Abstract
The ubiquity of top-down causal explanations within and across the sciences is prima facie evidence for the existence of top-down causation. Much debate has been focused on whether top-down causation is coherent or in conflict with reductionism. Less attention has been given to the question of whether these representations of hierarchical relations pick out a single, common hierarchy. A negative answer to this question undermines a commonplace view that the world is divided into stratified ‘levels’ of organization and suggests that attributions of causal responsibility in different hierarchical representations may not have a meaningful basis for comparison. Representations used in top-down and bottom-up explanations are primarily ‘local’ and tied to distinct domains of science, illustrated here by protein structure and folding. This locality suggests that no single metaphysical account of hierarchy for causal relations to obtain within emerges from the epistemology of scientific explanation. Instead, a pluralist perspective is recommended—many different kinds of top-down causation (explanation) can exist alongside many different kinds of bottom-up causation (explanation). Pluralism makes plausible why different senses of top-down causation can be coherent and not in conflict with reductionism, thereby illustrating a productive interface between philosophical analysis and scientific inquiry.
Keywords: hierarchy, causation, explanation, top-down, reductionism, pluralism
1. Top-down explanation (and causation): ubiquitous (and controversial)
One does not have to look very hard to find examples of scientists making appeals to top-down causation or emergent properties at higher ‘levels’ having causal effects at lower ‘levels’. They are ubiquitous.
The question of ‘Why is the world green?’ … has encouraged many ecologists to investigate whether herbivore population dynamics are limited by the availability of food plants and plant defence mechanisms or rather by top-down control through predators. In each case, herbivorous arthropods play a decisive role in ecosystem functioning because, as midtrophic level species, they are influenced by bottom-up and top-down forces [1, p. 1].
Molecular chaperones … have a number of functions [that] can be understood only by considering the emergent properties of cellular networks [such as] … genetic buffers stabilizing the phenotype of various cells and organisms [2, p. 45].
The terminology of ‘top-down control’, ‘top-down forces’ and ‘network functions’ is drawn from a causal vocabulary—predators exercise top-down control by eating prey and molecular chaperones biochemically interact with other proteins in a network to stabilize cellular phenotypes.
This causal vocabulary is used in the context of explaining biological phenomena, which requires that these phenomena be represented—symbolized, embodied, pictured, or designated in equations, scale miniatures or abstract diagrams. In cases where the representations are hierarchical, and properties of higher ‘levels’ influence or make a difference on lower ‘levels’, we have a top-down causal explanation. Top-down causal explanation is an epistemological concept because it refers to how research communities in the sciences try to comprehend the world by: (i) representing natural phenomena with hierarchical relations (i.e. with tops and bottoms) and (ii) taking temporal dependency relations (i.e. causation) between features of these representations to be explanatory. For example, an explanation of herbivore population dynamics will involve a hierarchical representation of trophic levels where the predators (birds, bats, etc.) are at a higher ‘level’ in the food web than the relevant herbivores (arboreal arthropods). The relation of dependency can be construed in terms of changes in the value of a variable for the population at the lower ‘level’ (e.g. prey abundance) at a later time owing to changes in the value of a variable for the population at the higher ‘level’ (e.g. predator foraging success) at an earlier time [3]. It is often assumed that causal explanations can be from the bottom-up if not also from the top-down (‘influenced by bottom-up and top-down forces’). To keep this in view, we can refer to these types of explanation more generally as hierarchical causal explanations.
Top-down versions of hierarchical causal explanation in the sciences exist alongside bottom-up (reductive) versions as a matter of fact. How to interpret them metaphysically is another question [4]. Top-down causation, or hierarchical causation more generally, is a metaphysical concept because it refers to the way our ontology might include causes that operate at higher ‘levels of organization’ and bring about effects on lower ‘levels of organization’ (or vice versa), quite apart from our inquiry into them [5,6]. ‘Downward causation … means that higher-level entities, by virtue of the emergent characteristics and capacities proper to their level and nature, sometimes possess the ability to act causally back down upon entities operating at lower levels of reality’ [7, p. 40]. This appeal to ‘levels’ is common and usually described as a discovery of the sciences: ‘I find the hierarchical picture of nature [‘as stratified into levels’] plausible as reflected in the structure and discoveries of the sciences’ [8, p. 498]. It is a persistent feature of discussions about emergent properties: ‘there are layered strata, or levels, of objects, based on increasing complexity’ [9, §3.1].
Although it is natural to take the ubiquity of top-down causal explanations as prima facie evidence for the existence of top-down causation, there is good reason to hesitate. Much of the philosophical debate surrounding top-down causation has focused on whether it is even possible or if it involves problematic assumptions [9–12]. These debates involve substantive disagreements about the nature of causation, such as whether causes necessitate their effects or whether causes can be understood sufficiently as difference makers. Although the possibility of pluralism about the nature of causation is an important question [13], it is not the focus herein. Another point of debate is the (seeming) incongruence of top-down causation with reductionism in the sciences. In one sense this is incorrect; sciences can use reductionist methods to investigate natural phenomena regardless of whether particular reductive explanations of those phenomena succeed or fail [14]. In another sense, the degree to which this incongruence is genuine turns on how top-down causation is interpreted metaphysically. If reductionism is understood as a claim about the asymmetry of hierarchical causation (i.e. causes always operate from the bottom up), then appeals to top-down causation will be in conflict because they invoke a contradictory asymmetry (i.e. causes sometimes operate from the top down).
These interpretations are conditional on a key question involved in deriving an account of top-down (hierarchical) causation from top-down (hierarchical) causal explanations: do these representations of hierarchical relations (i.e. tops and bottoms, and their temporal orderings) pick out a single, common hierarchy? If not, then it is possible that a top-down causal explanation that uses a particular hierarchical representation cannot be directly linked to another using a different representation of hierarchical relations; attributions of causal responsibility in different hierarchical representations may not have a meaningful basis for comparison. A top-down causal explanation of a natural phenomenon does not foreclose the possibility of a bottom-up causal explanation of the same phenomenon represented differently. This situation complicates the move from the ubiquity of top-down causal explanation to the existence of top-down causation, long before we invoke philosophical worries about the coherence of hierarchical causation or the possibility of pluralism about the nature of causation.
Whether the different representations of hierarchical relations in causal explanations found in diverse sciences can be combined or integrated is a question of hierarchical representation commensurability: is there a common standard or hierarchy to which heterogeneous hierarchical representations can be reduced or unified? (This differs from the Kuhnian sense of lacking a common standard to judge between two paradigms but retains the semantic import of incommensurability: ‘not able to be judged by the same standard or lacking a common standard of measurement’.) Actual scientific representations of hierarchical relationships often return a negative answer to this question, which throws doubt on the commonplace view that science has shown the world to be divided into stratified ‘levels’. This picture, reliant upon hierarchical representation commensurability, has long been a central component of claims that emergent properties with novel causal powers have been discovered by the sciences: ‘The organizational levels of molecule, cell, tissue, organ, organism, breeding population, species, … are accepted as factual realities rather than as arbitrary conveniences of classification, with each of the higher orders organizing the real units of the lower level’ ([5, p. 179]; cf. [9]). Instead, hierarchical representations deployed in top-down and bottom-up explanations are primarily ‘local’—not articulated in such a way as to ‘globally’ cohere or contrast with all other hierarchical representations—and the ‘locality’ is related to their application to specific domains of inquiry. For example, biological sub-disciplines can work with non-coincident spatial decompositions of organisms into parts, such as cell types, biochemical reactions or developmental fields [15]. These local ‘levels’ can be considered real but do not correspond to global, nominalized designations (e.g. the organismal ‘level’), in part because different representations of those ‘levels’ are available. This helps us to explain why seemingly similar hierarchical orderings can recur in different explanations; the hierarchical ordering of cells below organisms is widespread but the precise representation of hierarchical relations differs depending on the phenomena in view (e.g. whether intervening organizational structure is represented). Here I use layers of organization in protein structure and explanations of protein folding to illustrate the locality of hierarchical representations and how they operate in top-down causal explanations.
The locality of ‘levels’ and the incommensurability of hierarchical representations suggest that no unified metaphysical account of hierarchy for causal relations to obtain within emerges from the epistemology of scientific explanation. Therefore, a pluralist perspective might be warranted—many different kinds of top-down causation (explanation) can exist alongside many different kinds of bottom-up causation (explanation). Different sciences exhibit a plurality of representational schemes, explanatory strategies and models.
Pluralism is a view about this state of affairs: that plurality in science possibly represents an ineliminable character of scientific inquiry and knowledge (about at least some phenomena), that it represents a deficiency in knowledge only from a certain point of view, and that analysis of metascientific concepts (like theory, [hierarchical causal] explanation, evidence) should reflect the possibility that the explanatory and investigative aims of science can be best achieved by sciences that are pluralistic, even in the long run [16, p. ix–x].
Pluralism makes plausible why several different senses of top-causation can be coherent and why they do not conflict with reductionism, thereby illustrating a productive interface between philosophical analysis and scientific inquiry.
2. Local ‘levels’ of organization: protein structure
As is well known, proteins are composed of amino acids linked by covalent peptide bonds into a polypeptide chain. This linear chain of amino acids is the first structural ‘level’ of a protein (‘primary structure’). ‘Secondary structure’ refers to repeating patterns of coiling or folding (α helices or β pleated sheets) resulting from regular hydrogen bonding rather than specific chemical moieties of amino acid residues. Nearly all proteins adopt a three-dimensional structure (‘tertiary structure’) in order to be functional [17]. This conformation of the polypeptide is understood in terms of interactions among its amino acids (e.g. hydrophobic residues avoid interaction with surrounding water by segregating to internal regions). A major part of the protein-folding problem is explaining how this active conformation is achieved for polypeptides subsequent to translation from RNA in the cellular context. Finally, tertiary-structured proteins can further aggregate into ‘quaternary structure’ such as in haemoglobin, a tetramer composed of pairs of α and β subunits.
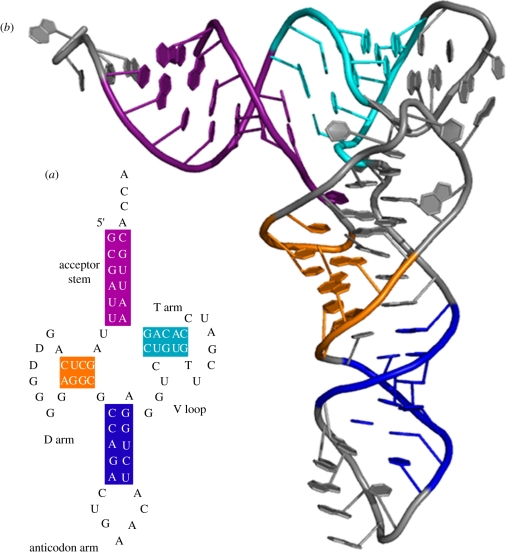
Proteins are represented regularly with these ‘four levels of structural organization’ [18, p. 78]. The structural features of proteins are depicted in a variety of ways, often with schematic diagrams that emphasize one or more of the ‘levels’ and their distinctive features (figure 1). Other biological macromolecules can be described with the same four layers, especially nucleic acids (DNA and RNA). But, here, we begin to see the manifestation of ‘locality’ because most of the utility in applying these ‘levels’ of structural organization to nucleic acids pertains to RNA, not DNA. DNA does have secondary structural organization—the famous double helix—as well as tertiary structure—three naturally occurring helical geometries (A, B and Z)—but these categories are less often applied. One reason is the lack of functional diversity exhibited among the structural ‘levels’ for DNA, making all four structural designations less useful in practice. In contrast, RNA adopts a wide variety of secondary structure (e.g. stem-loops) and tertiary structure (e.g. coaxial stacking; figure 2), although quaternary structure is less relevant [19,20]. This diversity at the different structural ‘levels’ is accompanied by a corresponding functional diversity in terms of the cellular roles RNA is able to play.
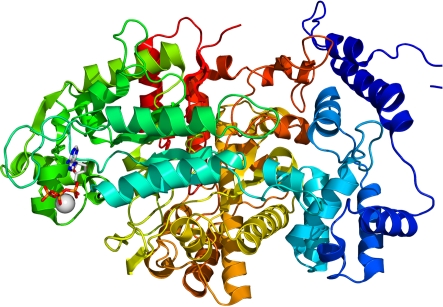
Figure 1.
One pictorial representation of a protein that emphasizes different structural ‘levels’. Ribonucleotide reductase protein R1E from Salmonella typhimurium. Adapted from Wikimedia Commons (http://commons.wikimedia.org/wiki/File:1PEU_R1E.png).
Figure 2.
Two different representations of a transfer RNA structure. (a) Secondary structure and (b) tertiary structure. Adapted from Wikimedia Commons (http://commons.wikimedia.org/wiki/File:TRNA_all2.png).
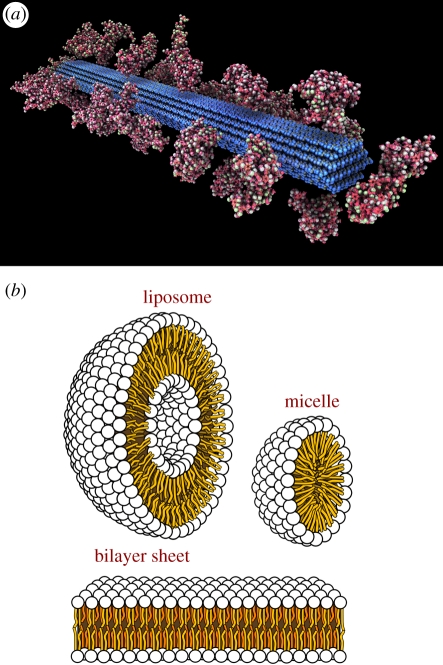
Different biological macromolecules, such as polysaccharides and lipids, have higher order structure but do not fit into the four layers of structural classification used for proteins and nucleic acids (figure 3). Polysaccharides are linked chains of repeating carbohydrate units (polymers), sometimes composed of the same building blocks (monosaccharides) and sometimes heterogeneously composed. They are often linear but also may display branching to varying degrees. Examples include cellulose and chitin (structural polysaccharides), which play critical roles in animal and plant morphologies, and starch and glycogen (storage polysaccharides), which are important in metabolism. Although they have a three-dimensional structure and are chains of (sometimes) heterogeneous elements, they are not described in terms of the four structural ‘levels’ for proteins and nucleic acids. Lipids include glycerides, phospholipids, waxes and some vitamins. They play roles in energy storage and signalling, as well as being structural components of membranes. Ketoacyl and isoprene groups are the two basic types of biochemical subunits of lipids, which can be further classified based on their structure and function (e.g. steroids, such as oestrogen, which share a structure of four core rings of carbon and function in hormonal signalling). Again, these biological macromolecules have three-dimensional structure and are built out of smaller subunits but are not classified with the four structural ‘levels’ for proteins and nucleic acids.
Figure 3.
Representations of biological macromolecules whose structural features are not captured by the four structural ‘levels’ of proteins and nucleic acids: (a) cellulose and (b) phospholipid arrangements in aqueous media. Adapted from Wikimedia Commons ((a) http://commons.wikimedia.org/wiki/File:Cellulose.jpg; (b) http://commons.wikimedia.org/wiki/File:Phospholipids_aqueous_solution_structures.svg).
The hierarchical representation of four structural layers of organization is applicable to proteins and nucleic acids. This categorization of hierarchical organization is extremely robust and well established empirically. But it quickly loses its significance when applied across the spectrum of biological macromolecules. In this sense, the ‘levels of structural organization’ are localized to a particular domain of inquiry (proteins and nucleic acids) and not reified into a nominalized designation (e.g. the tertiary structural level of biological macromolecules). Hierarchical representation incommensurability seems to obtain in the realm of macromolecules. The inability to align higher order polysaccharide or lipid structure with the four ‘levels’ of protein structure may not be a theoretical liability because these are not attempts to stratify macromolecular reality (i.e. an overall account of macromolecular levels of organization is not the scientific aim). There is no confusion in applying the hierarchical representations; there is no tendency among molecular biologists to assign a branched polysaccharide to the tertiary ‘level’ along with folded proteins or RNAs. At the same time, the four structural layers give a precise meaning to ‘higher’ and ‘lower’ for protein structure, and one that is empirically anchored. Tertiary structure is at a higher ‘level’ than primary or secondary structure; adjacent layers are stratified. This robustness and precision in the classification of ‘levels’ can facilitate hierarchical causal explanations, such as the dynamics of protein folding in the cell.
3. Top-down causal explanations of protein folding
The most familiar hierarchical causal explanation for protein structure is the linear sequence hypothesis: the three-dimensional folding of a protein (i.e. its tertiary structure) results from the properties of the amino acid residues in the polypeptide (i.e. the primary structure) and their interactions alone. A folded protein is purportedly explained by the chemical interactions of its components as ordered in a linear polypeptide—the whole is a ‘sum’ of the interaction of its parts over a (rapid) temporal duration. The primary, lower protein ‘level’ causally brings about the tertiary, higher protein ‘level’. This is a bottom-up (hierarchical) causal explanation in molecular biology focused on the kinetic, thermodynamic and structural characteristics of the protein-folding process [21,22], which differs from the question of whether one can accurately predict the three-dimensional shape of a protein from its primary structure [23].
Initial experiments on the denaturation and refolding of proteins in vitro appeared to confirm the linear sequence hypothesis [24]. Denatured ribonucleases refolded into their native conformation rather than a myriad of other biochemical possibilities, which could only occur if folding follows a limited number of pathways [25]. Although correct refolding happened as predicted by the linear sequence hypothesis, it took an hour or longer rather than several minutes or less without elements from the endoplasmic reticulum. Recent research has been able to characterize the role of these elements, and a more complex picture of protein folding has emerged [22]. ‘Cell biologists, having been taught that polypeptide chains can spontaneously fold to the native state, have been frustrated to discover that, although spontaneous folding can occur for small simple proteins … spontaneous, high-yield folding to the native state might be the exception, rather than the rule’ [26, p. 527]. Chaperone proteins guide folding during and after polypeptide synthesis [27], as well as in refolding subsequent to stressful conditions such as heat shock [28]: ‘Proteins need the assistance of molecular chaperones and folding enzymes to reach their native structure efficiently’ [29, p. 78].
One reason why molecular chaperones must provide oversight in the process of protein folding is that the cellular environment is crowded [29–31]. Another is that the rate of folding is faster than the rate of synthesis for a polypeptide chain in the ribosome, which is done in tandem for individual mRNAs (termed a ‘polyribosome’), thereby allowing for misfolding and cross-reactions subsequent to the completion of translation [32]. Distinct functional groups of chaperones monitor protein folding during de novo synthesis, quality control and the response to stress [33,34]. Chaperones work in different ways, often cooperatively and in conjunction with diverse client proteins [2,35], sometimes providing a sequestered environment for folding and at other times facilitating folding actively [32,36,37]. Experimental changes to the volume of the cavity inside a nanocage increase the folding speed for small proteins by modifying their energy landscapes [38]. Multiple amino acid residue interactions between an already functional, folded protein (the chaperone) and the unfolded polypeptide underlie the process of correct folding [39]. Even when mutations are introduced that lead to altered amino acid components, correct folding can be induced by the overproduction of molecular chaperones [40].
Explanations of protein folding that rely on chaperones are a form of top-down causal explanation. Chaperones are themselves folded proteins, at the tertiary (or higher) ‘level’ of structure. They operate on primary and secondary (or lower) ‘levels’ of structure. An entity at a ‘top’ or higher ‘level’ in the hierarchy causally brings about changes in a ‘bottom’ or lower ‘level’ entity as a consequence of having properties not found at the bottom ‘level’: ‘there is a need for molecular chaperones because the intrinsic properties of proteins assure that incorrect interactions are possible’ [41, p. 73]. The causal contribution of chaperones in protein folding is due to three-dimensional structure, a kind of property that the amino acid parts lack. ‘The manner in which a newly synthesized chain of amino acids transforms itself into a perfectly folded protein depends both on the intrinsic properties of the amino acid sequence and on multiple contributing influences from the crowded cellular milieu’ [42, p. 884].
The top-down causal explanation of protein folding is in terms of macromolecules and their components, and the hierarchical relations that apply to protein structure are delineated precisely (for a more complete discussion, see [43,44]). This does not prevent us from appealing to different sets of hierarchical relations where proteins are at a lower ‘level’ and causally explain higher level properties (e.g. with respect to organelles or the entire cell). It does require that we adopt a different representation of tops and bottoms because the four structural layers for proteins are not meant to be stratified ‘levels of organization’ across nature. An implication is that bottom-up and top-down causal explanations are not necessarily mutually exclusive options because they can be based on different representations of hierarchical relations rather than a single, correct stratification of the world.
4. Aspects of local ‘levels’ of organization
We have thus far observed that the hierarchical relations articulated for protein structure are not commensurate with the hierarchical relations invoked for other biological macromolecules, and that explanations of protein folding in terms of molecular chaperones constitute a well-defined (but ‘local’) top-down causal explanation. Hierarchical representation incommensurability of this kind is widespread within and across sciences and is displayed in the violation of expectations on the representation of part–whole relations (see also [45]). Instead of every part being assigned to a single ‘level’ and strictly nested within the whole at a higher level, some parts cannot be assigned to a ‘level’ and others occupy more than one. Organelles are represented as parts of cells but it is unclear that there is an ‘organelle level’ or simply an assortment of organelles within a cell. If cells are considered parts of organisms, then unicellular organisms are paradoxical since they are represented simultaneously at two ‘levels’. Ecological relationships among species suggest reversals of part–whole relationships. For example, microbiological populations and ecosystems are contained within our organs (e.g. the stomach and intestines). Ecosystem representations should not be nested within organs according to this expectation. Instead of a single hierarchy of part–whole relations where every part is a component of exactly one entity at each ‘level’ above it, proteins are often represented as cellular constituents but sometimes reside in the extracellular matrix. It is unclear whether the extracellular matrix should be represented at the cellular ‘level’. Finally, instead of every part being composed of parts at each ‘level’ beneath it, tissues are not composed exhaustively of cells, as they contain proteins, polysaccharides and other constituents external to the cells, and synapses are combinations of parts of two cells and intervening extracellular space (to highlight just two examples). The representation of compositional relations in these systems is not exhausted by immediately adjacent (stratified) ‘levels’. A primary reason why is that these hierarchically relations are articulated locally for particular domains of inquiry and are not designed to hold across all sciences (as universal, stratified levels should).
Although hierarchical representation incommensurability holds within and across different sciences to varying degrees, it is important to reiterate that the locality attributed to the structural ‘levels’ for proteins, and more generally for the violations of part–whole relations, is not equivalent to spatial locality. Proteins are everywhere in biological systems, so generalizations about their structural ‘levels’ license a variety of inferences and explanations. Here is one way to characterize this locality alongside its fecundity in scientific reasoning (e.g. the potential for general causal explanations): ‘Levels of organization can be thought of as local maxima of regularity and predictability in the phase space of alternative modes of organization of matter’ [15, p. 209]. The terms in this characterization pick out features we have already dwelt upon. ‘Local maxima of regularity and predictability’ refers to the domain specificity of ‘levels’ that are scientifically useful because they capture regular (robust) patterns of properties (e.g. the secondary structure of a protein highlights the significance of two organizational patterns resulting from hydrogen bonding), and facilitate generalizations and explanations (e.g. the role of molecular chaperones in folding). ‘Phase space’ is another way to refer to a representation where the variables map ‘modes of material [and functional] organization’ (e.g. the fourfold ‘levels’ of protein structure). This mapping always involves a selection of some set of variables from among a larger array: ‘we seek a set of reduced variables that efficiently capture the main features of … complicated behaviour’ [46, p. 186].
Different principles of selection yield different kinds of hierarchies, many of which are incommensurable across different sciences [15]. An organism might be decomposed according to physico-chemical principles or in terms of anatomical organs, cell types, developmental gradients or biochemical reactions. These decompositions sometimes yield parts with non-coincident spatial boundaries and thus no common measure for translating between them. The situation is compounded by the fact that these principles are often mixed: ‘This borrowing of criteria of individuation of parts from different and diverse theoretical perspectives is one factor that can make functional organization in general and biology in particular a conceptual morass at times’ [15, p. 184]. In summary, localized hierarchical representations facilitate scientific inquiry and causal explanation by extracting a subset of variables to define ‘levels of organization’ that exhibit regularity and predictability for natural phenomena represented in diverse ways.
Given that these hierarchical representations are primarily local and yet empirically robust, it is not advisable to seek a comprehensive account of ‘levels of organization’. A more germane philosophical strategy is to hunt for ‘aspects’ of hierarchical representations that operate within and across the sciences and characterize their associated assumptions [43,44]. One aspect is fundamentality: explanations that rely on hierarchical representations involve commitments to things being more or less fundamental [47]. This fundamentality is usually anchored to a particular hierarchical representation (e.g. ‘primary’ protein structure), which means that what counts as fundamental is a restricted set of properties depicted in the hierarchy (e.g. cell types or upstream metabolic reactions).
A second aspect involves the diversity of hierarchies: hierarchical relations can pertain to compositional (spatial) properties, functional (procedural) properties and/or abstract properties (e.g. directed graphs). Depending on the kinds of hierarchies involved, commitments of fundamentality vary. Other key factors manifested in the diversity of hierarchies include intrinsicality (how a system is represented as distinct from a surrounding environment) and temporality (how temporal relations are designated, whether via absolute chronology or via some type of staging or periodization). In the case of protein structure and folding, compositional relations in the structural hierarchy are salient and the representation of time is in terms of a very rapid chronology (milliseconds). Biochemical interactions arising from the distinctive side chains of amino acid residues in proteins are explanatorily fundamental. In contrast, the functional hierarchy of a gene network diagram indicates causal relations in terms of gene activation or repression, represented schematically and changing through temporal stages of specific embryonic processes (e.g. endomesoderm formation). Fundamentality is understood in terms of regulatory genes that reside high in cascades of network control.
Much more can be said about fundamentality and the diversity of hierarchies in biology [15,48–50]. In this context, the main message to take away from different commitments of fundamentality and diverse kinds of hierarchies is that the prevalence of hierarchical representation incommensurability modulates our interpretation of causation within hierarchical structures. Dependency relations can change as a consequence of adjusting abstract, spatial and temporal aspects of hierarchical representations, whether these pertain to compositional or functional organization. Deriving our understanding of top-down and bottom-up causation from the top-down and bottom-up causal explanations, which rely on empirically robust and yet ‘local’ hierarchical representations that are sometimes incommensurable, implies that an apposite metaphysical interpretation might be some form of pluralism.
5. ‘Levels of organization’ and pluralism
The assumption of hierarchical representation commensurability is a form of monism. In the face of diverse, local hierarchical classifications that are constructed according to different methods and for different aims, ‘monism holds that all such … accounts can be reconciled into a single unified account or that there is a single perspicuous representation system within which all correct accounts can be expressed’ [16, p. xv]. One rationale for this is a unified metaphysical perspective on nature: ‘The raison d'être of a useful metaphysics is to show how the separately developed and justified pieces of science (at a given time) can be fitted together to compose a unified world-view’ [51, p. 45]. Many philosophers seek to provide a unified, abstract characterization of ontology (e.g. the nature of composition or parts and wholes) from the concepts and theories found within and across different sciences.
If the materials that are used to construct this metaphysics are drawn from the epistemological successes of the sciences (a methodological stricture that can be debated), then hierarchical representation incommensurability presents an obstacle. A unified account of top-down causation does not emerge from the ubiquity of top-down causal explanations within and across the sciences. An initial construal of this ubiquity as a type of consilience—multiple, distinct lines of evidence supporting some unified explanation—does not withstand further scrutiny. These hierarchical representations do not offer a fully general account of features of reality; the four ‘levels’ of protein structure do not apply to all macromolecules and the top-down causal explanations that result from this hierarchical conception are localized to the domain of protein folding. They do not offer the prospect of a unified picture of ‘levels of organization’ in nature. Instead, the case of protein structure and folding point in the direction of a different metaphysical interpretation.
Some philosophers who hold a commitment to monism have drawn a negative conclusion about the metaphysical import of hierarchical representations: ‘We will deny … that the world comes in “levels”. Contemporary science … gives no interesting content to this metaphor, and so a metaphysics … should not reflect it’ [51, p. 54]. But this is simply untrue. Contemporary sciences give very interesting content to various conceptions of ‘levels’; they just do not yield a fully general or wholly unified account. Our discussion of protein structure and folding displays one example of interesting content—a robust fourfold conception of organizational ‘levels’ that is pertinent to understanding causation in the realm of macromolecules. If we recognize that these hierarchies are tailored to specific phenomena studied by different sciences with different methods, and are not meant to cohere in a global fashion, then the sciences affirm the existence (i.e. the reality) of manifold ‘levels’ or hierarchical layerings [52]. (One could debate whether the specific term ‘level’ should be retained or not.) The pervasiveness of hierarchical representation incommensurability could be a clue to the plurality of hierarchies present in the natural world.
What does it mean to offer a pluralist metaphysical interpretation of hierarchical causal explanations? Negatively, it means that there is no single, overarching picture of ‘levels of organization’ or hierarchical causation. Positively, it means that multiple accounts of ‘levels’ in different sciences capture significant features of reality and dependency relations in these hierarchical representations track a diversity of causal relationships. This perspective is motivated by the success of the epistemological practices in different sciences. It is not based on an appeal to our ignorance but from what we have learned about the world's diverse properties using divergent hierarchical representations in scientific reasoning. One might still argue that current science is incomplete or that pluralism is resolvable to monism in principle, even if it cannot be done in practice. Pluralism of the sort proffered here does not rule out these two possibilities, but both rejoinders move us away from the actual hierarchical representations and their discovered dependency relations that have been successful empirically within and across the sciences. In fact, to say that current scientific practices are incomplete with respect to interpreting the ubiquity of hierarchical explanations is to admit the absence of convergence on a univocal sense of causation within hierarchical structures.
One might object that the pluralist perspective laid out here is incoherent [51]. How can conflicting claims about hierarchical causation that emerge from incompatible hierarchical representations be considered a variety of realism? This might even be seen as a game-stopper or a form of quietism (i.e. there are severe limitations on drawing any metaphysical interpretations from the sciences). ‘If the world were fundamentally disunified, then discovery of this would be tantamount to discovering that there is not metaphysical work to be done’ [51, pp. 5–6]. ‘There is a legitimate role for metaphysics just insofar as the world is unified’ [53, p. 1818]. This is overly strict and an unwarranted view of metaphysical inquiry. It goes beyond a methodological dictum of seeking as unified a metaphysical picture as possible to the stronger claim that the absence of discovering one renders the project moot. Much turns on what is meant by ‘fundamentally’ disunified, as opposed to ‘locally’ disunified. Although the four structural ‘levels’ of protein and nucleic acid organization do not apply to all biological macromolecules, it does not follow that there is no legitimate metaphysical interpretation to be formulated from them. Pluralism is intended as a corrective to this ‘all or nothing’ attitude underwritten by monism (unification): ‘a commitment to avoid reliance on monist assumptions in interpretation or evaluation coupled with an openness to the ineliminability of multiplicity in some scientific contexts’ [16, p. xiii]. Pluralism as a methodology calls into question an expectation of unification, a presumption that the sciences aim at a (relatively) unified view of the world. And, as a consequence, we can explore a new array of possibilities that confront how realism and pluralism fit together given the variety of successful representational strategies contained in extant, mature sciences. This exploratory task will require a collaborative effort between scientists and philosophers because, instead of retreating to in principle considerations, questions about top-down causation must be tackled from actual, in practice scientific representation and explanation; i.e. how hierarchical relations and dependencies between ‘levels’ are established and facilitate powerful generalizations within and across sciences [3,52].
Pluralism also offers a possible resolution to the potential incongruence between reductionism and top-down causation; the apparent conflict between a claim that causes always operate from the bottom-up and a claim that causes sometimes operate from the top-down. If no unified metaphysical account of causation within hierarchies emerges from the epistemology of hierarchical causal explanations in scientific practice, then the contradiction can be illusory. Top-down causation and bottom-up causation can be fully compatible when the hierarchical representations are different, which encompasses the dependency relations among variables and what contours the aspects (such as intrinsicality and temporality) take in hierarchical explanations. Thus, pluralism respects the details of actual hierarchical causal explanations (including why several different senses might be extant), while offering a fruitful metaphysical interpretation of top-down causation that demonstrates why it is not in fundamental conflict with reductionism. Many different kinds of top-down causation (explanation) can coexist alongside of many different kinds of bottom-up causation (explanation) because there is a diversity of hierarchical relations in the world rather than a single, correct stratification of ‘levels’.
6. A productive interface for philosophy and science
Several consequences follow from the preceding discussion. In a recent critical evaluation of contemporary sociology, Christian Smith grounds his argument for personhood as an emergent aspect of human beings with a straightforward appeal to the ‘fact’ that the sciences have discovered a world stratified into ‘levels of organization’.
Reality…is not flat. Reality is stratified. It exists and operates, in fact, on many levels, each of which is governed by structures, processes and tendencies appropriate to its own level. The reality in which we live and participate (and of which we are partly composed) involves at the very least these many stratified levels: subatomic, atomic, molecular, chemical, biological, physiological, zoological, ecological, meteorological, mental, social, global, galactic and cosmological. At each of these levels different dynamics and mechanisms operate [7, pp. 34–35].
This is by no means a recent development. British emergentists also saw the world as ‘divided into discrete strata’ [9, §1.4]. Not surprisingly, Smith sees his project in terms of a choice between reductionism and emergence (‘the counter to reductionism is emergence’). We have now traced the inherent difficulties posed by hierarchical representation incommensurability for the layered view of nature. We also have observed how a pluralist stance calls into question whether there is always an either/or decision between reductionism and emergence. Much of Smith's project is built on a faulty understanding of the sciences and their utilization of hierarchical explanations. In this he is not alone.
Smith's argument shows that further issues are at stake. In addition to seeing the world as layered, there is an assumption that the sciences are organized to track this layering. ‘This is why we have the different scientific disciplines of physics, chemistry, biology, meteorology, physiology, psychology, sociology, astronomy and so on’ [7, p. 34]. Again, this not a new claim, and earlier emergentists were committed similarly: ‘To each level corresponds a special science, and the levels are arranged in terms of increasing organizational complexity of matter’ [9, §1.4]. Although there have been discussions about the hierarchical organization of empirical inquiry since Aristotle, infamously revived in Auguste Comte's positivist conception of scientific organization [54], the relationship between areas of science and hierarchical ‘levels’ is subtle and complex. For example, developmental biology tracks multiple ‘levels of organization’ simultaneously and classical mechanics applies to a variety of size scales that violate part–whole relationship requirements [55]. Hierarchical representation incommensurability and a pluralist interpretation of its metaphysical implications push us to capture, in a more adequate fashion, the ‘cross-level’ complexity of scientific disciplines.
Thus far, our analysis of hierarchical causal explanations and its implications for metaphysical interpretations of causation within hierarchies has produced two outcomes: (i) the undermining of arguments founded on faulty conceptions of stratified ‘levels’ in nature and (ii) a recognition that the organization of scientific disciplines is more complex than a layered cake of physics, chemistry, biology, psychology and sociology. Others include a reconfiguration of the task of philosophy, which is often assumed to demand a form of monism: ‘to understand how things in the broadest possible sense of the term hang together in the broadest possible sense of the term’ [56, p. 1]. The above analysis instantiates a model of the relationship between the sciences and philosophy termed reasoning explication: reconstructing and evaluating the kinds of reasoning used in scientific investigation, such as hierarchical causal explanation, to identify characteristic strengths and latent biases [57]. This model operates via a standard mode of inquiry in philosophical research, abstraction: the excluding of concrete particulars (e.g. bats and birds) in order to comprehend the significance of scientific claims and methodology over different degrees of exclusion (e.g. top-down control of prey in an ecological hierarchy). It is pursued with two aims in mind. First, methodologically, reasoning explication can indicate more or less fruitful lines of inquiry, modelling strategies and data gathering (e.g. the value of switching between hierarchical representations or adjusting their aspects in different ways). Second, epistemologically, reasoning explication can facilitate theory construction, data interpretation and the evaluation of explanations (e.g. the compatibility of hierarchical causal explanations and varying standards for explanations due to different representations).
Reasoning explication is therefore a productive interface between the sciences and philosophy. It is one in which the sciences benefit from the resources of philosophy through the advantages of abstraction, as seen here with respect to the locality of hierarchical explanations, their diverse aspects, and ways in which top-down causation can fit with a reductionist viewpoint. It is also one in which philosophy benefits from the resources of the sciences through a pluralist approach to metaphysics that can incorporate the empirically successful yet diverse findings within and across scientific disciplines. Additionally, philosophy is cautioned against too quickly assimilating diverse examples of hierarchical causal explanation into standard discussions about top-down causation, thereby obscuring the salient scientific issues in these examples that might suggest novel philosophical applications. A reciprocal relationship of this kind, where philosophical analyses of how science works offer genuine resources for ongoing empirical inquiry and epistemic dimensions of actual scientific practice inform metaphysical questions in new ways that advance our philosophical understanding, is exactly where interdisciplinary collaboration between philosophy and science is laudable.
Questions about the meaning and status of pluralism as a philosophical interpretation of scientific reasoning remain. There are clear differences between an integrative pluralism where the plurality exhibited by the sciences can be locally integrated when explaining phenomena [58] and the representational pluralism adumbrated here where the plurality often cannot be integrated due to hierarchical representation incommensurability. But arguments over these differences and other forms of pluralism not canvassed here (including radical versions, such as social constructionism), even if valuable in their own right, take us away from the key point. Top-down causal explanations rely on representing natural phenomena with hierarchical relations (i.e. with tops and bottoms), and taking a temporal ordering of dependency (i.e. causation) between features of these representations to be explanatory. This reliance, also found in bottom-up (reductive) causal explanations, means that any metaphysical interpretations derived from these hierarchical causal explanations (independent of disagreements about the nature of causation) must address the question of hierarchical representation commensurability. Thus, the burden of sorting through differences in pluralist positions, and the need to further articulate the details of specific viewpoints (integrative, representational or otherwise), should be undertaken with this question at the forefront.
We have observed one instance that suggests this incommensurability in the case of biological macromolecules even though a local top-down causal explanation of protein folding is precisely defined. In combination with more general considerations about mereological relationships, there is reason to doubt the commonplace view that science has shown the world to be divided into stratified layers. Hierarchical representations deployed in top-down explanations are not articulated in such a way as to cohere or contrast with others because they are related to specific domains of inquiry. A top-down causal explanation of some natural phenomena does not foreclose the possibility of a bottom-up causal explanation of the same phenomena; questions of congruence between reductionism and top-down causation turn on the details of hierarchical representation. On a pluralist metaphysical interpretation, these local ‘levels’ are real but do not correspond to global, nominalized designations and encourage a more tempered view of whether the sciences deliver a relatively unified world picture. The rejection of assumptions about causation across universal ‘levels’ of organization that do not correspond to the epistemological details found in scientific practice [52,59] and the development of more sophisticated accounts of hierarchical relations [60,61] are the most promising routes to inferring metaphysical conclusions from the local layers of organization that arise from diverse and incompatible hierarchies represented within and across the sciences. On the reasoning explication model of how science and philosophy interact [57], these types of analyses also hold methodological and epistemological promise for advancing ongoing investigation into the natural world.
Acknowledgements
Ingo Brigandt, Tom Doyle, Andreas Hüttemann, Marie Kaiser and Leia Rollag provided me with helpful criticisms and suggestions on a distant ancestor of this paper. Insightful comments on a more recent version were supplied by Robert Bishop, Tom Doyle, Alan Padgett, Leia Rollag and Michael Silberstein. I also benefited from the detailed comments provided by five anonymous reviewers for the journal. Thanks to the organizers of the September 2010 symposium ‘Top-Down Causation: An Integrating Theme Within and Across the Sciences’ and symposium participants for feedback. Critical comments and discussion were received from audiences at the workshop ‘Science Engages Metaphysics: Emergence, Reduction and Explanation’ (July 2010), and the Society for the Metaphysics of Science session at the Central Division meeting of the American Philosophical Association (March 2011), where some of these themes were advanced. I am grateful to Colin Klein for sharing his unpublished manuscript ‘Against levels’, which I profited from while revising the manuscript.
References
- 1.Böhm S., Wells K., Kalko E. 2011. Top-down control of herbivory by birds and bats in the canopy of temperate broad-leaved oaks (Quercus robur). PLoS ONE 6, e17857. 10.1371/journal.pone.0017857 (doi:10.1371/journal.pone.0017857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Csermely P., Korcsmáros T., Kovács I. A., Szalay M. S., Sőti C. 2008. Systems biology of molecular chaperone networks. In The biology of extracellular molecular chaperones (eds Chadwick D. J., Goode J.), pp. 45–58 Chichester, UK: Wiley; [DOI] [PubMed] [Google Scholar]
- 3.Woodward J. 2003. Making things happen: a theory of causal explanation. New York, NY: Oxford University Press [Google Scholar]
- 4.Craver C. F., Bechtel W. 2007. Top-down causation without top-down causes. Biol. Phil. 22, 547–563 10.1007/s10539-006-9028-8 (doi:10.1007/s10539-006-9028-8) [DOI] [Google Scholar]
- 5.Campbell D. T. 1974. ‘Downward causation’ in hierarchically organised biological systems. In Studies in the philosophy of biology: redution and related problems (eds Ayala F. J., Dobzhansky T.), pp. 179–186 Berkeley, CA: University of California Press [Google Scholar]
- 6.Corradini A., O'Connor T. 2010. Emergence in science and philosophy. New York, NY: Routledge [Google Scholar]
- 7.Smith C. 2010. What is a person? Rethinking humanity, social life, and the moral good from the person up. Chicago, IL: University of Chicago Press [Google Scholar]
- 8.Schaffer J. 2003. Is there a fundamental level? Noûs 37, 498–517 10.1111/1468-0068.00448 (doi:10.1111/1468-0068.00448) [DOI] [Google Scholar]
- 9.O'Connor T., Wong H. Y. 2009. Emergent properties. In The Stanford encylopedia of philosophy (ed. Zalta E. N.). See http://plato.stanford.edu/entries/properties-emergent/ [Google Scholar]
- 10.Kim J. 2005. Physicalism, or something near enough. Princeton, NJ: Princeton University Press [Google Scholar]
- 11.Marras A. 2007. Kim's supervenience argument and nonreductive physicalism. Erkenntnis 66, 305–327 10.1007/s10670-006-9023-0 (doi:10.1007/s10670-006-9023-0) [DOI] [Google Scholar]
- 12.Block N. 2003. Do causal powers drain away? Phil. Phenomenol. Res. 117, 133–150 10.1111/j.1933-1592.2003.tb00029.x (doi:10.1111/j.1933-1592.2003.tb00029.x) [DOI] [Google Scholar]
- 13.Godfrey-Smith P. 2009. Causal pluralism. In The Oxford handbook of causation (eds Beebee H., Hitchcock C., Menzies P.), pp. 326–337 New York, NY: Oxford [Google Scholar]
- 14.Brigandt I., Love A. C. 2008. Reductionism in biology. In The Stanford encyclopedia of philosophy (ed. Zalta E. N.). See http://plato.stanford.edu/entries/reduction-biology/ [Google Scholar]
- 15.Wimsatt W. C. 2007. Re-engineering philosophy for limited beings: piecewise approximations to reality. Cambridge, MA: Harvard University Press [Google Scholar]
- 16.Kellert S. H., Longino H. E., Waters C. K. 2006. Introduction: the pluralist stance. In Scientific pluralism. (eds Kellert S. H., Longino H. E., Waters C. K.), pp. vii–xxix Minneapolis, MN: University of Minnesota Press [Google Scholar]
- 17.Tompa P. 2009. Structure and function of intrinsically disordered proteins. New York, NY: CRC Press [Google Scholar]
- 18.Campbell N. A., Reece J. B. 2002. Biology, 6th edn. San Francisco, CA: Benjamin Cummings [Google Scholar]
- 19.Onoa B., Tinoco I. 2004. RNA folding and unfolding. Curr. Opin. Struct. Biol. 14, 374–379 10.1016/j.sbi.2004.04.001 (doi:10.1016/j.sbi.2004.04.001) [DOI] [PubMed] [Google Scholar]
- 20.Mathews D. H., Moss W. N., Turner D. H. 2010. Folding and finding RNA secondary structure. Cold Spring Harb. Perspect. Biol. 2, a003665. 10.1101/cshperspect.a003665 (doi:10.1101/cshperspect.a003665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedman R. B. 1999. Protein folding in the cell. In Protein folding (ed. Creighton T. E.), pp. 455–539 New York, NY: W. H. Freeman and Company [Google Scholar]
- 22.Hartl F., Hayer-Hartl M. 2009. Converging concepts of protein folding in vitro and in vivo. Nat. Struct. Mol. Biol. 16, 574–581 10.1038/nsmb.1591 (doi:10.1038/nsmb.1591) [DOI] [PubMed] [Google Scholar]
- 23.Service R. F. 2008. Problem solved* (*sort of). Science 321, 784–786 10.1126/science.321.5890.784 (doi:10.1126/science.321.5890.784) [DOI] [PubMed] [Google Scholar]
- 24.Anfinsen C. B. 1973. Principles that govern the folding of protein chains. Science 181, 223–230 10.1126/science.181.4096.223 (doi:10.1126/science.181.4096.223) [DOI] [PubMed] [Google Scholar]
- 25.Honig B. 1999. Protein folding: from the Levinthal paradox to structure prediction. J. Mol. Biol. 293, 283–293 10.1006/jmbi.1999.3006 (doi:10.1006/jmbi.1999.3006) [DOI] [PubMed] [Google Scholar]
- 26.Clark P. L. 2004. Protein folding in the cell: reshaping the folding funnel. Trends Biochem. Sci. 29, 527–534 10.1016/j.tibs.2004.08.008 (doi:10.1016/j.tibs.2004.08.008) [DOI] [PubMed] [Google Scholar]
- 27.Frydman J. 2001. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu. Rev. Biochem. 70, 603–647 10.1146/annurev.biochem.70.1.603 (doi:10.1146/annurev.biochem.70.1.603) [DOI] [PubMed] [Google Scholar]
- 28.Feder M. E., Hofmann G. E. 1999. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu. Rev. Physiol. 61, 243–282 10.1146/annurev.physiol.61.1.243 (doi:10.1146/annurev.physiol.61.1.243) [DOI] [PubMed] [Google Scholar]
- 29.Liscalijet I. M., Kleizen B., Braakmen I. 2005. Studying protein folding in vivo. In Protein folding handbook part II (eds Buchner J., Kiefhaber T.), pp. 73–104 Weinheim, Germany: Wiley-VCH [Google Scholar]
- 30.Ellis R. J. 2001. Macromolecular crowding: obvious but underappreciated. Trends Biochem. Sci. 26, 597–604 10.1016/S0968-0004(01)01938-7 (doi:10.1016/S0968-0004(01)01938-7) [DOI] [PubMed] [Google Scholar]
- 31.Homouz D., Perham M., Samiotakis A., Cheung M. S., Wittung-Stafshede P. 2008. Crowded, cell-like environment induces shape changes in aspherical protein. Proc. Natl Acad. Sci. USA 105, 11 754–11 759 10.1073/pnas.0803672105 (doi:10.1073/pnas.0803672105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lund P. A., Ellis R. J. 2008. The chaperone function: meanings and myths. In The biology of extracellular molecular chaperones (eds Chadwick D. J., Goode J.), pp. 23–44 Chichester, UK: Wiley; [DOI] [PubMed] [Google Scholar]
- 33.Albanese V., Yam A. Y.-W., Baughman J., Parnot C., Frydman J. 2006. Systems analyses reveal two chaperone networks with distinct functions in eukaryotic cells. Cell 124, 75–88 10.1016/j.cell.2005.11.039 (doi:10.1016/j.cell.2005.11.039) [DOI] [PubMed] [Google Scholar]
- 34.McClellan A. J., Scott M. D., Frydman J. 2005. Folding and quality control of the VHL tumor suppressor proceed through distinct chaperone pathways. Cell 121, 739–748 10.1016/j.cell.2005.03.024 (doi:10.1016/j.cell.2005.03.024) [DOI] [PubMed] [Google Scholar]
- 35.Zhao R., et al. 2005. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the Hsp90 chaperone. Cell 120, 715–727 10.1016/j.cell.2004.12.024 (doi:10.1016/j.cell.2004.12.024) [DOI] [PubMed] [Google Scholar]
- 36.Ellis R. J. 1998. Steric chaperones. Trends Biochem. Sci. 23, 43–45 10.1016/S0968-0004(98)01175-X (doi:10.1016/S0968-0004(98)01175-X) [DOI] [PubMed] [Google Scholar]
- 37.Siegers K., Bracher A., Hartl F. U. 2005. Structure and function of GimC/Prefoldin. In Protein folding handbook part II (eds Buchner J., Kiefhaber T.), pp. 756–767 Weinheim, Germany: Wiley-VCH [Google Scholar]
- 38.Tang Y.-C., Chang H.-C., Roeben A., Wischnewski D., Wischnewski N., Kerner M. J., Hartl F. U., Hayer-Hartl M. 2006. Structural features of the GroEL-GroES nano-cage required for rapid folding of encapsulated protein. Cell 125, 903–914 10.1016/j.cell.2006.04.027 (doi:10.1016/j.cell.2006.04.027) [DOI] [PubMed] [Google Scholar]
- 39.Tang Y.-C., Chang H.-C., Chakraborty K., Hartl F. U., Hayer-Hartl M. 2008. Essential role of the chaperonin folding compartment in vivo. EMBO J. 27, 1458–1468 10.1038/emboj.2008.77 (doi:10.1038/emboj.2008.77) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maisnier-Patin S., Roth J. R., Fredriksson A., Nystrom T., Berg O. G., Andersson D. I. 2005. Genomic buffering mitigates the effects of deleterious mutations in bacteria. Nat. Genet. 37, 1376–1379 10.1038/ng1676 (doi:10.1038/ng1676) [DOI] [PubMed] [Google Scholar]
- 41.van der Vies S. M., Gatenby A., Viitanen P. V., Lorimer G. H. 1993. Molecular chaperones and their role in protein assembly. In Protein folding in vivo and in vitro (ed. Cleland J. L.), pp. 72–83 Washington, DC: American Chemical Society [Google Scholar]
- 42.Dobson C. M. 2003. Protein folding and misfolding. Nature 426, 884–890 10.1038/nature02261 (doi:10.1038/nature02261) [DOI] [PubMed] [Google Scholar]
- 43.Hütteman A., Love A. C. 2011. Aspects of reductive explanation in biological science: intrinsicality, fundamentality, and temporality. Br. J. Phil. Sci. 62, 519–549 10.1093/bjps/axr006 (doi:10.1093/bjps/axr006) [DOI] [Google Scholar]
- 44.Love A. C., Hüttemann A. 2011. Comparing part-whole reductive explanations in biology and physics. In Explanation, prediction, and confirmation (eds Dieks D., Wenceslao J. G., Hartmann S., Uebel T., Weber M.), pp. 183–202 Dordrecht, The Netherlands: Springer [Google Scholar]
- 45.Beckner M. 1974. Reduction, hierarchies, and organicism. In Studies in the philosophy of biology: reduction and related problems (eds Ayala F. J., Dobzhansky T.), pp. 163–177 Berkeley, CA: University of California Press [Google Scholar]
- 46.Wilson M. 2006. Wandering significance: an essay on conceptual behavior. New York, NY: Oxford University Press [Google Scholar]
- 47.Sarkar S. 1998. Genetics and reductionism. New York, NY: Cambridge University Press [Google Scholar]
- 48.Valentine J. W., May C. L. 1996. Hierarchies in biology and paleontology. Paleobiology 22, 23–33 [Google Scholar]
- 49.Love A. C. 2006. Evolutionary morphology and Evo-devo: hierarchy and novelty. Theory Biosci. 124, 317–333 10.1016/j.thbio.2005.11.006 (doi:10.1016/j.thbio.2005.11.006) [DOI] [PubMed] [Google Scholar]
- 50.Salthe S. N. 1985. Evolving hierarchical systems: their structure and representation. New York, NY: Columbia University Press [Google Scholar]
- 51.Ross D., Ladyman J., Spurrett D. 2007. In defence of scientism. In Every thing must go: metaphysics naturalized (eds Ladyman J., Ross D.), pp. 1–65 New York, NY: Oxford University Press [Google Scholar]
- 52.Craver C. F. 2007. Explaining the brain: mechanisms and the mosaic unity of neuroscience. New York, NY: Oxford University Press [Google Scholar]
- 53.Ladyman J., Ross D. 2010. Authors' response. Metascience. 19, 179–185 [Google Scholar]
- 54.Comte A. 1988. Introduction to positive philosophy [transl. F. Frederick, 1830]. Indianapolis, IN: Hackett [Google Scholar]
- 55.Bishop R. C. 2008. Downward causation in fluid convection. Synthese 160, 229–248 10.1007/s11229-006-9112-2 (doi:10.1007/s11229-006-9112-2) [DOI] [Google Scholar]
- 56.Sellars W. 1963. Philosophy and the scientific image of man. In Science, perception and reality, pp. 1–40 London, UK: Routledge and Kegan Paul [Google Scholar]
- 57.Love A. C. 2008. From philosophy to science (to natural philosophy): evolutionary developmental perspectives. Quart. Rev. Biol. 83, 65–76 10.1086/529564 (doi:10.1086/529564) [DOI] [PubMed] [Google Scholar]
- 58.Mitchell S. D. 2003. Biological complexity and integrative pluralism. New York, NY: Cambridge University Press [Google Scholar]
- 59.Glennan S. 2010. Mechanisms, causes, and the layered model of the world. Phil. Phenomenol. Res. 131, 362–381 10.1111/j.1933-1592.2010.00375.x (doi:10.1111/j.1933-1592.2010.00375.x) [DOI] [Google Scholar]
- 60.Gillett C. 2010. Moving beyond the subset model of realization: the problem of qualitative distinctness in the metaphysics of science. Synthese 177, 165–192 10.1007/s11229-010-9840-1 (doi:10.1007/s11229-010-9840-1) [DOI] [Google Scholar]
- 61.Bishop R. C., Atmanspacher H. 2006. Contextual emergence in the description of properties. Found. Phys. 36, 1753–1777 10.1007/s10701-006-9082-8 (doi:10.1007/s10701-006-9082-8) [DOI] [Google Scholar]