Summary
Background
Nociceptive sensitization is a tissue damage response whereby sensory neurons near damaged tissue enhance their responsiveness to external stimuli. This sensitization manifests as allodynia (aversive withdrawal to previously nonnoxious stimuli) and/or hyperalgesia (exaggerated responsiveness to noxious stimuli). Although some factors mediating nociceptive sensitization are known, inadequacies of current analgesic drugs have prompted a search for additional targets.
Results
Here we use a Drosophila model of thermal nociceptive sensitization to show that Hedgehog (Hh) signaling is required for both thermal allodynia and hyperalgesia following ultraviolet irradiation (UV)-induced tissue damage. Sensitization does not appear to result from developmental changes in the differentiation or arborization of nociceptive sensory neurons. Genetic analysis shows that Hh signaling acts in parallel to tumor necrosis factor (TNF) signaling to mediate allodynia and that distinct transient receptor potential (TRP) channels mediate allodynia and hyperalgesia downstream of these pathways. We also demonstrate a role for Hh in analgesic signaling in mammals. Intrathecal or peripheral administration of cyclopamine (CP), a specific inhibitor of Sonic Hedgehog signaling, blocked the development of analgesic tolerance to morphine (MS) or morphine antinociception in standard assays of inflammatory pain in rats and synergistically augmented and sustained morphine analgesia in assays of neuropathic pain.
Conclusions
We demonstrate a novel physiological role for Hh signaling, which has not previously been implicated in nociception. Our results also identify new potential therapeutic targets for pain treatment.
Introduction
Nociceptive hypersensitivity often accompanies tissue damage. This hypersensitivity is a crucial defense mechanism that fosters protective behaviors, typically “nocifensive” aversive withdrawal responses, while the damaged tissue heals. Two common forms of hypersensitivity are allodynia, in which normally nonnoxious stimuli elicit a nociceptive response, and hyperalgesia, an exaggerated responsiveness to noxious stimuli. Normally, nociceptive hypersensitivity only persists until tissue repair is complete. Unfortunately, in certain cases, both allodynia and hyperalgesia can persist long after healing is complete, resulting in chronic pain. This disease is highly prevalent and difficult to treat, leading to widespread disability and suffering and a tremendous cost to society. Currently available analgesic drugs have serious limitations. In the case of opiates, which for many centuries have been a primary treatment for chronic pain, serious side effects such as tolerance (decreased analgesic effect over time), physical dependence, nausea, respiratory depression, and the perceived risk of addiction have limited the effectiveness of these drugs in chronic pain treatment. These limitations have prompted a broad search for new analgesic targets [1].
Mammalian studies have identified a plethora of molecular mediators of nociceptive sensitization. These include lipids, cytokines, neuropeptides, and ions that are secreted from cells within damaged tissue or from circulating blood cells recruited to the site of damage [2]. Ultimately, these factors are thought to modulate the activity of the ion channels present on nociceptive sensory neurons that normally gate in response to noxious thermal, mechanical, or chemical stimuli. One notable class of such receptors is the transient receptor potential (TRP) channel family. For example, transient receptor potential vanilloid-1 (TRPV1) plays an important role in both nociception and inflammatory hyperalgesia in mice [3, 4].
Might there be additional mediators that cause nociceptive sensitization? In addition to the factors noted above, secreted developmental morphogens such as Hedgehog (Hh) can be released from dying cells and can mediate compensatory proliferative responses following tissue damage [5–9]. Hh regulates diverse developmental processes in a variety of tissues including embryonic patterning [10], cell fate specification [11], axon guidance [12, 13], and proliferation [14]. However, other than involvement in cellular proliferation [15] and cancer [16], the roles of Hh in the physiology of differentiated tissues are not clear. In particular, it is currently unknown whether morphogens such as Hh, when released from damaged cells, can also alter the physiological sensitivity of nociceptive sensory neurons.
Drosophila has recently become a useful tool for identifying conserved genes required for nociception and nociceptive sensitization. For example, Drosophila larvae exhibit a unique nocifensive withdrawal response when challenged with a noxious thermal or mechanical stimulus. Such stimuli are detected by class IV dendritic arborization neurons, which are required for the behavioral response [17]. Various assays of nociception in flies have identified Painless, a TRP channel that is required for thermal and mechanical nociception [18]; Drosophila transient receptor potential channel A1 (dTRPA1), a TRP channel required for responses to noxious chemical stimuli [19]; Pickpocket, a degenerin epithelial sodium channel (DEG/ENaC) protein required for responsiveness to noxious mechanical stimuli [20]; and the straightjacket channel required for thermal nociception in adult flies [21]. We recently introduced a Drosophila model of ultraviolet irradiation (UV)-damage-induced nociceptive sensitization [22] in which the Drosophila tumor necrosis factor (TNF) ortholog and its receptor are required for sensitization, as in vertebrates [23]. This work demonstrated that the signaling mechanisms that modulate nociceptive sensitivity are also well conserved. In an effort to identify novel regulators of nociceptive signaling, we sought to test whether other classes of molecules released from damaged or dying cells play a role in the development of nociceptive hypersensitivity.
Here we establish that Hh can mediate two types of nociceptive sensitization, thermal allodynia and hyperalgesia. In Drosophila larvae, these actions occur in parallel to TNF signaling and appear to act via distinct TRP channels in nociceptive sensory neurons. Whereas Painless is required for the development of Hh- or TNF-induced thermal allodynia, dTRPA1 is required for Hh-induced thermal hyperalgesia. We also find that a pharmacological blockade of Sonic Hedgehog (Shh) signaling in rats interacts with opioid receptor signaling in standard models of both inflammatory and neuropathic pain. Our findings reveal a novel physiological role for Hedgehog signaling, and thus identify new potential therapeutic targets for the treatment of chronic pain.
Results
Lack of Nociceptive Sensitization in Drosophila hedgehog Mutants
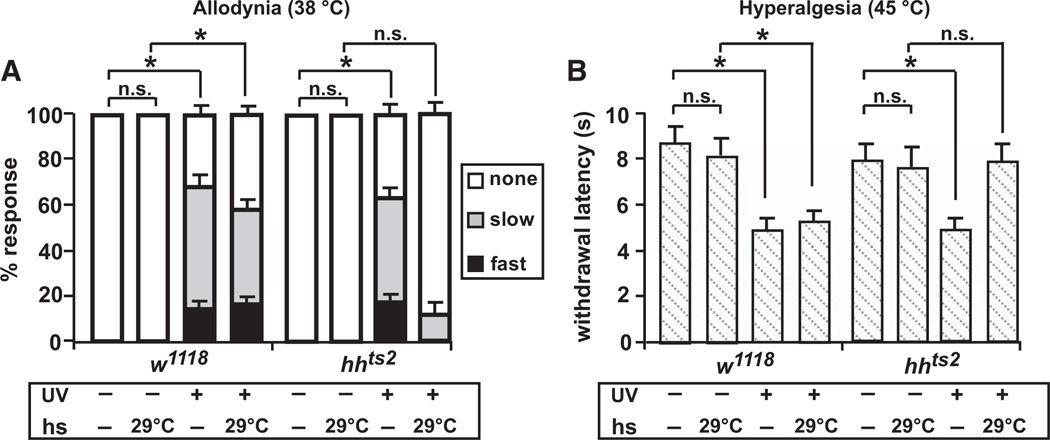
We first tested whether larvae bearing a temperature-sensitive (ts) allele of hedgehog (hhts2) [24] developed thermal allodynia and hyperalgesia following UV damage. As with control larvae (w1118), hhts2 mutant larvae did not display an aversive withdrawal behavior in response to a normally subthreshold stimulus of 38°C when maintained at a permissive temperature of 18°C. Twenty-four hours after UV exposure, thermal allodynia was observed in bothw1118 and hhts2 larvae because a normally subthreshold stimulus now elicited a withdrawal response from the majority of animals tested (Figure 1A). However, if the larvae were shifted to a restrictive temperature of 29°C after UV exposure, the development of thermal allodynia persisted only in w1118 controls. This suggests that the Hh ligand is required for thermal allodynia after UV damage.
Figure 1. hedgehog Mutants Fail to Develop Thermal Allodynia and Hyperalgesia.
(A) Behavioral responses of control (w1118) and hedgehog (hhts2) mutant larvae to a normally nonnoxious stimulus of 38°C. Behaviors were classified as no response (white, >20 s), slow withdrawal (gray, between 5 and 20 s), or fast withdrawal (black, <5 s). Responses were measured with and without ultraviolet irradiation (UV)-induced tissue damage, in the presence or absence of a 24 hr 29°C heat shock administered after UV treatment. n = triplicate sets of 30 larvae per condition. Brackets with asterisks represent statistically significant comparisons by Fisher’s exact test.
(B) Behavioral responses to a noxious suprathreshold stimulus of 45°C. Withdrawal latency was measured with and without UV-induced tissue damage in the presence or absence of an 8 hr 29°C heat shock administered after UV treatment. n = 50 larvae per condition. Error bars represent standard error of the mean (SEM). Brackets with asterisks represent statistically significant comparisons by two-way repeated-measures analysis of variance (ANOVA). n.s. indicates not significant.
In Drosophila, the only other secreted factor known to mediate the development of thermal allodynia is the TNF-like cytokine Eiger. Curiously, Eiger/TNF is not required for development of thermal hyperalgesia [25], which peaks 8 hr after UV irradiation in control larvae [22]. Therefore, we determined whether Hh might encode a missing signal required for the development of thermal hyperalgesia. In w1118 or hhts2 mutant larvae raised at the permissive temperature, a noxious stimulus of 45°C normally elicited a withdrawal response with an average latency of 8.7 s (Figure 1B). Eight hours after UV irradiation, control larvae withdrew nearly twice as fast. This exaggerated response was completely absent when the hhts2 larvae were shifted to the restrictive temperature following UV exposure. Together, these results identify Hh as a critical mediator of both thermal allodynia and hyperalgesia and distinguish this pathway from TNF signaling, which is required solely for development of thermal allodynia.
Nociceptive Sensory Neurons Require Hedgehog Signaling Components for Sensitization following Tissue Damage
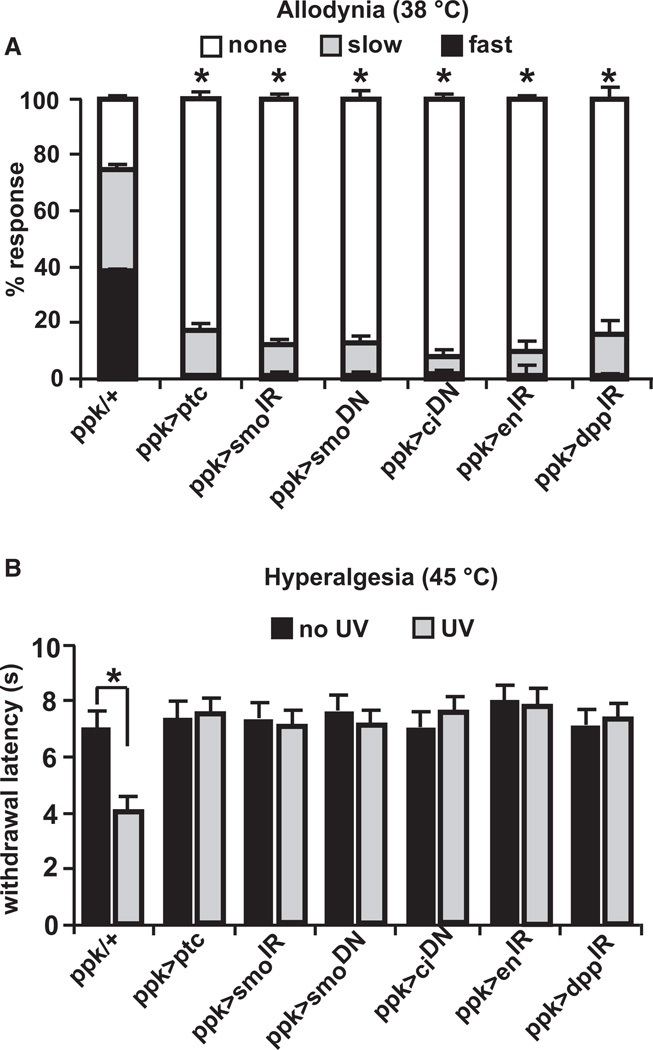
In canonical Hh signaling [26], Hh binds to and inhibits its receptor, Patched (Ptc). This binding relieves inhibition of the transmembrane protein Smoothened (Smo) and leads to signal transduction, the end result of which is activation of the transcription factor Cubitus interruptus (Ci). Activated Ci then turns on expression of target genes such as decapentaplegic (dpp) and engrailed (en). To examine whether Hh signaling acts within nociceptive sensory neurons and through the canonical pathway, we expressed various transgenes that target Hh signaling components using a Gal4 driver (ppk1.9-Gal4) [27] specific to these neurons. Overexpression of Patched (UAS-ptc) severely inhibited development of thermal allodynia 24 hr after UV (Figure 2A; see Figure S1 available online for all upstream activating sequence [UAS]-alone controls relevant to Figure 2). In control larvae (ppk1.9-Gal4 alone), about 70% of larvae exhibited aversive withdrawal, with about half of those responding in under 5 s. Interfering with Smoothened activity, using either tissue-specific RNA interference (UAS-smoIR) or expressing a dominant-negative form of the protein (UAS-smo.5A) [28], limited thermal allodynia to a similar extent as overexpression of Patched. The development of allodynia likely involves a transcriptional component, as expression of a dominant-negative form of the transcription factor Cubitus interruptus (UAS-ci76 = UAS-ciDN) [29] also prevented allodynia. Finally, canonical transcriptional targets of the Hh pathway, including engrailed and dpp, also played a role in thermal allodynia (Figure 2A).
Figure 2. Hedgehog Signaling Components Are Required in Nociceptive Sensory Neurons.
In both panels, ppk1.9-Gal4 drives (>) expression of the indicated upstream activating sequence (UAS) transgenes or no transgene (ppk1.9-Gal4 crossed to w1118 = ppk/+) in nociceptive sensory neurons.
(A) Behavioral responses of larvae of indicated genotypes to a stimulus of 38°C 24 hr after UV treatment. n = triplicate sets of 30 larvae per condition. Asterisk represents statistical significance (p < 0.05) versus ppk/+ control by Fisher’s exact test.
(B) Response of larvae of indicated genotypes to a 45°C stimulus without UV and 8 hr after UV treatment. n = 50 larvae. Bracket with asterisk represents statistically significant comparison by Fisher’s exact test. Error bars represent SEM. See also Figure S1.
We also tested whether larvae expressing these Hh-inhibitory transgenes in nociceptive sensory neurons could develop thermal hyperalgesia 8 hr after UV exposure. This analysis revealed that in no case did the withdrawal latency drop as in control larvae (Figure 2B). These data reinforce the concept that canonical Hh signaling within nociceptive sensory neurons is required for development of both thermal allodynia and hyperalgesia.
Normal Sensory Neuron Development and Baseline Nociception when Hh Signaling Is Blocked in Nociceptive Sensory Neurons
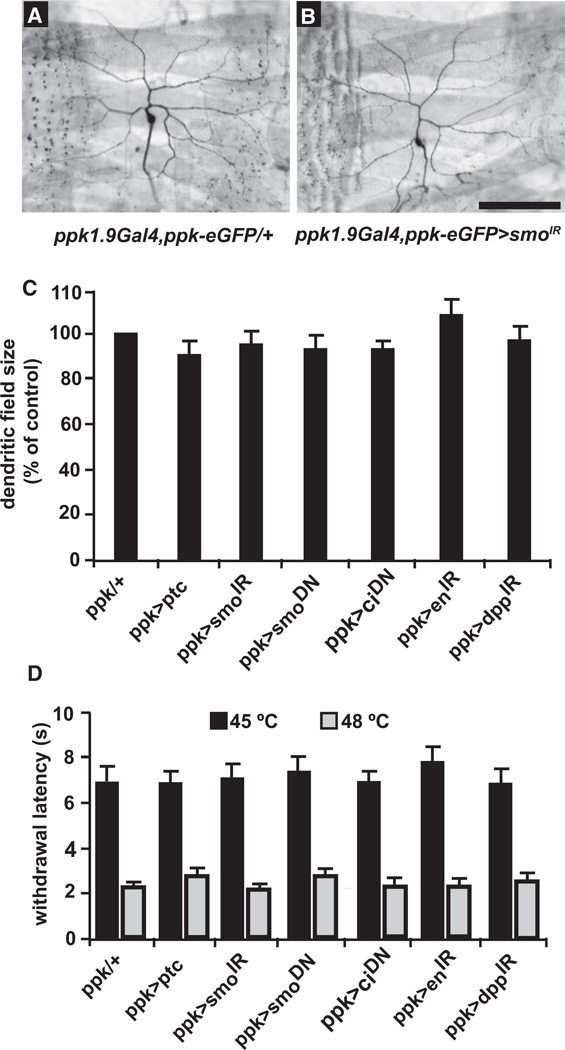
One concern, given its effects on axon guidance [12] and neuronal specification [11], was that the modulation of nociceptive sensitization by Hh might be the indirect result of defects in either differentiation or arborization of nociceptive sensory neurons during development. This appears not to be the case, however, because knockdown of Hh signaling components did not affect the presence or number of class IV nociceptive sensory neurons or the dendritic field size of their neuronal arbors (Figures 3A–3C; Figures S2A–S2G). Consistent with these normal neuronal numbers and morphology, blocking Hh signaling had no effect on baseline nociception without UV treatment (Figure 3D; see Figure S2H for all UAS-alone controls relevant to this panel). In the absence of tissue damage, there were no significant impairments to withdrawal latency measured at 45°C, the lowest temperature at which all larvae exhibit aversive withdrawal within our 20 s cutoff, and 48°C, the lowest temperature at which all larvae exhibit aversive withdrawal within 5 s [22] (Figure 3D). Thus, Hh signaling in nociceptive sensory neurons mediates tissue damage-induced changes in the behavioral response threshold without affecting nociceptive sensory neuron development or the baseline nociceptive threshold.
Figure 3. Blocking Hedgehog Signaling Components Does Not Affect Nociceptive Sensory Neuron Morphology or Baseline Nociception.
(A and B) Whole mounts of dissected L3 larvae of the indicated genotypes. Sensory neuron morphology (ppk1.9Gal4, ppk-eGFP) is shown.
(A) Control.
(B) UAS-smoIR. Scale bar represents 100 µm.
(C) Quantification of total dendritic arbor size (see Experimental Procedures) when ppk1.9-Gal4 drives expression of the indicated UAS transgenes in nociceptive sensory neurons. n = 10 larvae per genotype.
(D) Baseline nociception in response to a 45°C or 48°C stimulus in the absence of UV damage when ppk1.9-Gal4 drives expression of the indicated UAS transgenes in nociceptive sensory neurons. n = 50 larvae. Error bars represent SEM. See also Figures S2 and S3.
Ectopic Hh Signaling Causes Nociceptive Sensitization in the Absence of Tissue Damage
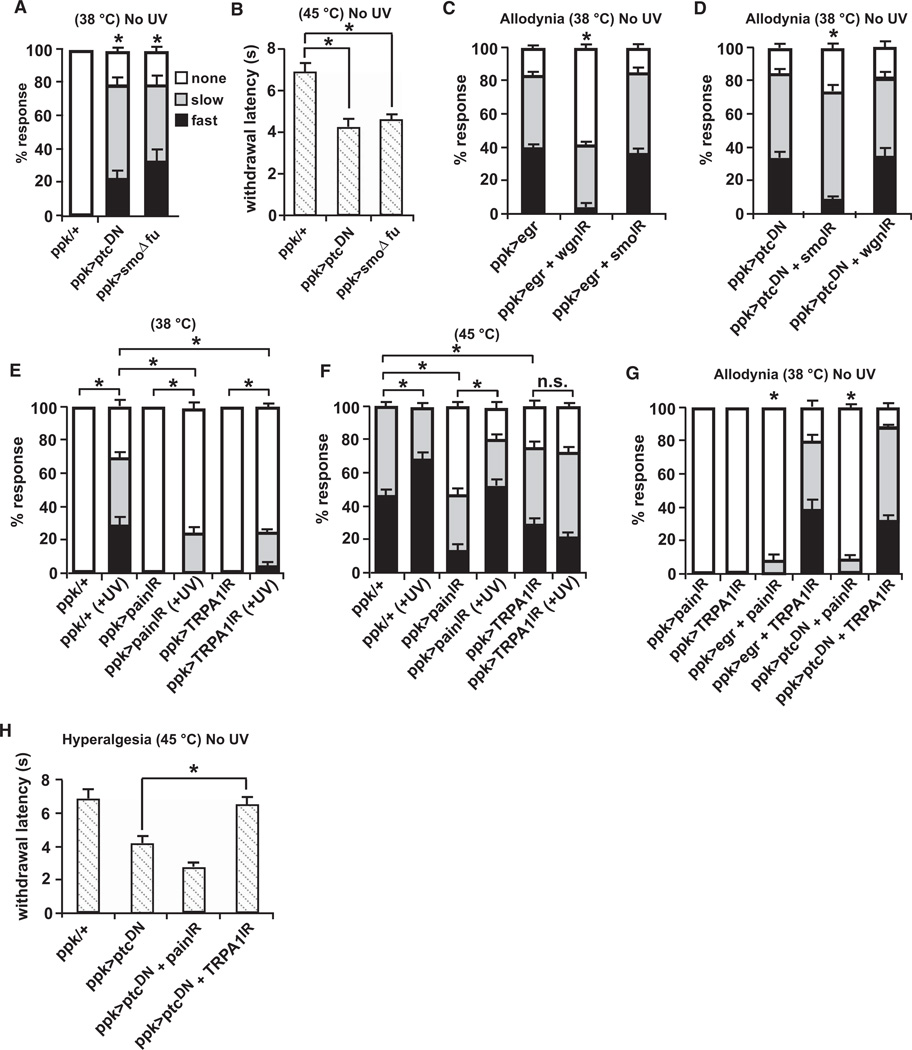
Does Hh require other factors released from damaged cells to mediate its effects on nociceptive sensitization? To test this, we asked whether activation of the Hh pathway in nociceptive sensory neurons was sufficient to cause hypersensitivity in the absence of tissue damage. Constitutive activation of the pathway was achieved by expression of a dominant-negative form of the Smoothened repressor Patched (UAS-ptc1130X) [30] or a form of Smoothened that cannot interact with the downstream kinase, Fused (UAS-SmoΔfu) [31]. In both cases, we found that nonirradiated larvae exhibited a robust response to a normally subthreshold stimulus of 38°C (Figure 4A) and displayed an exaggerated response to a normally noxious stimulus of 45°C (Figure 4B). Taken together, these results demonstrate that ectopic activation of the Hh signaling pathway can evoke thermal allodynia and hyperalgesia even in the absence of tissue damage.
Figure 4. Epistasis Analysis of Tumor Necrosis Factor versus Hedgehog and Tumor Necrosis Factor or Hedgehog versus Transient Receptor Potential Channels.
ppk1.9-Gal4 drives expression of the indicated UAS transgenes in nociceptive sensory neurons.
(A and B) Constitutive activation of Hedgehog (Hh) signaling in the absence of UV irradiation produced thermal allodynia to a 38°C stimulus (A) and thermal hyperalgesia to a 45°C stimulus (B).
(C) Constitutive activation of tumor necrosis factor (TNF) signaling causes allodynia that is reduced by knockdown of Wengen but not Smoothened.
(D) Constitutive activation of Hh signaling causes allodynia that is reduced by knockdown of Smoothened but not Wengen.
(E) RNAi directed against both Painless and TRPA1 reduce UV-induced thermal allodynia.
(F) RNAi directed against TRPA1 prevents UV-induced thermal hyperalgesia whereas Painless RNAi-expressing larvae develop thermal hyperalgesia. Both comparisons are to the altered thresholds for baseline 45C in RNAi-expressing larvae.
(G) Hh- and TNF-induced allodynia depend on Painless but not TRPA1.
(H) Both Painless and TRPA1 reduce responsiveness to a 45°C stimulus in unirradiated larvae.
Abbreviations are as follows: IR, inverted repeat; DN, dominant negative. n = triplicate sets of 30 larvae per condition. Error bars represent SEM.
Hh Acts in Parallel to Eiger/TNF in Development of Thermal Allodynia
Our previous studies demonstrated that the Drosophila TNF ortholog, Eiger, and its receptor, Wengen, were required for thermal allodynia following UV damage [22]. We next performed genetic epistasis experiments to test whether Hh-mediated thermal allodynia depends on TNF signaling or vice versa. To determine this, we constitutively activated one pathway while simultaneously interfering with the other. Like ectopic activation of Hh signaling, overexpression of Eiger/TNF in nociceptive sensory neurons induced thermal allodynia even in the absence of tissue damage [22] (Figure 4C). As expected, blocking TNF signaling by knocking down nociceptive sensory neuron expression of Wengen dampened ectopic Eiger/TNF-induced thermal allodynia (Figure 4C; see Figures S3A–S3D for all UAS-alone controls relevant to Figure 4). When Hh signaling is impaired via knockdown of Smoothened, however, the TNF-induced thermal allodynia was not affected, suggesting that Smoothened does not act downstream of Eiger/TNF in mediating thermal allodynia.
Constitutive activation of Hh signaling via a dominant-negative form of Patched caused UV-independent thermal allodynia that was dampened by knocking down expression of Smoothened (Figure 4D). However, when TNF signaling was blocked in the presence of Hh activation, Hh-induced thermal allodynia was not impaired (Figure 4D), suggesting that TNF signaling is not downstream of Hh in mediating thermal allodynia. Taken together, these results show that TNF- and Hh-induced thermal allodynia operate in parallel. This conclusion is strengthened by the observation that activation of both signaling pathways within nociceptive sensory neurons leads to ectopic allodynia that is more severe than that provoked by activation of either pathway alone (Figure S3E).
Distinct TRP Channels Mediate TNF- or Hh-Induced Thermal Allodynia and Thermal Hyperalgesia
Detection of noxious stimuli is mediated by TRP channels in organisms from worm to man [32, 33]. In Drosophila, the TRP channels Painless and dTRPA1 have been implicated in detection of noxious temperature [18] and establishing thermal preference [34], respectively. We find that both Painless and dTRPA1 are required for full development of UV-induced allodynia (Figure 4E). Although both channels are required for normal baseline responses to the suprathreshold noxious temperature of 45°C, only knockdown of dTRPA1 seems to block an increase in the percentage of fast and slow responders following UV-induced tissue injury (Figure 4G).
To investigate whether Hh and TNF signaling act via TRP channels for their effects on nociceptive sensitization, we tested whether expression of RNAi transgenes targeting Painless and dTRPA1 could block the allodynia induced by ectopic TNF or Hh activity or the hyperalgesia induced by ectopic Hh activity. We found that allodynia, whether induced by ectopic TNF or Hh signaling, was dependent on the Painless TRP channel and independent of dTRPA1 (Figure 4E). By contrast, Hh signaling-induced hyperalgesia was independent of Painless and dependent on dTRPA1 (Figure 4G) despite the fact that both TRP channels impact baseline nociception at temperatures that normally elicit a noxious response (Figure 4F). These results suggest that both TNF and Hh signaling act through TRP channels to effect their modulation of nociceptive sensitivity. They further suggest that during genetically-induced sensitization, Painless is active at the lower end (38°C–40°C) of the nociceptive threshold, whereas dTRPA1 is active at the higher end (45°C).
A Conserved Role for Hh Modulation of Nociception in Vertebrates
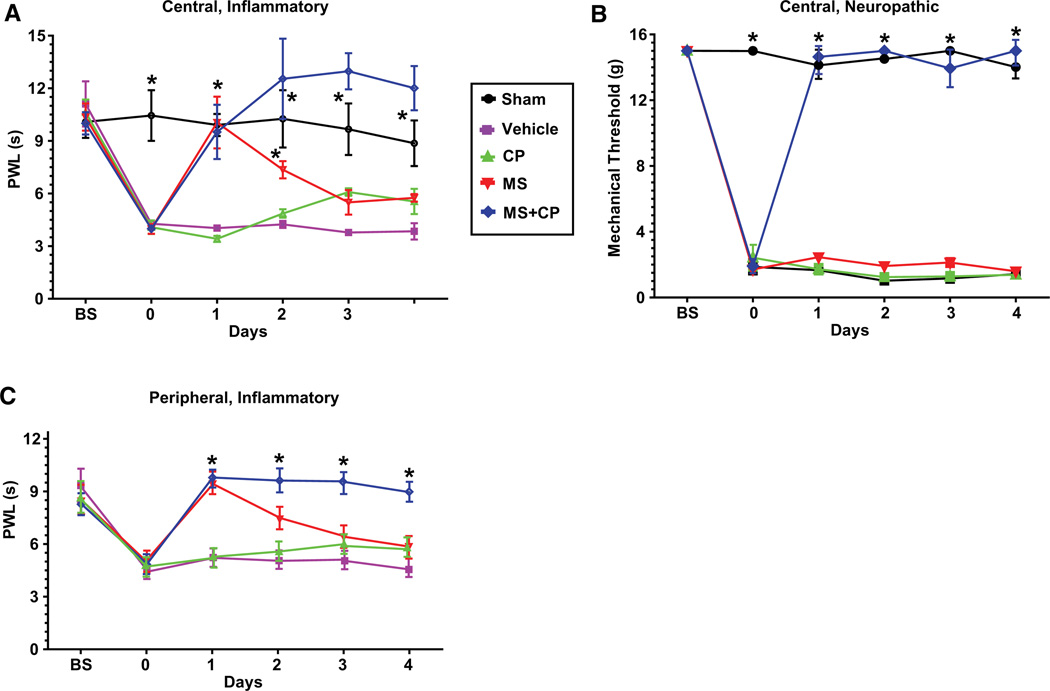
Is the role of Hh signaling in modulation of nociceptive sensitization conserved in mammals? To test this, we examined the effects of pharmacologically blocking Smoothened activity in rodent pain models. Injection of complete Freund’s adjuvant (CFA) into the rat hindpaw causes local inflammation, swelling, and decreased nociceptive thresholds [35]. After CFA injection, we observed a robust, sustained thermal hyperalgesia (Figure 5A), where the paw withdrawal latency (PWL) was decreased from 11.0 to 4.3 s. Intrathecal administration of morphine (MS) caused analgesia, although tolerance developed with repeated dosing. Intrathecal administration of cyclopamine (CP), a Smoothened inhibitor [36], had no analgesic effect. Combining cyclopamine with morphine did not alter the acute analgesic effect of morphine (Figure 5A; compare MS and MS + CP on day 1) but surprisingly, blocked either tolerance to the analgesic effect of morphine or enhanced morphine antinociception (Figure 5A; compare MS and MS + CP on days 2–4).
Figure 5. Cyclopamine Modulates Morphine Analgesia in Rat Models of Inflammatory and Neuropathic Pain.
(A) Inflammatory pain paradigm. Hindpaws were injected with saline (sham, n = 6), or complete Freund’s adjuvant (CFA). Thermal analgesia was assessed using paw withdrawal latency (PWL) following intrathecal administration of 10% Captisol (vehicle, n = 5), cyclopamine (CP, n = 6), morphine (MS, n = 6), or cyclopamine and morphine (MS + CP, n = 6). Abbreviations are as follows: BS, baseline before paw injection; 0, baseline after paw injection, prior to intrathecal drug administration. *p < 0.01 versus vehicle.
(B) Neuropathic pain paradigm. Rats underwent sciatic nerve ligation or sham (n = 7) operation. Animals received daily intrathecal injections of vehicle (n = 3) or drugs (CP, n = 6; MS, n = 6; or MS + CP, n = 5). Mechanical allodynia was assessed using Von Frey filaments. Abbreviations are as follows: BS, baseline measurement prior to operation; 0, baseline 2 weeks after operation, prior to intrathecal drug administration. *p < 0.001 versus vehicle. All data are ±SEM.
(C) Inflammatory pain paradigm with peripheral drug administration. Hindpaws were injected with CFA and cyclopamine, morphine, or a combination of both drugs. Animals received daily intraplantar injections of drugs. n = 9 animals per treatment group. Thermal analgesia was assessed using PWL. *p < 0.0001 versus vehicle (two-way repeated-measures ANOVA). All data are 6SEM.
Cyclopamine administration in a rat model of neuropathic pain [37] produced even more striking results. In this model, neither cyclopamine nor a low dose of morphine had analgesic effects alone. However, the combination of morphine and cyclopamine caused complete and sustained reversal of mechanical allodynia (Figure 5B). This suggests that in addition to blocking opioid tolerance, cyclopamine markedly augmented the analgesic effect of morphine against neuropathic pain. Taken together, these results suggest that in mammals, Smo signaling can affect both thermal and mechanical nociception and exerts its effects on nociceptive sensitivity through mechanisms that intersect with opioid receptor signaling.
To determine whether this effect could be mediated by actions on peripheral nociceptors, we injected CFA into the hindpaw as above. Animals were then treated with injections of low doses of morphine, cyclopamine, or the combination directly into the hindpaw. The effects on thermal hyperalgesia were very similar to those observed after intrathecal drug administration (Figure 5C; compare with Figure 5A). Cyclopamine alone had no analgesic effect but blocked the development of tolerance to the analgesic effect of peripherally administered morphine. This surprising result suggests that, as in Drosophila larvae, the locus of action of Hh signaling is in primary afferent nociceptors.
Discussion
Our results identify canonical Hh signaling as a major new pathway in modulation of nociception. Hh has long been known as a developmental signaling molecule responsible for patterning many developing tissues in both Drosophila and vertebrates. Many of these effects are exerted through Hh’s abilities to induce cell proliferation or the expression of specific target genes in signal-receiving cells. Hh can also signal through a noncanonical pathway to mediate cell migration and axon guidance as in the developing spinal cord [12, 13], where it helps guide spinal commissural neurons to the ventral midline. In our system, it is highly unlikely that Hh is exerting its effects through control of cell proliferation because both the UV-injured epidermis and nociceptive sensory neurons are postmitotic. It is also unlikely that Hh is exerting its effects through control of neuronal remodeling, because the nociceptive sensory neurons do not appreciably change their arborization or branching pattern following epidermal damage [22] and both baseline nociception and neuronal arbors are not affected when Hh signaling components are knocked down in nociceptive sensory neurons. The results described here thus represent a novel physiological function of Hh that is independent of its established roles in control of proliferation and neuronal morphology.
In both vertebrates and Drosophila, distinct TRP channels mediate the detection of both ambient and noxious temperatures across the full range that animals experience. For instance, in Drosophila, Painless in nociceptive sensory neurons detects stimuli at or near the nociceptive threshold [18], dTRPA1 mediates ambient thermal preference in the ambient range [34], and Brivido channels mediate cool avoidance in antennal sensory neurons [38]. Likewise, in vertebrates, TRPV1 mediates noxious detection of heat [39], whereas TRPM8 mediates detection of noxious cold [40, 41]. Our results provide evidence for a similar division of labor during sensitization (see model in Figure 6). Painless appears to be the target of both Eiger/TNF signaling and Hh signaling at the lower end of the nociceptive threshold because it is required for the allodynia induced by ectopic activation of either pathway. Unlike Eiger/TNF, Hh can also mediate thermal hyperalgesia, a response that appears to function through the TRP channel dTRPA1. The more natural case of UV-induced allodynia appears to be somewhat more complicated because it seems to require both Painless and dTRPA1. An interesting question for future studies is how dTRPA1 can mediate temperature preference in an ambient range, UV-induced allodynia at slightly subthreshold temperatures, and both UV- and genetically-induced hyperalgesia at much higher noxious temperatures.
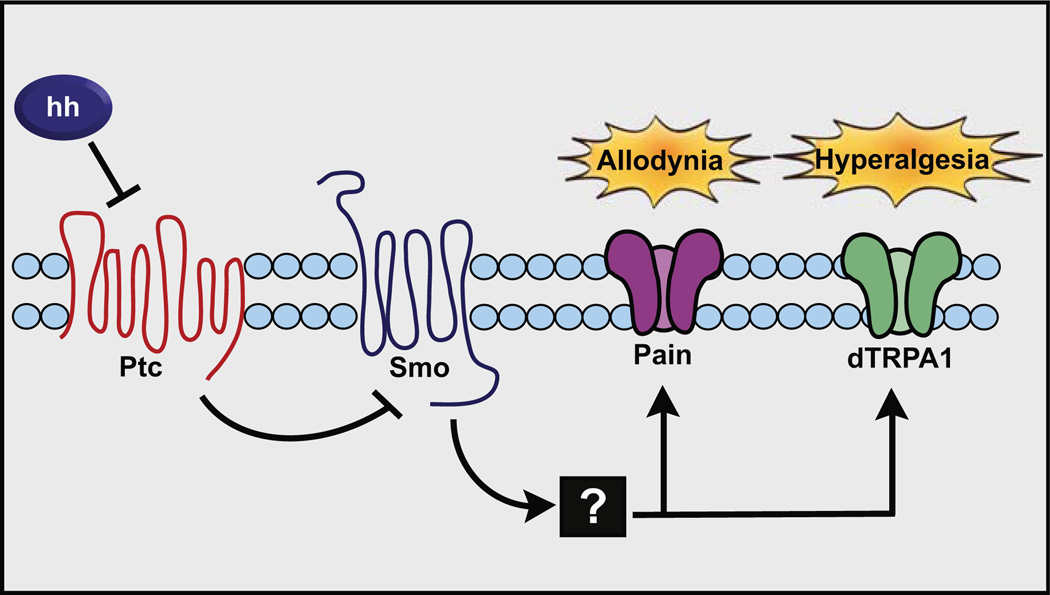
Figure 6. Model of Hedgehog-Induced Thermal Allodynia and Hyperalgesia.
Hh (from an as yet undetermined source tissue) acts through Patched (whose overexpression blocks sensitization), which subsequently inhibits Smoothened (required for sensitization) to activate signal transduction. The subsequent signaling steps likely involve the transcriptional activity of Cubitus interruptus (required for sensitization) and other components of the canonical Hh signaling pathway. For thermal allodynia, this pathway acts through the transient receptor potential (TRP) channel Painless, and for thermal hyperalgesia it requires the TRP channel dTRPA1.
It will also be important to determine how Hh signaling modifies Painless and dTRPA1 in the development of allodynia and hyperalgesia, respectively. In mammals, TRPV1 is phosphorylated by multiple kinases, which can increase the opening probability of the channel [42, 43] or reverse desensitization [44]. Activation of Hh signaling could cause similar effects. Other possible mechanisms for this effect could include an increase of transcription or translation of TRP channels, or perhaps increased translocation of channels to the plasma membrane. The requirement of two Hh-pathway transcription factors, Cubitus interruptus and Engrailed, for development of both thermal allodynia and hyperalgesia suggests that nociceptor-specific gene transcription is a component of the sensitization response, although the precise gene targets remain to be determined. It is not yet known whether there is a transcriptional component to Eiger/TNF-induced allodynia, but the shared requirement of Painless in thermal allodynia suggests that Hh and TNF must both somehow converge on this channel, possibly in mechanistically distinct manners, to alter its activity.
Our results and other recent studies [21] establish the power of using model genetic organisms to identify important new pathways in nociceptive biology. Once a pharmacologically targetable pathway is genetically identified in Drosophila, the translation to vertebrate systems using established nociceptive assays, as performed here, is both rapid and straightforward. In rats, central orperipheral administration of cyclopamine prevents the development of morphine analgesic tolerance or enhances morphine antinociception in models of both inflammatory and neuropathic pain. A particularly striking finding was that cyclopamine appeared to markedly augment the analgesic effect of morphine in the neuropathic pain model. This could have profound clinical implications, because neuropathic pain is often resistant to treatment with opioids in humans. These findings also suggest the intriguing possibility that central modulation of nociceptive signaling by Hh may be a conserved feature of sensitization in Drosophila and neuropathic pain in mammals. Although behavioral modulation of nociception by opiates has not been observed in Drosophila, the fly genome does contain G protein-coupled receptors with similarity to vertebrate opioid receptors [45].
In conclusion, these surprising findings establish a completely unexpected role for Hedgehog signaling in the modulation of nociception and analgesia. Our results suggest that blocking Hh signaling could eliminate the major shortcomings of opioids in chronic pain treatment, namely, poor efficacy against neuropathic pain, respiratory depression with increasing dose, and the development of analgesic tolerance [46]. Thus, Hh signaling components could represent a major new set of potential therapeutic targets for clinical pain treatment.
Experimental Procedures
Fly Stocks and Genetics
Almost all fly stocks were maintained at 25°C. The exceptions were the ts allele of hedgehog (hhts2) [24] and paired w1118 controls, which were kept at 18°C (permissive temperature, where Hh function is normal) and shifted to 29°C (restrictive temperature, where Hh function is impaired) under some experimental conditions (see Figure 1). For heat-shock experiments, UV- or mock-treated larvae were placed at either the permissive or restrictive temperature until assessment of thermal hyperalgesia (8 hr, 45°C) or thermal allodynia (24 hr, 38°C).
We used the GAL4/UAS system [47] to drive expression of UAS transgenes in larval nociceptive sensory neurons (ppk1.9-Gal4) [27]. UAS-smo.5A (=UAS-smoDN) [28], UAS-ptc [48], UAS-ci76 (=UAS-ciDN) [29], and UAS-dppIR [49] were used to inhibit Hh signaling, along with the following UAS-RNAi lines [50]: 11561 (9542) targeting Smoothened, 9015 (105678) targeting Engrailed, 15860 (39477) targeting Painless, and 5751 (37249) targeting dTRPA1. UAS-ptc1130X (=UAS-ptcDN) [30] and UAS-SmoΔfu [31] were used to constitutively activate Hh signaling. The UAS-bearing EP allele eigerGS9830 [51] was used to overexpress eiger. UAS-wengenIR [52] was used to inhibit TNF signaling.
UV Treatment and Assessment of Nociceptive Behavior in Drosophila
UV radiation of early third-instar larvae was carried out as previously reported [22]. Briefly, early third-instar larvae were etherized, immobilized, and exposed to 20 mJ/cm2 of 254 nm wavelength UV light in a Spectronics XL-1000 UV crosslinker. Noxious and nonnoxious thermal stimuli to both UV-treated, and untreated larvae were delivered using a custom-built heat probe [22]. Stimuli were presented along the dorsal midline in abdominal segments A4–A6. The withdrawal latency to each stimulus was recorded up to a 20 s cutoff. The withdrawal behavior is defined as at least one complete 360° roll in response to the stimulus. We tested for thermal allodynia at 38°C, the highest temperature at which control larvae do not respond in the absence of tissue damage. We tested for thermal hyperalgesia at 45°C, the lowest noxious temperature at which all control larvae respond with aversive withdrawal. Allodynia was assessed 24 hr after UV and hyperalgesia 8 hr post UV, the times at which these responses peak in control larvae [22].
Quantitation of Dendritic Arbor Size
Analysis of dendritic field size of Drosophila class IV sensory neurons was described previously [53]. Briefly, polygons were formed by connecting the distal-most tips of dendritic arbors from individual sensory neurons. The area of each polygon was calculated using ImagePro Plus (Media Cybernetics).
Vertebrate Neuropathic and Inflammatory Nociception Assays
Animals
Sprague-Dawley rats (male, 250–300 mg) were housed three to a cage with water and food ad libitum and kept in temperature-controlled rooms on a 12:12 hr light-dark cycle, with the dark cycle beginning at 7:00 p.m. Animals were habituated to the testing environment for one week prior to testing, and all tests were performed in the morning. All protocols were approved by our Institutional Animal Care and Use Committee.
Drugs, Paw Injection, and Intermittent Lumbar Puncture
Rats were anesthetized with 2% isoflurane in oxygen. The lumbar region was shaved, prepared with betadine solution, and the intervertebral spaces widened by placing the animal on a plexiglass tube. Animals then underwent lumbar puncture daily at the L5 to L6 interspace as previously described [54], using a half-inch 30G needle connected to a Hamilton syringe filled with 10% Captisol (CyDex) vehicle or drugs dissolved in Captisol: 0.4 nmol morphine sulfate (Mallinckrodt, Inc.), 10 mg cyclopamine (LG Laboratories), or the combination of morphine and cyclopamine. Correct subarachnoid positioning of the tip of the needle was verified by a tail and/or paw flick. For paw injection, animals were anesthetized as above and had vehicle, 10 µg cyclopamine, 1 µg morphine, or the combination injected in the plantar surface of the CFA-injected hindpaw. Animals recovered in their home cage for 45 min prior to analgesic testing.
Complete Freund’s Adjuvant Injection
Under isoflurane analgesia, the plantar surface of the left hindpaw was cleansed and 100 µl of CFA (Sigma) injected using a 25G needle. Animals recovered for 24 hr prior to analgesic testing.
Segmental Spinal Nerve Ligation
Under isoflurane anesthesia, rats underwent segmental ligation of the left L5 nerve root as previously described [37]. Animals were allowed to recover for 2 weeks before mechanical latency testing or drug administration was performed.
Paw Withdrawal Latency
PWL was measured by placing the animals in plexiglass cages (9 × 22 × 25 cm) on a modified Hargreaves’ device [55], consisting of a glass surface maintained at 30°C. A stimulus lamp was focused on the hindpaw with three measurements taken at 2 min intervals. Withdrawal of the paw secondary to the thermal stimulus was sensed by photodiodes, and this served to terminate the stimulus and stop the timer. Animals were habituated to the device for one week prior to testing and for 30 min before each test session. The intensity of the thermal stimulus used in the PWL test was adjusted so the baseline was between 10–12 s; 20 s was used as an automatic cutoff time to avoid paw damage (n = 6–8 per group).
Mechanical Latency Threshold
Animals were habituated to a plexiglass cage with wire mesh floor as described above. Animals were then tested using graded Von Frey filaments (Stoelting Corporation) in ascending stiffness.
Statistical Analysis
For Drosophila nociception assays, we used Fisher’s exact test to compare categorical responses: no response within 20 s cutoff, slow response (aversive withdrawal between 5 and 20 s), or fast response (aversive withdrawal in under 5 s). When the mean withdrawal latency was compared among genotypes, we used the Student’s t test or one-way analysis of variance (ANOVA). For rat nociception assays, results were reported as mean ± standard error of the mean. Between-group comparisons were made by ANOVA and Bonferroni post hoc tests. Differences were considered to be significant at p < 0.05.
Supplementary Material
Acknowledgments
We thank members of the Gutstein and Galko laboratories for comments on the manuscript. We thank Matthew Scott, Anne Plessis, Hirotaka Kanuka, Michael Welch, Andreas Bergmann, Paul Garrity, the Bloomington Stock Center, and the Vienna Drosophila RNAi Center for providing fly stocks. D.T.B. was supported by an American Heart Association predoctoral fellowship (0815339F). This work was funded by a University of Texas MD Anderson Cancer Center institutional research grant and National Institutes of Health (NIH) grant R01 NS069828 (to M.J.G.). H.B.G. was supported by NIH grants R01 DA05146 and R01 AA016157. D.T.B., H.B.G., and M.J.G. have applied for a United States patent relating to the use of Hh pathway-targeting drugs to treat pain.
Footnotes
Supplemental Information
Supplemental Information includes three figures and can be found with this article online at doi:10.1016/j.cub.2011.08.020.
References
- 1.Woolf CJ. Overcoming obstacles to developing new analgesics. Nat. Med. 2010;16:1241–1247. doi: 10.1038/nm.2230. [DOI] [PubMed] [Google Scholar]
- 2.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 3.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 4.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 5.Fan Y, Bergmann A. Distinct mechanisms of apoptosis-induced compensatory proliferation in proliferating and differentiating tissues in the Drosophila eye. Dev. Cell. 2008;14:399–410. doi: 10.1016/j.devcel.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huh JR, Guo M, Hay BA. Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase Dronc in a nonapoptotic role. Curr. Biol. 2004;14:1262–1266. doi: 10.1016/j.cub.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Pérez-Garijo A, Martín FA, Morata G. Caspase inhibition during apoptosis causes abnormal signalling and developmental aberrations in Drosophila. Development. 2004;131:5591–5598. doi: 10.1242/dev.01432. [DOI] [PubMed] [Google Scholar]
- 8.Pérez-Garijo A, Shlevkov E, Morata G. The role of Dpp and Wg in compensatory proliferation and in the formation of hyper-plastic overgrowths caused by apoptotic cells in the Drosophila wing disc. Development. 2009;136:1169–1177. doi: 10.1242/dev.034017. [DOI] [PubMed] [Google Scholar]
- 9.Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev. Cell. 2004;7:491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 11.Briscoe J. Making a grade: Sonic Hedgehog signalling and the control of neural cell fate. EMBO J. 2009;28:457–465. doi: 10.1038/emboj.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- 13.Yam PT, Langlois SD, Morin S, Charron F. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron. 2009;62:349–362. doi: 10.1016/j.neuron.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Heberlein U, Singh CM, Luk AY, Donohoe TJ. Growth and differentiation in the Drosophila eye coordinated by hedgehog. Nature. 1995;373:709–711. doi: 10.1038/373709a0. [DOI] [PubMed] [Google Scholar]
- 15.Amankulor NM, Hambardzumyan D, Pyonteck SM, Becher OJ, Joyce JA, Holland EC. Sonic hedgehog pathway activation is induced by acute brain injury and regulated by injury-related inflammation. J. Neurosci. 2009;29:10299–10308. doi: 10.1523/JNEUROSCI.2500-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodrich LV, Milenković L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 17.Hwang RY, Zhong L, Xu Y, Johnson T, Zhang F, Deisseroth K, Tracey WD. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr. Biol. 2007;17:2105–2116. doi: 10.1016/j.cub.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 19.Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, Garrity PA. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464:597–600. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong L, Hwang RY, Tracey WD. Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Curr. Biol. 2010;20:429–434. doi: 10.1016/j.cub.2009.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neely GG, Hess A, Costigan M, Keene AC, Goulas S, Langeslag M, Griffin RS, Belfer I, Dai F, Smith SB, et al. A genome-wide Drosophila screen for heat nociception identifies α2δ3 as an evolutionarily conserved pain gene. Cell. 2010;143:628–638. doi: 10.1016/j.cell.2010.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babcock DT, Landry C, Galko MJ. Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Curr. Biol. 2009;19:799–806. doi: 10.1016/j.cub.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br. J. Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma C, Zhou Y, Beachy PA, Moses K. The segment polarity gene hedgehog is required for progression of the morphogenetic furrow in the developing Drosophila eye. Cell. 1993;75:927–938. doi: 10.1016/0092-8674(93)90536-y. [DOI] [PubMed] [Google Scholar]
- 25.Babcock DT, Galko MJ. Two sides of the same coin no longer: Genetic separation of nociceptive sensitization responses. Commun. Integr. Biol. 2009;2:517–519. doi: 10.4161/cib.2.6.9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hooper JE, Scott MP. Communicating with Hedgehogs. Nat. Rev. Mol. Cell Biol. 2005;6:306–317. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- 27.Ainsley JA, Pettus JM, Bosenko D, Gerstein CE, Zinkevich N, Anderson MG, Adams CM, Welsh MJ, Johnson WA. Enhanced locomotion caused by loss of the Drosophila DEG/ENaC protein Pickpocket1. Curr. Biol. 2003;13:1557–1563. doi: 10.1016/s0960-9822(03)00596-7. [DOI] [PubMed] [Google Scholar]
- 28.Collins RT, Cohen SM. A genetic screen in Drosophila for identifying novel components of the hedgehog signaling pathway. Genetics. 2005;170:173–184. doi: 10.1534/genetics.104.039420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aza-Blanc P, Ramírez-Weber FA, Laget MP, Schwartz C, Kornberg TB. Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell. 1997;89:1043–1053. doi: 10.1016/s0092-8674(00)80292-5. [DOI] [PubMed] [Google Scholar]
- 30.Johnson RL, Milenkovic L, Scott MP. In vivo functions of the patched protein: Requirement of the C terminus for target gene inactivation but not Hedgehog sequestration. Mol. Cell. 2000;6:467–478. doi: 10.1016/s1097-2765(00)00045-9. [DOI] [PubMed] [Google Scholar]
- 31.Malpel S, Claret S, Sanial M, Brigui A, Piolot T, Daviet L, Martin-Lannerée S, Plessis A. The last 59 amino acids of Smoothened cytoplasmic tail directly bind the protein kinase Fused and negatively regulate the Hedgehog pathway. Dev. Biol. 2007;303:121–133. doi: 10.1016/j.ydbio.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 32.Stucky CL, Dubin AE, Jeske NA, Malin SA, McKemy DD, Story GM. Roles of transient receptor potential channels in pain. Brain Res. Brain Res. Rev. 2009;60:2–23. doi: 10.1016/j.brainresrev.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tobin DM, Bargmann CI. Invertebrate nociception: Behaviors, neurons and molecules. J. Neurobiol. 2004;61:161–174. doi: 10.1002/neu.20082. [DOI] [PubMed] [Google Scholar]
- 34.Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stein C, Millan MJ, Herz A. Unilateral inflammation of the hindpaw in rats as a model of prolonged noxious stimulation: Alterations in behavior and nociceptive thresholds. Pharmacol. Biochem. Behav. 1988;31:445–451. doi: 10.1016/0091-3057(88)90372-3. [DOI] [PubMed] [Google Scholar]
- 36.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung JM, Kim HK, Chung K. Segmental spinal nerve ligation model of neuropathic pain. Methods Mol. Med. 2004;99:35–45. doi: 10.1385/1-59259-770-X:035. [DOI] [PubMed] [Google Scholar]
- 38.Gallio M, Ofstad TA, Macpherson LJ, Wang JW, Zuker CS. The coding of temperature in the Drosophila brain. Cell. 2011;144:614–624. doi: 10.1016/j.cell.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 40.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 41.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 42.Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW., 4th Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc. Natl. Acad. Sci. USA. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J. Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW., 4th cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- 45.Hewes RS, Taghert PH. Neuropeptides and neuropeptide receptors in the Drosophila melanogaster genome. Genome Res. 2001;11:1126–1142. doi: 10.1101/gr.169901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gutstein H, Akil H. Opioid Analgesics. In: Brunton L, Lazo J, Parker K, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 11th Edition. New York: McGraw-Hill; 2006. pp. 547–590. [Google Scholar]
- 47.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 48.Johnson RL, Grenier JK, Scott MP. patched overexpression alters wing disc size and pattern: Transcriptional and post-transcriptional effects on hedgehog targets. Development. 1995;121:4161–4170. doi: 10.1242/dev.121.12.4161. [DOI] [PubMed] [Google Scholar]
- 49.Ni JQ, Markstein M, Binari R, Pfeiffer B, Liu LP, Villalta C, Booker M, Perkins L, Perrimon N. Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat. Methods. 2008;5:49–51. doi: 10.1038/nmeth1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 51.Igaki T, Kanda H, Yamamoto-Goto Y, Kanuka H, Kuranaga E, Aigaki T, Miura M. Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway. EMBO J. 2002;21:3009–3018. doi: 10.1093/emboj/cdf306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanda H, Igaki T, Kanuka H, Yagi T, Miura M. Wengen, a member of the Drosophila tumor necrosis factor receptor superfamily, is required for Eiger signaling. J. Biol. Chem. 2002;277:28372–28375. doi: 10.1074/jbc.C200324200. [DOI] [PubMed] [Google Scholar]
- 53.Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- 54.Xu JJ, Walla BC, Diaz MF, Fuller GN, Gutstein HB. Intermittent lumbar puncture in rats: A novel method for the experimental study of opioid tolerance. Anesth. Analg. 2006;103:714–720. doi: 10.1213/01.ane.0000226100.46866.ea. [DOI] [PubMed] [Google Scholar]
- 55.Dirig DM, Salami A, Rathbun ML, Ozaki GT, Yaksh TL. Characterization of variables defining hindpaw withdrawal latency evoked by radiant thermal stimuli. J. Neurosci. Methods. 1997;76:183–191. doi: 10.1016/s0165-0270(97)00097-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.