Abstract
Stem cell therapy is an emerging technique which is being translated into treatment of degenerated tissues. However, the success of translation relies on the stem cell lineage commitment in the degenerated regions of interest. This commitment is precisely controlled by the stem cell microenvironment. Engineering a biomimetic three-dimensional microenvironment enables a thorough understanding of the mechanisms of governing stem cell fate. We review the individual microenvironment components, including soluble factors, extracellular matrix, cell–cell interaction and mechanical stimulation. The perspectives in creating the biomimetic microenvironments are discussed with emerging techniques.
Keywords: stem cell, microenvironment, stem cell niche, microscale technologies, bioprinting, optical tweezers
1. Introduction
Since the potential of stem cells for therapeutic application was discovered by Evans and Kaufman [1], stem cells have been anticipated to treat cancer, type 1 diabetes, Parkinson's disease, Huntington's disease, coeliac disease, cardiac failure, muscle damage, neurological disorders and many other conditions [2]. Stem cell-based therapies may represent the next generation of biologics to treat or cure many diseases for which adequate therapies do not yet exist. Currently, over 500 cell-therapy-based companies worldwide [3] are translating the experimental research into clinical therapeutics. The example of a re-engineered trachea transplanted into a patient in Spain in 2008 has demonstrated the great opportunities for applying stem cells to the repair of degenerative tissues [4].
Nevertheless, before stem cell-based therapies are applied in clinics, stem cell behaviour upon transplantation should be precisely controlled and the mechanisms of stem cell interactions with its microenvironment need to be elucidated [2]. Upon transplantation, stem cells and their derived lineages experience a multitude of biochemical, structural, mechanical and stimulatory cues that influence cell behaviour [5]. For example, a fundamental understanding of the implications of the interplay between stem cell microenvironment components or stem cell niche factors (growth factors, cell–cell contact and cell–matrix interaction) and external forces will enable better control of therapeutic cells and effective regeneration of functional tissues [6].
The unique function of stem cells inside the human body can be achieved possibly by the specialized, three-dimensional microenvironment that surrounds them in native tissues. The microenvironment components have to be placed to their anatomical and functional locations; the interactions between those components are crucial for regulating stem cell functions [7]. When stem cells are removed from their microenvironments, their functionality, phenotype and responses to environmental cues can often be altered. For example, muscle stem cells grown on the standard tissue culture plastic support have been found to lose their regenerative potential [8,9]. This poses great uncertainty in the clinical application of stem cells when the appropriate characterization, manipulation, proliferation and physio-chemical environment control are not adequately performed [10,11]. On the other hand, the risk associated with tumorigenesis has been demonstrated by the case in which a 13 year old boy with a hereditary neurodegenerative disease developed a multifocal brain tumour after being treated three times with stem cell implantations in Moscow [12].
The potential risks highlight the gap that still exists between our current knowledge of stem cell performance in vivo in the niche and its intractability outside of that niche. To regulate stem cell differentiation into the right phenotype, an appropriate microenvironment should be created in a precisely controlled spatial and temporal manner [13]. The creation of such a microenvironment requires applying engineering design principles and emerging technologies to mimic the complex three-dimensional biological structure. Engineered biomimetic microenvironments have been designed to regulate the balance between stem cell differentiation and self-renewal. Comprehensive reviews have addressed the underlying biological principles driving the biomimetic microenvironments [6,14,15]. In contrast, this review highlights the progress in creating microenvironments from an engineering perspective. This paper will discuss engineering methods for identifying and controlling the release and delivery of soluble factors, analysing and mimicking the extracellular matrix (ECM), probing cell–cell interactions and applying mechanical stimulation. The emerging techniques for creating biomimetic microenvironments are highlighted.
2. Controlling stem cell fate
Inside the human body, stem cells are surrounded by niche cells and embedded in an ECM, which defines the geometric configuration, signalling pathways and biomechanical characteristics of the microenvironment. Stem cell functions are determined by a variety of biochemical, structural, hydrodynamic, mechanical and electrical cues at spatial and temporal levels as shown in figure 1. The stem cell niche consists of a group of supporting cells, ECM and soluble environment factors at the specific sites. Cells respond to their immediate microenvironment wherein remodelling consequently occurs, via homotypic or heterotypic interactions with neighbouring cells, and with the tissue matrix. The components contributing to the stem cell niche can be classified into: (i) soluble factors secreted by the stem cells or niche cells, present in the surrounding tissue or culture media; (ii) ECM or cell substrate; (iii) direct cell-to-cell interactions to elicit cellular signals; and (iv) external mechanical and electrical forces such as fluid-induced shear stress, dynamics tensile and compressive loading.
Figure 1.
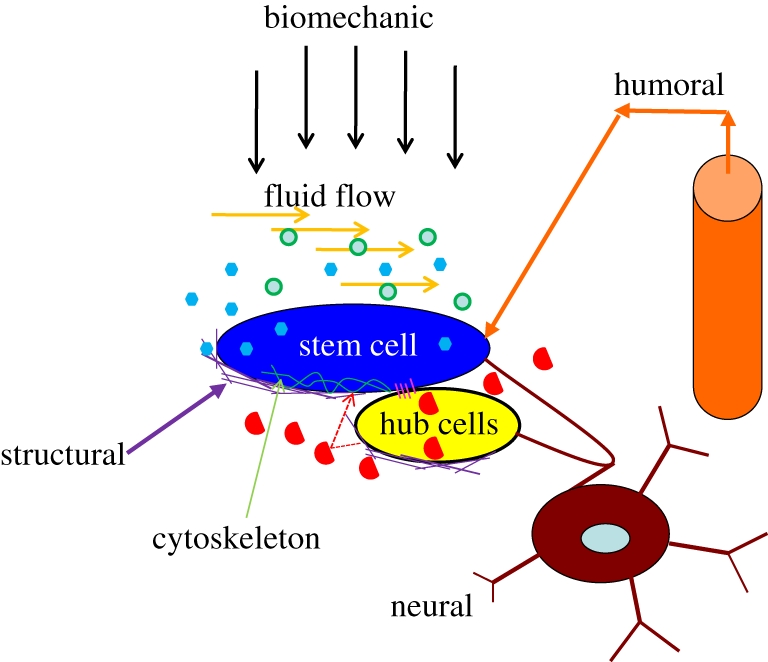
Stem cell microenvironment. Elements are identified for regulating the system of a stem cell, including the constraints of the architectural space, physical engagement of the cell membrane, signalling interactions, neural input and metabolic products of tissue activity. Green circles, oxygen soluble; blue hexagon, factors autocrine; red semi-circles, soluble factors paracrine; pink lines, cell–cell contact; purple lines, ECM.
The regulation of stem cell activities by its microenvironment has been validated in a number of different systems, such as germline stem cells from Caenorhabditis elegans and Drosophila melanogaster, and haematopoietic and neural stem cells from mammalian organisms, as shown in table 1. These elements function as a physical anchor to constrain stem cells, to adjust the concentration of extrinsic factors, and to regulate the intracellular signalling pathways. Thus, after cell division, one daughter cell is maintained in the niche while the other one migrates out of the niche to the target site and differentiates into a functional mature cell. Although progress has been made to elucidate the mechanisms for regulating stem cells in their microenvironment in different systems in vivo, the highly dynamic and complex structures of stem cell niches will be revealed through elegantly engineering biomimetic microenvironments in vitro. More importantly, the engineering system, through accurately controlling physical and molecular interactions, enables directed regulation of stem cell behaviour, which extends our capabilities in engineering functional tissue substitutes in vitro. The engineering approach for mimicking the four key components of the stem cell microenvironment will be discussed below.
Table 1.
Stem cell niche components in different systems in vivo. The references for the table can be found in [7,16,17] and their cited papers.
| stem cell | species | location | niche cells/support cells | extracellular matrix | soluble factors | cell–cell contact |
|---|---|---|---|---|---|---|
| germ stem cell | D. melanogaster | testis/ovary | hub/cap cells | basement membrane | BMP and JAK/STATs pathway | gap junction, adherens junction |
| germ stem cell | C. elegans | gonad | distal tip cells | Wnt pathway | notch signalling | |
| neural stem cell | mammalian | brain | endothelial cells, astrocytes | physical contact structure; tenascin C; N-sulphate heparin sulphate proteoglycan; integrin α6β1 for laminin. | BMP pathway, growth factors, such as EGF, FGF2 | tight junction; adherens junction; notch signalling |
| haematopoietic stem cell | mammalian | bone marrow (osteoblastic or vascular) | osteoblast, osteoclasts, mesenchymal progenitors, reticular cells, or endothelial cells | osteopontin; integrin α4β1 for fibronectin | Wnt pathway; O2, | notch signalling |
2.1. Soluble factors
Soluble factors have been demonstrated to regulate stem cell growth and differentiation, including growth factors, morphogenetic factors, cytokines, enzymes and small cell-permeable molecules, such as basic fibroblast growth factor (bFGF), members of the transforming growth factor-β family (TGF-β), vascular endothelial growth factor (VEGF), bone morphogenetic factor (BMP), vitamin C, sodium pyruvate, retinoic acids (RAs) and other small molecules. For instance, bFGF plays a significant role in several pathways, including Akt [18] and mitogen-activated protein kinase (MAPK) for a number of stem cell lineages [19], and all-trans RA is a strong differentiation agent to enhance expression of the neural crest [20] and reduces mesodermal differentiation [21]. The nutrient components including oxygen are also considered as soluble factors in the biomimetic system.
These factors added to the cell culture system or secreted by stem cells or niche cells are potent in their effects on stem cell fate. For example, cytokine leukaemia inhibitory factor (LIF) is able to maintain the self-renewal capacity of embryonic stem cells (ESCs) in the presence of serum without embryonic fibroblast feeder cells [22]. It has also been noticed that the combined effect of these factors can vary substantially. For instance, co-presence of BMP4 and LIF in serum-free media promotes ESC self-renewal, while BMP4 alone induces ventral mesodermal differentiation [23].
The above addition method in the cell culture media provides clues to the biological functions of soluble factors in the microenvironment; however, it cannot control the delivery of these factors in a spatial and temporal way. Several approaches have been employed to design the spatial concentration gradient of soluble factor(s) of interest. Among these, microfluidic technology is an emerging method to generate molecular gradients in soluble form through laminar flow within the microfluidic channel. Microfluidics enables massive and parallel manipulation of a tiny volume of solutions containing multiple soluble factors or combinations in a multi-dimensional space to determine stem cell function. This approach is exemplified by a microfluidic alginate hydrogel controlling release of soluble factors in three-dimensional microenvironments [24]. A lithographic technique was used to build functional networks within a calcium alginate hydrogel seeded with cells. Temporal and spatial control of the distribution of non-reactive solutes and reactive solutes (metabolites) within the bulk of the scaffold has been demonstrated. This approach can control the chemical environment on a micrometre scale within a macroscopic scaffold and is useful in engineering complex tissues.
In the biological system, these soluble factors are often bound to ECM to slow their diffusion and fine-tune their local concentrations and gradients. The biomimicking approach is to conjugate or bind soluble factors to the surface of biomaterials such as ECM, three-dimensional scaffolds, hydrogels, macro- or microporous foams and woven and non-woven fabrics in a spatial and temporal manner. Soluble factors released from biomaterials are normally controlled by diffusion, cell-mediated proteolysis and external physical stimulation. For example, growth factors can be localized in multicomponent, spatially patterned and photo-cross-linked hydrogels [25]. Human ESCs were encapsulated in a dextran-based hydrogel with two immobilized regulatory factors, a tethered (arg-gly-asp) RGD peptide and microencapsulated VEGF165. The fraction of cells expressing VEGF receptor KDR/Flk-1, a vascular marker, increased up to 20-fold, which demonstrated that this approach can be used to induce vascular differentiation of human ESCs [26].
2.2. Extracellular matrix
The ECM forms a complex architecture containing proteins, polysaccharides and proteoglycans, and these molecules constantly undergo dynamic change owing to assembly, remodelling and degradation events. The ECM not only provides architectural guides for tissue development, but also defines and maintains cellular phenotype and drives cell fate decisions. At a molecular level, the ECM has been demonstrated to influence stem cell fate by mechanical traction forces and cell adhesion. When cells anchor to the ECM, they exert inward-directed traction (tensional) forces generated by the contractile cytoskeleton because of adhesion to substrate surfaces. The deformation induced by the traction force activates transmembrane integrin signal pathways that affect the cell fate [27]. The magnitude of these traction forces depends on the mechanical properties of the ECM, such as stiffness. At cell adhesion sites, stem cells adhered to the specific component of the ECM via integrins, cadherins and discoidin, etc., activate unique signalling pathways. The adhesion also determines orientation of the stem cell division plane and cell morphology through the constraints imposed by the surrounding ECM, and consequently influences stem cell self-renewal and differentiation.
2.2.1. Identification of extracellular matrix components
The identification of ECM molecules and their roles in stem cell regulation are critical steps towards creating biomimetic microenvironments. Combinations of specific molecular interactions between numerous isoforms, ratios and geometrical arrangements of collagen, elastin, proteoglycans and adhesion proteins (such as fibronectin and laminins) for identifying and mimicking the natural ECM composition are extremely challenging. Furthermore, the composition of the ECM is normally tissue specific. For instance, laminin is the major component in basement membranes, while stromal ECMs in connective tissues consist mainly of collagen [28]. The ECM composition is also dynamic, varying as the tissue is being developed. Fibronectin-rich ECM can promote proliferation of immature capillaries, while as the capillary matures, the composition of the ECM changed to become laminin-rich [29]. To screen an ECM component or combinations, novel methods, such as robotic spotting [30] and microfabricated wells [31], have been developed to make protein arrays. In a single experiment, typically more than 1000 combinations of ECM molecules can be screened simultaneously [32]. For example, Flaim et al. [30] prepared an ECM microarray that combined five different ECM molecules in varying ratios and demonstrated that combinations of ECM components were more effective than single ECM components for differentiating mouse embryonic stem cells (mESCs) into liver cells. Anderson et al. [33] developed arrays consisting of 192 unique combinations of ECM. Signalling factors were printed onto slides containing a thin coating of polydimethylsiloxane, and ECM molecules were identified to maintain the progenitor state, and to guide progenitor differentiation towards myoepithelial and luminal lineages.
Functional components of ECM in vivo are summarized in table 2. However, natural ECMs are variable in their composition and mechanical properties. Naturally derived or synthetic gels or scaffolds shown in table 2 have been tailored to mimic specific ECMs for the creation of well-controllable and reproducible microenvironments. Naturally derived three-dimensional structures can be from protein-based (collagen, fibrin, silk, gelatin and Matrigel), or from polysaccharide-based (hyaluronan, alginate, agarose and chitosan), or protein- and polysaccharide-combined decellularized matrices. Synthetic materials include poly(ethylene glycol) (PEG), poly(ethylene oxide) (PEO), polyacrylamide, poly(vinyl alcohol) (PVA), poly(l-lactic acid) (PLA), poly(glycolic acid) (PGA), poly(l-lactic-co-glycolic acid) (polyPLGA), poly(hydroxyl ethyl mathacrylate) (PHEMA), poly(anhydride) and functional peptide self-assembly three-dimensional structures. ECM-derived peptides or protein fragments need to be covalently linked to polymer backbones via their hydroxyl-, carboxyl- or amino-termini to improve their biological functions.
Table 2.
Functional ECM components and the biomimetic counterparts.
| ECM components in vivo | functions in vivo (see [34]) | biomimetic materials |
|
|---|---|---|---|
| collagen: fibrillar (I, II, III, V, XI, XXIV, XXVII) | forms structural cues; controls stiffness, resists tension; binds adhesion factors; binds some growth factors; allows amoeboid migration in porous structure | naturally derived biomaterials | collagen derivatives; fibrin derivatives; porous gelatin-derived; silk derived; Matrigel; agarose-based; chitosan-based; alginate-based; hyaluronan (HA)-based; de-cellularized matrices |
| collagen: non-fibrillar (I–XXVII, except fibrillar types) | serves many ECM and cell-adhesion functions, including binding other ECM proteins and proteoglycans to aid ECM organization and stability, aiding fibrillar collagen formation; forming networks as barriers for solute transport; modulating cell migration and proliferation | ||
| fibrin | functions as structural matrix in wound healing; controls stiffness, resists tension; binds adhesion molecules; easily degraded and remodelled | ||
| elastin | provides elastic recoil | synthetic and biosynthetic materials | poly(ethylene glycol) (PEG); poly(ethylene oxide) (PEO); polyacrylamide; poly(vinyl alcohol) (PVA); poly(l-lactic acid) (PLA or PLLA); poly(glycolic acid) (PGA); poly(l-lactic-co-glycolic acid) (PLGA); poly(hydroxyl ethyl mathacrylate) (PHEMA); poly(anhydride); functional peptide self-assembly three-dimensional structures |
| proteoglycans | resists compression; hinders water transport; hinders macromolecular transport; binds growth factors and chemokines; electrokinetic effects | ||
| matricellular proteins | intermediate, weak adhesion | ||
2.2.2. Ligand presentation of extracellular matrix
Apart from the composition of natural ECMs, the surface and mechanical properties, such as ligand density, surface nanotopography and substrate elasticity also influence stem cell fate. The RGD peptide in various ECM molecules such as fibronectin and vitronectin is the prevailing adhesive ligand because the binding of most cells to ECM is dependent on RGD density [35]. Other adhesive peptides, such as the YIGSR and IKVAV peptides of laminin and the VPGIG sequence of elastin, have also been studied [36,37]. Cell behaviour can be influenced by the coexistence of multiple peptides. For example, a spacing of 4 nm between RGD and the synergy site pro-his-ser-arg-asn (PHSRN) resulted in an increase in indicators of osteoblastic cell function, such as metabolic activity and alkaline phosphatase production, while showing a decrease in ECM production [38]. Although some adhesive ligands have been integrated with PEG-based hydrogels [39], the incorporation of ligands into artificial ECMs and control of the distribution of those ligands on the surface in a spatio-temporal manner are still very challenging.
2.2.3. Extracellular matrix nanotopography
Nanotopography of the ECM has a great impact on cell adhesion. Not only the scale of topography (5 nm to micrometre scale) but also the type of ordered topography, such as ridges, steps, grooves, pillars and pits can modulate cell behaviour [40]. Ruiz & Chen [41] micropatterned fibronectin in various geometries (circle, square, rectangle and ellipses), and measured the tractive force experienced by human mesenchymal stem cells (hMSCs) on these fibronectin patterns. They concluded that cells on the concave surface experience greater tractive forces than those on convex surfaces, and the forces direct cells into osteogenic instead of adipogenic lineages. The tractive forces also guide cytoskeletal formation, hence, to determine the cell shape. Evidence has suggested that physical control of cell shape alone can act as a potent regulator of stem cell fate [42]. For example, single mesenchymal stem cells (MSCs) cultured on small micropatterned islands adhered poorly, displayed a rounded morphology and acquired an adipogenic fate, whereas those cultured on larger islands adhered strongly, spread out, exhibited increased focal adhesion and cytoskeletal reorganization, and acquired osteogenic fate [43]. Mechanisms for the sensing of matrices as well as for stem cell trafficking are still to be clarified. The ability of cells to recognize the nano-scale topographic features requires developing synthetic ECMs with nano-structure. Self-assembly of amphilic peptides and electrospinning have been used to form nanofibres in biomimetic cell substrates [44,45].
2.2.4. Extracellular matrix mechanical stiffness
The mechanical stiffness or elasticity of the ECM has a major influence on cell behaviours, such as migration, apoptosis and proliferation. Cells have been attached to various gel matrices with controllable elasticity by varying degrees of cross-linking and non-limiting ligand density, and most of the cells have been found to anchor more strongly to stiff substrates, building focal adhesions and forming actin–myosin stress fibres [46]. MSCs cultured on the rigid substrate preferentially differentiated into osteoblasts instead of adipocytes [47], while muscle stem cells on soft hydrogel substrates at a similar elasticity of muscle maintained self-renewal in vitro [43].
2.3. Cell–cell interactions
Cell–cell interactions have been widely studied in vivo in different systems as shown in table 1. They not only act as a physical anchor to constrain stem cells in a defined space, but also to provide instructive secreted signalling cues or to send signals through transmembrane proteins or bound matrix proteins. Interactions between cell populations can be divided into homotypic or heterotypic interactions. Homotypic interactions are between stem cells themselves, while heterotypic interactions are between stem cells and their neighbouring cells. Stem cells communicate with niche cells through the tight junctions, adherens junctions, notch signalling pathways and gap junctions [7,16]. Signalling molecules generated by the niche cells pass the channels between the stem cell and the niche cell to regulate the stem cell behaviour. Alternatively, they interact with each other via paracrine signalling, and diffuse soluble signal molecules from the neighbouring cells to the stem cells, such as Wnt, BMP, JAK/STAT pathways [7,16]. In addition to the extensive in vivo study of cell–cell interactions, biomimetic approaches for these interactions have been explored to induce stem cell differentiation [48–51] and promote expansion and self-renewal [52,53].
A common approach to investigating homotypic interactions is to plate cells with different cell seeding density [53]. However, as the cell density is not able to be controlled locally, it is difficult to differentiate the effect of paracrine signalling from actual physical contact between the cells in the culture dishes. The effects of heterotypic interactions are usually investigated by placing two cell types in close contact or co-culturing them [49–51]. Nevertheless, particular signalling molecules generated by niche cells in co-culture methods may be neglected. A smart design to distribute cells in a micropattern allows for control of the cell–cell contact between cells adherent on RGD microdomains [49]. In this way, the interference of soluble factors or cell seeding concentration can be overcome. Traditionally, the cell–cell interactions occur in two-dimensional cell culture systems, which may not be able to generate the same channels or signalling molecules as those in three-dimensional in vivo.
The study of cell–cell interactions in three-dimensional microenvironments in vitro is still a great challenge as it is difficult to precisely control cell–cell contact in a spatial dimension. To overcome this challenge, physical micromanipulation techniques have been used to accurately manipulate, move and organize cells in three-dimensional and to control cell–cell contact. For example, bioprinting can be used to generate spatially oriented co-culture by printing layer-by-layer bioactive molecules with a well-defined gap [54]; laser-deposited human umbilical vein endothelial cells (HUVECs) self-assembled into vascular structures, while a combination of primary rat hepatocytes heterogeneously patterned with HUVECs resulted in tubular structures [55]; mammalian cells were electropatterned within three-dimensional hydrogels with dielectrophoretic forces; and large numbers of multicellular clusters of precise size and shape were formed in three-dimensional on one focal plane [56]. The cell–cell interactions were affected by clusters of various sizes: smaller clusters influenced the biosynthesis of bovine articular chondrocytes, while larger clusters produced smaller amounts of sulphated glycosaminoglycan per cell.
2.4. Mechanical stimulation
Mechanical forces play an important role in cell attachment, spreading, proliferation, migration and differentiation. External applied forces not only directly transmit to cells, but also change the relative distance between cells, ECM components and soluble factors. Several molecular bases, not exclusive, for mediating cellular activity include: (i) activating ion channels by stretching; (ii) inducing conformational change of proteins or unfolding of ECM proteins, leading to changes in protein activity; (iii) inducing conformational change of cytoskeletal elements such as filaments, cross-linkers or motor proteins; (iv) direct action on gene expression owing to forces transmitted to the nucleus; and (v) changing the intercellular space by stretching or compression, leading to changes in the local concentration and gradient of secreted signal molecules, and ECM ligand presentation and nanotopology [15,57]. Local variations of ECM elasticity at the micro-scale result in different abilities to resist cell traction forces, and thereby regulate cell and tissue development in vivo. It is difficult to precisely determine the biomechanical and biochemical mechanisms in vivo [27] owing to the above inter-connected molecular changes and also dynamic cellular mechanical properties. Biomimetic systems have, therefore, been developed to characterize stem cell responses to more highly controllable mechanical stimulation and to determine the biophysical mechanisms and biochemical signal transduction pathways [58]. Biomimetic mechanical stimulation includes deformation loading (compression, stretching and tension) and fluid-induced forces (pressure or shear stress).
2.4.1. Mechanical strains
Mechanical strain from applied loading, either cyclic or uniform biaxial, can direct specific stem cell lineages. For example, cyclic stretch has been demonstrated to commit MSCs to a myogenic phenotype [59] and mESCs to a vascular smooth muscle cell phenotype [60]. Cyclic compression is able to alter MSC phenotype as well. MSCs subjected to dynamic compression or hydrostatic pressure showed increased chondrocyte lineage differentiation and enhanced ECM deposition [61,62]. Mechanical strain can also increase proliferation and inhibit differentiation in mouse and human ESCs [60,63] as well as foster cell alignment with respect to the direction of strain [64].
2.4.2. Fluid-induced shear stress
Fluid-induced shear stress (FISS) has a significant impact on the fate of stem cells as well. In two-dimensional systems, FISS induces osteogenic differentiation of stem cells by activating multiple intracellular signalling pathways, such as the signalling pathways of nitric oxide (NO)/cyclic guanosine monophosphate-dependent protein kinase (PKG) [65], prostaglandin E2 (PGE2)/cyclic adenosine monophosphate-dependent protein kinase (PKA) [66], Ca2+/calmodulin-dependent protein kinase (PKC) [67] and MAPK [68]. Interestingly, while expression levels of osteogenesis-related genes, alkaline phosphatase (ALP) [69], osteopontin (OPN) [67], collagen type 1 (Coll1), PGE2 and NO [66] have been promoted in multiple studies, some other studies showed statistically insignificant changes in the levels of ALP [70], Coll1 [71] and PGE2 [72]. The contradictory conclusions may be due to the variation in cell types used, culture conditions, and experimental platforms.
Stem cells may also be differentiated towards endothelial cell fate in response to shear stress via the VEGF signalling pathway. Most of the studies showed fluid shear stress can direct ESCs or endothelial precursors towards positive endothelial differentiation [73]; however, shear force alone seems insufficient to differentiate adult MSCs or human adipose-derived stem cells into endothelial cells [74].
In three-dimensional culture systems, both osteogenic and angiogenic differentiation have been induced by FISS for stem cells in a range of 1 × 10−4 to 1.2 Pa, and the majority of work was focused on in a range 0.01–0.05 Pa [75], which is at least an order of magnitude below the average shear stress for two-dimensional culture and up to two orders of magnitude lower in some cases. These values are also orders of magnitude below those expected to cause differentiation in vivo. This may be due to morphological deformation when attaching to the three-dimensional surface. However, these findings are not only dependent on the magnitude of FISS, but also on the soluble environment, cell–ECM interactions, three-dimensional surface topology and material stiffness, as these will alter cell focal adhesions and stem cell colony shape. For example, a low flow rate resulted in cell clumping on aggregation supporting embryo body culture owing to a lower rate of mass transfer to cells [76]. More synergistic effects are discussed in the next section.
2.5. Synergistic effects
The above individual niche factors rarely function alone in the stem cell microenvironment, which further complicates the elucidation of the mechanisms of controlling stem cell fate. For instance, soluble factors first diffuse through the ECM and then reach the cell membrane surface. The ECM structure may block or delay the delivery of these soluble factors. It is of great interest and importance to investigate the synergistic effects by optimally combining those niche factors. However, this will require multi-factorial experimental designs and sophisticated statistical analyses.
The synergistic effects can be caused by a combination of the same or different categories of niche factors. For example, MSCs were exposed to a combination of three stresses: a pulsatile pressure, radial distension and FISS. They exhibited a similar mechanosensitive response to that of endothelial cells. However, gene expression results show the cells expressed greater levels of smooth muscle cell-associated markers [77]. This result highlights the synergistic effects of physiological flow and stretch on cell behaviour. However, this also increases the complexity of characterizing the individual mechanical force. A mathematical modelling approach may be integrated with the experimental methods, so the synergistic effects of biological phenomena can be quantitatively correlated with the individual contributing factors. Another example from Salvi et al. [78] combined the mechanical stimulation and the ECM topology. They investigated the effect of FISS when MSCs were seeded and cultured on randomly distributed nano-island surfaces with varying island heights. Their observation suggests that specific scale nanotopographies provide an optimal milieu for promoting stem cell mechanotransduction activity. The mechanical signals and substrate nanotopography may synergistically regulate cell function.
The soluble factors were also combined with mechanical stresses to determine stem cell fate. A combination of an electrical stimulation and soluble factors induced synergistic osteogeneric differentiation of MSCs, while use of electric stimulation alone fails to differentiate MSCs [79]. Endothelial progenitor cells were differentiated into endothelial cells by using a combination of VEGF and shear stress [80], while chrondrogenic differentiation of MSCs was achieved by TGF-β3 and a hydraulic pressure [81]. Once the mechanisms of the synergistic effects are well characterized, strategies to optimize the combination of the niche factors can be formulated to differentiate stem cells into the lineages of interest.
3. Perspectives and emerging technologies
From the above discussion, it demonstrates that cellular activities are mediated by a variety of molecular, structural, hydrodynamic, mechanical and electrical cues and combinations in a spatial and temporal manner. Before stem cells can be used for clinical applications, the interplay between these cues must be understood. It is strongly recommended that an engineered biomimetic three-dimensional system should be developed to closely mimic the human system to correctly promote differentiation or proliferation of stem cells. Such an elaborate system would range from nano-scale to millimetre-scale, involve multiple cues and combinations, and require multi-disciplinary collaboration. Four future trends in designing the biomimetic system, which can be envisaged are reported here.
3.1. Synthetic biomaterials as instructive extracellular microenvironments
A biomaterials approach has been employed to identify the composition of the stem cell ECM and to synthesize biomimetic three-dimensional scaffolds or hydrogels [82]. However, well-defined synthetic three-dimensional systems are far more challenging and require mimicking the mechanical and biological properties of the ECM, such as ligand presentation, nanotopography, substrate elasticity, growth-factor binding, degradation and remodelling. Furthermore, currently three-dimensional cell culture structures are normally prepared without cells. When seeding cells onto the three-dimensional system, cells are not uniformly distributed. Ideally cells are homogeneously suspended in the polymer solution, which will then form the three-dimensional materials. A cell-compatible and highly specific cross-linking reaction will be required to maintain cell viability. DeForest's group [83] has prepared a hydrogel from its monomers in the presence of cells using a ‘click’ reaction, so that the cells were encapsulated in the gel. When irradiated with light, the chemical group reacted in a second click reaction with molecules acting as signals that monitor or dictate the behaviour of the encapsulated cells. The in situ synthesis and remodelling of functional biomaterials to incorporate clusters of ligands and growth-factor binding sites will enable cells to evenly distribute within the three-dimensional network.
3.2. Microscale technologies for fine tuning and screening niche factors
Microscale technologies have been extensively used to examine the three-dimensional microenvironment in vitro. They have two important features for stem cell studies. First, they allow fine tuning of stem cell niche factors at the scale of from 1 µm to 1 cm. Examples of employing microscale technologies are generating soluble factor concentration gradients in the laminar-flow microfluidic channel; creating micropatterning for confinement of individual cells to control the cell shape; and designing microbioreactors or microwells for investigating cell–cell contact and mechanical stimulation. Secondly, microscale technologies offer high-throughput platforms because they consume a minute amount of volume and operate automatically by integrating with a robotic liquid dispensing system. Owing to the complexity of the factors affecting stem cell differentiation, it is essential to analyse the stem cell microenvironment in a high-throughput manner.
Microscale technologies for stem cell research and tissue engineering have been excellently reviewed by Khademhosseini et al. [84] and Toh et al. [85]. One can access the two reviews for more information.
3.3. Micromanipulation techniques for probing cell–cell interactions in three-dimensional architecture
Three-dimensional manipulation techniques can be valuable for precisely controlling the three-dimensional architecture and cell attachment, and probing cell–ECM interaction and cell–cell interaction. These techniques include the use of contact-dependent atomic force microscopes, non-contact optical tweezers, dieletrophoretic traps [56] and magnetic tweezers. Optical tweezers, for instance, have emerged as an essential tool for manipulating single biological cells and performing sophisticated biophysical/biomechanical characterizations without any contact [86]. The ability to manipulate cells in a three-dimensional architecture will help to probe cell–cell and cell–ECM interactions in a biomimetic system.
Another way to probe cell–cell interactions is by bioprinting. Bioprinting is a rapid prototyping strategy to produce three-dimensional structures through computer-aided, localized deposition of multiple types of cells and biomaterials, which can create three-dimensional cellular microenvironments to mimic the natural physiological, geometrical, mechanical and regulatory cues. Currently, there are two common types of bioprinting: ink-jet printing (IJP) or laser-based direct writing (LBDW). By applying the LBDW technique, hMSCs have been directly written on a Matrigel-coated substrate using a 1064 nm wavelength laser. Two-dimensional patterns consisting of one or more cell types were generated and the cell viability was around 90 per cent [87]. Similarly, mESCs were deposited into defined arrays of spots, and stem cell pluripotency was maintained [88].
This technique can also be integrated with CAD/CAM at a single cell resolution using a minute amount of volume. For example, HUVECs were direct-written in a three-line pattern on a collagen-coated surface. The media was aspirated and a collagen gel (0.5 mm high) was carefully layered on top of the first pattern. An additional pattern of three lines of cells was written and oriented perpendicularly to the first pattern on top of the gel to create a three-dimensional pattern. This technique also demonstrates the ability to place one cell on top of another in a true three-dimensional pattern [89], offering the potential to investigate the cell–cell interactions in three-dimensional. Bioprinting can also precisely construct the ECM layer-by-layer to form a well-defined biomimetic anchor for stem cells.
3.4. Mathematical modelling for quantitative analysis of cell behaviour and microenvironment parameters
After an engineered three-dimensional microenvironment is built, it is essential to assess the performance of the microenvironment by cellular responses to individual components including cell growth, migration, differentiation or apoptosis, as well as to characterize the parameters associated with the engineered microenvironment. Cellular activities depend not only on the presence or absence of cues, but also on their quantity, their spatial arrangements and the temporal order in which they are presented [90]. Parameters associated with the microenvironmental cues include the substrate elasticity, soluble factor concentration and distribution inside the microenvironment, mechanical stress on the cells and the ECM geometrical information (nanofibre size, porosity, surface chemistry, etc.). These parameters are unfortunately difficult to achieve. Computational models have been developed to characterize the engineering microenvironment as well as dynamic change in cellular activities.
The flow-related parameters, such as oxygen, soluble factor concentration and shear stress, can be characterized by computational fluid dynamics, which reveal the local flow information in the microenvironment in a complex geometry [90–94]. The dynamic nature of the cellular activities can be described by cellular automata models. Both continuous [95,96] and discrete [97,98] models have been employed to capture tissue level dynamics with varying levels of success. Many of the latter type are emerging to explicitly model a large number of individual cells, which behave within a certain biological framework with respect to cell adhesion, division and migration [99]. Future models will combine the above two models, accounting for force-dependent molecular switches, signal transduction pathways, cell deformation and tension and reciprocal interactions with the microenvironment [100].
Quantitative prediction through both multi-level (from molecules to tissue) and multi-scale (from nano- to millimetre) computational modelling can help to correlate the cellular performance with parameters of engineered microenvironments. It will play a critical role in elucidating the mechanisms for governing stem cell behaviour in the stem cell microenvironment [90].
4. Conclusions
The extracellular microenvironment plays a significant role in control of stem cell fate. A biomimetic approach is proposed to construct an artificial microenvironment by engineering its components, such as soluble factors, ECM, cell–cell interaction and external mechanical stimulation. Advances in materials science, micro-/nano-fluidics, micromanipulation, nanofabrication and multi-scale modelling will facilitate the design and creation of a well-controlled biomimetic three-dimensional microenvironment, which enables the elucidation of the mechanisms governing stem cell activities. Ultimately, such a microenvironment will benefit stem cell science to be successfully translated into the clinical treatment for tissue repairing.
Acknowledgements
We would like to acknowledge Mr Ray Adams for proof-reading the manuscript. Financial support from the Faculty of Engineering, Computer and Mathematical Sciences of the University of Adelaide for H.Z., S.D. and J.X.B. was also appreciated.
References
- 1.Martin G. R., Evans M. J. 1975. The morphology and growth of a pluripotent teratocarcinoma cell line and its derivatives in tissue culture. Cell 2, 163–172 10.1016/0092-8674(74)90090-7 (doi:10.1016/0092-8674(74)90090-7) [DOI] [PubMed] [Google Scholar]
- 2.Singec I., Jandial R., Crain A., Nikkhah G., Snyder E. Y. 2007. The leading edge of stem cell therapeutics. Annu. Rev. Med. 58, 313–328 10.1146/annurev.med.58.070605.115252 (doi:10.1146/annurev.med.58.070605.115252) [DOI] [PubMed] [Google Scholar]
- 3.Parson A. B. 2008. Stem cell biotech: seeking a piece of action. Cell 132, 511–513 10.1016/j.cell.2008.02.004 (doi:10.1016/j.cell.2008.02.004) [DOI] [PubMed] [Google Scholar]
- 4.Macchiarini P., et al. 2008. Clinical transplantation of a tissue-engineered airway. Lancet 372, 2023–2030 10.1016/S0140-6736(08)61598-6 (doi:10.1016/S0140-6736(08)61598-6) [DOI] [PubMed] [Google Scholar]
- 5.Wozniak P., El Haj A. J. 2007. Bone regeneration and repair using tissue engineering. In Tissue engineering using ceramics and polymers (eds Boccacani A. R., Gough J. E.), pp. 298–318 Cambridge, UK: Woodhead Publishers [Google Scholar]
- 6.Discher D. E., Mooney D. J., Zandstra P. W. 2009. Growth factors, matrices, and forces combine and control stem cells. Science 326, 1673–1677 10.1126/science.1171643 (doi:10.1126/science.1171643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scadden D. T. 2006. The stem-cell niche as an entity of action. Nature 441, 1075–1079 10.1038/nature04957 (doi:10.1038/nature04957) [DOI] [PubMed] [Google Scholar]
- 8.Montarras D., Morgan J., Collins C., Relaix F., Zaffran S., Cumano A., Partridge T., Buckingham M. 2005. Direct isolation of satellite cells for skeletal muscle regeneration. Science 309, 2064–2067 10.1126/science.1114758 (doi:10.1126/science.1114758) [DOI] [PubMed] [Google Scholar]
- 9.Sacco A., Doyonnas R., Kraft P., Vitorovic S., Blau H. M. 2008. Self-renewal and expansion of single transplanted muscle stem cells. Nature 456, 502–506 10.1038/nature07384 (doi:10.1038/nature07384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Björklund L. M., et al. 2000. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc. Natl Acad. Sci. USA 99, 2344–2349 10.1073/pnas.022438099 (doi:10.1073/pnas.022438099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reubinoff B. E., Pera M. F., Fong C. Y., Trounson A., Bongso A. 2000. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat. Biotechnol. 18, 399–404 10.1038/74447 (doi:10.1038/74447) [DOI] [PubMed] [Google Scholar]
- 12.Amariglio N., et al. 2009. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med 6, e1000029. 10.1371/journal.pmed.1000029 (doi:10.1371/journal.pmed.1000029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burdick J. A., Vunjak-Novakovic G. 2009. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng. Part A 15, 205–219 10.1089/ten.tea.2008.0131 (doi:10.1089/ten.tea.2008.0131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lund A. W., Yener B., Stegemann J. P., Plopper G. E. 2009. The natural and engineered 3-D microenvironment as a regulatory cue during stem cell fate determination. Tissue Eng. Part B 15, 371–380 10.1089/ten.teb.2009.0270 (doi:10.1089/ten.teb.2009.0270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh K., Ingber D. E. 2007. Micromechanical control of cell and tissue development: implications for tissue engineering. Adv. Drug Deliv. Rev. 59, 1306–1318 10.1016/j.addr.2007.08.014 (doi:10.1016/j.addr.2007.08.014) [DOI] [PubMed] [Google Scholar]
- 16.Walker M. R., Patel K. K., Stappenbeck T. S. 2009. The stem cell niche. J. Pathol. 217, 169–180 10.1002/path.2474 (doi:10.1002/path.2474) [DOI] [PubMed] [Google Scholar]
- 17.Morrison S. J., Spradling A. C. 2008. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611 10.1016/j.cell.2008.01.038 (doi:10.1016/j.cell.2008.01.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y., Li X., Eswarakumar V. P., Seger R., Lonai P. 2000. Fibroblast growth factor (FGF) signalling through PI-3-kinase and Akt/PKB is required for embryoid body differentiation. Oncogene 19, 3750–3756 10.1038/sj.onc.1203726 (doi:10.1038/sj.onc.1203726) [DOI] [PubMed] [Google Scholar]
- 19.Numakawa T., Yokomaku D., Kiyosue K., Adachi N., Matsumoto T., Numakawa Y., Taguchi T., Hatanaka H., Yamada M. 2002. Basic growth factor evokes a rapid glutamate release through activation of the MAPK pathway in cultured cortical neuron. J. Biol. Chem. 277, 28 861–28 869 10.1074/jbc.M202927200 (doi:10.1074/jbc.M202927200) [DOI] [PubMed] [Google Scholar]
- 20.Lee G. S., Kochhar D. M., Collins M. D. 2004. Retinod-induced limb malformations. Curr. Pharm. Des. 10, 2657–2699 10.2174/1381612043383728 (doi:10.2174/1381612043383728) [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi J., Mee P. J., Smith A. G. 2005. Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone 36, 758–769 10.1016/j.bone.2004.07.019 (doi:10.1016/j.bone.2004.07.019) [DOI] [PubMed] [Google Scholar]
- 22.Smith A. G. 2001. Embryo-derived stem cells: of mice and men. Annu. Rev. Cell Dev. Biol. 17, 435–462 10.1146/annurev.cellbio.17.1.435 (doi:10.1146/annurev.cellbio.17.1.435) [DOI] [PubMed] [Google Scholar]
- 23.Johansson B., Wiles M. 1995. Evidence for involvement of activin A and bone morphogenetic protein 4 in mammalian mesoderm and hematopoietic development. Mol. Cell Biol. 15, 141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho N. W., Cabodi M., Held B., Gleghorn J. P., Bonassar L. J., Stroock A. D. 2007. Microfluidic scaffolds for tissue engineering. Nat. Mater. 6, 908–915 10.1038/nmat2022 (doi:10.1038/nmat2022) [DOI] [PubMed] [Google Scholar]
- 25.Mapili G., Lu Y., Chen S., Roy K. 2005. Laser-layered microfabrication of spatially patterned functionalized tissue-engineering scaffolds. J. Biomed. Mater. Res. B 75, 414–424 10.1002/jbm.b.30325 (doi:10.1002/jbm.b.30325) [DOI] [PubMed] [Google Scholar]
- 26.Ferreira L., Gerecht-Nir S., Shieh H., Vunjak-Novakovic G., Langer R. 2007. Bioactive hydrogel scaffolds for controllable vascular differentiation of human embryonic stem cells. Biomaterials 28, 2706–2717 10.1016/j.biomaterials.2007.01.021 (doi:10.1016/j.biomaterials.2007.01.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guilak F., Cohen D. M., Estes B. T., Gimble J. M., Liedtke W., Chen C. S. 2009. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5, 17–26 10.1016/j.stem.2009.06.016 (doi:10.1016/j.stem.2009.06.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francesco R., Antonio G., Manlio B., Alfonso B. 2004. From cell–ECM interactions to tissue engineering. J. Cell Physiol. 199, 174–180 10.1002/jcp.10471 (doi:10.1002/jcp.10471) [DOI] [PubMed] [Google Scholar]
- 29.Risau W., Lemmon V. 1988. Changes in the vascular extracellular matrix during embryonic vasculogenesis and angiogenesis. Dev. Biol. 125, 441–450 10.1016/0012-1606(88)90225-4 (doi:10.1016/0012-1606(88)90225-4) [DOI] [PubMed] [Google Scholar]
- 30.Flaim C. J., Chien S., Bhatia S. N. 2005. An extracellular matrix microarray for probing cellular differentiation. Nat. Methods 2, 119–125 10.1038/nmeth736 (doi:10.1038/nmeth736) [DOI] [PubMed] [Google Scholar]
- 31.Griffith L. G., Swartz M. A. 2006. Capturing complex 3-D tissue physiology in vitro. Nat. Rev. Mol. Cell. Biol. 7, 211–224 10.1038/nrm1858 (doi:10.1038/nrm1858) [DOI] [PubMed] [Google Scholar]
- 32.Khademhosseini A., Ferreira L., Blumling J., Yeh J., Karp J. M., Fukuda J., Langer R. 2006. Co-culture of human embryonic stem cells with murine embryonic fibroblasts on microwell-patterned substrates. Biomaterials 27, 5968–5977 10.1016/j.biomaterials.2006.06.035 (doi:10.1016/j.biomaterials.2006.06.035) [DOI] [PubMed] [Google Scholar]
- 33.Anderson D. G., Levenberg S., Langer R. 2004. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat. Biotechnol. 22, 863–866 10.1038/nbt981 (doi:10.1038/nbt981) [DOI] [PubMed] [Google Scholar]
- 34.LaBarge M. A., Nelson C. M., Villadsen R., Fridriksdottir A., Ruth J. R., Stampfer M. R., Petersen O. W., Bissell M. J. 2009. Human mammary progenitor cell fate decisions are products of interactions with combinatorial microenvironments. Integr. Biol. 1, 70–79 10.1039/b816472j (doi:10.1039/b816472j) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hersel U., Dahmen C., Kessler H. 2003. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials 24, 4385–4415 10.1016/S0142-9612(03)00343-0 (doi:10.1016/S0142-9612(03)00343-0) [DOI] [PubMed] [Google Scholar]
- 36.Panitch A., Yamaoka T., Fournier M. J., Mason T. L., Tirrell D. A. 1999. Design and biosynthesis of elastin-like artificial extracellular matrix proteins containing periodically spaced fibronectin CS5 domains. Macromolecules 32, 1701–1703 10.1021/ma980875m (doi:10.1021/ma980875m) [DOI] [Google Scholar]
- 37.Lin X., Takahashi K., Liu Y., Zamora P. O. 2006. Enhancement of cell attachment and tissue integration by a IKVAV containing multi-domain peptide. Biochim. Biophys. Acta 1760, 1403–1410 10.1016/j.bbagen.2006.05.010 (doi:10.1016/j.bbagen.2006.05.010) [DOI] [PubMed] [Google Scholar]
- 38.Benoit D. S., Anseth K. S. 2005. The effect on osteoblast function of colocalized RGD and PHSRN epitopes on PEG surfaces. Biomaterials 26, 5209–5220 10.1016/j.biomaterials.2005.01.045 (doi:10.1016/j.biomaterials.2005.01.045) [DOI] [PubMed] [Google Scholar]
- 39.Yang F., Williams C. G., Wang D. A., Lee H., Manson P. N., Elisseeff J. 2005. The effect of incorportating RGD adhensive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials 26, 5991–5998 10.1016/j.biomaterials.2005.03.018 (doi:10.1016/j.biomaterials.2005.03.018) [DOI] [PubMed] [Google Scholar]
- 40.Stevens M. M., George J. H. 2005. Exploring and engineering the cell surface interface. Science 310, 1135–1138 10.1126/science.1106587 (doi:10.1126/science.1106587) [DOI] [PubMed] [Google Scholar]
- 41.Ruiz S. A., Chen C. S. 2008. Emergence of patterned stem cell differentiation within multicellular structures. Stem Cells 26, 2921–2927 10.1634/stemcells.2008-0432 (doi:10.1634/stemcells.2008-0432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wozniak M. A., Chen C. S. 2009. Mechanotransduction in development: a growing role for contractility. Nat. Rev. Mol. Cell Biol. 10, 34–43 10.1038/nrm2592 (doi:10.1038/nrm2592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McBeath R., Pirone D. M., Nelson C. M., Bhadriraju K., Chen C. S. 2004. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell. 6, 483–495 10.1016/S1534-5807(04)00075-9 (doi:10.1016/S1534-5807(04)00075-9) [DOI] [PubMed] [Google Scholar]
- 44.Garreta E., Genove E., Borros S., Semino C. E. 2006. Osteogenic diffentiation of mouse embryoic stem cells and mouse embryoic fibroblasts in a three-dimensional self-assembling peptide scaffold. Tissue Eng. 12, 2215–2217 10.1089/ten.2006.12.2215 (doi:10.1089/ten.2006.12.2215) [DOI] [PubMed] [Google Scholar]
- 45.Nur E. K. A., Ahmed I., Kamal J., Schindler M., Meiners S. 2006. Three-dimensional nanofibrilar surfaces promote self-renewal in mouse embryoic stem cells. Stem Cells 24, 426–433 10.1634/stemcells.2005-0170 (doi:10.1634/stemcells.2005-0170) [DOI] [PubMed] [Google Scholar]
- 46.Discher D. E., Janmey P., Wang Y. L. 2005. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 10.1126/science.1116995 (doi:10.1126/science.1116995) [DOI] [PubMed] [Google Scholar]
- 47.Gibert P. M., et al. 2010. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329, 1078–1081 10.1126/science.1191035 (doi:10.1126/science.1191035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faucheux N., Zahm J. M., Bonnet N., Legeay G., Nagel M. D. 2004. Gap junction communication between cells aggregated on a cellulose-coated polystyrene: influence of connexin 43 phosphorylation. Biomaterials 25, 2501–2506 10.1016/j.biomaterials.2003.09.036 (doi:10.1016/j.biomaterials.2003.09.036) [DOI] [PubMed] [Google Scholar]
- 49.Tang J., Peng R., Ding J. 2010. The regulation of stem cell differentiation by cell-cell contact on micropatterned material surfaces. Biomaterials 31, 2470–2476 10.1016/j.biomaterials.2009.12.006 (doi:10.1016/j.biomaterials.2009.12.006) [DOI] [PubMed] [Google Scholar]
- 50.Tsai R. Y., McKay R. D. 2000. Cell contact regulates fate choice by cortical stem cells. J. Neurosci. 20, 3725–3735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hwang N., Varghese S., Puleo C., Zhang Z., Elisseeff J. 2007. Morphogenic signals from chondrocytes promote chondrogenic and osteogenic differentiation of mesenchymal stem cells. J. Cell Physiol. 212, 281–284 10.1002/jcp.21052 (doi:10.1002/jcp.21052) [DOI] [PubMed] [Google Scholar]
- 52.Wagner W., Saffrich R., Wirkner U., Eckstein V., Blake J., Ansorge A. 2005. Hematopoietic progenitor cells and cellular microenvironment: behavioral and molecular changes upon interaction. Stem Cells 23, 1180–1191 10.1634/stemcells.2004-0361 (doi:10.1634/stemcells.2004-0361) [DOI] [PubMed] [Google Scholar]
- 53.Purpura K. A., Aubin J. E., Zandstra P. W. 2004. Sustained in vitro expansion of bone progenitors is cell density dependent. Stem Cells 22, 39–50 10.1634/stemcells.22-1-39 (doi:10.1634/stemcells.22-1-39) [DOI] [PubMed] [Google Scholar]
- 54.Fukuda J., Khademhosseini A., Yeh J., Eng G., Cheng J., Farokhzad O. C., Langer R. 2006. Micropatterned cell co-culture using layer-by-layer deposition of extraceullar matrix components. Biomaterials 27, 1479–1486 10.1016/j.biomaterials.2005.09.015 (doi:10.1016/j.biomaterials.2005.09.015) [DOI] [PubMed] [Google Scholar]
- 55.Leach J., Howard D., Roberts S., Gibson G., Gothard D., Cooper J., Shakesheff K., Padgett M., Buttery L. 2009. Manipulation of live mouse embryonic stem cells using holographic optical tweezers. J. Modern Opt. 56, 448–452 10.1080/09500340802488565 (doi:10.1080/09500340802488565) [DOI] [Google Scholar]
- 56.Albrecht D. R., Underhill G. H., Wassermann T. B., Sah R. L., Bhatia S. N. 2006. Probing the role of multicellular organization in three-dimensional microenvironments. Nat. Methods 3, 369–375 10.1038/nmeth873 (doi:10.1038/nmeth873) [DOI] [PubMed] [Google Scholar]
- 57.Butler D. L., et al. 2009. The impact of biomechanics in tissue engineering and regenerative medicine. Tissue Eng. Part B 15, 477–484 10.1089/ten.teb.2009.0340 (doi:10.1089/ten.teb.2009.0340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J. H., Thampatty B. P. 2008. Mechanobiology of adult and stem cells. Int. Rev. Cell Mol. Biol. 271, 301–346 10.1016/S1937-6448(08)01207-0 (doi:10.1016/S1937-6448(08)01207-0) [DOI] [PubMed] [Google Scholar]
- 59.Gong Z., Niklason L. E. 2008. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs). FASEB J. 22, 1635–1648 10.1096/fj.07-087924 (doi:10.1096/fj.07-087924) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimizu N., et al. 2008. Cyclic strain induces mouse embryonic stem cell differentiation into vascular smooth muscle cells by activating PDGF receptor beta. J. Appl. Physiol. 104, 766–772 10.1152/japplphysiol.00870.2007 (doi:10.1152/japplphysiol.00870.2007) [DOI] [PubMed] [Google Scholar]
- 61.Saha S., Ji L., de Pablo J., Palecek S. 2006. Inhibition of human embryonic stem cell differentiation by mechanical strain. J. Cell. Physiol. 206, 126–137 10.1002/jcp.20441 (doi:10.1002/jcp.20441) [DOI] [PubMed] [Google Scholar]
- 62.Mauck R., Byers B., Yuan X., Tuan R. 2007. Regulation of cartilaginous ECM gene transcription by conondrocytes and MSCs in 3-D culture in response to dynamic loading. Biomech. Model Mechanobiol. 6, 113–125 10.1007/s10237-006-0042-1 (doi:10.1007/s10237-006-0042-1) [DOI] [PubMed] [Google Scholar]
- 63.Huang C., Hagar K., Frost L., Sun Y., Cheung H. 2004. Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells 22, 313–323 10.1634/stemcells.22-3-313 (doi:10.1634/stemcells.22-3-313) [DOI] [PubMed] [Google Scholar]
- 64.Altman G., Horan R. L., Martin I., Farhadi J. M., Stark P. R. H., Volloch V., Richmond J. C., Vunjak-Novakovic G., Kaplan D. L. 2002. Cell differentiation by mechanical stress. FASEB J. 16, 270–272 [DOI] [PubMed] [Google Scholar]
- 65.Knippenberg M., Helder M. N., Doulabi B. Z., Semeins C. M., Wuisman P. I., Klein-Nulend J. 2005. Adipose tissue-derived mesenchymal stem cells acquire bone cell-like responsiveness to fluid shear stress on osteogenic stimulation. Tissue Eng. 11, 1780–1788 10.1089/ten.2005.11.1780 (doi:10.1089/ten.2005.11.1780) [DOI] [PubMed] [Google Scholar]
- 66.McGarry J. G., Klein-Nulend J., Mullender M. G., Prendergast P. J. 2004. A comparison of strain and fluid shear stress in stimulating bone cell responses—a computational and experimental study. FASEB J. 19, 482–484 10.1096/fj.04-2210fje (doi:10.1096/fj.04-2210fje) [DOI] [PubMed] [Google Scholar]
- 67.Riddle R. C., Taylor A. F., Genetos D. C., Donahue H. J. 2006. MAP kinase and calcium signaling mediate fluid flow-induced human mesenchymal stem cell proliferation. Am. J. Physiol. Cell Physiol. 290, C776–C784 10.1152/ajpcell.00082.2005 (doi:10.1152/ajpcell.00082.2005) [DOI] [PubMed] [Google Scholar]
- 68.Glossop J. R., Cartmell S. H. 2009. Effect of fluid flow-induced shear stress on human mesenchymal stem cells: differential gene expression of IL1B and MAP3K8 in MAPK signaling. Gene Express. Patterns 9, 381–388 10.1016/j.gep.2009.01.001 (doi:10.1016/j.gep.2009.01.001) [DOI] [PubMed] [Google Scholar]
- 69.Zhao F., Chella R., Ma T. 2007. Effects of shear stress on 3-D human mesenchymal stem cell construct development in a perfusion bioreactor system: experiments and hydrodynamic modeling. Biotechnol. Bioeng. 96, 584–595 10.1002/bit.21184 (doi:10.1002/bit.21184) [DOI] [PubMed] [Google Scholar]
- 70.Kreke M. R., Huckle W. R., Goldstein A. S. 2005. Fluid flow stimulates expression of osteopontin and bone sialoprotein by bone marrow stromal cells in a temporally dependent manner. Bone 36, 1047–1055 10.1016/j.bone.2005.03.008 (doi:10.1016/j.bone.2005.03.008) [DOI] [PubMed] [Google Scholar]
- 71.Li Y. J., Batra N. N., You L., Meier S. C., Coe I. A., Yellowley C. E., Jacobs C. R. 2004. Oscillatory fluid flow affects human marrow stromal cell proliferation and differentiation. J. Orthop. Res. 22, 1283–1289 10.1016/j.orthres.2004.04.002 (doi:10.1016/j.orthres.2004.04.002) [DOI] [PubMed] [Google Scholar]
- 72.McGarry J. G., Klein-Nulend J., Prendergast P. J. 2005. The effect of cytoskeletal disruption on pulsatile fluid flowinduced nitric oxide and prostaglandin E2 release in osteocytes and osteoblasts. Biochem. Biophys. Res. Commun. 330, 341–348 10.1016/j.bbrc.2005.02.175 (doi:10.1016/j.bbrc.2005.02.175) [DOI] [PubMed] [Google Scholar]
- 73.Adamo L., et al. 2009. Biomechanical forces promote embryonic haematopoiesis. Nature 459, 1131–1135 10.1038/nature08073 (doi:10.1038/nature08073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bai K., Huang Y., Jia X., Fan Y., Wang W. 2010. Endothelium oriented differentiation of bone marrow mesenchymal stem cells under chemical and mechanical stimulations. J. Biomech. 43, 1176–1181 10.1016/j.jbiomech.2009.11.030 (doi:10.1016/j.jbiomech.2009.11.030) [DOI] [PubMed] [Google Scholar]
- 75.McCoy R. J., O'Brien F. J. 2010. Influence of shear stress in perfusion bioreactor cultures for the development of three-dimensional bone tissue constructs: a review. Tissue Eng. Part B 16, 1–15 10.1089/ten.teb.2010.0370 (doi:10.1089/ten.teb.2010.0370) [DOI] [PubMed] [Google Scholar]
- 76.King J., Miller W. 2007. Bioreactor development for stem cell expansion and controlled differentiation. Curr. Opin. Chem. Biol. 11, 1–5 10.1016/j.cbpa.2007.05.034 (doi:10.1016/j.cbpa.2007.05.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O'Cearbhaill E. D., Punchard M. A., Murphy M., Barry F. P., McHugh P. E., Barron V. 2008. Response of mesenchymal stem cells to the biomechanical environment of the endothelium on a flexible tubular silicone substrate. Biomaterials 29, 1610–1619 10.1016/j.biomaterials.2007.11.042 (doi:10.1016/j.biomaterials.2007.11.042) [DOI] [PubMed] [Google Scholar]
- 78.Salvi J. D., Lim J. Y., Donahue H. J. 2010. Increased mechanosensitivity of cells cultured on nanotopographies. Biomaterials 43, 3058–3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun S., Liu Y. M., Lipsky S., Cho M. R. 2007. Physical manipulation of calcium oscillations facilitates osteodifferentiation of human mesenchymal stem cells. FASEB J. 21, 1472–1480 10.1096/fj.06-7153com (doi:10.1096/fj.06-7153com) [DOI] [PubMed] [Google Scholar]
- 80.Wu C.-C., Chao Y.-C., Chen C.-N., Chien S., Chen Y. C., Chien C. C., Chiu J. J., Linju Yen B. 2008. Synergism of biochemical and mechanical stimuli in the differentiation of human placenta-derived multipotent cells into endothelial cells. J. Biomech. 41, 813–821 10.1016/j.jbiomech.2007.11.008 (doi:10.1016/j.jbiomech.2007.11.008) [DOI] [PubMed] [Google Scholar]
- 81.Miyanishi K., Trindade M. C. D., Lindsey D. P., Beaupré G. S., Carter D. R., Goodman S. B., Schurman D. J., Smith R. L. 2006. Effects of hydrostatic pressure and transforming growth factor-β3 on adult human mesenchymal stem cell chondrogenesis in vitro. Tissue Eng. 12, 1419–1428 10.1089/ten.2006.12.1419 (doi:10.1089/ten.2006.12.1419) [DOI] [PubMed] [Google Scholar]
- 82.Lutolf M. P., Gilbert P. M., Blau H. M. 2009. Designing materials to direct stem-cell fate. Nature 462, 433–441 10.1038/nature08602 (doi:10.1038/nature08602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DeForest C. A., Polizzotti B. D., Anseth K. S. 2009. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat. Mater. 8, 659–664 10.1038/nmat2473 (doi:10.1038/nmat2473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khademhosseini A., Langer R., Borenstein J., Vacanti J. P. 2006. Microscale technologies for tissue engineering and biology. Proc. Natl Acad. Sci. USA 103, 2480–2487 10.1073/pnas.0507681102 (doi:10.1073/pnas.0507681102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Toh Y.-C., Blagovic K., Voldman J. 2010. Advancing stem cell research with microtechnologies: opportunities and challenges. Integr. Biol. 2, 305–325 10.1039/c0ib00004c (doi:10.1039/c0ib00004c) [DOI] [PubMed] [Google Scholar]
- 86.Zhang H., Liu K. K. 2008. Optical tweezers for single cells. J. R. Soc. Interface 5, 671–690 10.1098/rsif.2008.0052 (doi:10.1098/rsif.2008.0052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koch L., et al. 2010. Laser printing of skin cells and human stem cells. Tissue Eng. Part C 16, 847–854 10.1089/ten.tec.2009.0397 (doi:10.1089/ten.tec.2009.0397) [DOI] [PubMed] [Google Scholar]
- 88.Abdul Raof N., Schiele N. R., Xie Y., Chrisey D. B., Corr D. T. The maintenance of pluripotency following laser direct-write of mouse embryonic stem cells. Biomaterials 32, 1802–1808 10.1016/j.biomaterials.2010.11.015 (doi:10.1016/j.biomaterials.2010.11.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nahmias Y., Schwartz R. E., Verfaillie C. M., Odde D. J. 2005. Laser-guided direct writing for three-dimensional tissue engineering. Biotechnol. Bioeng. 92, 129–136 10.1002/bit.20585 (doi:10.1002/bit.20585) [DOI] [PubMed] [Google Scholar]
- 90.Griffith L. G., Swartz M. A. 2006. Capturing complex 3-D tissue physiology in vitro. Nat. Mol. Cell Biol. 7, 211–224 10.1038/nrm1858 (doi:10.1038/nrm1858) [DOI] [PubMed] [Google Scholar]
- 91.Jungreuthmayer C., Donahue S. W., Jaasma M. J., Al-Munajjed A. A., Zanghellini J., Kelly D. J., O'Brien F. J. 2009. A comparative study of shear stresses in collagen-glycosaminoglycan and calcium phosphate scaffolds in bone tissue-engineering bioreactors. Tissue Eng. Part A 15, 1141–1149 10.1089/ten.tea.2008.0204 (doi:10.1089/ten.tea.2008.0204) [DOI] [PubMed] [Google Scholar]
- 92.Bilgen B., Uygun K., Bueno E. M., Sucosky P., Barabino G. A. 2009. Tissue growth modelling in a wavy-walled bioreactor. Tissue Eng. Part A 15, 761–771 10.1089/ten.tea.2008.0078 (doi:10.1089/ten.tea.2008.0078) [DOI] [PubMed] [Google Scholar]
- 93.Boschetti F., Raimondi M. T., Migliavacca F., Dubini G. 2006. Prediction of the micro-fluid dynamic environment imposed to three-dimensional engineered cell systems in bioreactors. J. Biomech. 39, 418–425 10.1016/S0021-9290(06)85506-5 (doi:10.1016/S0021-9290(06)85506-5) [DOI] [PubMed] [Google Scholar]
- 94.Maes F., Van Ransbeeck P., Van Oosterwyck H., Verdonck P. 2009. Modelling fluid flow through irregular scaffolds for perfusion bioreactor. Biotechnol. Bioeng. 103, 621–630 10.1002/bit.22277 (doi:10.1002/bit.22277) [DOI] [PubMed] [Google Scholar]
- 95.Galbusera F., Cioffi M., Raimondi M. T., Pietrabissa R. 2007. Computational modeling of combined cell population dynamics and oxygen transport in engineered tissue subject to interstitial perfusion. Comput. Methods Biomech. Biomed. Eng. 10, 279–287 10.1080/10255840701318404 (doi:10.1080/10255840701318404) [DOI] [PubMed] [Google Scholar]
- 96.Chung C. A., Yang C. W., Chen C. W. 2006. Analysis of cell growth and diffusion in a scaffold for cartilage tissue engineering. Biotechnol. Bioeng. 94, 1138–1146 10.1002/bit.20944 (doi:10.1002/bit.20944) [DOI] [PubMed] [Google Scholar]
- 97.Trewenack A. J., Please C. P., Landman K. A. 2009. A continuum model for the development of tissue-engineered cartilage around a chondrocyte. Math. Med. Biol. 26, 241–262 10.1093/imammb/dqp013 (doi:10.1093/imammb/dqp013) [DOI] [PubMed] [Google Scholar]
- 98.Binder B. J., Landman K. A., Simpson M. J., Mariani M., Newgreen D. F. 2008. Modeling proliferative tissue growth: a general approach and an avian case study. Phys. Rev. E 78, 031912. 10.1103/PhysRevE.78.031912 (doi:10.1103/PhysRevE.78.031912) [DOI] [PubMed] [Google Scholar]
- 99.Pancrazio J. J., Wang F., Kelley C. A. 2007. Enabling tools for tissue engineering. Biosens. Bioelectron. 22, 2803–2811 10.1016/j.bios.2006.12.023 (doi:10.1016/j.bios.2006.12.023) [DOI] [PubMed] [Google Scholar]
- 100.Engler A. J., Humbert P. O., Wehrle-Haller B., Weaver V. M. 2009. Multiscale modeling of form and function. Science 324, 208–212 10.1126/science.1170107 (doi:10.1126/science.1170107) [DOI] [PMC free article] [PubMed] [Google Scholar]


