Abstract
Previous studies have shown that substrate surface chemistry and topography exhibit significant impact on haematopoietic progenitor cell adhesion, proliferation and differentiation. In the present study, the effect of surface amine density and structure of grafted polymer chains on the adhesion and expansion of haematopoietic progenitor cells was investigated. Cryopreserved human umbilical cord blood CD133+ cells were expanded in cytokine-supplemented medium on ethylenediamine (EDA)- or 2-aminoethyl methacrylate hydrochloride (AEMA)-grafted polyethersulphone (PES) nanofibre scaffolds for 10 days. Although the percentage of CD34+ cells among the expanded cells increased with the surface amine density, the maximum fold expansion of CD34+ cells was obtained at a moderate amine density of 20–80 nmol cm−2. When comparing nanofibre matrices with similar amine densities, but prepared with two different methods, cells cultured on the AEMA-grafted PES nanofibre matrix showed lower fold expansion in terms of total cell number (300 ± 84 fold) and CD34+ cell number (68 ± 19-fold) in comparison with those cultured on EDA-grafted nanofibres (787 ± 84-fold and 185 ± 84-fold, respectively). These results indicate that the surface amine density and the conjugate structure are important determinants for the preservation of CD34 surface marker and expansion efficiency of CD34+ cells.
Keywords: haematopoietic progenitors, expansion, nanofibre, matrix, surface chemistry
1. Introduction
Over the past two decades, allogeneic umbilical cord blood (UCB) transplantation has been increasingly used for the treatment of malignant or non-malignant disorders [1–4]. Compared with other sources of haematopoietic stem/progenitor cells (HSPCs), such as bone marrow or peripheral blood, UCB provides a readily available source of HSPCs for allogeneic cell therapy applications and has a higher degree of immune tolerance of human leucocyte antigen (HLA) mismatch with a potentially reduced risk of graft versus host disease (GvHD) [5–7]. However, the small number of primitive cells available from a single collected placenta limits the therapeutic efficacy for transplantation in adults, as cell dose has been shown to be a major determinant of engraftment and survival [2,8,9]. An ex vivo HSPC expansion paradigm has been employed to overcome the insufficient number of primitive cells from cord blood, to minimize the delay of engraftment and the risk of neutropenia or thrombocytopenia [10]. Early acting cytokines, such as stem cell factor (SCF), Flt3-ligand, thrombopoietin (TPO) and lineage-specific growth factors, such as interleukin (IL)-3 and IL-6 have been used in various combinations in serum or serum-free culture systems: they are known to play key roles in the regulation of early stages of haematopoiesis by maintaining the self-renewal capability and proliferation of HSPCs [11].
CD133 and CD34 are routinely used as cell surface antigens to identify, select and characterize haematopoietic progenitors. Previous studies indicate that in addition to the signalling molecules supplemented in the medium, substrate surface properties including topographical, mechanical and biochemical properties can significantly influence the proliferation and differentiation of CD34+ cells [12–18]. For example, Li et al. [12] designed non-woven polyester matrices to facilitate cell–cell and cell–matrix interactions, resulting in increased expansion of CD34+ cells. LaIuppa et al. [13] showed that the ex vivo expansion of haematopoietic progenitors in a serum-free culture system was significantly affected by various types of materials. Most recently, a report by Holst et al. [14] showed that substrate elasticity played an important role in regulating haematopoietic progenitor proliferation and phenotype maintenance.
Accumulating evidence indicates that cell adhesion plays a crucial role in haematopoietic progenitor proliferation and differentiation. We and others have previously reported that haematopoietic progenitor adhesion in expansion culture can promote the survival and proliferation of haematopoietic progenitors. For example, we previously demonstrated that covalently immobilized fibronectin [15] and cell adhesion peptides CS-1 and RGD [16] on polymeric substrate-mediated CD34+ cell adhesion, with higher corresponding expansion efficiency on the functionalized substrate surface. We have recently reported that chemically modifying nanofibre topography with covalently linked primary amine groups can synergistically improve HSPC adhesion, and influence the proliferation characteristics of cultured UCB-derived CD34+ cells. We have shown that CD34+ cells are enriched on the surface of these aminated nanofibres through direct adhesion [17], and the spacer-linking primary amino groups to the surface-grafted polyacrylic acid chains significantly influence the expansion outcomes [18]. Das et al. [19] have recently shown that the aminated nanofibre matrix can effectively expand haematopoietic progenitors from UCB CD133+ cells and demonstrated that these aminated nanofibre-expanded cells can improve vasculogenesis in ischaemic tissue. In the present study, we further investigated the effects of surface primary amine density and grafted polymer structure on the ex vivo expansion of UCB CD133+ cells, with respect to cell proliferation and the expression of progenitor surface markers (CD34 and CD133) and homing-related surface marker CXCR4.
2. Experimental
2.1. Materials
All chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA) unless otherwise specified.
2.2. Electrospinning of polyethersulphone nanofibre scaffolds
Polyethersulphone (PES) nanofibre scaffolds were prepared according to the method we reported previously [17]. Briefly, PES (average molecular weight: 55 000 g mol−1, Goodfellow Cambridge Limited, Cambridge, UK) solution (20.0 w/w%) in dimethylsulphoxide (DMSO) was loaded in a 1 ml plastic syringe fitted with a 27-gauge blunt needle. Polymer solution was fed into the needle tip at a flow rate of 0.5 ml h−1, controlled using a syringe pump (KD Scientific, Holliston, MA, USA). A high-voltage power supply (Gamma High Voltage Research, Ormand Beach, FL, USA) was connected to the needle through an alligator clip and a voltage differential of 12 kV was applied between the needle (polymer solution) and the collector plate. The PES nanofibre was collected directly onto grounded 15 mm diameter glass coverslips (Fisher Scientific Co., Silver Spring, MD, USA) affixed to the collector plate at a distance of 15 cm from the needle tip, over a collection time of 2 min per scaffold. The deposited nanofibre samples were then thoroughly dried in a desiccator.
2.3. The two-step amination method for polyethersulphone nanofibre modification
The two-step surface functionalization of PES nanofibre meshes was conducted according to our published protocol (figure 1a) [17,18]. Briefly, acrylic acid was distilled and stored at −20°C prior to use. Poly(acrylic acid) (PAAc) was grafted onto the PES nanofibre mesh surface by photo-activated surface-grafting free radical polymerization. Samples were immersed in acrylic acid solution containing 0.5 mM NaIO4 in a 10 cm Petri dish. The temperature of the solution was maintained at 20°C by cooling the dish in a water bath. Samples were then exposed to ultraviolet (UV) light from a 400 W mercury lamp (5000-EC; Dymax, Germany). The intensity and the energy of UV light were measured using a radiometer (Accu-Cal 50, Dymax, Germany). The PAAc-grafted meshes were then thoroughly washed with deionized water to remove any un-grafted PAAc from the surface of the scaffold.
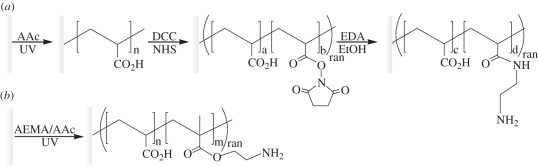
Figure 1.
Schematic of PES surface modification: (a) PES nanofibre was grafted with poly(acrylic acid) followed by conjugation of EDA and (b) one-step grafting of poly(AEMA-co-AAc) copolymer. PES, polyether sulphone; AAc, acrylic acid; DCC, N,N′-dicyclohexylcarbodiimide; NHS, N-hydroxysuccinimide; EDA, ethylenediamine; AEMA, 2-aminoethyl methacrylate; ran, random copolymer.
A batch of 20 PAAc-grafted PES nanofibre scaffolds were further reacted with 10 ml of 100 mM N-hydroxysuccinimide and 100 mM dicyclohexylcarbodiimide in acetonitrile in a glass dish. After 3 h, the reaction solution was carefully aspirated and 15 ml of 50 mM 1,2-ethanediamine (EDA) in ethanol was immediately added. After 12 h of incubation, the reaction solution was carefully aspirated and scaffolds were thoroughly washed in absolute ethanol to remove any excess reagents. All substrates were subsequently dried in a laminar flow hood and loaded into a 24-well tissue culture plate (Nunc, Denmark). Scaffolds were rinsed with sterile phosphate-buffered saline before use.
2.4. The one-step polyethersulphone nanofibre amination method
To simplify the surface amination steps, we developed a protocol to conjugate amino groups onto the substrate surface in a single step (figure 1b). 2-Aminoethyl methacrylate hydrochloride (AEMA, Polysciences Inc., Warrington, PA, USA) was dissolved in acrylic acid solution and was grafted onto the PES nanofibre mesh surface by photo polymerization according to the method described above. Various ratios of monomers and UV intensities were tested to determine the amine density outputs. The AEMA–PAAc-grafted scaffolds were then thoroughly washed with deionized water to remove any un-grafted polymers from the scaffold surface. All substrates were sterilized in 70 per cent ethanol, and loaded into 24-well tissue culture plates. Scaffolds were rinsed with sterile phosphate-buffered saline before use.
2.5. Surface analysis
The PAAc grafting densities on PES nanofibre scaffolds were directly quantified by toluidine blue O colorimetric assay [20]. Surface amine densities were quantified as previously described. Primary amino groups were converted into sulphydryl groups by reaction with 2-iminothiolane. The thiols were quantified by bicinchoninic acid assay. A multi-plate reader was used to measure the absorbance at 562 nm [21]. The modified and unmodified nanofibres were imaged using scanning electron microscopy (SEM). Nanofibre samples were platinum sputter-coated before viewing under a field-emission scanning electron microscope (FE-SEM; JEOL JSM-6700F, Japan). The fibre diameters were measured directly from calibrated images at a screen magnification of 5000-fold.
2.6. Ex vivo haematopoietic stem/progenitor cells expansion culture
Frozen human UCB CD133+ cells were purchased from AllCells, LLC (Emeryville, CA, USA). The CD133+ purity in the post-thawed HSPCs was determined by flow cytometry to be 97 per cent and the viability was determined by Trypan blue exclusion assay to be more than 95 per cent. Recombinant human SCF, Flt-3 ligand (FL3), TPO and IL-3 were purchased from Peprotech Inc. (Rocky Hill, NJ, USA). Low-density lipoprotein (LDL) was purchased from Athens Research & Technology Inc. (Athens, GA, USA). HSPCs were seeded onto fibre-matrix-loaded 24-well plates, at a density of 600 cells per well, and were cultured 10 days in 0.6 ml of StemSpan serum-free medium (StemCell Technologies Inc., Vancouver, BC, Canada) supplemented with 0.04 mg ml−1 of LDL, 100 ng ml−1 of SCF, 100 ng ml−1 of FL3, 50 ng ml−1 of TPO and 20 ng ml−1 of IL-3 at 37°C in 5 per cent CO2. Tissue culture polystyrene (TCPS) 24-well plates were used as controls. All cells were harvested by repeated gentle pipetting on day 10 and counted using a haemacytometer. Cells were further stained for flow cytometry analysis.
2.7. Flow cytometry
Before and after expansion culture, cell samples were stained with phycoerythrin (PE)-conjugated anti-human CD133 (Miltenyi Biotech; Bergisch Gladbach, Germany), fluorescein isothiocyanate (FITC)-conjugated anti-human CD34 or PE-conjugated CXCR4 (BD Biosciences, San Jose, CA, USA) antibodies according to previously described protocols. Cells were analysed by flow cytometry (FACSCalibur, BD Biosciences) with CellQuest software. Relevant isotype controls (FITC-mouse IgG1 and PE-mouse IgG1) were also included to ensure specificity and proper compensation. The Milan–Mulhouse gating method was used for cell enumeration.
2.8. Preparation of culture samples for scanning electron microscopy
Selected cell samples were gently rinsed with phosphate-buffered saline (PBS), fixed with 3 per cent glutaraldehyde in 0.1 M PBS (pH 7.2) containing 3 per cent sucrose. Samples were then dehydrated using a graded series of ethanol, followed by hexamethyldisilazane critical-point drying. Samples were platinum sputter-coated before viewing under FE-SEM (JEOL JSM-6700F).
2.9. Statistical analysis
The statistical analysis was performed using the Student's t-test, and comparisons with p < 0.05 were considered significant.
3. Results
3.1. Polyethersulphone nanofibre surface modification and characterization
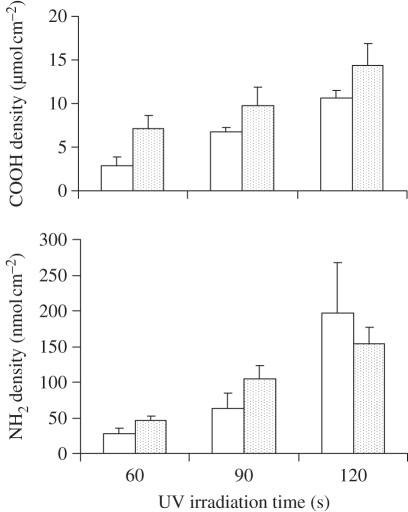
In the two-step surface amination scheme (figure 1a), PES nanofibre matrices were initially carboxylated by UV-initiated PAAc grafting and subsequently activated using N,N′-dicyclohexylcarbodiimide (DCC)/N-hydroxysuccinimide (NHS) chemistry. The reactive intermediates were then incubated with an excess amount of ethylene diamine to produce aminated fibres. Figure 2 shows that the amine density can be effectively adjusted by controlling the primary carboxylic group density. A strong correlation was observed between the density of carboxylic groups on the intermediate fibre surface and the resulting amino group density on the aminated fibres. The surface carboxylic group density was easily controlled by the concentration of acrylic acid (AAc) or UV irradiation time. Under the conditions described, only a small fraction (0.72–1.67%) of carboxylic groups on nanofibre surfaces were converted to primary amines. The surface amine density of the final nanofibre matrices ranged from 5 to 200 nmol cm−2.
Figure 2.
Effect of UV irradiation time on the surface concentration of grafted carboxyl groups and primary amine density on the PES nanofibre mesh. open bars, 3% acrylic acid; dotted bars, 6% acrylic acid.
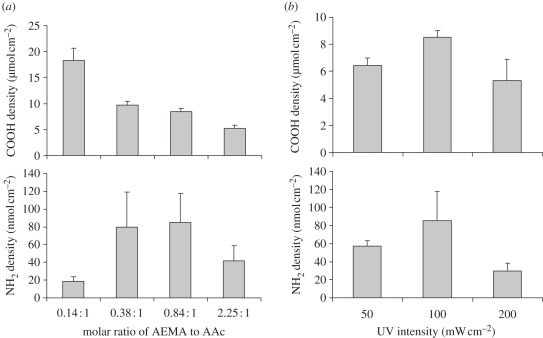
To simplify the surface functionalization process, an amine-containing monomer, 2-aminoethyl metharylate (AEMA), was used to introduce primary amine groups onto the PES nanofibres using a similar photo-initiated surface-grafting polymerization method (figure 1b). Direct grafting of poly(AEMA) only generated a very low density of amine groups on the PES nanofibre surface (data not shown). To increase the surface amine density to levels comparable to those acquired using two-step grafting, a co-monomer, AAc was used to increase the grafting efficiency of AEMA. Under the optimized ranges of AEMA/AAc ratio and UV exposure time, fibres were generated with AEMA density as high as those prepared by a two-step process (figure 3). When the total vinyl concentration was maintained at 0.55 M, the density of the surface-grafted AAc increases with the AAc monomer concentration used for grafting copolymerization. However, the amino group density was limited by the onset of acrylic gelation when the scaffolds were exposed to a UV light with intensity higher than 200 mW cm−2. Thus, the focus was solely on surface grafting at 100 mW cm−2, under which condition the highest density of amino group achieved was approximately 80 nmol cm−2.
Figure 3.
Effect of (a) molar ratio of AEMA and AAc (the total vinyl concentration is maintained at 0.55 M) and (b) UV intensity (UV dosage is kept at 10.0 J cm−2) on the surface concentration of grafted carboxyl groups and represent the primary amine density on the PES nanofibre mesh.
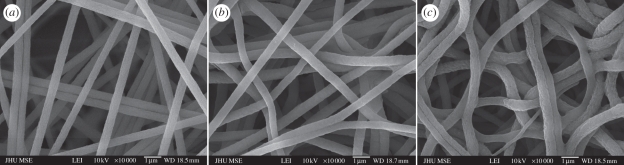
A moderate increase in fibre diameter was observed for matrices produced by both modification methods, indicating the occurrence of surface grafting. The average diameter of electrospun PES nanofibre was 517 ± 102 nm (figure 4a), whereas the aminated fibres generated by the two-step and one-step processes showed average diameters of 608 ± 82 nm and 784 ± 121 nm, respectively (figure 4b,c).
Figure 4.
SEM images of electrospun PES nanofibre. (a) unmodified; (b) grafted with poly(acrylic acid) followed by conjugation of ethylenediamine (EDA); (c) grafted with poly(AEMA-co-AAc) copolymer.
3.2. Ex vivo expansion of haematopoietic progenitors on ethylenediamine-grated aminated nanofibre matrix (two-step method)
We first examined the effect of surface amine density on haematopoietic progenitor adhesion and expansion. The nanofibre substrates were evaluated based on culture of cryopreserved human UCB CD133+ cells in serum-free medium for 10 days; the surface marker expression of expanded cells was analysed by FACScan after non-adherent and attached cells were removed from the fibre matrix by repeated pipetting. We have previously shown that the attached cells can be effectively removed from the surface, suggesting that the adhesion strength of these cells on aminated nanofibres was moderate [17,18]. Cell expansion efficiency was expressed as the fold expansion of total cell number and CD34+ cell number on each surface normalized by initial cell seeding. After the 10 day expansion culture, cell viability was measured using the Trypan blue exclusion test and was found to be greater than 95 per cent under all conditions.
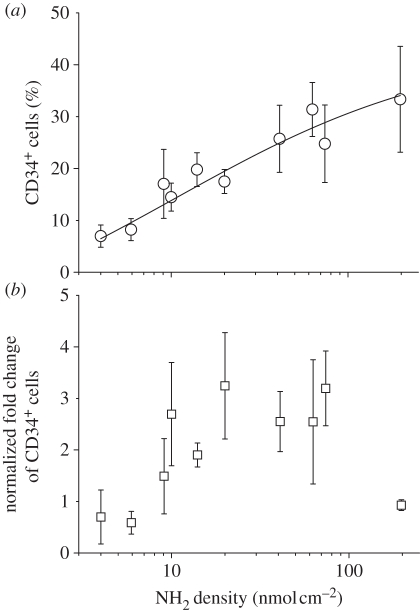
We found that after 10 days of culture, the percentage of CD34+ cells increased with the surface amine density in the range 5–200 nmol cm−2 (figure 5a). However, the fold expansion of the total cells decreased dramatically with highest amine density (197 nmol cm−2), thus leading to a decrease in fold expansion of CD34+ cells. Since the fibre-cultured expansion results deviated slightly from batch to batch owing to normal experimental variation, we normalized the expansion efficiency to the TCPS control group fold expansion of CD34+ cells, which ranged from 20 to 60 in these experiments. Figure 5b shows the normalized fold expansion of CD34+ cells as a function of fibre surface amine density. The maximal fold expansion of CD34+ cells was obtained at an amine density of 20–80 nmol cm−2 in comparison with the TCPS control group.
Figure 5.
(a) Effect of amine density on EDA-grafted PES nanofibre surface on percentage of CD34-expressing cells among CB CD133+ cells expanded for 10 days; (b) Effect of amine density on EDA-grafted PES nanofibre surface on fold expansion of CD34+ cells that is normalized to CD34+ cell expansion fold on TCPS control. Levels of CD34 antigen expression on cell surface were analysed by flow cytometry. Each data point represents the average of four or eight replicates in a single experiment.
3.3. Ex vivo haematopoietic progenitor expansion on 2-aminoethyl methacrylate hydrochloride-grafted nanofibre matrix (one-step method)
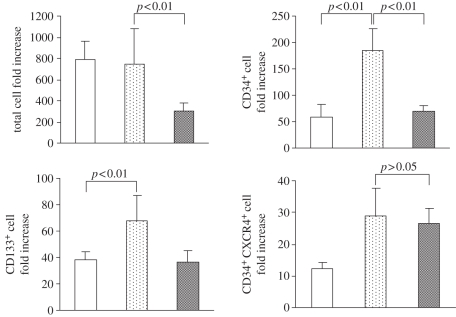
In order to assess haematopoietic progenitor expansion efficiency of aminated fibres prepared by the one-step grafting process, we repeated the expansion of UCB CD133+ cells using the same protocol as described above. Based on the previous results, we chose nanofibre matrices prepared by both one and two-step grafting methods for direct comparison, with an optimal surface amine density in the range 50–80 nmol cm−2. Figure 6 shows the fold expansion of total nucleated cells and CD34+ cells cultured on these two sets of nanofibres, along with a TCPS control.
Figure 6.
Fold expansion of total nucleated cells, CD34+ cells and CD34+CXCR4+ cells after 10 day culture of CB CD133+ cells on different substrates. EDA- and AEMA-modified PES nanofibre scaffolds with 50–80 nmol cm−2 amine density were used for cell culture. Results were expressed as the mean ± s.d. (n = 4). A comparison with a p-value of less than 0.05 was considered statistically significant. Open bars, TCPS; dotted bars, EDA-grafted PES nanofibre; grey bars, AEMA-grafted PES nanofibre.
All three substrates supported the proliferation of the total nucleated cells. EDA-modified PES nanofibre scaffolds (two-step grafting) showed comparable total fold cell expansion to the TCPS surface (787 ± 170 fold and 749 ± 333-fold, respectively), whereas cells cultured on AEMA-grafted nanofibres (one-step grafting) yielded a lower proliferation of total nucleated cells (300 ± 84-fold), with an CD34+ cell fold expansion of 70 ± 11. Haematopoietic progenitors cultured on EDA-grafted nanofibres exhibited the highest CD34+ and CD133+ cell expansion (185 ± 42-fold and 68 ± 19-fold, respectively), whereas AEMA-grafted matrix showed similar expansion outcomes as TCPS surface in terms of CD34+ cells (70 ± 11 versus 58 ± 22-fold, respectively, p > 0.05) and CD133+ cells (36 ± 9 versus 38 ± 6-fold).
3.4. Comparison of surface marker expression on expanded haematopoietic progenitors
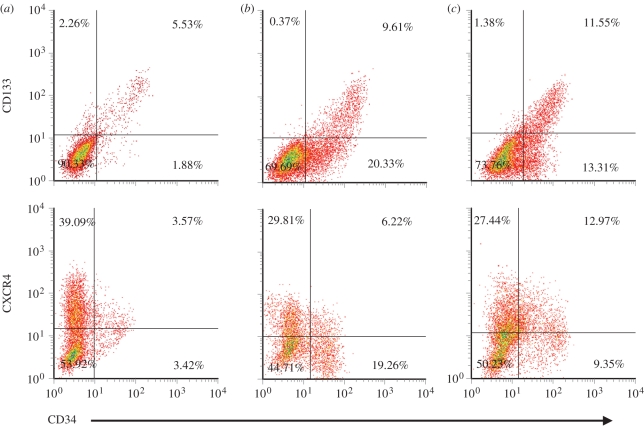
Surface marker expression on expanded haematopoietic progenitors was further analysed for these three groups and compared with freshly thawed CD133+ cells, which were 97 per cent CD133+ and 86 per cent CD34+, with over 98 per cent CD34+ cells co-expressing CD133. Representative flow cytometry scatter plots for expanded cultures are showed in figure 7 and detailed results are listed in table 1. After the 10 day expansion culture, CD34+ and CD133+ cell fractions for cells cultured on TCPS decreased to 7.8 ± 0.8% and 8.1 ± 0.8%. In contrast, cultures on aminated nanofibres prepared with the two-step and one-step methods yielded 25.3 ± 6.6% and 21.6 ± 4.1% of CD34+ cells, and 11.6 ± 2.1% and 13.8 ± 2.5% of CD133+ cells, respectively. For all three groups, nearly 100 per cent of CD133+ cells co-expressed the CD34 antigen.
Figure 7.
Representative density plots for the expression of CD133 and CXCR4 on CD34+ cell subsets after 10 days of culture in serum-free culture medium supplemented with SCF/Flt3L/TPO/IL-3 on (a) TCPS, (b) aminated PES nanofibres and (c) AEMA-grafted PES nanofibres.
Table 1.
Percentage of surface marker expression on cells expanded for 10 days on different nanofibre matrices and tissue culture polystyrene surface. Values were expressed as the mean ± s.d. (n = 3 independent experiments). Asterisk (*) denotes p < 0.05 when compared with TCPS control.
| cells expanded on various substrate | CD34+(%) | CD133+(%) | CXCR4+ (%) | CD34+/CXCR4+ (%) |
|---|---|---|---|---|
| unexpanded cells | 85.7 ± 3.3 | 97.4 ± 1.3 | ||
| tissue culture polystyrene (TCPS) | 7.8 ± 0.7 | 8.1 ± 0.8 | 38.0 ± 6.1 | 1.8 ± 2.0 |
| EDA-grafted nanofibres (two-step grafting) | 25.3 ± 6.6* | 11.6 ± 2.1* | 37.5 ± 4.5 | 3.9 ± 2.5 |
| AEMA-grafted nanofibres (one-step grafting) | 21.6 ± 4.1* | 13.8 ± 2.5* | 40.6 ± 7.2 | 8.0 ± 2.6* |
CXCR4 is known to be essential to HSPC homing following cell transplantation, and is essential to establish long-term haematopoiesis or recruitment to inflamed tissue [22,23]. Therefore, we also analysed the expression of CXCR4 on expanded cells. Although there was no significant difference in the proportions of expanded cells expressing CXCR4 (approx. 40%) on all surfaces, the average CD34+/CXCR4+ cell population was significantly higher (p<0.05) on cells expanded on AEMA-grafted nanofibres (8.0 ± 2.6%) than those expanded on EDA-grafted fibres prepared by the two-step process (3.9 ± 2.5%) and those on TCPS (1.8 ± 2.0%). However, owing to the lower total cell expansion obtained on AEMA-grafted nanofibres, the expansion of the CD34+CXCR4+ cell population (26 ± 5-fold) was similar to the EDA-grafted nanofibres (29 ± 9-fold); both were significantly higher than that obtained among cells expanded on TCPS surface (12 ± 2-fold).
3.5. Morphology of adherent haematopoietic progenitors on aminated nanofibres
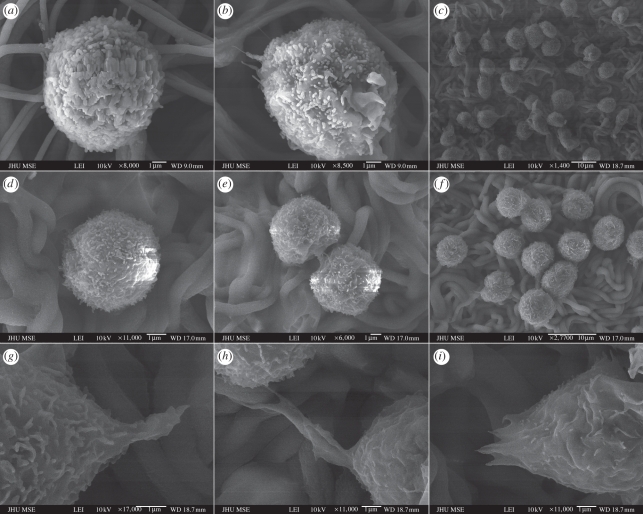
SEM imaging was used to observe the adherent haematopoietic progenitor population on aminated nanofibre matrices. On days 1, 3 and 10, samples were fixed and dehydrated, and their interactions with the nanofibre surface were analysed using SEM, as shown in figure 8. On days 1 and 3, fewer cells were visible under SEM imaging owing to low cell numbers and weaker cell adhesion; the attached cells on both sets of fibre matrices remained largely spherical (figure 8a,b). More pronounced difference was observed on day 10 with more cells and larger colonies observed on the cells cultured on EDA-grafted fibres showing extended filopodia (figure 8c,g,h,i), in comparison with AEMA-grafted nanofibres prepared with similar amine density. It was apparent that adherent haematopoietic progenitors interacted with the aminated nanofibres through these filopodia. In contrast, adherent cells were rarely observed on TCPS surface (data not shown).
Figure 8.
SEM images of haematopoietic progenitors cultured on (a–c) EDA-grafted nanofibre matrix, (d–f) AEMA-grafted nanofibre matrix and (g–i) the local magnification of (c) for 1 day (a,d), 3 days (b,e) and 10 days (c, f, h–i), respectively.
4. Discussion
Here, we reported the effect of nanofibre surface amine density on the ex vivo expansion efficiency of cryo-preserved human UCB CD133+ cells in serum-free culture, and the effect of conjugate chemistry on cell adhesion and proliferation. Despite the fact that only a small fraction of surface carboxylic groups were converted to primary amino groups, the surface amine density greatly influenced haematopoietic progenitor expansion. The percentage of CD34+ cells among expanded cells increased gradually with the surface amine density, but the overall expansion fold decreased. Therefore, the highest CD34+ cell expansion was obtained with intermediate surface amine densities (20–80 nmol cm−2).
It is worth noting that fibre diameters in the range of 200 nm to 1.2 µm with similar amine density (40–60 nmol cm−2) did not significantly influence the expansion outcome in terms of fold expansion of total cells, CD133+ cells and CD34+ cells, when tested under the same expansion culture conditions as described above (data not shown). Based on this observation, we attributed the differences in expansion outcomes primarily to the differences in surface amine density and conjugation chemistry on CD34+ cell expansion, even though the diameters of the EDA-grafted (approx. 608 nm) and AEMA-grafted nanofibres (approx. 784 nm) were about 18 and 51 per cent larger than unmodified nanofibres (approx. 517 nm), respectively.
To simplify the two-step amination process, we attempted to develop a method for single-step nanofibre amination, with the same amine functional groups on nanofibre surface. Our first attempt aiming at surface grafting of AEMA alone was unsuccessful, since insufficient amine density could be detected. Copolymerization of AEMA with AAc has allowed for successful AEMA grafting with amine densities comparable to the two-step process. Using this protocol, we anticipate that the fibre surface-grafted poly(AEMA-co-AAc) chains are random copolymers. This chain structure could be different from that obtained in the two-step process, in which selective conversion of carboxylic groups in the top surface layer of grafted PAAc to amines, thus yielding a heterogeneous distribution of amino groups on the fibre surface. Such a difference in amino group distribution likely influenced the cell–fibre interaction and resulted in the different haematopoietic progenitor expansion efficiencies observed between the two different aminated fibres (figure 6).
Although the mechanism remains an area of interest for further studies, it is intriguing that such a subtle difference in chain structure can significantly alter haematopoietic progenitor adhesion and expansion. In a previous study, it was hypothesized that the initial adhesion of haematopoietic progenitors may be mediated by CD34 antigen directly interacting with amine groups on the nanofibre surface since CD34 carries negative charges [17]. Primary amine groups presented on the nanofibre surface provided non-specific binding, promoting weak cell adhesion and enhancing the proliferation of CD34+ cells. Those studies also revealed that very late antigen-4 (VLA-4) and VLA-5 expression were not altered for both the adherent fraction and suspension fraction of the cells expanded on aminated nanofibres, whereas there was a significantly higher expression of CD34 among the adherent fraction than non-adherent fraction of cells in the same culture. This suggests that haematopoietic progenitor adhesion to the aminated fibres promoting the preservation of CD34+ cells was mediated through an integrin-independent mechanism [18].
Another possible contribution to the increased progenitor adhesion and proliferation on the aminated fibre matrices may come from medium components being selectively absorbed onto the positively charged surface. The surface amino groups have the potential to selectively enrich some haematopoietic cytokines from the culture medium. SCF has an isoelectric point of 5.76 and is negatively charged at physiological pH; this makes it a likely candidate for selective adsorption to an aminated fibre surface, likely yielding higher local concentrations at the cell–fibre interface. Such an adsorption may also mimic a bound form of SCF, which has been shown to be more potent than its soluble equivalent in promoting HSPC proliferation [24,25].
In summary, our results highlight the importance of surface conjugation chemistry and cell–substrate interaction in promoting haematopoietic progenitor adhesion and proliferation. The aminated nanofibre matrix may act to improve the preservation of CD34+ cell population among the expanded cells. However, the density and conjugate structure of surface amine groups significantly influence the expansion and phenotype maintenance of human CD34+ cells. The EDA-grafted aminated nanofibre matrix prepared by the two-step method offers the highest fold expansion of CD34+ cells compared with AEMA-grafted fibres and TCPS surface.
Acknowledgements
We thank Pauline Che of Johns Hopkins University for the technical assistance. This work was partially supported by Arteriocyte Inc. through a grant to Johns Hopkins University.
References
- 1.Wagner J. E., Kernan N. A., Steinbuch M., Broxmeyer H. E., Gluckman E. 1995. Allogeneic sibling umbilical-cord-blood transplantation in children with malignant and non-malignant disease. Lancet 346, 214–219. 10.1016/S0140-6736(95)91268-1 ( 10.1016/S0140-6736(95)91268-1) [DOI] [PubMed] [Google Scholar]
- 2.Rubinstein P., et al. 1998. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N. Engl. J. Med. 339, 1565–1577. 10.1056/NEJM199811263392201 ( 10.1056/NEJM199811263392201) [DOI] [PubMed] [Google Scholar]
- 3.Gluckman E. 2000. Current status of umbilical cord blood hematopoietic stem cell transplantation. Exp. Hematol. 28, 1197–1205. 10.1016/S0301-472X(00)00540-3 ( 10.1016/S0301-472X(00)00540-3) [DOI] [PubMed] [Google Scholar]
- 4.Ballen K. K. 2005. New trends in umbilical cord blood transplantation. Blood 105, 3786–3792. 10.1182/blood-2004-10-4125 ( 10.1182/blood-2004-10-4125) [DOI] [PubMed] [Google Scholar]
- 5.Rocha V., et al. 2001. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood 97, 2962–2971. 10.1182/blood.V97.10.2962 ( 10.1182/blood.V97.10.2962) [DOI] [PubMed] [Google Scholar]
- 6.Kleen T. O., et al. 2005. Recipient-specific tolerance after HLA-mismatched umbilical cord blood stem cell transplantation. Transplantation 80, 1316–1322. 10.1097/01.tp.0000188172.26531.6f ( 10.1097/01.tp.0000188172.26531.6f) [DOI] [PubMed] [Google Scholar]
- 7.Grewal S. S., Barker J. N., Davies S. M., Wagner J. E. 2003. Unrelated donor hematopoietic cell transplantation: marrow or umbilical cord blood? Blood 101, 4233–4244. 10.1182/blood-2002-08-2510 ( 10.1182/blood-2002-08-2510) [DOI] [PubMed] [Google Scholar]
- 8.Laughlin M. J., et al. 2001. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N. Engl. J. Med. 344, 1815–1822. 10.1056/NEJM200106143442402 ( 10.1056/NEJM200106143442402) [DOI] [PubMed] [Google Scholar]
- 9.Cairo M. S., Wagner J. E. 1997. Placental and/or umbilical cord blood: an alternative source of hematopoietic stem cells for transplantation. Blood 90, 4665–4678. [PubMed] [Google Scholar]
- 10.Dravid G., Rao S. G. 2002. Ex vivo expansion of stem cells from umbilical cord blood: expression of cell adhesion molecules. Stem Cells 20, 183–189. 10.1634/stemcells.20-2-183 ( 10.1634/stemcells.20-2-183) [DOI] [PubMed] [Google Scholar]
- 11.Heike T., Nakahata T. 2002. Ex vivo expansion of hematopoietic stem cells by cytokines. Biochim. Biophys. Acta 1592, 313–321. 10.1016/S0167-4889(02)00324-5 ( 10.1016/S0167-4889(02)00324-5) [DOI] [PubMed] [Google Scholar]
- 12.Li Y., Ma T., Kniss D. A., Yang S. T., Lasky L. C. 2001. Human cord cell hematopoiesis in three-dimensional nonwoven fibrous matrices: in vitro simulation of the marrow microenvironment. J. Hematother. Stem Cell Res. 10, 355–368. 10.1089/152581601750288966 ( 10.1089/152581601750288966) [DOI] [PubMed] [Google Scholar]
- 13.LaIuppa J. A., McAdams T. A., Papoutsakis E. T., Miller W. M. 1997. Culture materials affect ex vivo expansion of hematopoietic progenitor cells. J. Biomed. Mater. Res. 36, 347–359. () [DOI] [PubMed] [Google Scholar]
- 14.Holst J., et al. 2010. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat. Biotechnol. 28, 1123–1128. 10.1038/nbt.1687 ( 10.1038/nbt.1687) [DOI] [PubMed] [Google Scholar]
- 15.Feng Q., Chai C., Jiang X. S., Leong K. W., Mao H. Q. 2006. Expansion of engrafting human hematopoietic stem/progenitor cells in three-dimensional scaffolds with surface-immobilized fibronectin. J. Biomed. Mater. Res. A 78, 781–791. 10.1002/jbm.a.30829 ( 10.1002/jbm.a.30829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang X. S., Chai C., Zhang Y., Zhuo R. X., Mao H. Q., Leong K. W. 2006. Surface-immobilization of adhesion peptides on substrate for ex vivo expansion of cryopreserved umbilical cord blood CD34+ cells. Biomaterials 27, 2723–2732. 10.1016/j.biomaterials.2005.12.001 ( 10.1016/j.biomaterials.2005.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chua K. N., Chai C., Lee P. C., Tang Y. N., Ramakrishna S., Leong K. W., Mao H. Q. 2006. Surface-aminated electrospun nanofibers enhance adhesion and expansion of human umbilical cord blood hematopoietic stem/progenitor cells. Biomaterials 27, 6043–6051. 10.1016/j.biomaterials.2006.06.017 ( 10.1016/j.biomaterials.2006.06.017) [DOI] [PubMed] [Google Scholar]
- 18.Chua K. N., Chai C., Lee P. C., Ramakrishna S., Leong K. W., Mao H. Q. 2007. Functional nanofiber scaffolds with different spacers modulate adhesion and expansion of cryopreserved umbilical cord blood hematopoietic stem/progenitor cells. Exp. Hematol. 35, 771–781. 10.1016/j.exphem.2007.02.002 ( 10.1016/j.exphem.2007.02.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das H., Abdulhameed N., Joseph M., Sakthivel R., Mao H. Q., Pompili V. J. 2009. Ex vivo nanofiber expansion and genetic modification of human cord blood-derived progenitor/stem cells enhances vasculogenesis. Cell Transplant. 18, 305–318. 10.3727/096368909788534870 ( 10.3727/096368909788534870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin C., Ying L., Zhang P. C., Zhuo R. X., Kang E. T., Leong K. W., Mao H. Q. 2003. High density of immobilized galactose ligand enhances hepatocyte attachment and function. J. Biomed. Mater. Res. A 67, 1093–1104. 10.1002/jbm.a.10033 ( 10.1002/jbm.a.10033) [DOI] [PubMed] [Google Scholar]
- 21.Kakabakos S. E., Tyllianakis P. E., Evangelatos G. P., Ithakissios D. S. 1994. Colorimetric determination of reactive solid-supported primary and secondary amino groups. Biomaterials 15, 289–297. 10.1016/0142-9612(94)90054-X ( 10.1016/0142-9612(94)90054-X) [DOI] [PubMed] [Google Scholar]
- 22.Sharma M., Afrin F., Satija N. K., Thripati R. K., Gangenahalli G. U. 2010. SDF-1/CXCR4 Signaling: indispensable role in homing and engraftment of hematopoietic stem cells in bone marrow. Stem Cells Dev. 20, 933–946. ( 10.1089/scd.2010.0263) [DOI] [PubMed] [Google Scholar]
- 23.Peled A., et al. 1999. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 283, 845–848. 10.1126/science.283.5403.845 ( 10.1126/science.283.5403.845) [DOI] [PubMed] [Google Scholar]
- 24.Doran M. R., Markway B. D., Aird I. A., Rowlands A. S., George P. A., Nielsen L. K., Cooper-White J. J. 2009. Surface-bound stem cell factor and the promotion of hematopoietic cell expansion. Biomaterials 30, 4047–4052. 10.1016/j.biomaterials.2009.04.043 ( 10.1016/j.biomaterials.2009.04.043) [DOI] [PubMed] [Google Scholar]
- 25.Miyazawa K., Williams D. A., Gotoh A., Nishimaki J., Broxmeyer H. E., Toyama K. 1995. Membrane-bound steel factor induces more persistent tyrosine kinase activation and longer life span of c-kit gene-encoded protein than its soluble form. Blood 85, 641–649. [PubMed] [Google Scholar]