Abstract
The myelodysplastic Syndromes (MDS) are a group of clonal hematopoietic stem cell diseases characterized by cytopenia(s), dysplasia in one or more of the major myeloid cell lines, ineffective hematopoiesis, and increased risk of development of acute myeloid leukemia. The classification and the diagnostic criteria have been redefined by the recent World Health Organization Classification of Tumors – International Agency for Research on Cancer for Hematopoietic and Lymphoid Tissues. The myelodysplastic syndromes are now classified into the following categories – refractory cytopenia with unilineage dysplasia, refractory anemia with ring sideroblasts, refractory cytopenia with multilineage dysplasia, refractory anemia with excess blasts, myelodysplastic syndrome associated with isolated del (5q), myelodysplastic syndrome – unclassifiable, and childhood myelodysplastic syndrome. The clinicopathologic features, morphology, differential diagnosis, immunophenotyping, cytogenetics, prognosis and predictive factors are presented in the light of recent World Health Organization Classification of Tumors – International Agency for Research on Cancer.
Keywords: myelodysplastic syndromes, leukemia
Introduction
The myelodysplastic syndromes (MDS) are a group of clonal hematopoietic stem cell diseases characterized by cytopenia(s), dysplasia in one or more of the major myeloid cell lines, ineffective hematopoiesis, and increased risk of development of acute myeloid leukemia (AML).1–3 The thresholds for cytopenia(s) as recommended in the International Prognostic Scoring System (IPSS) for risk stratification in the MDS are hemoglobin <10 g/dl, absolute neutrophil count (ANC) <1.8 × 109/L and platelets <100 × 109/L.4,5 Values above these thresholds are not exclusionary for a diagnosis of MDS if definitive morphologic or cytogenetic findings are present.6 Myeloblasts in the peripheral blood and bone marrow are <20%. A working group of the World Health Organization (WHO) recently proposed a new classification of MDS (Table 1), based on a significant modification of the original FAB proposals. The classification system as proposed by FAB included the following subtypes – refractory anemia (RA), refractory anemia with ring sideroblasts (RARS), refractory anemia with excess blasts (RAEB), refractory anemia with excess blasts in transformation (RAEBT), and chronic myelomonocytic leukemia (CMML). In the recent WHO classification, CMML and RAEB-T were removed from the MDS classification and RAEB was split into two groups with medullary blast counts below and above 10%. In addition, a group of patients with less than 5% medullary blasts but evidence of multilineage dysplasia was defined. MDS patients with 5q – as the sole chromosomal anomaly were also considered a separate group. There is a significant difference in prognosis between RAEB I and RAEB II, as well as a difference between refractory anemia and multilineage dysplasia. Furthermore, patients with 5q – anomaly had a much better prognosis than other WHO subtypes, but this was only true for patients with a medullary blast count below 5%. In summary, the WHO classification appears to define morphological subgroups that are more homogeneous with respect to prognosis than the FAB subtypes.
Table 1.
Recent WHO 2008 classification of myelodysplastic syndromes11
| Disease | Blood findings | Bone marrow findings |
|---|---|---|
| Refractory cytopenia with unilineage dysplasia (RCUD) | Unicytopenia or bicytopenia$ | Unilineage dysplasia: ≥10% of cells in one myeloid lineage |
| No or rare blasts (<1%)# | ||
| Refractory anemia (RA); | <5% blasts | |
| Refractory neutropenia (RN); | <15% erythroid precursors are ring sideroblasts | |
| Refractory thrombocytopenia (RT) | ||
| Refractory anemia with ring sideroblasts (RARS) | Anemia | >15% erythroid precursors are ring sideroblasts |
| No blasts | Erythroid dysplasia only <5% blasts | |
| Refractory cytopenia with multilineage dysplasia (RCMD) | Cytopenia(s) | Dysplasia in >10% of cells in ≥two myeloid lineages |
| No or rare blasts (<1%)# | ||
| No Auer rods | <5% blasts in marrow No Auer rods | |
| <1 × 109/L monocytes | ±15% ring sideroblasts | |
| Refractory anemia with excess blasts-1 (RAEB-1) | Cytopenia(s) | Unilineage or multilineage dysplasia |
| <5% blasts# | 5%–9% blasts# | |
| No Auer rods | No Auer rods | |
| <1 × 109/L monocytes | ||
| Refractory anemia with excess blasts-2 (RAEB-2) | Cytopenia(s) | Unilineage or multilineage dysplasia |
| 5%–19% blasts | 10%–19% blasts | |
| Auer rods ± | Auer rods ± | |
| <1 × 109/L monocytes | ||
| Myelodysplastic syndrome – unclassified (MDS-U) | Cytopenias | Unequivocal dysplasia in less than 10% of cells in one or more myeloid cell lines when accompanied by cytogenetic abnormality considered as presumptive evidence for diagnosis of MDS <5% blasts |
| <1% blasts# | ||
| MDS associated with isolated del (5q) | Anemia | Normal to increased megakaryocytes with hypolobated nuclei <5% blasts Isolated del (5q) cytogenetic abnormality |
| Usually normal or increased platelet count | ||
| No or rare blasts (<1%) | No Auer rods |
Notes:
Bicytopenia may occasionally be observed. Cases with pancytopenia should be classified as MDS-U.
If the marrow myeloblast percentage is <5% but there are 2%–4% myeloblasts in the blood, the diagnostic classification is RAEB-1. Cases of RCUD and RCMD with 1% myeloblasts in the blood should be classified as MDS, U.
Although progression to AML is the natural course in many cases of MDS, the percentage of patients who progress varies substantially in the various subtypes. A higher percentage of patients with increased myeloblasts transforms into AML.7,8 The recurring chromosomal abnormalities and their frequency in MDS at diagnosis is illustrated in Table 2A scoring system for predicting survival and evolution to AML based on percent of BM blasts, type of cytogenetic abnormalities, and degree and number of cytopenias has been proposed by International Prognostic Scoring System (IPSS) for MDS4,5 (Table 3). Treatment is based on several factors including age, prior history of a myelodysplastic syndrome, overall clinical assessment and tempo of the process.
Table 2.
Recurring chromosomal abnormalities and their frequency in myelodysplastic syndromes at diagnosis11
| Abnormality | MDS | t-MDS |
|---|---|---|
| Unbalanced | ||
| +8 | 10% | |
| −7 or del (7q) | 10% | 50% |
| −5 or del (5q) | 10% | 40% |
| del (20q) | 5%–8% | |
| −Y | 5% | |
| i(17q) or t(17p) | 3%–5% | |
| −13 or del (13q) | 3% | |
| del (11q) | 3% | |
| del (12p) or t(12p) | 3% | |
| del (9q) | 1%–2% | |
| idic(X)(q13) | 1%–2% | |
| Balanced | ||
| t(11;16)(q23;p13.3) | 3% | |
| t(3;21)(q26.2;q22.1) | 2% | |
| t(1;3)(p36.3;q21.2) | 1% | |
| t(2;11)(p21;q23) | 1% | |
| inv(3)(q21q26.2) | 1% | |
| t(6;9)(p23;q34) | 1% |
Abbreviation: MDS, myelodysplastic syndrome.
Table 3.
| Score | 0 | 0.5 | 1 | 1.5 | 2 |
|---|---|---|---|---|---|
| Prognostic variables | |||||
| % bone marrow blasts | <5% | 5%–10% | 11%–19% | 20%–30%* | |
| Karyotype** | Good | Intermediate | Poor | ||
| Cytopenias*** | 0–1 | 2–3 | |||
Notes:
This group is recognized as AML in the WHO classification;
Karyotype: Good = normal, −Y, del (5q), del (20q); Poor = complex (≥3 abnormalities) or chromosome 7 anomalies; Intermediate = other abnormalities;
Cytopenias: Hgb <10 g/dL; Neutrophils <1.8 × 109/L; Platelets <100 × 109/L.
Abbreviations: MDS, myelodysplastic syndrome; AML, acute myeloid leukemia; WHO, world health organization.
The annual incidence of myelodysplastic syndromes is 3–5/100000 persons but rising to >20/100000 among those over the age of 70 years.9,10 MDS principally occurs in older adults with a median age of 70 years. There is a male predominance.
Clinical features
The majority of patients present with symptoms related to cytopenia(s) – hemorrhage, recurrent infection, fatigue, dyspnea, gingival bleeding and hematomas. Most patients are anemic and transfusion dependent.11 Less frequent are neutropenia or thrombocytopenia. Organomegaly is infrequently observed. Approximately 10%–40% of the patients progress to AML and 20%–40% of the patients die of infection or bleeding, or both.
Etiology
Primary MDS occurs without a known history of chemotherapy or radiation exposure. Possible etiologies for primary MDS include benzene exposure at levels above the minima allowed by most government agencies, cigarette smoking, exposure to agricultural chemicals or solvents and family history of hematopoietic neoplasms.12 Secondary MDS is usually seen under 50 years of age and is due to chemotherapy, radiation therapy or environmental mutagens.
Morphology
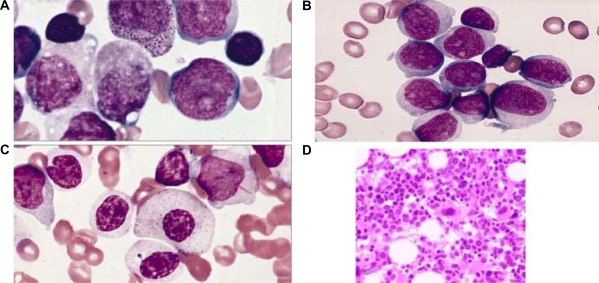
The morphological classification of MDS is principally based on the percent of blasts in the bone marrow (BM) and peripheral blood (PB), the type and degree of dysplasia and the presence of ring sideroblasts.1 To determine the blast percentage in the BM, a 500-cell differential of all nucleated cells in a smear or trephine imprint is recommended and in the PB, a 200-leukocyte differential. In severely cytopenic patients, buffy coat smears of PB may facilitate performing the differential. The characteristics of dysplasia are relevant when distinguishing between various types of MDS and may be important in predicting biology. Persistent cytopenia without dysplasia and without one of the specific cytogenetic abnormalities considered as presumptive evidence of MDS should be viewed as the recently described “idiopathic cytopenia of undetermined significance” (ICUS).13 The recommended requisite percentage of cells manifesting dysplasia to qualify as significant is ≥10% in the erythroid precursors and granulocytes.14 Significant megakaryocyte dysplasia is defined as ≥10% dysplastic megakaryocytes based on evaluation of at least 30 megakaryocytes in smears or sections. Dyserythropoiesis is manifest principally by alterations in the nucleus including budding, internuclear bridging, karyorrhexis, multinuclearity (Figure 2A, 2B and 2C) and megaloblastoid changes (Figure 2A and 2B); cytoplasmic features include ring sideroblasts, vacuolization and periodic acid-Schiff positivity, either diffuse or granular. Dysgranulopoiesis is characterized primarily by nuclear hypolobation (pseudo Pelger–Huet) and hypersegmentation, cytoplasmic hypogranularity, pseudo Chediak–Higashi granules and small size. Megakaryocyte dysplasia is characterized by micro-megakaryocytes with hypolobated nuclei, nonlobated nuclei in megakaryocytes of all sizes, and multiple, widely separated nuclei. The characteristics of dysplasia may be relevant in predicting biology of myelodysplastic disorder and the relationship to specific cytogenetic abnormalities, eg, 5q-syndrome.6 The significance of Auer rod in myeloid disorders is uncertain. In the revised FAB classification of 1982 it was viewed as evidence of a high grade MDS, refractory anemia with excess of blasts in transformation (RAEBT), irrespective of the blast percentage in the PB or BM.1 In the prior WHO Classification of the MDS it was considered as evidence of RAEB-2 or CMML-2 in the context of MDS/MPN regardless of blast percentage. This concept is retained in the present classification. The BM in MDS is usually hypercellular or normocellular; the cytopenias result from the ineffective hematopoiesis. Histologically, aggressive MDS may be characterized by the presence of aggregates (3–5 cells) or clusters (>5 cells) of blasts in BM biopsies usually localized in the central portion of the BM away from the vascular structures and endosteal surfaces of the bony trabeculae. These are frequently present in RAEB.
Figure 2.
A) Leishman Stain (×100 magnification). Showing dyserythropoiesis. B) May–Grunwald Giemsa Stain (×100 magnification). Arrow showing binucleate erythroblast. C) Leishman Stain (×200 magnification). Showing multinucleate erythroblast and erythroid dysplasia.
Other investigations reveal normal-to-high serum iron and folic acid levels. The Total Iron Binding Capacity (TIBC) may be normal-to-decreased. There is a decrease in the number of T-lymphocytes (CD4 and CD8) while the B-lymphocytes are quantitatively normal. Serum immunoglobulins are increased and circulating immune complexes are frequently present.15
Differential diagnosis
Several nutritional, toxic and other factors including essential element deficiencies and exposure to heavy metals, particularly arsenic and several commonly used drugs and biologic agents may cause myelodysplastic changes.16 Other causes for dyserythropoiesis include congenital hematological disorders, Parvovirus B19 infection and immunosuppressive agent mycophenolate mofetil. Chemotherapeutic agents may result in marked dysplasia of all myeloid cell lineages. Paroxysmal nocturnal hemoglobinuria may also present with features similar to MDS.
Variants
Hypoplastic MDS
In approximately 10% of the cases the BM is hypocellular. These cases have been referred to as hypoplastic MDS. The BM cellularity is <30% or <20% in patients over 60 years of age. This group has no individual prognostic significance. The differential diagnosis for this group includes aplastic anemia, toxic myelopathy and autoimmune disorders. Antithymocyte globulin and other therapies used for aplastic anemia are used as treatment options in this subgroup.16
MDS with myelofibrosis
Significant degrees of myelofibrosis are observed in approximately 10% of the cases of MDS. These cases have been referred to as MDS with fibrosis.17 Most of these cases have an excess of blasts and an aggressive clinical course. Laboratory investigations reveal pancytopenia, hypocellular bone marrow with fibrosis, trilineage dysplasia, small megakaryocytes with hypolobated nuclei and absence of hepatomegaly with prominent splenomegaly. Increase in fibrosis is due to liberation of cytokines such as TGF-β and PDGF from dysplastic megakaryocytes.15 The differential diagnosis includes myelofibrosis with myeloid metaplasia, chronic myelocytic leukemia and acute megakaryocytic leukemia.
Classification
Refractory cytopenia with unilineage dysplasia
Refractory anemia with unilineage dysplasia (RCUD) is intended to encompass those myelodysplastic syndromes which present with a refractory cytopenia with unilineage dysplasia and includes refractory anemia (RA), refractory neutropenia (RN) and refractory thrombocytopenia (RT). Refractory bicytopenia may be included in the RCUD category if accompanied by unilineage dysplasia. Refractory pancytopenia is placed in a category of myelodysplastic syndrome, unclassifiable (MDS-U). The recommended level for dysplasia is ≥10% of the cell lineage affected. The recommended levels for defining cytopenias are hemoglobin <10 g/dL, absolute neutrophil count (ANC) <1.8 × 109/L and platelet count <100 × 109/L.4,5 The presence of peripheral blood blasts essentially excludes a diagnosis of RCUD although in an occasional case a rare blast may be identified.
Refractory cytopenia with unilineage dysplasia comprises 10%–20% of the cases of MDS.18 The median age of presentation is around 65–70 years. There is no significant sex predilection. Majority of the RCUD cases are RA. Refractory neutropenia and refractory thrombocytopenia are rare. The presenting symptoms are related to the type of cytopenia.
Refractory anemia
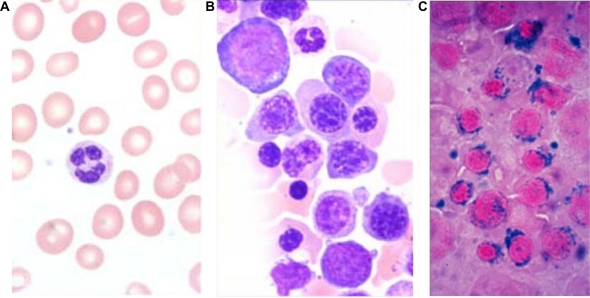
The peripheral blood picture in refractory anemia shows normocytic or macrocytic with normochromic red blood cells. There is variable anisopoikilocytosis. Blasts are rarely seen and if present, account for <1% of the white blood cells. The neutrophils and platelets are usually normal in number and morphology. The bone marrow is usually hypercellular with erythroid hyperplasia. Dyserythropoiesis varies from slight to moderate with evidence of dysplasia in 10% or more of the erythroid precursors (Figure 1A, 2A, 2B, 2C). Ring sideroblasts may be present but are <15% of erythroid precursors. Myeloblasts account for <5% of the nucleated BM cells. The neutrophils and megakaryocytes are normal or may show minimal dysplasia, but always <10% in either cell line.
Figure 1.
Refractory cytopenia with unilineage dysplasia (RCUD). Bone marrow aspirate (Wright–Giemsa – 100x). A) Refractory anemia – showing dyserythropoiesis with cytoplasmic vacuolation. B) Refractory thrombocytopenia – showing dysmegakaryopoiesis with multinucleation.
Cytogenetic abnormalities associated with RA include del (20q), +8 and abnormalities of chromosome 5 and/or chromosome 7. Approximately 90%–95% of patients with refractory anemia have low or intermediate International Prognostic Scoring System (IPSS).4,5
Refractory neutropenia
Refractory neutropenia is characterized by ≥10% dysplastic neutrophils in the peripheral blood or bone marrow. The dysplasia principally manifests as nuclear hypolobation and hypogranulation. The other myeloid cell lines do not show significant dysplasia (<10%).
Refractory thrombocytopenia
Refractory thrombocytopenia is characterized by ≥10% dysplastic megakaryocytes of at least 30 megakaryocytes evaluated. The dysplasia principally manifests as hypolobate megakaryocytes, binucleate and multinucleate megakaryocytes and micromegakaryocytes (Figure 1B). The other myeloid cell lines do not show significant dysplasia (<10%).
Most patients with refractory thrombocytopenia have low IPSS scores and 90% of the patients live more than two years.19
Refractory anemia with ring sideroblasts
Refractory anemia with ring sideroblasts (RARS) is a myelodysplastic syndrome (MDS) characterized by anemia, morphologic dysplasia in the erythroid lineage and ring sideroblasts comprising ≥15% of the bone marrow erythroid precursors. There is no significant dysplasia in the nonerythroid lineages. Myeloblasts comprise <5% of the nucleated BM cells and are not present in the peripheral blood.
RARS accounts for 3%–11% of the MDS cases. The median age of presentation is 60–73 years with no sex predilection. Primary defects of heme synthesis can largely be excluded because protoporphyrin IX, the end product of porphyrin synthesis, is not decreased in RARS.20 Acquired mutations in genes of the heme synthetic pathway have not been demonstrated in RARS. Thus a primary defect of mitochondrial iron metabolism is suspected. RARS represents a clonal stem cell defect that manifests as abnormal iron metabolism in the erythroid lineage and results in ineffective erythropoiesis. The PB and BM are the principal sites of involvement. The liver and spleen may show signs of iron overload. Patients present with symptoms related to anemia and progressive iron overload.
The peripheral blood picture shows a normochromic macrocytic or normochromic normocytic anemia. Blasts are not present in the PB (Figure 3A). The BM aspirate smear shows increase in the number of erythroid precursors with erythroid lineage dysplasia (Figure 3B). Granulocytes and megakaryocytes show no significant dysplasia (<10% dysplastic forms). Hemosiderin laden macrophages are often abundant. On an iron stained aspirate smear, 15% or more of the red cell precursors are ring sideroblasts (Figure 3C). The BM biopsy is normocellular to markedly hypercellular, usually with marked erythroid proliferation. Megakaryocytes are normal in number and morphology.
Figure 3.
Refractory anemia with ringed sideroblasts (RARS). A) Peripheral blood smear (Wright–Giemsa – 100x). Occasional macro-ovalocytes seen. B) Bone marrow biopsy specimen (H&E stain – 100x). Erythroid hyperplasia with immature erythroid cells. C) Iron stain – 100x. With ringed sideroblasts.
Clonal chromosomal abnormalities are seen in 5%–20% of cases of RARS and when present, typically involve a single chromosome.21 Approximately 1%–2% of cases of RARS evolve to acute myeloid leukemia. The overall median survival is 69–108 months.
Refractory cytopenia with multilineage dysplasia
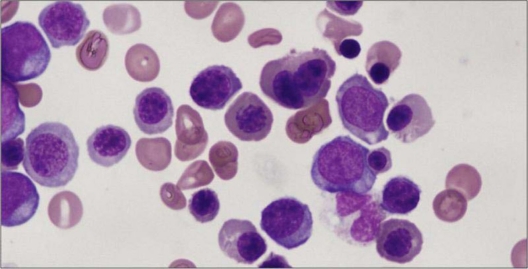
Refractory cytopenia with multilineage dysplasia (RCMD) is a type of myelodysplastic syndrome with one or more cytopenias and dysplastic changes in two or more of the myeloid lineages: erythroid, granulocytic, megakaryocytic.14 There are <1% blasts in the peripheral blood and <5% in the bone marrow. Auer rods are not present and the monocytes in the peripheral blood are less than 1 × 109/L. The recommended levels for defining cytopenias are hemoglobin <10 g/dL, absolute neutrophil count <1.8 × 109/L and platelet count <100 × 109/L.4,5 The thresholds for dysplasia are ≥10% in each of the affected cell lines. At least 30 megakaryocytes should be evaluated for dysplasia in BM smears or sections.
RCMD accounts for 30% of cases of MDS. The median age is approximately 70 years. There is a slight predominance of males. The peak incidence for males is 70–74 years, for females 75–79 years.18 Blood and bone marrow are the principal sites of involvement. Most of the patients present with evidence of BM failure with cytopenia of two or more myeloid cell lines.
The BM is usually hypercellular. Myeloblasts account for <5% of the BM cells. Neutrophil dysplasia is characterized by hypogranulation and nuclear hyposegmentation with marked clumping of nuclear chromatin (pseudo Pelger–Huet nuclei) (Figure 4). Erythroid precursors may show cytoplasmic vacuoles and marked nuclear irregularity. The vacuoles may be periodic acid-Schiff (PAS) positive. There may also be diffuse cytoplasmic PAS positivity. Megakaryocyte abnormalities include nonlobated nuclei, hypolobated nuclei, binucleate or multinucleate megakaryocytes and micromegakaryocytes. Micromegakaryocytes are the most reliable and reproducible dysplastic feature in the megakaryocyte series.22
Figure 4.
Refractory cytopenia with multilineage dysplasia (RCMD). Bone marrow aspirate (Wright–Giemsa stain – 100x). Erythroid precursors with nuclear irregularity and myeloid precursors with hypogranulation and hyposegmentation.
Clonal cytogenetic abnormalities including trisomy 8, monosomy 7, del (7q), monosomy 5, del (5q), and del (20q) as well as complex karyotypes, may be found in up to 50% of patients with RCMD.18,4
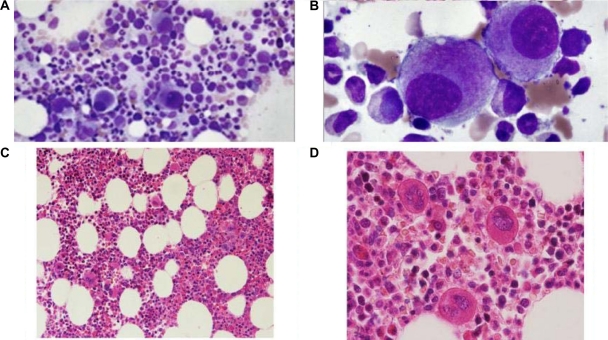
Refractory anemia with excess blasts
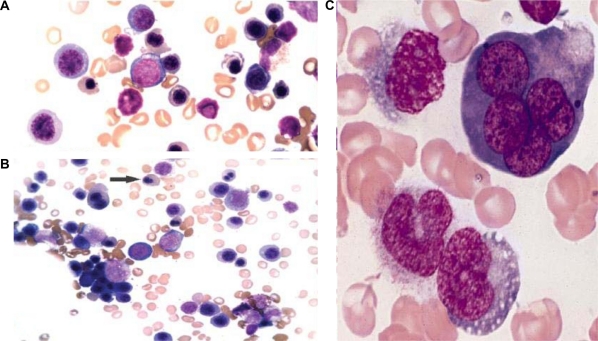
Refractory anemia with excess blasts (RAEB) is a myelodysplasyic syndrome with 5%–19% myeloblasts in the bone marrow or 2%–19% blasts in the peripheral blood. Due to differences in survival and incidence of evolution to acute myeloid leukemia (AML), two categories of RAEB are recognized: RAEB-1, defined by 5%–9% blasts in the BM (Figure 5A, 5B) or 2%–4% blasts in the PB, and RAEB-2, defined by 10%–19% blasts in the BM or 5%–19% blasts in the PB.4 The presence of Auer rods in blasts qualifies a case as RAEB-2 (Figure 5C) irrespective of the blast percentage.7
Figure 5.
Refractory anemia with excess blasts (RAEB). Bone Marrow (A, B) Aspirate (Wright–Giemsa – 100x) showing blasts, RAEB-1. C) Aspirate (Wright–Giemsa – 100x) Blasts with Auer rods, RAEB-2. D) Biopsy (H&E stain – 40x), Abnormal localization of immature precursors (ALIP).
RAEB accounts for approximately 40% of all patients with MDS. It affects individuals above 50 years of age. Risk factors include exposure to environmental toxins; such as pesticides, petroleum derivatives, some heavy metals, and cigarette smoking. RAEB principally involves the blood and bone marrow. Patients present with symptoms related to BM failure, including anemia, thrombocytopenia and neutropenia.
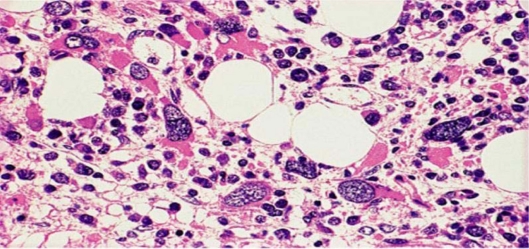
The peripheral blood smear frequently shows abnormalities in all three myeloid cell lines, including red cell anisopoikilocytosis; large, giant or hypogranular platelets and abnormal cytoplasmic granularity and nuclear segmentation of the neutrophils. Blasts are commonly present in the PB. The bone marrow is usually hypercellular with varying degrees of dysplasia. Erythropoiesis may be increased with macrocytic or megaloblastoid changes. Granulopoiesis and megakaryopoiesis are frequently increased with varying degrees of dysplasia. The megakaryocytes often show a tendency to cluster. In the BM biopsy, both erythropoiesis and megakaryopoiesis appear frequently dislocated towards the paratrabecular areas that are normally predominantly occupied by granulopoietic cells. Blasts in RAEB often tend to form cell clusters or aggregates that are usually located away from bone trabeculae and vascular structures, a histologic finding commonly referred to as abnormal localization of immature precursors (ALIP) (Figure 5D). These may be identified by immunohistochemical staining for CD34.
Flow cytometry shows increased number of cells positive for CD34 and CD117. These cells are usually positive for CD38, HLA-DR and myeloid associated antigens CD13 and CD33. Asynchronous expression of granulocytic maturation antigens CD15, CD11b and CD65 can be seen in the blast population. Antibodies such as CD61 or CD42b can aid in the identification of micromegakaryocytes and other small dysplastic forms, which are particularly numerous in cases of RAEB-F.17
Clonal cytogenetic abnormalities are present in 30%–50% cases of RAEB. These include +8, −5, del (5q), −7, del (7q) and del (20q). Approximately 25% of cases of RAEB-1 and 33% of patients with RAEB-2 progress to AML, the remainder succumb to sequelae of BM failure. The median survival is approximately 16 months for RAEB-1 and 9 months for RAEB-2.
RAEB with fibrosis (RAEB-F)
The current working definition of MDS with fibrosis (MDS-F) requires diffuse coarse reticulin fibrosis with or without concomitant collagenization, associated with dysplasia in at least two cell lineages.23 Most of the cases defined as MDS-F belong to the RAEB category. The presence of excess blasts can be demonstrated using immunohistochemistry for CD34. A characteristic finding in RAEB-F is an increased number of megakaryocytes of different cell sizes and a high degree of dysplasia.17 The differential diagnosis includes therapy-related MDS, myeloproliferative neoplasms, acute panmyelosis with myelofibrosis (APMF) and rarely reactive dyshematopoietic conditions.
Myelodysplastic syndrome with isolated del (5q)
Myelodysplastic syndrome with isolated del (5q) is a MDS characterized by anemia with or without other cytopenias and/or thrombocytosis and in which the sole cytogenetic abnormality is del (5q). Myeloblasts comprise <5% of nucleated BM cells and <1% of peripheral blood leukocytes and Auer rods are absent.
The median age of presentation is 67 years and occurs more often in women. The etiology includes presumed loss of tumor suppressor gene in the deleted region. Possible candidates include early growth response 1 (EGR1) and α-catenin (CTNNA1) genes. The RPS 14 gene that encodes a ribosomal protein (40S subunit) has been proposed as a candidate in the 5q-syndrome, raising the possibility that a defect in the ribosomal protein function causes the disorder.11 The gene haploinsufficiency blocks the processing of the preribosomal RNA and the formation of 40S subunit. Forced expression of RPS 14 in primary bone marrow cells from patients with the 5q-syndrome rescues the phenotype.24
The principal sites of involvement are blood and bone marrow. The most common symptoms are related to anemia, which is often severe and usually macrocytic. Thrombocytosis is present in one-third to one-half of patients, while thrombocytopenia is uncommon.25
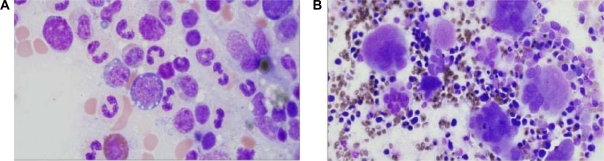
The bone marrow is usually hypercellular or normocellular and frequently exhibits erythroid hypoplasia. Megakaryocytes are increased in number and are normal to slightly decreased in size with nonlobated or hypolobated nuclei (Figure 6). In contrast, dysplasia in erythroid and myeloid lineages is uncommon.25
Figure 6.
Myelodysplastic syndrome with isolated del (5q). A, B) Bone marrow aspirate (40x and 100x). Hypolobated megakaryocyte seen. C, D) Bone marrow biopsy – (10x and 40x – H&E stain). Hypercellular bone marrow with myeloid proliferation associated with hypolobated or nonlobated megakaryocytes.
The sole cytogenetic abnormality involves an interstitial deletion of chromosome 5; the size of the deletion and breakpoints are variable, but bands q31–q33 are invariably deleted. Any additional cytogenetic abnormality if present, (with the exception of loss of Y chromosome), the case should not be placed in this category. It has been recently reported that a small subset of patients with isolated del (5q) may show concomitant JAK2 V617F mutation.11 The disease is associated with a median survival of 145 months, with transformation to AML occurring in <10% of patients.25 The thalidomide analog lenalidomide has been shown to benefit MDS patients with isolated del (5q) as well as del (5q) with additional cytogenetic abnormalities.
Myelodysplastic syndrome, unclassifiable
Myelodysplastic syndrome, unclassifiable (MDS-U) is a subtype of MDS which initially lacks findings appropriate for classification into any other MDS category.
The incidence of MDS-U is unknown. The peripheral blood and bone marrow are the principal sites of involvement. Patients present with symptoms similar to those seen in other myelodysplastic syndromes.
There are no specific morphologic findings. The diagnosis of myelodysplastic syndrome, unclassifiable is made in the following three instances: i) Patients with findings of refractory cytopenia with unilineage dysplasia or refractory cytopenia with multilineage dysplasia but with 1% blasts in the peripheral blood, ii) cases of MDS with unilineage dysplasia which are associated with pancytopenia, iii) patients with persistent cytopenia with 1% or fewer blasts in the blood and fewer than 5% in the BM, unequivocal dysplasia in less than 10% of the cells in one or more myeloid lineages, and who have cytogenetic abnormalities considered as a presumptive evidence of MDS.26
In cases diagnosed as MDS-U, it is unknown both percentage of patients which transform to acute myeloid leukemia as well as the disease survival.
Childhood myelodysplastic syndrome
Myelodysplastic syndrome is very uncommon in children, accounting for less than 5% of all hematopoietic neoplasms in patients less than 14 years of age. Refractory cytopenia of childhood (RCC) is a myelodysplastic syndrome characterized by persistent cytopenia with <5% blasts in the bone marrow and <2% blasts in the peripheral blood.27
RCC is the most common subtype of MDS in childhood accounting for about 50% of the cases. It is diagnosed in all age groups and affects boys and girls with equal frequency. Blood and bone marrow are always affected. Generally, spleen, liver and lymph nodes are not sites of initial manifestation. The most common symptoms are malaise, bleeding, fever and infection. Lymphadenopathy may be present. In up to 20% of patients no clinical signs or symptoms are reported.28 Congenital abnormalities of different organ systems may be present.
The classical picture of RCC is a peripheral blood smear that shows red blood cell anisopoikilocytosis and macrocytosis. Anisochromia may be present. Hemoglobin <10 g/dL is noted in about half of the affected children. Three quarters of patients have a platelet count below 150 × 109/L. Platelets often display anisocytosis and occasionally giant platelets may be detected. Neutropenia with pseudo-Pelger–Huet nuclei or hypogranularity of neutrophil cytoplasm may be noted. Blasts are absent or account for less than 2% of the white blood cells. On bone marrow aspirate smears dysplastic changes should be present in two different myeloid cell lineages, or exceed 10% in one single cell line. Myeloblasts account for fewer than 5% of bone marrow cells. Megakaryocytes are usually absent or very low in number. The detection of micromegakaryocytes is a strong indicator of RCC (Figure 7). Ring sideroblasts are not found. About 75% of children with RCC show considerable hypocellularity of the bone marrow, down to 5%–10% of the normal age matched value. The morphologic findings are similar to those observed in the normally cellular or hypercellular cases.
Figure 7.
Childhood myelodysplastic syndrome. Bone marrow biopsy (H and E – 100X). Dysplastic megakaryocytes.
Monosomy 7 is the most common cytogenetic abnormality. Other cytogenetic abnormalities including complex karyotypes may also be observed. Patients with monosomy 7 have a significantly higher probability of progression than patients with other chromosomal abnormalities or normal karyotype. Immunosuppressive therapy can be a successful therapy strategy for improving outlook in some children with RCC.
Micromegakaryocytes are readily appreciated by the expression of platelet glycoproteins like CD61 (glycoprotein IIIa), CD41 (glycoprotein IIb/IIIa) or von Willebrand factor. Myeloblasts are positive for CD34, myeloperoxidase, lysozyme and CD117.
Cytochemistry and immunophenotyping
Peroxidase and Sudan black B identify blasts with a myeloid origin, however, in MDS the blasts may have a lower peroxidase activity than normal blasts. Combined esterase stain may be performed on both peripheral blood and bone marrow for a more accurate assessment of monocytic cells. Iron stain may reveal abnormal iron metabolism in erythroblasts with the presence of increased iron stores and ringed sideroblasts. Abnormal carbohydrate metabolism is indicated by the presence of blocks of PAS-positive material in erythrocyte precursors. Abnormal small megakaryocytes are identified by immunochemistry with antibodies against platelet-specific glycoproteins IIb/IIIa (CD41), GPIIIa (CD61), or by antibody against factor VIII in histologic sections. Diaminobenzidine can be utilized to identify platelet peroxidase in electron micrographs.15,17 Erythroid abnormalities can be determined by the pattern of expression of H-Ferritin, CD71 and CD105 in glycophorin A (GPA) positive nucleated cells. Increased expression of markers found on immature myeloid cells such as CD13, CD33, CD34, and HLA-DL and decreased expression of NAT-9 (found on mature myeloid cells) may be associated with a worse prognosis and with progression to AML.15
Cytogenetics
Cytogenetic and molecular studies have a major role in the evaluation of patients with MDS in regard to prognosis, determination of clonality, and the recognition of cytogenetic, morphologic, and clinical correlates. Clonal cytogenetic abnormalities are observed in around 50% of MDS cases. The recurring chromosomal abnormalities and their frequency in MDS at diagnosis is illustrated in Table 2. The types of cytogenetic abnormalities seen in MDS are usually unbalanced in contrast to inversions or translocations seen in AML. The more frequent cytogenetic abnormalities involve structural or numeric abnormalities of chromosome 5 and 7 and trisomy 8.15 New techniques such as fluorescence in situ hybridization (FISH) have improved the identification of chromosome abnormalities using specific DNA probes. Three major risk categories of cytogenetic findings have been defined by the International Myelodysplastic Syndrome Working Group.4 These include: i) good risk – normal karyotype, isolated del (5q), isolated del (20q) and – Y; ii) poor risk – complex abnormalities, ie, ≥3 abnormalities, or abnormalities of chromosome 7; and iii) intermediate risk – all other abnormalities.
Prognosis and predictive factors
A scoring system for predicting survival and evolution to AML based on percent of BM blasts, type of cytogenetic abnormalities, and degree and number of cytopenias has been proposed by International Prognostic Scoring System (IPSS) for MDS (Table 3).4,5 Four risk groups are recognized based on this scoring system: low, 0; INT (intermediate) −1, 0.5–1.0; INT −2, 1.5–2.0; and high, ≥2.5. The median survival for the low risk group is 5.7 years, for intermediate −1 is 3.5 years, for intermediate −2 is 1.2 years and for the high risk group is 0.4 years.15 In general, the higher risk groups are related to higher BM blast percentage, more unfavorable cytogenetic findings and more severe degree of cytopenia. Patients younger than 60 years of age have improved survival in the individual risk categories compared with patients older than 60 years. The cytogenetic subgrouping of the IPSS system also has independent value in predicting the outcome of allogeneic stem cell transplantation in patients with MDS.29
Treatment
The therapy of MDS has been unsatisfactory. Only stem cell transplantation offers cure: survival rates of 50% at 3 years have been reported, but older patients are particularly prone to develop treatment-related mortality and morbidity. Results of transplant using matched unrelated donors are comparable, although most series contain younger and more highly selected cases.30
MDS has been regarded as particularly refractory to cytotoxic chemotherapy regimens but is probably no more resistant to effective treatment than acute myeloid leukemia in the elderly, in whom drug toxicity is often fatal and remissions, if achieved, are brief.
Low doses of cytotoxic drugs have been administered for their “differentiating” potential, and from this experience has emerged drug therapies based on pyrimidine analogs. Azacitidine is directly cytotoxic but also inhibits DNA methylation, thereby altering gene expression. Azacitidine improves blood counts and modestly improves survival in about 16% of MDS patients, compared to best supportive care.30 Decitabine is closely related to azacitidine and more potent. The major toxicity of both azacitidine and decitabine is myelosuppression, leading to worsened blood counts. Other symptoms associated with cancer chemotherapy frequently occur.
Thalidomide, a drug with many activities including anti-angiogenesis and immunomodulation, has modest biologic activity in MDS. Lenalidomide, a thalidomide derivative with a more favorable toxicity profile, is particularly effective in reversing anemia in MDS patients with 5q-syndrome; not only do a high proportion of these patients become transfusion-independent with normal or near-normal hemoglobin levels, but their cytogenetics also become normal.29 Toxicities include myelosuppression (worsening thrombocytopenia and neutropenia, necessitating blood count monitoring) and an increased risk of deep vein thrombosis and pulmonary embolism.
Other treatments for MDS include amifostine, an organic thiophosphonate that blocks apoptosis; it can improve blood counts but has significant toxicities. Antithymocyte globulin and cyclosporine, also may produce sustained independence from transfusion, especially in younger MDS patients with more favorable International Prognostic Scoring System (IPSS) scores.30
Hematopoietic growth factors can improve blood counts but, as in most other marrow failure states, have been most beneficial to patients with the least severe pancytopenia. Erythropoietin alone or in combination with G-CSF can improve hemoglobin levels, but mainly in those with low serum erythropoietin levels who have no or only a modest need for transfusions. RBC transfusion support should be accompanied by iron chelation in order to prevent secondary hemochromatosis.30
Conclusion
In 1982, the French–American–British (FAB) cooperative group proposed a classification of myelodysplastic syndromes (MDS) based on morphological features in blood and bone marrow, namely on medullary and peripheral blast count, Auer rods, ring sideroblasts and the number of monocytes in the peripheral blood. This classification has been used for numerous studies regarding morphology, prognosis and treatment of MDS. Some details of this morphological classification remained unclear, and some patients were unclassifiable. Myelodysplastic syndromes (MDS) are spectrum of bone marrow failure disorders that share a common pathologic feature: cytologic dysplasia. The classification of MDS reflects the understanding of the disease. The WHO 2008 Classification for MDS has been summarized in Table 1. It is hoped that in the future classification and risk stratification will be based on underlying pathobiology of different disease subsets and molecular signatures where the pathologic classification represents their phenotype. This article reviews MDS classification and risk stratification highlighting differences between the various systems.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and not under consideration by any other publication and has not been published elsewhere. The authors report no conflicts of interest in this work.
References
- 1.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189–199. [PubMed] [Google Scholar]
- 2.Cazzola M, Malcovati L. Myelodysplastic syndromes-coping with ineffective hematopoiesis. N Eng J Med. 2005;352:536–538. doi: 10.1056/NEJMp048266. [DOI] [PubMed] [Google Scholar]
- 3.Vardiman JW. The new World Health Organization classification of myeloid neoplasms: Q&A with James W. Vardiman MD. Clin Adv Hematol Oncol. 2003;1:18, 21. [PubMed] [Google Scholar]
- 4.Greenberg P, Cox C, Lebeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 5.Greenberg P, Cox C, Lebeau MM, et al. Erratum Blood. 1998;91:1100. [Google Scholar]
- 6.Verburgh E, Achten R, Louw VJ, et al. A new disease categorization of low-grade myelodysplastic syndromes based on the expression of cytopenia and dysplasia in one versus more than one lineage improves on the WHO classification. Leukemia. 2007;21:668–677. doi: 10.1038/sj.leu.2404564. [DOI] [PubMed] [Google Scholar]
- 7.Germing U, Strupp C, Knendgen A, et al. Refractory anemia with excess blasts (RAEB): analysis of reclassification according to the WHO proposals. Br J Haematol. 2006;132:162–167. doi: 10.1111/j.1365-2141.2005.05853.x. [DOI] [PubMed] [Google Scholar]
- 8.Malcovati L, Porta MG, Pascutto C, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23:7594–7603. doi: 10.1200/JCO.2005.01.7038. [DOI] [PubMed] [Google Scholar]
- 9.Aul C, Gattermann N, Schneider W. Age-related incidence and other epidemiological aspects of myelodysplastic syndromes. Br J Haematol. 1992;82:358–367. doi: 10.1111/j.1365-2141.1992.tb06430.x. [DOI] [PubMed] [Google Scholar]
- 10.Germing U, Strupp C, Knendgen A, et al. No increase in age-specific incidence of myelodysplastic syndromes. Hematological. 2004;89:905–910. [PubMed] [Google Scholar]
- 11.Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues. IARC; Lyon: 2008. [Google Scholar]
- 12.Strom SS, Gu Y, Gruschkus SK, Pierce SA, Estey EH. Risk factors of myelodysplastic syndromes: a case-control study. Leukemia. 2005;19:1912–1918. doi: 10.1038/sj.leu.2403945. [DOI] [PubMed] [Google Scholar]
- 13.Wimazal F, Fonatsch C, Thalhammer R, Schwarzinger I, et al. Idiopathic cytopenia of undetermined significance (ICUS) versus low risk MDS: The diagnostic interface. Leuk Res. 2007;31:1461–1468. doi: 10.1016/j.leukres.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Rosati S, Mick R, Xu F, Stonys E. Refractory cytopenia with multilineage dysplasia: further characterization of an ‘unclassifiable’ myelodysplastic syndrome. Leukemia. 1996;10:20–26. [PubMed] [Google Scholar]
- 15.Lawrence LW, McKenzie SB, Williams JL. Myelodysplastic Syndromes. 2nd edition. Prentice Hall; 2004. Clinical Laboratory Hematology. [Google Scholar]
- 16.Bowen D, Culligan D, Jowitt S, Kelsey S, et al. Guidelines for diagnosis and therapy of adult myelodysplastic syndromes. Br J Haematol. 2003;120:187–200. doi: 10.1046/j.1365-2141.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- 17.Lambertenghi-Detiliers G, Orazi A, Luksch R, Annaloro C, et al. Myelodysplastic syndrome with increased marrow fibrosis: a distinct clinic-pathological entity. Br J Haematol. 1991;78:163–166. doi: 10.1111/j.1365-2141.1991.tb04411.x. [DOI] [PubMed] [Google Scholar]
- 18.Germing U, Strupp C, Knendgen A, et al. Prospective validation of the WHO proposals for the classification of myelodysplastic syndromes. Hematologica. 2006;91:1596–1604. [PubMed] [Google Scholar]
- 19.Sashida G, Takaku TI, Shoji N, et al. Clinic-hematologic features of myelodysplastic syndrome presenting as isolated thrombocytopenia: an entity with relatively favorable prognosis. Leuk Lymphoma. 2003;44:653–658. doi: 10.1080/1042819031000063507. [DOI] [PubMed] [Google Scholar]
- 20.Kushner JP, Lee GR, Wintrobe MM, Cartwight GE. Idiopathic refractory sideroblastic anemia: clinical and laboratory investigation of 17 patients and review of literature. Medicine (Baltimore) 1971;50:139–159. doi: 10.1097/00005792-197105000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Breccia M, Carmosino I, Biondo F, et al. Usefulness and prognostic impact on survival of WHO reclassification in FAB low risk myelodysplastic syndromes. Leuk Res. 2006;30:178–182. doi: 10.1016/j.leukres.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda A, Germing U, Jinnai I, et al. Improvement of criteria for refractory cytopenia with multilineage dysplasia according to the WHO classification based on prognostic significance of morphological features in patients with refractory anemia according to the FAB classification. Leukemia. 2007;21:678–686. doi: 10.1038/sj.leu.2404571. [DOI] [PubMed] [Google Scholar]
- 23.Steensma DP, Hanson CA, Letendre L, Tefferi A. Myelodysplasia with fibrosis: a distinct entity? Leuk Res. 2001;25:829–838. doi: 10.1016/s0145-2126(01)00055-8. [DOI] [PubMed] [Google Scholar]
- 24.Ebert BL, Pretz J, Bosco J, Chang CY, Tamayo P, Galili N, et al. Identification of RPS 14 as a 5q- syndrome gene by RNA interference screen. Nature. 2008;451:335–339. doi: 10.1038/nature06494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giagounidis AA, Germing U, Haase S, et al. Clinical, morphologic, cytogenetic, and prognostic features of patients with myelodysplastic syndromes and del (5q) including band q31. Leukemia. 2004;18:113–119. doi: 10.1038/sj.leu.2403189. [DOI] [PubMed] [Google Scholar]
- 26.Knipp S, Strupp C, Gattermann N, Hilderbrandt B, et al. Presence of peripheral blasts in refractory anemia and refractory cytopenia with multilineage dysplasia predicts an unfavorable outcome. Leuk Res. 2008;32:33–37. doi: 10.1016/j.leukres.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 27.Hasle H, Niemeyer CM, Chessells JM, et al. A pediatric approach to the WHO classification of myelodysplastic and myeloproliferative diseases. Leukemia. 2003;17:277–282. doi: 10.1038/sj.leu.2402765. [DOI] [PubMed] [Google Scholar]
- 28.Kardos G, Baumann I, Passmore SJ, et al. Refractory anemia in childhood: a retrospective analysis of 67 patients with particular reference to monosomy 7. Blood. 2003;102:1997–2003. doi: 10.1182/blood-2002-11-3444. [DOI] [PubMed] [Google Scholar]
- 29.De Witte T, Oosterveld M, Muus P. Autologous and allogeneic stem cell transplantation for myelodysplastic syndrome. Blood Rev. 2007;21:49–59. doi: 10.1016/j.blre.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Fauci, Braunwald, Kasper, et al. Aplastic anemia, Myelodysplasia, and related bone marrow failure syndromes. 17th edition. McGraw-Hill’s; 2008. Harrison’s-Principles of Internal Medicine. [Google Scholar]