr. 55, 1921–1934 10.1109/TUFFC.884 (doi:10.1109/TUFFC.884) [DOI] [PubMed] [Google Scholar]
- 33.Hasegawa H., Kanai H. 2008. Simultaneous imaging of artery-wall strain and blood flow by high frame rate acquisition of RF signals. IEEE Trans. Ultrason. Ferroelectr. Freq. Contr. 55, 2626–2639 10.1109/TUFFC.2008.978 (doi:10.1109/TUFFC.2008.978) [DOI] [PubMed] [Google Scholar]
- 34.Kanai H., Hasegawa H., Ichiki M., Tezuka F., Koiwa Y. 2003. Elasticity imaging of atheroma with transcutaneous ultrasound: preliminary study. Circulation 107, 3018–3021 10.1161/01.CIR.0000078633.31922.8A (doi:10.1161/01.CIR.0000078633.31922.8A) [DOI] [PubMed] [Google Scholar]
- 35.Shi H., Mitchell C. C., McCormick M., Kliewer M. A., Dempsey R. J., Varghese T. 2008. Preliminary in vivo atherosclerotic carotid plaque characterization using the accumulated axial strain and relative lateral shift strain indices. Phys. Med. Biol. 53, 6377–6394 10.1088/0031-9155/53/22/008 (doi:10.1088/0031-9155/53/22/008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasegawa H., Kanai H. 2009. Phase-sensitive lateral motion estimator for measurement of artery-wall displacement—phantom study. IEEE Trans. Ultrason. Ferroelectr. Freq. Contr. 56, 2450–2462 10.1109/TUFFC.2009.1332 (doi:10.1109/TUFFC.2009.1332) [DOI] [PubMed] [Google Scholar]
- 37.Ribbers H., Lopata R. G., Holewijn S., Pasterkamp G., Blankensteijn J. D., de Korte C. L. 2007. Noninvasive two-dimensional strain imaging of arteries: validation in phantoms and preliminary experience in carotid arteries in vivo. Ultrasound Med. Biol. 33, 530–540 10.1016/j.ultrasmedbio.2006.09.009 (doi:10.1016/j.ultrasmedbio.2006.09.009) [DOI] [PubMed] [Google Scholar]
- 38.Hansen H. H. G., Lopata R. G. P., de Korte C. L. 2009. Noninvasive carotid strain imaging using angular compounding at large beam steered angles: validation in vessel phantoms. IEEE Trans. Med. Imaging 28, 872–880 10.1109/TMI.2008.2011510 (doi:10.1109/TMI.2008.2011510) [DOI] [PubMed] [Google Scholar]
- 39.Céspedes E. I., de Korte C. L., van der Steen A. F. W. 1999. Echo decorrelation from displacement gradients in elasticity and velocity estimation. IEEE Trans. Ultrason. Ferroelectr. Freq. Contr. 46, 791–801 10.1109/58.775642 (doi:10.1109/58.775642) [DOI] [PubMed] [Google Scholar]
- 40.Maurice R. L., Ohayon J., Fretigny Y., Bertrand M., Soulez G., Cloutier G. 2004. Noninvasive vascular elastography: theoretical framework. IEEE Trans. Med. Imaging 23, 164–180 10.1109/TMI.2003.823066 (doi:10.1109/TMI.2003.823066) [DOI] [PubMed] [Google Scholar]
- 41.Schmitt C., Soulez G., Maurice R. L., Giroux M. F., Cloutier G. 2007. Noninvasive vascular elastography: toward a complementary characterization tool of atherosclerosis in carotid arteries. Ultrasound Med. Biol. 33, 1841–1858 10.1016/j.ultrasmedbio.2007.05.020 (doi:10.1016/j.ultrasmedbio.2007.05.020) [DOI] [PubMed] [Google Scholar]
- 42.Nakagawa N., Hasegawa H., Kanai H. 2004. Cross-sectional elasticity imaging of carotid arterial wall in short-axis plane by transcutaneous ultrasound. Jpn. J. Appl. Phys. 1 Regul. Pap. Short Notes Rev. Pap. 43, 3220–3226 [Google Scholar]
- 43.Hansen H. H. G., Lopata R. G. P., Idzenga T., de Korte C. L. 2010. An angular compounding technique using displacement projection for noninvasive ultrasound strain imaging of vessel cross-sections. Ultrasound Med. Biol. 36, 1947–1956 10.1016/j.ultrasmedbio.2010.06.008 (doi:10.1016/j.ultrasmedbio.2010.06.008) [DOI] [PubMed] [Google Scholar]
- 44.Hansen H. H. G., Lopata R. G. P., Idzenga T., de Korte C. L. 2010. Full 2D displacement vector and strain tensor estimation for superficial tissue using beam steered ultrasound imaging. Phys. Med. Biol. 5, 3201–3218 10.1088/0031-9155/55/11/014 (doi:10.1088/0031-9155/55/11/014) [DOI] [PubMed] [Google Scholar]
- 45.Hansen H. H. G., Lopata R. G. P., Keijts M., Idzenga T., de Korte C. L. In press. Noninvasive strain imaging in pulsating vessels using beam steered ultrasound acquisitions. In Proc. IEEE Int. Symp. on Biomedical Imaging, Chicago, IL, USA, 30 March–2 April 2011 [Google Scholar]
- 46.Céspedes E. I., de Korte C. L., van der Steen A. F. W. 2000. Intraluminal ultrasonic palpation: assessment of local and cross-sectional tissue stiffness. Ultrasound Med. Biol. 26, 385–396 10.1016/S0301-5629(99)00169-6 (doi:10.1016/S0301-5629(99)00169-6) [DOI] [PubMed] [Google Scholar]
- 47.de Korte C. L., Céspedes E. I., van der Steen A. F. W., Lancée C. T. 1997. Intravascular elasticity imaging using ultrasound: feasibility studies in phantoms. Ultrasound Med. Biol. 23, 735–746 10.1016/S0301-5629(97)00004-5 (doi:10.1016/S0301-5629(97)00004-5) [DOI] [PubMed] [Google Scholar]
- 48.Ryan L. K., Foster F. S. 1997. Ultrasonic measurement of differential displacement and strain in a vascular model. Ultrason. Imaging 19, 19–38 [DOI] [PubMed] [Google Scholar]
- 49.Shapo B. M., Crowe J. R., Erkamp R., Emelianov S. Y., Eberle M., O'Donnell M. 1996. Strain imaging of coronary arteries with intraluminal ultrasound: experiments on an inhomogeneous phantom. Ultrason. Imaging 18, 173–191 10.1006/uimg.1996.0010 (doi:10.1006/uimg.1996.0010) [DOI] [PubMed] [Google Scholar]
- 50.de Korte C. L., Pasterkamp G., van der Steen A. F. W., Woutman H. A., Bom N. 2000. Characterization of plaque components using intravascular ultrasound elastography in human femoral and coronary arteries in vitro. Circulation 102, 617–623 [DOI] [PubMed] [Google Scholar]
- 51.de Korte C. L., Sierevogel M., Mastik F., Strijder C., Velema E., Pasterkamp G., van der Steen A. F. W. 2002. Identification of atherosclerotic plaque components with intravascular ultrasound elastography in vivo: a Yucatan pig study. Circulation 105, 1627–1630 10.1161/01.CIR.0000014988.66572.2E (doi:10.1161/01.CIR.0000014988.66572.2E) [DOI] [PubMed] [Google Scholar]
- 52.Schaar J. A., de Korte C. L., Mastik F., Strijder C., Pasterkamp G., Serruys P., van der Steen A. F. W. 2001. Vulnerable plaque detection with intravascular elastography: a sensitivity and specificity study. Circulation 104, II-459 [DOI] [PubMed] [Google Scholar]
- 53.Schaar J. A., et al. 2004. Incidence of high strain patterns in human coronary arteries: assessment with three-dimensional intravascular palpography and correlation with clinical presentation. Circulation 109, 2716–2719 10.1161/01.CIR.0000131887.65955.3B (doi:10.1161/01.CIR.0000131887.65955.3B) [DOI] [PubMed] [Google Scholar]
- 54.van Mieghem C. A., et al. 2006. Noninvasive detection of subclinical coronary atherosclerosis coupled with assessment of changes in plaque characteristics using novel invasive imaging modalities: the Integrated Biomarker and Imaging Study (IBIS). J. Am. Coll. Cardiol. 47, 1134–1142 10.1016/j.jacc.2005.09.075 (doi:10.1016/j.jacc.2005.09.075) [DOI] [PubMed] [Google Scholar]
- 55.Serruys P. W., et al. 2008. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation 118, 1172–1182 10.1161/CIRCULATIONAHA.108.771899 (doi:10.1161/CIRCULATIONAHA.108.771899) [DOI] [PubMed] [Google Scholar]
- 56.Nicolaides A., et al. 2003. The asymptomatic carotid stenosis and risk of stroke (ACSRS) study: aims and results of quality control. Int. Angiol. 22, 263–272 [PubMed] [Google Scholar]
- 57.O'Leary D. H., Polak J. F., Kronmal R. A. 1999. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke: reply. N. Engl. J. Med. 340, 1763. 10.1056/NEJM199901073400103 (doi:10.1056/NEJM199901073400103) [DOI] [PubMed] [Google Scholar]
- 58.Feinstein S. B. 2004. The powerful microbubble: from bench to bedside, from intravascular indicator to therapeutic delivery system, and beyond. Am. J. Physiol Heart Circ. Physiol. 287, H450–H457 10.1152/ajpheart.00134.2004 (doi:10.1152/ajpheart.00134.2004) [DOI] [PubMed] [Google Scholar]
- 59.Rajaram V., et al. 2004. Role of surrogate markers in assessing patients with diabetes mellitus and the metabolic syndrome and in evaluating lipid-lowering therapy. Am. J. Cardiol. 93, 32C–48C 10.1016/j.amjcard.2004.02.004 (doi:10.1016/j.amjcard.2004.02.004) [DOI] [PubMed] [Google Scholar]
- 60.Magnoni M., Coli S., Marrocco-Trischitta M. M., Melisurgo G., De D. D., Cianflone D., Chiesa R., Feinstein S. B., Maseri A. 2009. Contrast-enhanced ultrasound imaging of periadventitial vasa vasorum in human carotid arteries. Eur. J. Echocardiogr. 10, 260–264 10.1093/ejechocard/jen221 (doi:10.1093/ejechocard/jen221) [DOI] [PubMed] [Google Scholar]
- 61.Shah F., et al. 2007. Contrast-enhanced ultrasound imaging of atherosclerotic carotid plaque neovascularization: a new surrogate marker of atherosclerosis? Vasc. Med. 12, 291–297 10.1177/1358863X07083363 (doi:10.1177/1358863X07083363) [DOI] [PubMed] [Google Scholar]
- 62.Vicenzini E., Giannoni M. F., Puccinelli F., Ricciardi M. C., Altieri M., Di P. V., Gossetti B., Valentini F. B., Lenzi G. L. 2007. Detection of carotid adventitial vasa vasorum and plaque vascularization with ultrasound cadence contrast pulse sequencing technique and echo-contrast agent. Stroke 38, 2841–2843 10.1161/STROKEAHA.107.487918 (doi:10.1161/STROKEAHA.107.487918) [DOI] [PubMed] [Google Scholar]
- 63.Coli S., et al. 2008. Contrast-enhanced ultrasound imaging of intraplaque neovascularization in carotid arteries: correlation with histology and plaque echogenicity. J. Am. Coll. Cardiol. 52, 223–230 10.1016/j.jacc.2008.02.082 (doi:10.1016/j.jacc.2008.02.082) [DOI] [PubMed] [Google Scholar]
- 64.Papaioannou T. G., Vavuranakis M., Androulakis A., Lazaros G., Kakadiaris I., Vlaseros I., Naghavi M., Kallikazaros I., Stefanadis C. 2009. In-vivo imaging of carotid plaque neoangiogenesis with contrast-enhanced harmonic ultrasound. Int. J. Cardiol. 134, e110–e112 10.1016/j.ijcard.2008.01.020 (doi:10.1016/j.ijcard.2008.01.020) [DOI] [PubMed] [Google Scholar]
- 65.Giannoni M. F., et al. 2009. Contrast carotid ultrasound for the detection of unstable plaques with neoangiogenesis: a pilot study. Eur. J. Vasc. Endovasc. Surg. 37, 722–727 10.1016/j.ejvs.2008.12.028 (doi:10.1016/j.ejvs.2008.12.028) [DOI] [PubMed] [Google Scholar]
- 66.Vicenzini E., Giannoni M. F., Benedetti-Valentini F., Lenzi G. L. 2009. Imaging of carotid plaque angiogenesis. Cerebrovasc. Dis. 27(Suppl. 2), 48–54 10.1159/000203126 (doi:10.1159/000203126) [DOI] [PubMed] [Google Scholar]
- 67.Xiong L., Deng Y. B., Zhu Y., Liu Y. N., Bi X. J. 2009. Correlation of carotid plaque neovascularization detected by using contrast-enhanced US with clinical symptoms. Radiology 251, 583–589 10.1148/radiol.2512081829 (doi:10.1148/radiol.2512081829) [DOI] [PubMed] [Google Scholar]
- 68.Staub D., Partovi S., Schinkel A. F., Coll B., Uthoff H., Aschwanden M., Jaeger K. A., Feinstein S. B. 2011. Correlation of carotid artery atherosclerotic lesion echogenicity and severity at standard US with intraplaque neovascularization detected at contrast-enhanced US. Radiology 258, 618–626 10.1148/radiol.10101008 (doi:10.1148/radiol.10101008) [DOI] [PubMed] [Google Scholar]
- 69.Staub D., et al. 2010. Contrast-enhanced ultrasound imaging of the vasa vasorum: from early atherosclerosis to the identification of unstable plaques. JACC: Cardiovasc. Imaging 3, 761–771 10.1016/j.jcmg.2010.02.007 (doi:10.1016/j.jcmg.2010.02.007) [DOI] [PubMed] [Google Scholar]
- 70.Goertz D. E., Frijlink M. E., de Jong N., van der Steen A. F. W. 2006. High frequency nonlinear scattering from a micrometer to submicrometer sized lipid encapsulated contrast agent. Ultrasound Med. Biol. 32, 569–577 10.1016/j.ultrasmedbio.2006.01.002 (doi:10.1016/j.ultrasmedbio.2006.01.002) [DOI] [PubMed] [Google Scholar]
- 71.Frijlink M. E., Goertz D. E., Bouakaz A., van der Steen A. F. 2006. Intravascular ultrasound tissue harmonic imaging: a simulation study. Ultrasonics 44(Suppl. 1), e185–e188 10.1016/j.ultras.2006.06.044 (doi:10.1016/j.ultras.2006.06.044) [DOI] [PubMed] [Google Scholar]
- 72.Vos H. J., Frijlink M. E., Droog E., Goertz D. E., Blacquiere G., Gisolf A., de Jong N., van der Steen A. F. 2005. Transducer for harmonic intravascular ultrasound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Contr. 52, 2418–2422 10.1109/TUFFC.2005.1563286 (doi:10.1109/TUFFC.2005.1563286) [DOI] [PubMed] [Google Scholar]
- 73.van der Steen A. F. W., et al. 2006. IVUS beyond the horizon. EuroIntervention 2, 132–142 [PubMed] [Google Scholar]
- 74.Degertekin F. L., Guldiken R. O., Karaman M. 2006. Annular-ring CMUT arrays for forward-looking IVUS: transducer characterization and imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Contr. 53, 474–482 10.1109/TUFFC.2006.1593387 (doi:10.1109/TUFFC.2006.1593387) [DOI] [PubMed] [Google Scholar]
- 75.Granada J. F., Feinstein S. B. 2008. Imaging of the vasa vasorum. Nat. Clin. Pract. Cardiovasc. Med. 5(Suppl. 2), S18–S25 10.1038/ncpcardio1157 (doi:10.1038/ncpcardio1157) [DOI] [PubMed] [Google Scholar]
- 76.Frijlink M. E., Goertz D. E., Bouakaz A., van der Steen A. F. 2006. A simulation study on tissue harmonic imaging with a single-element intravascular ultrasound catheter. J. Acoust. Soc. Am. 120, 1723–1731 10.1121/1.2226069 (doi:10.1121/1.2226069) [DOI] [PubMed] [Google Scholar]
- 77.Goertz D. E., et al. 2006. Contrast harmonic intravascular ultrasound: a feasibility study for vasa vasorum imaging. Invest. Radiol. 41, 631–638 10.1097/01.rli.0000229773.11715.da (doi:10.1097/01.rli.0000229773.11715.da) [DOI] [PubMed] [Google Scholar]
- 78.Goertz D. E., Frijlink M. E., Tempel D., Bhagwandas V., Gisolf A., Krams R., de Jong N., van der Steen A. F. 2007. Subharmonic contrast intravascular ultrasound for vasa vasorum imaging. Ultrasound Med. Biol. 33, 1859–1872 10.1016/j.ultrasmedbio.2007.05.023 (doi:10.1016/j.ultrasmedbio.2007.05.023) [DOI] [PubMed] [Google Scholar]
- 79.Plantinga Y., Dogan S., Grobbee D. E., Bots M. L. 2009. Carotid intima-media thickness measurement in cardiovascular screening programmes. Eur. J. Cardiovasc. Prev. Rehabil. 16, 639–644 10.1097/HJR.0b013e3283312ece (doi:10.1097/HJR.0b013e3283312ece) [DOI] [PubMed] [Google Scholar]
- 80.Muntendam P., McCall C., Sanz J., Falk E., Fuster V. 2010. The BioImage study: novel approaches to risk assessment in the primary prevention of atherosclerotic cardiovascular disease—study design and objectives. Am. Heart J. 160, 49–57 10.1016/j.ahj.2010.02.021 (doi:10.1016/j.ahj.2010.02.021) [DOI] [PubMed] [Google Scholar]
- 81.Gronholdt M. L. M., Nordestgaard B. G., Schroeder T. V., Vorstrup S., Sillesen H. 2001. Ultrasonic echolucent carotid plaques predict future strokes. Circulation 104, 68–73 10.1161/hc2601.091704 (doi:10.1161/hc2601.091704) [DOI] [PubMed] [Google Scholar]
- 82.Renaud G., ten Kate G. L., Akkus Z., van der Oord S. C. H., Schinkel A. F. L., Bosch J. G., van der Streen A. F. W., de Jong N. 2011Distal arterial wall pseudoenhancement in ultrasound contrast imaging of carotids. In Proc. 16th European Symp. on Ultrasound Contrast Imaging, Rotterdam, The Netherlands, 21–22 January 2011, pp. 91–92 [Google Scholar]
- 83.Maresca D., Emmer M., Springeling G., Mastik F., van Soest G., de Jong N., van der Steen A. F. W. 2011. Contrast-enhanced intravascular ultrasound 3D reconstruction of a vasa vasorum mimicking model. In Proc. IEEE Int. Ultrasonics Symp., San Diego, CA, USA, 11–14 October 2010 [Google Scholar]