Abstract
Post-operative atrial fibrillation (POAF) is one of the most frequent complications of cardiac surgery and an important predictor of patient morbidity as well as of prolonged hospitalization. It significantly increases costs for hospitalization. Insights into the pathophysiological factors causing POAF have been provided by both experimental and clinical investigations and show that POAF is ‘multi-factorial’. Facilitating factors in the mechanism of the arrhythmia can be classified as acute factors caused by the surgical intervention and chronic factors related to structural heart disease and ageing of the heart. Furthermore, some proarrhythmic mechanisms specifically occur in the setting of POAF. For example, inflammation and beta-adrenergic activation have been shown to play a prominent role in POAF, while these mechanisms are less important in non-surgical AF. More recently, it has been shown that atrial fibrosis and the presence of an electrophysiological substrate capable of maintaining AF also promote the arrhythmia, indicating that POAF has some proarrhythmic mechanisms in common with other forms of AF. The clinical setting of POAF offers numerous opportunities to study its mechanisms. During cardiac surgery, biopsies can be taken and detailed electrophysiological measurements can be performed. Furthermore, the specific time course of POAF, with the delayed onset and the transient character of the arrhythmia, also provides important insight into its mechanisms.
This review discusses the mechanistic interaction between predisposing factors and the electrophysiological mechanisms resulting in POAF and their therapeutic implications.
Keywords: Atrial fibrillation, Post-operative atrial fibrillation, Inflammation, Sympathetic activation, Oxidative stress, Atrial remodelling, Fibrosis, Ageing
Introduction
Atrial arrhythmias and atrial fibrillation (AF) in particular are well-known complications after cardiac surgery with a reported incidence between 10 and 60%.1–17 The incidence is higher in patients undergoing valve surgery than in patients undergoing coronary artery bypass surgery (CABG).1,2,7,8,10,11,17,18 Post-operative atrial arrhythmias also occur after non-cardiac surgery, especially after oesophagectomy,19 lung surgery,20–24 and large abdominal surgery.25–27 The incidence after non-cardiac surgery is, however, lower with incidences ranging from 0.3 to 29%.22,28,29 Postoperative atrial arrhythmias are associated with prolonged hospital stay, haemodynamic instability, an increased risk of stroke, and increased mortality.2–4,6,10,12,13,24,28,30–33
The exact pathophysiological mechanisms responsible for the onset and perpetuation of post-operative atrial arrhythmias are incompletely understood. Factors facilitating post-operative AF (POAF) can be classified in acute factors directly related to surgery (e.g. adrenergic stimulation) and factors that are reflecting a chronic and progressive process of remodelling or ageing of the heart (e.g. left atrial enlargement).14,15 These predisposing factors can on the one hand provoke triggers able to initiate the arrhythmia and on the other hand enhance the development of a substrate capable of perpetuating AF.
The association of POAF with specific kinds of surgery and the time course of the arrhythmia can help to better understand its mechanisms. First, the association of POAF with cardiac surgery and degree of structural heart disease suggests a direct role for cardiac surgical trauma and pre-existing cardiac pathology in the occurrence of POAF. Secondly, the arrhythmia follows a specific time course. In most studies, the incidence peaks on the second day after surgery and rapidly declines to around 2% at discharge,32 suggesting that some of the pro-arrhythmic mechanisms require some time to become operative. Furthermore, the transient nature of the arrhythmia suggests a reversible mechanism, caused by factors which come into play shortly after surgery, but seem to subside on the long run.
This review discusses the mechanistic interaction between predisposing factors, alterations in intracellular signalling, and the electrophysiological mechanisms of POAF.
Epidemiology
The incidence of POAF after cardiac surgery varies considerably between different studies (Table 1). This variation in incidence is due to differences in patient demographics, techniques for rhythm monitoring and criteria for diagnosis.5,34 Mathew et al.3 found diverging POAF incidences according to different regions. The authors reported a similar POAF incidence among patients in the USA (33.7%), Canada (36.6%), Europe (34%), and the Middle East (41.6%), but a lower POAF incidence in South America (17.4%) and Asia (15.7%).3 Another example that stresses the importance of patient demographics is the identification of Caucasian race as an independent predictor of POAF in several studies.17,34 Fluctuation in reported POAF incidence due to differences in rhythm follow-up is illustrated by the comparison of the following three studies. Siebert et al.11 found an incidence as low as 9.8% after isolated CABG. However, only AF occurrence during stay on the intensive care unit was studied, with a mean period of 2.3 days.11 In another example, Leitch et al. found an incidence of 17.2% after isolated CABG. Again, only during the first 48 h AF and atrial flutter (AFL) were detected by continuous electrocardiogram (ECG) monitoring. After these 48 h, AF and AFL were solely identified if clinical symptoms occurred.9 On the other hand, in a large retrospective study Shen et al.17 found an incidence of 29% after isolated CABG. In this report, all patients received continuous 24 h telemetry with arrhythmia-detection algorithms during their entire hospital stay. The difference in reported POAF incidence between these studies emphasizes that a more systematic ECG monitoring results in a better identification and thus a higher detected incidence.17 Finally, the definition of POAF also influences its reported incidence. For example, in one study POAF is defined as any documented AF >5 min, while in another study only episodes of >10 min are counted.7,16
Table 1.
Incidence of post-operative atrial fibrillation
| Author | Fuller et al. | Leitch et al. | Creswell et al. | Aranki et al. | Almassi et al. | Siebert et al. | Mahoney et al. | Mathew et al. | Villareal et al. | Banach et al. | Mariscalco et al. | Ahlsson et al. | Shen et al. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year of publication | 1989 | 1990 | 1993 | 1996 | 1997 | 2001 | 2002 | 2004 | 2004 | 2006 | 2009 | 2009 | 2010 |
| n (% male) | 1666 (88.6) | 5807 (NS) | 3983 (66.7) | 570 (69) | 3855 (98.4) | 821 (74.4) | 10550 (71) | 4657 (79.8) | 6477 (73.8) | 1200 (66.6) | 9495 (73.2) | 571 (78) | 10390 (65) |
| Age (overall) | NS | 62.2 ± 12.3 | 67 | 63.7 ± 9.6 | NS | NS | 61 ± 2.4 | 66.2 ± 9.5 | 62.3 ± 12.9 | ||||
| Age (AF group) | 60.9 ± 7.3 | 71 | 66.8 ± 8.3 | 67.8 | 67.9 ± 9.6 | 66 ± 7.8 | 69.2 ± 7.6 | ||||||
| Study type | Prospective | Prospective | Prospective | Prospective | Prospective | Prospective | Retrospective | Prospective | Retrospective | Prospective | Retrospective | Prospective | Retrospective |
| Multicentre (number) | No | No | No | No | Yes (14) | No | No | Yes (70) | No | Yes(12) | No | No | No |
| Definition of POAF | AF detected by continuous telemetry | detected by continuous monitoring (48 h) or by clinical symptoms and signs | New onset AF, AFL, PAT | AF requiring medication/pacing | NS | Any AF detected via continuous ECG monitoring (only) during intensive care unit stay | NS | Entry in case report form/AF detected by ECG | AF of any duration, any time based on ECG | NS | AF–AFL>15 min | ECG verified episode >1 min during first 7 days | AF(L) during post-operative recovery period and requiring treatment |
| CPB (% of patients) | 100 | 100 | 100 | 100 | 100 | 85.6 (703/821) | 100 | NS | 87.5 | 100 | 93.9 | NS | |
| History of AF | None | None | None | None | None | None | None | 0.09% (424/4657) | None | NS (28% arrhythmias) | None | None | None |
| Incidence of POAF (%) | 28.4 | 17.2 | 34.6 (1378/3983) | 33 | 29.6 (1143/3885) | NS | NS | 32.3 (1503/4657) | 16 | 23.2 | 26.7 | 28.9 | 30 |
| CABG Alone | 28.4 (476/1666) | 17.2 | 31.9 (905/2833) | 33 | 27.6 | 9.8 (64/650) | 17.7 | 30.9 (1349/4371) | 16 (994/6477) | 23.2 (278/1200) | 22.9 | 28.9 (165/571) | 29 (2098/7284) |
| OPCAB | 10.2 (12/118) | ||||||||||||
| CABG+AVR | 60.1 (95/158) | 36.4 | 25.0 (9/36) | |33.8 | |53.9 (154/286) | |45.2 | |49 (436/887) | ||||||
| CABG+MVR | 63.1 (65/103) | 60 | 17.6 (3/17) | ||||||||||
| AVR | 48.8 (83/170) | 32.9 | |24.6 | |39.8 | |33 (459/1399) | ||||||||
| MVR | 44.3 (43/97) | 48.8 | |||||||||||
| Transplantation | 11.0 (15/136) |
Table showing incidences for POAF in different studies.
n, number of patients included; CABG, coronary artery bypass grafting; AVR, aortic valve replacement, MVR, mitral valve repair/replacement, NS, not stated; CPB, cardiopulmonary bypass; AFL, atrial flutter; PAT, paroxysmal atrial tachycardia; OPCAB, off-pump coronary artery bypass.
Despite the methodical differences between studies, the incidence of POAF could be shown to be strongly determined by the kind of surgery. In general, the reported incidence of POAF after CABG ranges between 16 and 50%.4,6,8,9,11–17 The incidence is higher after valve surgery and highest after a combination of CABG and valve surgery.1,2,7,10,11,17,18 Remarkably, after heart transplantation the incidence of post-operative atrial arrhythmias is reported to be very low (4%).35 However, due to the surgical cut and sew lines in the atria during heart transplantation, the atrial surface is significantly reduced and pulmonary veins are isolated.
In the group of non-cardiac surgery, the incidence of POAF is higher after thoracic surgery than after non-thoracic surgical procedures.36,37 In non-cardiac, non-thoracic surgery, POAF occurs relatively infrequently (0.37% for ophthalmic surgery up to 13% for large colorectal surgery).25–30 After thoracic surgery POAF is more frequent, with reported incidences of 9–29%.20,21,23,24,38–42 Some studies report no differences in incidence between more invasive and less invasive types of thoracic surgical procedures,20,21 although other studies do.22,38–40
The time course of the onset of POAF after cardiac surgery is very typical, with 70% of the patients developing POAF in the first 4 post-operative days and only 6% developing AF after the 6th day.4,7,43 Post-operative atrial fibrillation has its peak incidence on the 2nd post-operative day and recurrence of POAF is highest on the 3th post-operative day, with only 22% of the patients experiencing >2 episodes of POAF.3,8
Different risk factors for development of POAF after cardiac surgery have been identified. The strongest predictor of POAF is advancing age.1,2,4,6–9,15–18,44–48 Association with other risk factors shows a large degree of variability between different studies (Table 2). For example, Zacharias et al.47 found body mass index to be an important determinant of POAF, while other studies failed to show this.6,7,15 Furthermore, left atrial enlargement is a predictor of POAF in some studies.15,46 However, sometimes left atrial enlargement is not predictive even when mitral valve surgery in the same study is a risk factor. This might indicate a role for tissue trauma as a consequence of a more invasive procedure during mitral valve repair/replacement.3,48
Table 2.
Risk factors for post-operative atrial fibrillation
| Author | Fuller et al. | Leitch et al. | Creswell et al. | Aranki et al. | Almassi et al. | Zaman et al. | Hakala et al. | Mathew et al. | Auer et al. | Zacharias et al. | Banach et al. | Shen et al. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year of publication | 1989 | 1990 | 1993 | 1996 | 1997 | 2000 | 2002 | 2004 | 2005 | 2005 | 2006 | 2010 |
| Number of Patients | 1666 | 5807 | 3983 | 570 | 3855 | 326 | 88 | 4657 | 253 | 8051 | 1200 | 10390 |
|
Risk Factors | ||||||||||||
| Age | P = 0.0001 | OR = 1.7, P<0.001 (10 yr decile) | P < 0.001 | OR = 2.0 P = 0.002 (age 70 to 80 yr) | OR = 1.61 P = 0.0001 (10 yr decile) | OR = 1.53 P < 0.0005 (per 5 yr increase) | OR = 1.07, P = 0.02 (each increasing yr above lower border) | OR = 1.75 P < 0.001 (10 yr decile) | OR = 2.6, P < 0.01 (above vs. below median) | OR = 1.52 P < 0.001 (10 yr decile) | OR = 2.6 P < 0.001 (age>70 yr) | OR = 5.34 (age>72 yr) |
| History of AF | OR = 2.11, P < 0.001 | OR = 6.1, P < 0.002 | ||||||||||
| COPD | OR = 1.5, P = 0.006 | P < 0.001 | ns | OR = 1.37, P = 0.0016 | ns | OR = 1.43, P = 0.009 | OR = 1.28, P < 0.001 | ns | ns | |||
| Hypertension | ns | ns | ns | OR = 1.6, P = 0.03 | OR = 1.19, P = 0.027 | ns | ns | ns | ns | ns | OR = 1.15 | |
| Male gender | P = 0.02 | ns | OR = 1.7, P = 0.01 | ns | OR = 2.88, P = 0.009 | ns | ns | ns | OR = 1.24, P = 0.001 | ns | ns | |
| Diabetes | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ||
| Prior MI | ns | ns | OR = 1.6, P = 0.01 | ns | ns | ns | ns | ns | ||||
| CHF | ns | ns | ns | ns | OR = 4.8, P < 0.005 | OR = 1.28 | ||||||
| BMI | ns | ns | ns | OR = 1.36, p < 0.001 BMI = >30–35 kg/m2 | ns | |||||||
| No pre-operative ß-blocker therapy | ns | OR = 1.2, P = 0.011 | ns | ns | ns | ns | ns | OR = 1.7, P < 0.05 | OR = 1.17, P = 0.005 | OR = 0.79, P < 0.01 | ns | |
| Left atrial enlargement | ns | OR = 1.29, P = 0.01 | ns | |||||||||
| RCA stenosis | ns | ns | ns | ns | ||||||||
| Mitral valve surgery | OR = 2.86, P = 0.0001 | OR = 1.74, P < 0.001 | OR = 2.8, P < 0.01 | OR = 2.42, P < 0.001 | OR = 1.91 | |||||||
| Postoperative withdrawal of ß-blocker | ns | OR = 1.91, P < 0.001 | ||||||||||
| Postoperative withdrawal of ACE-I | OR = 1.69, P < 0.001 | |||||||||||
| (No) post-operative ß-blocker therapy | P = 0.001 | ns | OR = 0.32, P < 0.001 | OR = 0.79, P < 0.01 | ||||||||
| Postoperative ACE-I therapy | OR = 0.62, P < 0.001 | |||||||||||
This table shows an overview of risk factors for POAF in different studies. The numbers in the boxes are statistical values (P value, odds ratio, relative risk). ns means not significant after multivariate analysis. If risk factors are not mentioned in the study, the boxes are empty.
COPD, chronic obstructive pulmonary disease, ACE-I, angiotensin-converting enzyme inhibitor; RCA, right coronary artery; MI, myocardial infarction; CHF, chronic heart failure, yr=year.
In 2002, Ferguson et al.49 confirmed the wide-spread perception among cardiac surgeons that the population of patients currently referred for isolated CABG are older, sicker, and have a higher surgical risk than a decade ago. As age represents an important risk factor for the onset of POAF, one would expect an increase in incidence of POAF over time which indeed in one study has been reported.1 Other studies, however, failed to identify an increase or even reported a trend towards a decreasing prevalence.9,10,17,50,51 Whether the lack of increase in POAF incidence over the past years is due to more frequent use of beta-blockers or amiodarone is currently unknown. In the study of Shen et al.,17 the annual percentage of aortic and mitral valve procedures increased over two decades. Considering that these surgical procedures are associated with a higher risk of POAF, and that the incidence remained approximately 30%, the authors concluded that some progress in treating POAF has been made.17
Mechanisms based on acute factors
Inflammation
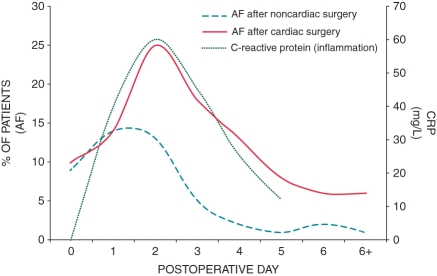
The similarity between the time course of AF occurrence after cardiac surgery and the activation of the complement system with the release of pro-inflammatory cytokines suggests an inflammatory component in the mechanism triggering POAF.52–57 Complement activation during cardiac surgery with cardiopulmonary bypass (CPB) occurs in two steps. The first phase occurs during CPB, results from interaction of blood with the surface of the extracorporeal circuit, and is mediated via the ‘alternative pathway’ involving tumour necrosis factor α. The second phase acts via the ‘classical pathway’ which is initiated by protamine usually administered after CPB. Interestingly, fever and POAF do not occur before the first post-operative days and thus coincide with the second phase rather than with the first. Their time course corresponds to changes in activity of markers indicating complement activation and inflammation, such as C-reactive protein (CRP), complement-CRP complexes,52 interleukin-2,58 and interleukin-6.53 The similarity in the post-operative time course of POAF incidence and CRP is illustrated in Figure 1. Also, a more pronounced increase in post-operative white blood cell count as a marker of inflammatory response independently predicts the development of post-operative AF in some studies, but not in others.56,59 Furthermore, patients developing POAF have up-regulated monocyte activation and higher monocyte and neutrophil levels post-CPB.60,61
Figure 1.
Time course of atrial fibrillation incidence after cardiac and non-cardiac surgery and time course of C-reactive protein after cardiac surgery. Atrial fibrillation incidence after non-cardiac surgery peaks at post-operative day 1 and then rapidly declines to 2% at day 6, while atrial fibrillation incidence after cardiac surgery peaks at post-operative day 2 and slowly declines to around 6 % at day 6. This suggests a ‘cardiac factor’, related to the specific setting of cardiac surgery. The time course of C-reactive protein is surprisingly similar to that of atrial fibrillation incidence after cardiac surgery, supporting the role for inflammation in the mechanism of post-operative atrial fibrillation (modified from references 4,30,52).
Besides the systemic inflammatory reaction caused by use of CPB, also local inflammation caused by surgical incision contributes to the occurrence of POAF. It is known that the degree of atrial inflammation increases with the invasiveness of surgery, but even after pericardiotomy alone the atrium becomes mildly inflamed. This transient sterile pericarditis, which is part of the healing process, might help to explain the temporal occurrence of POAF. Comparison of AF incidence after off-pump and on-pump surgery facilitates to distinguish the importance of systemic inflammation from that of surgical incision and manipulation. As such, off-pump CABG (OPCAB) is believed to elicit less systemic inflammation than on-pump surgery because of reduced cytokine responses and less myocardial injury.62 However, several studies failed to show statistical association between OPCAB and a lower incidence of POAF.11,63–68 This lack of association suggests that surgical stress as such is a more important determinant than systemic inflammation in triggering POAF. It has to be noted, however, that some of these studies are limited by their retrospective nature and sample size, and that they all showed at least a non-significant trend towards lower AF incidence in off-pump surgery. Other controlled randomized studies do reveal CPB in combination with cardioplegic arrest as the main predictor of POAF, especially in elderly and high-risk individuals.69–72 For example, Panesar et al.73 performed an extensive meta-analysis including 4921 patients aged 70 years and older and reported a significantly lower AF incidence in the OPCAB group compared with on-pump surgery. It could be argued that the influence of on-pump surgery compared with off-pump surgery on AF occurrence is rather small and that this effect only emerges in the older patient population, where the risk of POAF is known to be higher.1–4 Furthermore, minimal invasive OPCAB resulted in lower AF incidence compared with conventional, more extensive OPCAB in one study, but surprisingly failed to reach significance in another.74,75 This might indicate that the trauma and the successive inflammation of the pericardium that easily spreads within the pericardial sac rather than the manipulation of the myocardial tissue itself renders the atria more prone to AF.
Several experimental and clinical studies have been undertaken to explore how inflammation enhances AF susceptibility of atrial tissue. A prominent example of involvement of inflammation in the development of AF is the study by Frustaci et al.76, showing lymphomononuclear infiltrates compatible with atrial myocarditis in atrial tissue of 66% of patients with lone AF.76 Also Chen et al.77 found CD45-positive cells to be independently and significantly higher in right atrial appendages of patients with AF compared with patients with sinus rhythm. An excellent experimental model to study post-operative AF/AFL is the canine sterile pericarditis model of Page et al.78 In this model, sterile pericarditis is created by epicardial application of sterile talcum. The time course of atrial arrhythmias in patients after open heart surgery is consistent with inducibility of AF/AFL in this model, both peaking between day 2 and 4 after surgery.79 In response to sterile pericarditis, proliferation, and activation of epicardial fibroblasts takes place in the atria, with loss of epicardial myocytes and altered distribution of connexins 40 and 43.80 These changes are associated with non-uniform slowing of conduction and promote induction and maintenance of AF/AFL. The causative association between inflammation and post-operative AF/AFL was further studied in the canine pericarditis model by suppression of the inflammatory response with steroids and HMG-CoA reductase inhibitors (statins).81,82 Administration of prednisone inhibited tissue inflammation and reduced serum CRP and AF inducibility.81 Also atorvastatin, an HMG-CoA reductase inhibitor, significantly reduced CRP levels and AF duration, and attenuated perimyocarditis.82 Finally, the use of n-3 polyunsaturated fatty acids in the sterile pericarditis model was associated with lower levels of inflammatory markers, a reduction in AF inducibility and AF duration, prolongation of the refractory period, and shortening of intra-atrial conduction times.83
Atrial inflammation is known to cause conduction disturbances. For example, in a study with mongrel canines, Ishii et al.84 measured myeloperoxidase activity and neutrophil cell infiltration in atrial myocardium. The degree of atrial inflammation was associated with a proportional increase in the heterogeneity of atrial conduction after experimental cardiac surgery and increased the incidence and duration of AF.84 In another canine study, acute inflammation provoked by arachidonic acid produced slowing and enhanced anisotropy of conduction but did not affect atrial refractoriness.85
In several clinical trials, drugs with anti-inflammatory effects have shown to be effective in lowering AF incidence after CABG and/or valve surgery. Corticosteroids reduce the incidence of new-onset POAF by inhibition of cytokine release (tumour necrosis factor α and interleukin-6), thereby reducing complement activation.86,87 A recent meta-analysis of 17 643 patients undergoing cardiac surgery suggests that pre-operative use of statins significantly reduces POAF incidence.88 The exact mechanism by which statins lower POAF incidence is, however, likely pleiotropic. Besides its lipid-lowering effect, pre-operative statin therapy is known to decrease inflammation markers,89 and also to attenuate myocardial reperfusion injury after cardiac surgery.90 According to the European guidelines, corticosteroids (class IIb recommendation) may be and statins (class IIa recommendation) should be considered for prevention of POAF after CABG and/or valve surgery.91 Treatment with n-3 polyunsaturated fatty acids to prevent POAF has been reported with success in some studies;92,93 however, placebo-controlled, double-blinded, randomized trials have failed to reproduce this protective effect of fish oil.94,95
Finally, it is known that chronic inflammation in patients can cause atrial structural remodelling. C-reactive protein is associated with and predicts patients at risk of developing future non-surgical AF.54,55 This chronic inflammation might also predispose to the occurrence of POAF. Some studies found elevated pre-operative CRP levels to be associated with an increased risk of the arrhythmia after CABG.32,96 Others, on the contrary, failed to find an association between pre-operative CRP and POAF.60,97,98 This controversy suggests that the induction of inflammation during surgery, rather than a pre-existing inflammation process, contributes more to the development of the arrhythmia.
Sympathetic activation
In the heart, sympathetic stimulation is mediated by β-adrenoreceptors and leads to an increase in heart rate and contractile force, but also to enhanced excitability and automaticity.99 Several findings support a role for sympathetic activation in the pathogenesis of atrial arrhythmias after cardiac surgery. Advanced age, the most important risk factor for POAF, is associated with increasing circulating norepinephrine levels.100 Patients who develop POAF also have significantly elevated norepinephrine levels post-operatively compared with patients without POAF.14 This association is further reflected by the fact that the onset of POAF is preceded by an increase in sinus rate and atrial ectopic activity. Also, studies on post-operative heart rate variability (HRV) showed an increase in time- and frequency-domain parameters of HRV prior to the onset of POAF, consistent with increasing sympathetic activity.101,102 However, controversy remains if this sympathetic activation is accompanied by either increased activity or loss of vagal tone.101–103 Finally, sympathetic activation has been reported to shorten atrial refractoriness non-uniformly, thereby favouring the perpetuation of the arrhythmia.104 On the other hand, there is a slight discrepancy between the peak of sympathetic activation, which occurs within 24 h post-operatively, and the onset of POAF, mostly developing between 48 and 72 h after surgery.105
If sympathetic activation plays an important role in onset of POAF, one would expect that cardiac denervation reduces the incidence of the arrhythmia. This was first studied by Melo et al.106 They performed ventral cardiac denervation in 207 patients undergoing low-risk CABG, and indeed found that this intervention significantly reduced the incidence and severity of POAF.106 It should be noted, however, that only 15% of the patients in their study underwent telemetric monitoring and some patients with asymptomatic AF might not have been identified. Other studies failed to show the benefit of cardiac denervation or found even an increase in POAF.107–109 These findings, however, do not exclude a role for sympathetic activation in the arrhythmia substrate, as both sympathetic and parasympathetic activation alter atrial refractoriness.109,110 Hogue et al.103 detected a higher HRV in some patients, but a lower HRV in others in the hour before onset of POAF, suggesting the possibility of divergent autonomic conditions shortly before onset of the arrhythmia. Cardiac denervation obviously interrupts both sympathetic and parasympathetic regulation of heart function.
Drugs mimicking sympathetic activation are also pro-arrhythmic. Administration of milrinone, a phosphodiesterase inhibitor that increases cardiac cyclic adenosine monophosphate (cAMP), dobutamine, and dopamine, both binding on the β-adrenoreceptor, is associated with an increased incidence of POAF.111–113 Activation of protein kinase A by cAMP can lead to stimulation of multiple cardiac currents, including the L-type calcium current (ICaL), thereby promoting the occurrence of early and delayed afterdepolarizations.114,115 Mechanisms by which inotropic drugs can promote POAF consist of abbreviation of atrial refractoriness (presumably due to activation of the slowly activating delayed rectifier current (IKs)) and increased ectopic activity.114–116
In theory, blocking the sympathetic activation by β-adrenoreceptor blocking drugs should reduce the incidence of POAF. Indeed, patients receiving β-blockers post-operatively have fewer episodes of AF compared with patients receiving placebo.50,117,118 However, these results must be interpreted with caution as arrhythmia detection varies between studies and some of the patients, assigned to the placebo group, were withdrawn from their pre-operative β-blocker therapy.105 The withdrawal of pre-operative β-blockade after CABG is associated with a more than two-fold increase in POAF.119 The peak effect of this rebound phenomenon correlates well with the time course of POAF, suggesting that the continuity of pre-operative β-blocking therapy after surgery has a stronger reductive effect on POAF incidence than β-blocking treatment started de novo after surgery.14 The increase in POAF incidence after β-blocker withdrawal might be due to the synergistic effect of the rebound phenomenon and the higher sympathetic tone post-operatively.
Workman et al.50 found pre-operative β-blockade to be associated with significant prolongation of atrial cell action potential duration (APD) and atrial effective refractory period (AERP) in isolated cells of patients undergoing open heart surgery.120 The authors called this adaptive response ‘pharmacological remodelling’, as it appeared to be caused by the previous exposure to but not by the acute presence of β-blocker. Contribution of this prolongation of refractoriness to the anti-arrhythmic effect of β-blockers can act via lengthening the minimum pathlength for reentry. In their study, however, this β-blocker-induced AERP prolongation was identical between patients who did and did not developed POAF.50 The authors concluded that, as pre-operative β-blockade did reduce POAF incidence in their study without involvement of β-blocker-induced AERP prolongation, attenuation of triggered atrial extrasystoles may also underlie the antiarrhythmic effect of β-blockers.50
In conclusion, it seems that sympathetic activation, by altering atrial refractoriness and promoting ectopic activity, contributes to the onset of POAF. The fact that β-blockade does not abolish all episodes of POAF once more stresses the multifactorial aetiology of POAF.121 However, oral β-blocker therapy started at least 1 week before surgery remains the first choice in preventing POAF after cardiac surgery.91
Oxidative stress
Oxidative stress occurs from an imbalance between pro-oxidants and antioxidants in favour of pro-oxidants. The use of CPB in cardiac surgery involves controlled ischaemia followed by reperfusion of the heart. During reperfusion, increased production of reactive oxygen species takes place, leading to myocardial stunning, tissue damage, and cell death.122,123
The interaction between oxidative stress and electrical remodelling has been studied in experimental studies. In a canine rapid atrial pacing (RAP) model of AF, pacing-induced reduction of AERP was attenuated by ascorbate, a potent antioxidant.124 Production of atrial peroxynitrite, a free radical, was enhanced while endogenous atrial ascorbate levels were diminished during RAP. Supplementation of vitamin C prevented atrial tissue ascorbate depletion and the increased peroxynitrite formation. These results suggest a direct effect of oxidative stress on early electrical remodelling (24–48 h after RAP).124 In another canine RAP study, AF promotion after 7 days of RAP was attenuated by simvastatine but not by antioxidant vitamins C and E.125 The dosages in both studies were comparable. Therefore, these results might indicate that vitamin C can attenuate AF promotion in very early remodelling, but that it loses its protective effect during later stages of the electrical remodelling process. The fact that simvastatine attenuated AF promotion can be partly due to an anti-inflammatory mechanism. As discussed before, statins possess antioxidant as well as anti-inflammatory properties.126
Atrial myocytes of patients with persistent AF show oxidative damage following cardiac production of peroxynitrite, which oxidizes cellular lipids, proteins, and DNA and promotes death of cardiomyocytes via necrosis/apoptosis.127 This oxidation contributes to the loss of fibrillar protein function and thus to atrial contractile dysfunction. Moreover, atrial nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity is increased in right atrial appendages of patients with non-surgical forms of AF compared with patients without AF.128 Nicotinamide adenine dinucleotide phosphate oxidase is known to be an important source of reactive oxygen species in human atrial myocytes.128 Direct measurement of free radicals in atrial tissue of patients during episodes of POAF is impossible. Therefore, evidence for the role of oxidative stress has been obtained by measuring concentrations of antioxidants as lipid peroxidation products and by administering antioxidant substances.129,130
Indications that oxidative stress plays a role in the occurrence of POAF can be summarized as follows. First of all, reperfusion of patients undergoing CABG results in oxidative stress and the amount of oxidative stress depends on the severity of the ischaemic period and left ventricular ejection fraction.129,130 Secondly, Ramlawi et al.131 confirmed that patients with POAF, compared with patients without the arrhythmia, have a larger increase in systemic oxidative stress as well as at the myocardial level. Third, in a follow-up study Kim et al.132 measured NADPH oxidase activity in right atrial appendage samples from patients undergoing CABG. The authors identified NADPH oxidase activity as the most important independent predictor of POAF. Surprisingly, in their preliminary data there was no difference in NADPH oxidase activity before CPB and after reperfusion. They hypothesized that the perioperative inflammatory response, rather than ischaemia and reperfusion, stimulates atrial NADPH oxidase activity, thereby increasing oxidative stress. Interestingly, also Clermont et al.133 argue that oxidative stress related to myocardial ischaemia/reperfusion might be overwhelmed by systemic radical activation, which is due to the activation of neutrophils and high-oxygen tension level during CPB.
The involvement of oxidative stress in the multifactorial mechanism of POAF has been further studied by administrating antioxidant drugs to patients undergoing heart surgery. Indeed, antioxidant drugs are reported to lower the incidence of POAF after cardiac surgery involving CPB use. For example, Carnes et al.124 showed that administration of ascorbate to patients undergoing CABG decreased the incidence of POAF. Moreover, combination of ascorbic acid and β-blockers seems to be more effective than β-blockers alone in reducing post-CABG AF.134 Another example is the administration of the antioxidant N-acetylcysteine. N-acetylcysteine lowered the incidence of POAF after CABG and/or valve surgery significantly.135 By scavenging reactive oxygen species with N-acetylcysteine, myocardial oxidative stress is attenuated in patients undergoing CABG with CPB and cardioplegic arrest.136 Furthermore, nitric oxide (NO) gas has been reported to significantly inhibit oxidative stress when administered to patients undergoing CABG.137 Sodium nitroprusside (SNP), an NO donor, significantly lowered the incidence and duration of post-CABG AF in a recent pilot study.138 Finally, also statins are known to lower the incidence of POAF, presumably in part through their antioxidative properties.88
Another argument supporting a causative relation between oxidative stress and POAF is the higher occurrence of the arrhythmia in the elderly.4 Ageing hearts are more susceptible for ischaemia/reperfusion injury.139 It can be hypothesized that cellular damage due to oxidation is more important in older patients undergoing cardiac surgery and that this, in part, explains the higher incidence of POAF in this population.
Finally, the relation between the specific setting of off-pump surgery and oxidative stress has also been studied. Off-pump surgery not only allows avoidance of ischaemia/reperfusion, but has also been associated with a reduced systemic inflammatory reaction.62 Furthermore, inflammation seems to be at least as important as ischaemia/reperfusion in producing oxidative radicals during on-pump surgery.132,133 Indeed, some studies indicate that off-pump surgery is associated with less oxidative stress. For example, Fontaine et al.140 reported that only plasmas isolated after on-pump, but not after OPCAB, induce superoxide generation in the vascular wall of rat aorta, leading to oxidative stress. Moreover, levels of oxidative stress markers (lipid hydroperoxides, protein carbonyls, and nitrotyrosine) in peripheral plasma of patients undergoing CABG were significantly lower in OPCAB compared with on-pump CABG.141 In another study, Orhan et al.142 found reduced systemic inflammation in patients undergoing OPCAB compared with on-pump CABG. However, they failed to show a reduction in myocardial oxidative stress in the off-pump group.142
Mechanisms based on pre-existing factors
Presence of a substrate
Besides AF promotion by acute, surgery-induced factors (Table 3), also the pre-existence of a substrate for AF can predispose to onset of the arrhythmia in the post-operative setting (Table 4). Development of such an AF substrate can involve (A) ion channel alterations resulting in shortening and/or enhanced dispersion of atrial refractoriness and (B) heterogeneities in conduction due to interstitial alterations like, for example, accumulation of collagen fibres, inflammatory infiltration or amyloidosis. Both mechanisms and their relationship with POAF will be separately discussed.
Table 3.
Overview of studies with important findings regarding the role of acute surgery-induced factors in the mechanism of post-operative atrial fibrillation
| Author, year | Acute factor | Species | Main finding |
|---|---|---|---|
| White (1984) | Adrenergic Activation | Human | Prophylactic use of timolol after CABG decreases frequency and severity of supraventricular arrhythmias. |
| Kalman (1995) | Adrenergic Activation | Human | Significant association between norepinephrine levels and the development of POAF |
| Bruins (1997) | Inflammation | Human | The second phase of complement activation during CPB involves CRP and is associated with POAF |
| Frustaci (1997) | Inflammation | Human | Lymphomononuclear infiltrates compatible with atrial myocarditis in atrial tissue of 66% of patients with lone AF |
| Carnes (2001) | Oxidative stress | Canine/human | Ascorbate attenuates rapid pacing-induced atrial ERP shortening and decreases the incidence of POAF after CABG. |
| Kumagai (2004) | Inflammation/oxidative stress | Canine | Atorvastatin prevents AF by inhibiting inflammation in the sterile pericarditis model |
| Shiroshita-Takeshita (2004) | Oxidative Stress/inflammation | Canine | AF promotion by atrial tachycardia is attenuated by simvastatin, but not by antioxidant vitamins. |
| Ishii (2005) | Inflammation | Canine | Atrial inflammation after cardiac surgery is associated with inhomogeneity of atrial conduction |
| Workman (2006) | Adrenergic activation | Human | Chronic β-blocker therapy is associated with reduced POAF incidence, unrelated to pre-operative ERP-prolonging |
| Kim (2008) | Oxidative stress | Human | NADPH oxidase activity in right atrial appendage is the most important independent predictor of POAF. |
| Fleming (2008) | Adrenergic Activation | Human | Perioperative milrinone use is associated with an increased incidence of POAF |
| Ozaydin (2008) | Oxidative stress | Human | Treatment with N-acetylcysteine, an antioxidant, decreases the incidence of post-operative AF. |
| Ho (2009) | Inflammation | Human | Corticosteroid prophylaxis is effective in reducing the risk of atrial fibrillation |
Table 4.
Overview of studies with important findings regarding the pre-existence of a substrate in the mechanism of post-operative atrial fibrillation
| Author, year | Substrate factor | Species | Main finding |
|---|---|---|---|
| Steinberg (1993) | Structural alteration | Human | Signal-averaged surface P-wave duration is a potent, accurate, and independent predictor of POAF |
| Von Wagoner (1999) | Alteration in ion channels | Human | Positive correlation between ICaL measured at the time of surgery and the occurrence of POAF. |
| Brandt (2000) | Alteration in Ion channels | Human | No difference in Ito and a non-significant trend towards a decrease in Ikur between patients with and without POAF. |
| Ad (2001) | Structural alteration | Human | Atrial myolysis and lipofuscin levels identified as an independent histologic finding associated with POAF |
| Goette (2002) | Structural alteration | Human | Amount of atrial fibrosis in association with prolongation of the surface P-wave or ageing correlates with POAF |
| Dobrev (2002) | Alteration in Ion channels | Human | Atrial myocytes of patients developing POAF have no alterations in IK1 and IK,ACH. |
| Ak (2005) | Structural alteration | Human | Degree of atrial myolysis and increased apoptotic pattern are significant predictors for development POAF. |
| Mariscalco (2006) | Structural alteration | Human | Atrial histology is similar in patients undergoing on- or off-pump surgery and is similar before and after CPB. |
| Workman (2006) | Alteration in ion channels | Human | No differences in ICaL, Ito, IK1, and ISUS in right atrial biopsies of patients who do and do not develop POAF |
| Kanagaratnam (2008) | Structural alteration | Human | Only patients with sustained induced AF develop POAF and have prolonged unipolar electrograms. |
A. Alterations in electrical ion channels as predisposing factor for POAF
The question as to whether propensity to POAF can be explained by pre-existing alterations of ion-channel function in these patients has been addressed by several investigators.
Calcium (Ca2+) influx through the L-type Ca2+ channels is the main current to produce the plateau phase of the atrial action potential. High atrial rates as they occur during AF or RAP are known to down-regulate ICaL which contributes to shortening of atrial refractoriness as a consequence of AF.143 Some studies have investigated whether changes of this current can also predispose to AF in the setting of cardiac surgery. In a study by Van Wagoner et al.,144 ICaL in isolated atrial myocytes of non-AF patients was larger in patients developing POAF compared with those without the arrhythmia. Also, a higher sympathetic tone after surgery14 will further increase calcium influx through L-type Ca2+ channels. Enhanced calcium load might elicit triggered activity (e.g. delayed afterdepolarizations) potentially initiating POAF.144 However, a more recent and very detailed study by Workman et al.50 could not confirm any differences in ICaL between patients with and without POAF. This recent study and the significant overlap in the Ca2+ current density data for most patients in the earlier report144 suggest that changes in L-type Ca2+ channel might have contributed to POAF initiation in some patients, but certainly not in all.
Potassium (K+) channels, which are altered in patients with persistent AF, are apparently not involved in the occurrence of POAF. First, Brandt et al.145 reported that the ultra-rapid delayed rectifier K+ current (IKur) is reduced in human persistent AF. In non-AF patients developing AF after cardiac surgery, however, only a non-significant trend towards a decrease in IKur and no difference in the transient outward K+ current (Ito) were detected compared with patients without POAF.145 Dobrev et al.146,147 found the larger basal inward rectifying K+ current in patients with persistent AF to consist of increased activity of the inward rectifier K+ current (IK1) and constitutive activity of the acetylcholine-activated K+ current (IK,ACH). Again, both IK1 and IK,ACH were not altered in non-AF patients developing POAF compared with patients not having AF after surgery. Thirdly, Workman et al.50 found no differences in Ito, in IK1, or in the sustained outward K+ current (ISUS) between patients who did and did not develop POAF. These findings are consistent with unaltered APD or ERP in their study50 and with results in other reports.145,147 Finally, a recent study of Swartz et al.148 confirmed the lack of difference in K+ channels in atrial biopsies of patients who did and did not develop AF after cardiac surgery.
Altogether, these data suggest that, unlike in persistent AF, pre-operative changes in cellular Ca2+ and K+ channels do not play an important role in the occurrence of POAF.
B. Alterations of the atrial interstitium and extracellular matrix predisposing to POAF
Ageing is an important risk factor for POAF and slowing of conduction is known to occur as atria structurally remodel with age. Spach and Dolber149 were the first to report that progressive electrical uncoupling of the side-to-side connections between parallel-orientated atrial fibres occurs in atrial muscle with advancing age. This uncoupling results in a decrease of transverse conduction and enhances anisotropy of conduction velocity.149 Such an alteration in conduction is often associated with the presence of extensive collagenous septa and favours reentry.149 The relationship between this age-dependent remodelling and the occurrence of POAF is strengthened by several studies. First, Ad et al.150 reported that the severity of pre-operative atrial myolysis in right atrial biopsies of non-AF patients undergoing CABG correlated well with the occurrence of POAF. In this study and in a study by Mariscalco et al.,151 no histological differences were noted in atrial specimens before and after CPB. This suggests that any contribution of CPB to POAF must be independent from histological changes. Secondly, Ak et al.152 found that pre-operative morphologic alterations such as atrial myocardial vacuolization and increased myocardial apoptosis may constitute a pathologic substrate for post-operative AF. Third, a study of Goette et al.153 further strengthens this role for pre-existent structural alterations. In this report, the incidence of POAF increased with the amount of fibrosis in right atrial appendages of patients undergoing cardiac surgery. Moreover, atrial fibrosis was not only age dependent, but also correlated with P-wave duration suggesting macroscopic slowing of conduction.153 Finally, a larger amount of fibrosis was found in left atria of patients developing POAF.148 On the other hand, one study reported no differences in right atrial histology between patients who do and do not develop POAF.154
By comparing POAF incidences between different types of cardiac surgery, the important contribution of atrial structural alterations to the mechanisms of POAF is further supported. For example, Anné et al.155 found more profound structural changes in patients with mitral valve disease than in patients undergoing CABG: larger atria, hypertrophied cells, more interstitial fibrosis, and signs of cellular degeneration. Left atrial fibrosis was more pronounced in patients undergoing mitral valve surgery compared with patients undergoing CABG, independently of the underlying heart rhythm. It appears reasonable to assume that the higher AF incidence after mitral valve surgery is due to these structural alterations. Also, Asher et al.156 found left atrial enlargement to be a independently associated with POAF in patients undergoing only valve surgery.
Other studies more directly demonstrate the pre-existence of an arrhythmogenic substrate in patients who do develop POAF. For example, Lowe et al.45 screened patients at risk for developing POAF by electrical stimulation of the mid–right atrium during surgery. Of a total of 36 patients in whom AF was inducible, 17 patients developed POAF. One patient was not inducible, but did develop POAF. Another example is the study by Kanagaratnam et al.,157 where AF was induced during cardiac surgery by burst pacing in patients without a history of AF. Only patients with sustained induced AF developed any episodes of POAF.157 Also in the same study, patients with sustained AF had prolonged unipolar electrograms compared with patients not able to sustain AF and this prolongation was more marked in the region of the crista terminalis than in the trabeculated right atrium. The authors stated that this prolongation of local electrograms is suggestive of microscopic conduction abnormalities.157 Finally, connexin 40 expression, one of the three connexins present in atrial myocytes, is significantly higher in patients who develop POAF compared with sinus rhythm patients.158 Cell-to-cell conduction properties are determined by gap junctions, which are clusters of transmembrane channels built up from connexins. As such, enhanced expression and heterogeneous distribution of connexin 40 could result in local conduction heterogeneities.158
Some indirect evidence for the existence of a structural substrate for AF comes from studies investigating surface-ECG parameters in patients with POAF. In a study of Steinberg et al.,159 measurement of P-wave duration on the standard ECG was longer in patients with POAF, but this did not reach significance. In the same study, however, signal-averaged P-wave duration proved to be an independent predictor of AF after cardiac surgery.159 In another study, increase in P-wave dispersion post-operatively predicted POAF after CABG.160
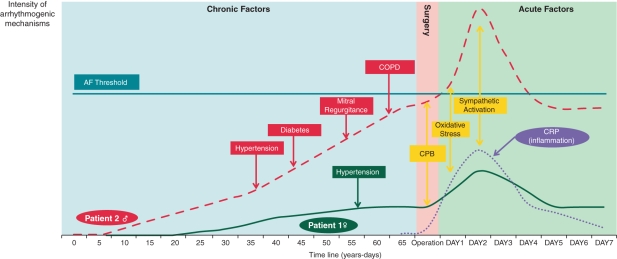
The concept of a pre-existing substrate for AF as an important predictor of POAF is also supported by a study of Ahlsson et al.12 who recently published the remarkable finding that one-fourth of patients with POAF developed AF of any form during a follow-up of 5 years. A possible explanation is that these patients already had a pre-existing substrate for AF at the time of surgery, that this substrate was unmasked by occurrence of acute factors increasing the activity of pro-arrhythmic factors in the perioperative period and eventually led to a non-surgical form of AF later on. The hypothetical relationship between acute and chronic factors is illustrated in Figure 2. In this figure, the time course of two hypothetical patients is depicted. Both patients have no AF history at the time of surgery and undergo on-pump CABG at the same age. In patient 1, acute surgery-related factors enhance the AF susceptibility, but the ‘AF threshold’161 is not reached and sinus rhythm is maintained in the post-operative phase. In patient 2, synergistic interaction of acute, surgery-induced factors and the pre-existence of a substrate for AF due to structural heart disease enhances AF susceptibility that much that the ‘AF threshold’161 is exceeded. In this sense, the post-operative setting can be regarded as a ‘stress test’ for the propensity to the arrhythmia.
Figure 2.
Time course of substrate development and surgery-related factors in the occurrence of atrial fibrillation. Time course of pro-arrhythmic mechanisms is depicted in two hypothetical patients undergoing cardiac surgery. Both chronic as well as acute factors related to the operation on day 0 are shown. When the intensity of pro-arrhythmic factors reaches a certain threshold,161 atrial fibrillation will occur. Patient 1 has no relevant cardiovascular history, only hypertension (green) at the age of 57. Patient 2 already developed hypertension (red) at a younger age, followed by diabetes (red), mitral regurgitation (red), and COPD (red) at an older age, respectively. Both patients have no history of AF and undergo on-pump coronary artery bypass grafting at the same age. However, patient 2 has developed an AF substrate by the time of operation due to above mentioned cardiovascular diseases. Acute, surgery-related factors occur in both patients: cardiopulmonary bypass (CPB, yellow), inflammation (CRP, purple), oxidative stress (yellow), and sympathetic activation (yellow). Patient 2 develops post-operative atrial fibrillation (exceeds the ‘AF threshold’), while patient 1 remains with sinus rhythm. AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease.
Risk factors for POAF and the development of an AF substrate
After having discussed the mechanisms predisposing to POAF, this section describes the relation between these mechanisms and clinical risk factors for AF and POAF.
Age
Advancing age correlates strongly with the occurrence of new onset AF162,163 and POAF.1,2,4,6–9,15–18,44–48 Moreover, several arguments support that ageing enhances the development of a substrate capable of perpetuating AF.149 As discussed above, ageing goes along with fibrosis151,153,164 and is associated with slowing of conduction.165 Surprisingly, in one study endocardial AF inducibility in patients without any AF history did not increase with age and even decreased in elderly patients (>70 years).166 These older patients had a significant longer AERP compared with younger patients (40 years). However, as wavelength, measured as the product of conduction velocity and ERP, is a more reliable predictive index for induction of atrial arrhythmias than conduction velocity or ERP alone,167 the pro-arrhyhtmic effect of slowing of conduction likely outweighs the protective effect of prolongation of atrial refractoriness.
Structural heart disease
Left atrial enlargement, mitral valve disease, congestive heart failure, and hypertension are well-known risk factors for non-surgical AF.162,163,168 Atrial structural remodelling consequent to these risk factors can predispose to the onset of POAF. Indeed, in large epidemiological studies,2,4,17 these risk factors are also associated with the incidence of POAF. This suggests that underlying mechanisms enhance the propensity to AF similarly in cardiac surgery patients as in patients not undergoing cardiac surgery. However, it appears that the association of structural heart disease with POAF is weaker than with persistent non-surgical AF.1,8,9,47 It can be hypothesized that in the case of non-surgical AF, structural alterations enhanced the development of an AF substrate so far that AF occurs ‘spontaneously’. In the setting of POAF, however, superimposition of acute surgery-induced factors is required to exceed the ‘AF threshold’. As such, a weaker association would be expected between these risk factors and POAF incidence compared with non-surgical AF incidence.
Left atrial enlargement and mitral valve disease
Chronic structural alterations in the left atrium, rather than changes in ion channels, seem responsible for the higher POAF susceptibility in patients with enlargement of the left atrium. In non-AF patients with mitral regurgitance, prolongation rather than shortening of AERP is seen in the left atrium.169 Moreover, a line of conduction block runs vertically between the pulmonary veins in the posterior left atrium.170 In patients with greater left atrial enlargement, this line of block is more extensive compared with ‘unremodelled’ patients. Furthermore, complex-fractionated electrograms, which can be found at a line of block, are relatively stable in this region and thus most likely related to the underlying architecture of the atrial wall.169 Finally, also experimental data support this rationale. In a canine study of chronic left atrial dilatation due to mitral regurgitation, persistence of induced AF went hand in hand with the degree of left atrial dilatation.171 Histological analysis of these atria revealed areas of chronic inflammation and increased interstitial fibrosis.171
Congestive heart failure
Congestive heart failure is known to cause (i) left atrial dilatation due to increased atrial filling pressures secondary to decreased ventricular function, (ii) increased atrial fibrosis, and (iii) regional conduction abnormalities.172,173
Hypertension
Elevated blood pressure causes left ventricular hypertrophy, left atrial dilatation, and modifications of atrial mechanical function, all promoting AF.174 However, administration of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker has not yet been clearly associated with a decrease in POAF incidence.91,175,176
History of atrial fibrillation
High propensity to POAF in patients with previous episodes of AF is not surprising3,6. The fact that spontaneous episodes of AF already occurred shows that the activity of pro-arrhythmic mechanisms in these patients exceeded the ‘AF threshold’.161 Superimposition of acute surgery-induced factors will only facilitate new episodes of the arrhythmia. On the other hand, AF itself might have contributed to the development of an AF substrate secondary to electrical and structural remodelling of the atria.
Risk factors for atrial fibrillation but not for post-operative atrial fibrillation
Some but not all studies identified diabetes as independent risk factor for AF.162,163,177,178 Also in POAF, the predictive value of diabetes for the incidence of the arrhythmia is low.1–4,6–8,15,17,47 In a recent meta-analysis reviewing 100 217 patients, no difference in POAF incidence was found between patients with and without diabetes.179 Furthermore, men have a 1.5 times greater likelihood of developing new onset AF compared with women.162 The mechanism behind the higher AF susceptibility remains unclear. In POAF, male gender fails to reach significance in many studies,1–3,6,7,15,17 and in studies that find male gender to be associated with POAF, the number of women included is often low.4,8,47,48 Association between chronic obstructive pulmonary disease (COPD) and new onset AF is still under discussion.162,178 COPD has been identified as an independent predictor of AF progression.180 In the occurrence of POAF, COPD is often identified as a risk factor.1–3,9,47 The pathogenesis of AF in patients with COPD is unclear,3 but pulmonary hypertension, inflammation, hypoxia, acidosis, and right atrial and ventricular dilatation might contribute to the formation of a substrate for AF in these patients.181
Conclusions
From the numerous experimental and epidemiological studies addressing the mechanisms of POAF several conclusions can be drawn.
-
Both transient factors related to surgery as well as factors developing slowly and progressively contribute to the occurrence of POAF.
The time course of POAF4,7,43 unmasks the importance of temporary surgery-induced factors as inflammation, sympathetic stimulation, and oxidative stress. However, transient factors cannot be the only responsible mechanism for the occurrence of the arrhythmia, as many patients in whom one or even several of these factors are clearly operative do not develop POAF.
-
Among the transient predisposing mechanisms of POAF, sympathetic activation appears to be more relevant than inflammation and oxidative stress.
Withdrawal from and treatment with β-blockers has been shown to largely affect POAF incidence,50,117–119 while reducing oxidative stress,124,135 or inflammation86,87 were less effective. In line with this the 2010 European Society of Cardiology guidelines recommend β-blocker treatment as first-line therapy of POAF.91
-
Occurrence of POAF is strongly determined by the pre-existence of an AF substrate.
Despite the importance of transient surgery-induced factors, the majority of POAF cases occur in atria with a pre-existing AF substrate due to a long-lasting structural remodelling process. Moreover, patients developing POAF have an eight-fold increased risk of developing AF in the future.12 If transient factors were the only cause of onset of POAF, AF after surgery would not be expected to be associated with occurrence of AF later on. This emphasizes the important role for more chronic factors, not directly related to surgery.
-
AF after cardiac surgery and non-surgical AF have common clinical risk factors.
Patients developing AF after surgery have pre-operatively increased intra-atrial conduction times (longer signal-averaged P-wave duration)159 and more profound atrial structural changes like fibrosis148,153 compared with patients who maintain sinus rhythm after surgery. This presence of structural ‘remodelling’ of atria before the onset of POAF is very similar to the setting of non-surgical AF. In agreement with this hypothesis, the risk factors for POAF are surprisingly similar to the classical risk factors identified for AF as such.
-
Susceptibility to AF after surgery is not to due to changes in ionic currents.
In non-surgical AF, alterations in ion channels as a consequence of AF enhance the perpetuation of AF.143,145–147 However, the function of these ion channels is not altered in pre-operative atrial biopsies of patients developing AF after cardiac surgery.50,145,147,148
-
Progress in the preventive treatment of POAF has been made during the past decade.
As age is the strongest risk factor for POAF1,2,4,6–9,15–18,44–48 and cardiac surgery nowadays is performed in older patients,49 one would expect POAF incidence to rise over time. The fact that recent epidemiological studies10,17 were not able to confirm this trend suggests significant progress in preventive treatment of POAF.
Reviewing mechanisms predisposing to the occurrence of AF after cardiac surgery clearly reveals that the pathogenesis of POAF is multifactorial. Therefore, subclassification of POAF based on these mechanisms does not appear adequate. Identifying leading mechanisms in individual patients, however, might improve treatment in the future. The clinical setting of POAF offers numerous opportunities to study not only mechanisms of POAF but also of AF in general, as cardiac surgery enables direct access to the heart. This opportunity appears to be underused so far.
Conflict of interest: none declared.
Funding
This work was supported by the Dutch Research Organization (NOW, VIDI-grant 016.086.379) and the Foundation Leducq (07 CVD 03). Funding to pay the Open Access publication charges for this article was provided by the University of Maastricht.
References
- 1.Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56:539–49. doi: 10.1016/0003-4975(93)90894-n. [DOI] [PubMed] [Google Scholar]
- 2.Almassi GH, Schowalter T, Nicolosi AC, Aggarwal A, Moritz TE, Henderson WG, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997;226:501–11. doi: 10.1097/00000658-199710000-00011. discussion 11–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–9. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 4.Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVliet M, et al. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. 1996;94:390–7. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 5.Frost L, Molgaard H, Christiansen EH, Hjortholm K, Paulsen PK, Thomsen PE. Atrial fibrillation and flutter after coronary artery bypass surgery: epidemiology, risk factors and preventive trials. Int J Cardiol. 1992;36:253–61. doi: 10.1016/0167-5273(92)90293-c. [DOI] [PubMed] [Google Scholar]
- 6.Banach M, Rysz J, Drozdz JA, Okonski P, Misztal M, Barylski M, et al. Risk factors of atrial fibrillation following coronary artery bypass grafting: a preliminary report. Circ J. 2006;70:438–41. doi: 10.1253/circj.70.438. [DOI] [PubMed] [Google Scholar]
- 7.Auer J, Weber T, Berent R, Ng CK, Lamm G, Eber B. Risk factors of postoperative atrial fibrillation after cardiac surgery. J Card Surg. 2005;20:425–31. doi: 10.1111/j.1540-8191.2005.2004123.x. [DOI] [PubMed] [Google Scholar]
- 8.Fuller JA, Adams GG, Buxton B. Atrial fibrillation after coronary artery bypass grafting. Is it a disorder of the elderly? J Thorac Cardiovasc Surg. 1989;97:821–5. [PubMed] [Google Scholar]
- 9.Leitch JW, Thomson D, Baird DK, Harris PJ. The importance of age as a predictor of atrial fibrillation and flutter after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1990;100:338–42. [PubMed] [Google Scholar]
- 10.Mariscalco G, Engstrom KG. Postoperative atrial fibrillation is associated with late mortality after coronary surgery, but not after valvular surgery. Ann Thorac Surg. 2009;88:1871–6. doi: 10.1016/j.athoracsur.2009.07.074. [DOI] [PubMed] [Google Scholar]
- 11.Siebert J, Anisimowicz L, Lango R, Rogowski J, Pawlaczyk R, Brzezinski M, et al. Atrial fibrillation after coronary artery bypass grafting: does the type of procedure influence the early postoperative incidence? Eur J Cardiothorac Surg. 2001;19:455–9. doi: 10.1016/s1010-7940(01)00621-2. [DOI] [PubMed] [Google Scholar]
- 12.Ahlsson A, Fengsrud E, Bodin L, Englund A. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur J Cardiothorac Surg. 2010;37:1353–59. doi: 10.1016/j.ejcts.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 13.Villareal RP, Hariharan R, Liu BC, Kar B, Lee VV, Elayda M, et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43:742–8. doi: 10.1016/j.jacc.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Kalman JM, Munawar M, Howes LG, Louis WJ, Buxton BF, Gutteridge G, et al. Atrial fibrillation after coronary artery bypass grafting is associated with sympathetic activation. Ann Thorac Surg. 1995;60:1709–15. doi: 10.1016/0003-4975(95)00718-0. [DOI] [PubMed] [Google Scholar]
- 15.Hakala T, Hedman A, Turpeinen A, Kettunen R, Vuolteenaho O, Hippelainen M. Prediction of atrial fibrillation after coronary artery bypass grafting by measuring atrial peptide levels and preoperative atrial dimensions. Eur J Cardiothorac Surg. 2002;22:939–43. doi: 10.1016/s1010-7940(02)00565-1. [DOI] [PubMed] [Google Scholar]
- 16.Zangrillo A, Landoni G, Sparicio D, Benussi S, Aletti G, Pappalardo F, et al. Predictors of atrial fibrillation after off-pump coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2004;18:704–8. doi: 10.1053/j.jvca.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Shen J, Lall S, Zheng V, Buckley P, Damiano RJ, Jr., Schuessler RB. The persistent problem of new-onset postoperative atrial fibrillation: A single-institution experience over two decades. J Thorac Cardiovasc Surg. 2011;141:559–70. doi: 10.1016/j.jtcvs.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahoney EM, Thompson TD, Veledar E, Williams J, Weintraub WS. Cost-effectiveness of targeting patients undergoing cardiac surgery for therapy with intravenous amiodarone to prevent atrial fibrillation. J Am Coll Cardiol. 2002;40:737–45. doi: 10.1016/s0735-1097(02)02003-x. [DOI] [PubMed] [Google Scholar]
- 19.Ma JY, Wang Y, Zhao YF, Wu Z, Liu LX, Kou YL, et al. Atrial fibrillation after surgery for esophageal carcinoma: clinical and prognostic significance. World J Gastroenterol. 2006;12:449–52. doi: 10.3748/wjg.v12.i3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amar D, Zhang H, Heerdt PM, Park B, Fleisher M, Thaler HT. Statin use is associated with a reduction in atrial fibrillation after noncardiac thoracic surgery independent of c-reactive protein*. Chest. 2005;128:3421–27. doi: 10.1378/chest.128.5.3421. [DOI] [PubMed] [Google Scholar]
- 21.Passman RS, Gingold DS, Amar D, Lloyd-Jones D, Bennett CL, Zhang H, et al. Prediction rule for atrial fibrillation after major noncardiac thoracic surgery. Ann Thorac Surg. 2005;79:1698–703. doi: 10.1016/j.athoracsur.2004.10.058. [DOI] [PubMed] [Google Scholar]
- 22.Curtis JJ, Parker BM, McKenney CA, Wagner-Mann CC, Walls JT, Demmy TL, et al. Incidence and predictors of supraventricular dysrhythmias after pulmonary resection. Ann Thorac Surg. 1998;66:1766–71. doi: 10.1016/s0003-4975(98)00942-4. [DOI] [PubMed] [Google Scholar]
- 23.Lanza LA, Visbal AI, DeValeria PA, Zinsmeister AR, Diehl NN, Trastek VF. Low-dose oral amiodarone prophylaxis reduces atrial fibrillation after pulmonary resection. Ann Thorac Surg. 2003;75:223–30. doi: 10.1016/s0003-4975(02)04285-6. discussion 30. [DOI] [PubMed] [Google Scholar]
- 24.Amar D, Roistacher N, Burt M, Reinsel RA, Ginsberg RJ, Wilson RS. Clinical and echocardiographic correlates of symptomatic tachydysrhythmias after noncardiac thoracic surgery. Chest. 1995;108:349–54. doi: 10.1378/chest.108.2.349. [DOI] [PubMed] [Google Scholar]
- 25.Walsh SR, Oates JE, Anderson JA, Blair SD, Makin CA, Walsh CJ. Postoperative arrhythmias in colorectal surgical patients: incidence and clinical correlates. Colorectal Dis. 2006;8:212–6. doi: 10.1111/j.1463-1318.2005.00881.x. [DOI] [PubMed] [Google Scholar]
- 26.Batra GS, Molyneux J, Scott NA. Colorectal patients and cardiac arrhythmias detected on the surgical high dependency unit. Ann R Coll Surg Engl. 2001;83:174–6. [PMC free article] [PubMed] [Google Scholar]
- 27.Siu CW, Tung HM, Chu KW, Jim MH, Lau CP, Tse HF. Prevalence and predictors of new-onset atrial fibrillation after elective surgery for colorectal cancer. Pacing Clin Electrophysiol. 2005;28(Suppl 1):S120–3. doi: 10.1111/j.1540-8159.2005.00024.x. [DOI] [PubMed] [Google Scholar]
- 28.Christians KK, Wu B, Quebbeman EJ, Brasel KJ. Postoperative atrial fibrillation in noncardiothoracic surgical patients. Am J Surg. 2001;182:713–15. doi: 10.1016/s0002-9610(01)00799-1. [DOI] [PubMed] [Google Scholar]
- 29.Kahn RL, Hargett MJ, Urquhart B, Sharrock NE, Peterson MG. Supraventricular tachyarrhythmias during total joint arthroplasty. Incidence and risk. Clin Orthop Relat Res. 1993;296:265–9. [PubMed] [Google Scholar]
- 30.Brathwaite D, Weissman C. The new onset of atrial arrhythmias following major noncardiothoracic surgery is associated with increased mortality. Chest. 1998;114:462–8. doi: 10.1378/chest.114.2.462. [DOI] [PubMed] [Google Scholar]
- 31.Auer J, Weber T, Berent R, Ng CK, Lamm G, Eber B. Postoperative atrial fibrillation independently predicts prolongation of hospital stay after cardiac surgery. J Cardiovasc Surg. 2005;46:583–8. [PubMed] [Google Scholar]
- 32.Lo B, Fijnheer R, Nierich AP, Bruins P, Kalkman CJ. C-reactive protein is a risk indicator for atrial fibrillation after myocardial revascularization. Ann Thorac Surg. 2005;79:1530–5. doi: 10.1016/j.athoracsur.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Lahtinen J, Biancari F, Salmela E, Mosorin M, Satta J, Rainio P, et al. Postoperative atrial fibrillation is a major cause of stroke after on-pump coronary artery bypass surgery. Ann Thorac Surg. 2004;77:1241–44. doi: 10.1016/j.athoracsur.2003.09.077. [DOI] [PubMed] [Google Scholar]
- 34.Nazeri A, Razavi M, Elayda MA, Lee V-V, Massumi A, Wilson JM. Race/ethnicity and the incidence of new-onset atrial fibrillation after isolated coronary artery bypass surgery. Heart Rhythm. 2010;7:1458–63. doi: 10.1016/j.hrthm.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 35.Vaseghi M, Boyle NG, Kedia R, Patel JK, Cesario DA, Wiener I, et al. Supraventricular tachycardia after orthotopic cardiac transplantation. J Am Coll Cardiol. 2008;51:2241–9. doi: 10.1016/j.jacc.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 36.Goldman L. Supraventricular tachyarrhythmias in hospitalized adults after surgery. Clinical correlates in patients over 40 years of age after major noncardiac surgery. Chest. 1978;73:450–4. doi: 10.1378/chest.73.4.450. [DOI] [PubMed] [Google Scholar]
- 37.Rogers WR, Wroblewski F, Ladue JS. Supraventricular tachycardia complicating surgical procedures; a study of the contributing causes, course, and treatment of this complication in fifty patients. Circulation. 1953;7:192–9. doi: 10.1161/01.cir.7.2.192. [DOI] [PubMed] [Google Scholar]
- 38.Vaporciyan AA, Correa AM, Rice DC, Roth JA, Smythe WR, Swisher SG, et al. Risk factors associated with atrial fibrillation after noncardiac thoracic surgery: analysis of 2588 patients. J Thorac Cardiovasc Surg. 2004;127:779–86. doi: 10.1016/j.jtcvs.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Asamura H, Naruke T, Tsuchiya R, Goya T, Kondo H, Suemasu K. What are the risk factors for arrhythmias after thoracic operations? A retrospective multivariate analysis of 267 consecutive thoracic operations. J Thorac Cardiovasc Surg. 1993;106:1104–10. [PubMed] [Google Scholar]
- 40.Harpole JDH, Liptay MJ, DeCamp JMM, Mentzer SJ, Swanson SJ, Sugarbaker DJ. Prospective analysis of pneumonectomy: risk factors for major morbidity and cardiac dysrhythmias. Ann Thorac Surg. 1996;61:977–82. doi: 10.1016/0003-4975(95)01174-9. [DOI] [PubMed] [Google Scholar]
- 41.Materazzo C, Piotti P, Mantovani C, Miceli R, Villani F. Atrial fibrillation after non-cardiac surgery: P-wave characteristics and Holter monitoring in risk assessment. Eur J Cardiothorac Surg. 2007;31:812–6. doi: 10.1016/j.ejcts.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Park BJ, Zhang H, Rusch VW, Amar D. Video-assisted thoracic surgery does not reduce the incidence of postoperative atrial fibrillation after pulmonary lobectomy. J Thorac Cardiovasc Surg. 2007;133:775–79. doi: 10.1016/j.jtcvs.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 43.Auer J, Weber T, Berent R, Puschmann R, Hartl P, Ng CK, et al. A comparison between oral antiarrhythmic drugs in the prevention of atrial fibrillation after cardiac surgery: the pilot study of prevention of postoperative atrial fibrillation (SPPAF), a randomized, placebo-controlled trial. Am Heart J. 2004;147:636–43. doi: 10.1016/j.ahj.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 44.Butler J, Chong JL, Rocker GM, Pillai R, Westaby S. Atrial fibrillation after coronary artery bypass grafting: a comparison of cardioplegia versus intermittent aortic cross-clamping. Eur J Cardiothorac Surg. 1993;7:23–5. doi: 10.1016/1010-7940(93)90143-y. [DOI] [PubMed] [Google Scholar]
- 45.Lowe JE, Hendry PJ, Hendrickson SC, Wells R. Intraoperative identification of cardiac patients at risk to develop postoperative atrial fibrillation. Ann Surg. 1991;213:388–91,. doi: 10.1097/00000658-199105000-00002. discussion 91–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ducceschi V, D'Andrea A, Liccardo B, Alfieri A, Sarubbi B, De Feo M, et al. Perioperative clinical predictors of atrial fibrillation occurrence following coronary artery surgery. Eur J Cardio-Thorac Surg. 1999;16:435–39. doi: 10.1016/s1010-7940(99)00217-1. [DOI] [PubMed] [Google Scholar]
- 47.Zacharias A, Schwann TA, Riordan CJ, Durham SJ, Shah AS, Habib RH. Obesity and risk of new-onset atrial fibrillation after cardiac surgery. Circulation. 2005;112:3247–55. doi: 10.1161/CIRCULATIONAHA.105.553743. [DOI] [PubMed] [Google Scholar]
- 48.Zaman AG, Archbold RA, Helft G, Paul EA, Curzen NP, Mills PG. Atrial fibrillation after coronary artery bypass surgery: a model for preoperative risk stratification. Circulation. 2000;101:1403–08. doi: 10.1161/01.cir.101.12.1403. [DOI] [PubMed] [Google Scholar]
- 49.Ferguson TB, Jr., Hammill BG, Peterson ED, DeLong ER, Grover FL. A decade of change—risk profiles and outcomes for isolated coronary artery bypass grafting procedures, 1990–1999: a report from the STS National Database Committee and the Duke Clinical Research Institute. Society of Thoracic Surgeons. Ann Thorac Surg. 2002;73:480–9,. doi: 10.1016/s0003-4975(01)03339-2. discussion 89–90. [DOI] [PubMed] [Google Scholar]
- 50.Workman AJ, Pau D, Redpath CJ, Marshall GE, Russell JA, Kane KA, et al. Post-operative atrial fibrillation is influenced by beta-blocker therapy but not by pre-operative atrial cellular electrophysiology. J Cardiovasc Electrophysiol. 2006;17:1230–8. doi: 10.1111/j.1540-8167.2006.00592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olshansky B. Management of atrial fibrillation after coronary artery bypass graft. Am J Cardiol. 1996;78:27–34. doi: 10.1016/s0002-9149(96)00563-2. [DOI] [PubMed] [Google Scholar]
- 52.Bruins P, te Velthuis H, Yazdanbakhsh AP, Jansen PG, van Hardevelt FW, de Beaumont EM, et al. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation. 1997;96:3542–8. doi: 10.1161/01.cir.96.10.3542. [DOI] [PubMed] [Google Scholar]
- 53.Gaudino M, Andreotti F, Zamparelli R, Di Castelnuovo A, Nasso G, Burzotta F, et al. The -174G/C interleukin-6 polymorphism influences postoperative interleukin-6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication? Circulation. 2003;108(Suppl 1):II195–9. doi: 10.1161/01.cir.0000087441.48566.0d. [DOI] [PubMed] [Google Scholar]
- 54.Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–91. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 55.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, et al. Inflammation as a Risk Factor for Atrial Fibrillation. Circulation. 2003;108:3006–10. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 56.Abdelhadi RH, Gurm HS, Van Wagoner DR, Chung MK. Relation of an exaggerated rise in white blood cells after coronary bypass or cardiac valve surgery to development of atrial fibrillation postoperatively. Am J Cardiol. 2004;93:1176–8. doi: 10.1016/j.amjcard.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 57.Anselmi A, Possati G, Gaudino M. Postoperative inflammatory reaction and atrial fibrillation: simple correlation or causation? Ann Thorac Surg. 2009;88:326–33. doi: 10.1016/j.athoracsur.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 58.Hak Ł, Myśliwska J, Wickiewicz J, Szyndler K, Siebert J, Rogowski J. Interleukin-2 as a predictor of early postoperative atrial fibrillation after cardiopulmonary bypass graft (CABG) J Interferon Cytokine Res. 2009;29:327–32. doi: 10.1089/jir.2008.0082.2906. [DOI] [PubMed] [Google Scholar]
- 59.Lamm G, Auer J, Weber T, Berent R, Ng C, Eber B. Postoperative white blood cell count predicts atrial fibrillation after cardiac surgery. J Cardiothorac Vasc Anesth. 2006;20:51–56. doi: 10.1053/j.jvca.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 60.Fontes ML, Mathew JP, Rinder HM, Zelterman D, Smith BR, Rinder CS. Atrial fibrillation after cardiac surgery/cardiopulmonary bypass is associated with monocyte activation. Anesth Analgesia. 2005;101:17–23. doi: 10.1213/01.ANE.0000155260.93406.29. [DOI] [PubMed] [Google Scholar]
- 61.Gibson PH, Cuthbertson BH, Croal BL, Rae D, El-Shafei H, Gibson G, et al. Usefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2010;105:186–91. doi: 10.1016/j.amjcard.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 62.Wan S, Izzat MB, Lee TW, Wan IYP, Tang NLS, Yim APC. Avoiding cardiopulmonary bypass in multivessel CABG reduces cytokine response and myocardial injury. Ann Thorac Surg. 1999;68:52–56. doi: 10.1016/s0003-4975(99)00315-x. [DOI] [PubMed] [Google Scholar]
- 63.Enc Y, Ketenci B, Ozsoy D, Camur G, KayacIoglu I, Terzi S, et al. Atrial fibrillation after surgical revascularization: is there any difference between on-pump and off-pump? Eur J Cardio-Thorac Surg. 2004;26:1129–33. doi: 10.1016/j.ejcts.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 64.Siebert J, Lewicki L, Mlodnicki M, Rogowski J, Lango R, Anisimowicz L, et al. Atrial fibrillation after conventional and off-pump coronary artery bypass grafting:two opposite trends in timing of atrial fibrillation occurrence? Med Sci Monit. 2003;9:CR137–41. [PubMed] [Google Scholar]
- 65.van Dijk D, Nierich AP, Jansen EWL, Nathoe HM, Suyker WJL, Diephuis JC, et al. Early outcome after off-pump versus on-pump coronary bypass surgery: results from a randomized study. Circulation. 2001;104:1761–66. doi: 10.1161/hc4001.097036. [DOI] [PubMed] [Google Scholar]
- 66.Legare J-F, Buth KJ, King S, Wood J, Sullivan JA, Friesen CH, et al. Coronary bypass surgery performed off pump does not result in lower in-hospital morbidity than coronary artery bypass grafting performed on pump. Circulation. 2004;109:887–92. doi: 10.1161/01.CIR.0000115943.41814.7D. [DOI] [PubMed] [Google Scholar]
- 67.Zamvar V, Williams D, Hall J, Payne N, Cann C, Young K, et al. Assessment of neurocognitive impairment after off-pump and on-pump techniques for coronary artery bypass graft surgery: prospective randomised controlled trial. BMJ. 2002;325:1268. doi: 10.1136/bmj.325.7375.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Czerny M, Baumer H, Kilo J, Zuckermann A, Grubhofer G, Chevtchik O, et al. Complete revascularization in coronary artery bypass grafting with and without cardiopulmonary bypass. Ann Thorac Surg. 2001;71:165–69. doi: 10.1016/s0003-4975(00)02230-x. [DOI] [PubMed] [Google Scholar]
- 69.Ascione R, Caputo M, Calori G, Lloyd CT, Underwood MJ, Angelini GD. Predictors of atrial fibrillation after conventional and beating heart coronary surgery : a prospective, randomized study. Circulation. 2000;102:1530–35. doi: 10.1161/01.cir.102.13.1530. [DOI] [PubMed] [Google Scholar]
- 70.Murphy GJ, Ascione R, Caputo M, Angelini GD. Operative factors that contribute to post-operative atrial fibrillation: insights from a prospective randomized trial. Card Electrophysiol Rev. 2003;7:136–9. doi: 10.1023/a:1027407431834. [DOI] [PubMed] [Google Scholar]
- 71.Boyd WD, Desai ND, Del Rizzo DF, Novick RJ, McKenzie FN, Menkis AH. Off-pump surgery decreases postoperative complications and resource utilization in the elderly. Ann Thorac Surg. 1999;68:1490–93. doi: 10.1016/s0003-4975(99)00951-0. [DOI] [PubMed] [Google Scholar]
- 72.Van Belleghem Y, Caes F, Maene L, Van Overbeke H, Moerman A, Van Nooten G. Off-pump coronary surgery: surgical strategy for the high-risk patient. Cardiovasc Surg. 2003;11:75–79. doi: 10.1016/s0967-2109(02)00119-9. [DOI] [PubMed] [Google Scholar]
- 73.Panesar SS, Athanasiou T, Nair S, Rao C, Jones C, Nicolaou M, et al. Early outcomes in the elderly: a meta-analysis of 4921 patients undergoing coronary artery bypass grafting—comparison between off-pump and on-pump techniques. Heart. 2006;92:1808–16. doi: 10.1136/hrt.2006.088450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stamou SC, Dangas G, Hill PC, Pfister AJ, Dullum MKC, Boyce SW, et al. Atrial fibrillation after beating heart surgery. Am J Cardiol. 2000;86:64–67. doi: 10.1016/s0002-9149(00)00829-8. [DOI] [PubMed] [Google Scholar]
- 75.Scherer M, Sirat A, Dogan S, Aybek T, Moritz A, Wimmer-Greinecker G. Does totally endoscopic access for off-pump cardiac surgery influence the incidence of postoperative atrial fibrillation in coronary artery bypass grafting? a preliminary report. Cardiovasc Engg. 2006;6:118–21. doi: 10.1007/s10558-006-9015-3. [DOI] [PubMed] [Google Scholar]
- 76.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–84. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 77.Chen M-C, Chang J-P, Liu W-H, Yang C-H, Chen Y-L, Tsai T-H, et al. Increased inflammatory cell infiltration in the atrial myocardium of patients with atrial fibrillation. Am J Cardiol. 2008;102:861–65. doi: 10.1016/j.amjcard.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 78.Page PL, Plumb VJ, Okumura K, Waldo AL. A new animal model of atrial flutter. J Am Coll Cardiol. 1986;8:872–9. doi: 10.1016/s0735-1097(86)80429-6. [DOI] [PubMed] [Google Scholar]
- 79.Kumagai K, Khrestian C, Waldo AL. Simultaneous multisite mapping studies during induced atrial fibrillation in the sterile pericarditis model: insights into the mechanism of its maintenance. Circulation. 1997;95:511–21. doi: 10.1161/01.cir.95.2.511. [DOI] [PubMed] [Google Scholar]
- 80.Ryu K, Li L, Khrestian CM, Matsumoto N, Sahadevan J, Ruehr ML, et al. Effects of sterile pericarditis on connexins 40 and 43 in the atria: correlation with abnormal conduction and atrial arrhythmias. Am J Physiol Heart Circ Physiol. 2007;293:H1231–41. doi: 10.1152/ajpheart.00607.2006. [DOI] [PubMed] [Google Scholar]
- 81.Goldstein RN, Ryu K, Khrestian C, van Wagoner DR, Waldo AL. Prednisone prevents inducible atrial flutter in the canine sterile pericarditis model. J Cardiovasc Electrophysiol. 2008;19:74–81. doi: 10.1111/j.1540-8167.2007.00970.x. [DOI] [PubMed] [Google Scholar]
- 82.Kumagai K, Nakashima H, Saku K. The HMG-CoA reductase inhibitor atorvastatin prevents atrial fibrillation by inhibiting inflammation in a canine sterile pericarditis model. Cardiovasc Res. 2004;62:105–11. doi: 10.1016/j.cardiores.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Z, Zhang C, Wang H, Zhao J, Liu L, Lee J, et al. n-3 polyunsaturated fatty acids prevents atrial fibrillation by inhibiting inflammation in a canine sterile pericarditis model. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2010.08.024. online publish ahead of print 15 September 2010, doi:10.1016/j.ijcard.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 84.Ishii Y, Schuessler RB, Gaynor SL, Yamada K, Fu AS, Boineau JP, et al. Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. 2005;111:2881–8. doi: 10.1161/CIRCULATIONAHA.104.475194. [DOI] [PubMed] [Google Scholar]
- 85.Tselentakis EV, Woodford E, Chandy J, Gaudette GR, Saltman AE. Inflammation effects on the electrical properties of atrial tissue and inducibility of postoperative atrial fibrillation. J Surg Res. 2006;135:68–75. doi: 10.1016/j.jss.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 86.Ho KM, Tan JA. Benefits and risks of corticosteroid prophylaxis in adult cardiac surgery: a dose-response meta-analysis. Circulation. 2009;119:1853–66. doi: 10.1161/CIRCULATIONAHA.108.848218. [DOI] [PubMed] [Google Scholar]
- 87.Bourbon A, Vionnet M, Leprince P, Vaissier E, Copeland J, McDonagh P, et al. The effect of methylprednisolone treatment on the cardiopulmonary bypass-induced systemic inflammatory response. Eur J Cardio-Thoracic Surg. 2004;26:932–38. doi: 10.1016/j.ejcts.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 88.Liakopoulos OJ, Choi Y-H, Kuhn EW, Wittwer T, Borys M, Madershahian N, et al. Statins for prevention of atrial fibrillation after cardiac surgery: a systematic literature review. J Thorac Cardiovasc Surg. 2009;138:678–86. doi: 10.1016/j.jtcvs.2009.03.054. e1. [DOI] [PubMed] [Google Scholar]
- 89.Chello M, Anselmi A, Spadaccio C, Patti G, Goffredo C, Di Sciascio G, et al. Simvastatin increases neutrophil apoptosis and reduces inflammatory reaction after coronary surgery. Ann Thorac Surg. 2007;83:1374–80. doi: 10.1016/j.athoracsur.2006.10.065. [DOI] [PubMed] [Google Scholar]
- 90.Wagner AH, Kohler T, Ruckschloss U, Just I, Hecker M. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler Thromb Vasc Biol. 2000;20:61–69. doi: 10.1161/01.atv.20.1.61. [DOI] [PubMed] [Google Scholar]
- 91.Camm AJ, Kirchhof P, Lip GYH, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation. Europace. 2010;12:1360–420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 92.Calò L, Bianconi L, Colivicchi F, Lamberti F, Loricchio ML, de Ruvo E, et al. N-3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial. J Am Coll Cardiol. 2005;45:1723–28. doi: 10.1016/j.jacc.2005.02.079. [DOI] [PubMed] [Google Scholar]
- 93.Mariscalco G, Sarzi Braga S, Banach M, Borsani P, Bruno VD, Napoleone M, et al. Preoperative n-3 polyunsatured fatty acids are associated with a decrease in the incidence of early atrial fibrillation following cardiac surgery. Angiology. 2010;61:643–50. doi: 10.1177/0003319710370962. [DOI] [PubMed] [Google Scholar]
- 94.Saravanan P, Bridgewater B, West AL, O'Neill SC, Calder PC, Davidson NC. Omega-3 fatty acid supplementation does not reduce risk of atrial fibrillation after coronary artery bypass surgery: a randomized, double-blind, placebo-controlled clinical trial. Circ Arrhythm Electrophysiol. 2010;3:46–53. doi: 10.1161/CIRCEP.109.899633. [DOI] [PubMed] [Google Scholar]
- 95.Heidarsdottir R, Arnar DO, Skuladottir GV, Torfason B, Edvardsson V, Gottskalksson G, et al. Does treatment with n-3 polyunsaturated fatty acids prevent atrial fibrillation after open heart surgery? Europace. 2010;12:356–63. doi: 10.1093/europace/eup429. [DOI] [PubMed] [Google Scholar]
- 96.Kaireviciute D, Blann AD, Balakrishnan B, Lane DA, Patel JV, Uzdavinys G, et al. Characterisation and validity of inflammatory biomarkers in the prediction of post-operative atrial fibrillation in coronary artery disease patients. Thromb Haemost. 2010;104:122–7. doi: 10.1160/TH09-12-0837. [DOI] [PubMed] [Google Scholar]
- 97.Ahlsson AJ, Bodin L, Lundblad OH, Englund AG. Postoperative Atrial Fibrillation is Not Correlated to C-Reactive Protein. Ann Thorac Surg. 2007;83:1332–37. doi: 10.1016/j.athoracsur.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 98.Gaudino M, Nasso G, Andreotti F, Minniti G, Iacoviello L, Donati M, et al. Preoperative C-reactive protein level and outcome following coronary surgery. Eur J Cardiothorac Surg. 2002;22:521–6. doi: 10.1016/s1010-7940(02)00436-0. [DOI] [PubMed] [Google Scholar]
- 99.Workman A. Cardiac adrenergic control and atrial fibrillation. Naunyn-Schmiedeberg's Arch Pharmacol. 2010;381:235–49. doi: 10.1007/s00210-009-0474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoeldtke RD, Cilmi KM. Effects of aging on catecholamine metabolism. J Clin Endocrinol Metab. 1985;60:479–84. doi: 10.1210/jcem-60-3-479. [DOI] [PubMed] [Google Scholar]
- 101.Dimmer C, Tavernier R, Gjorgov N, Van Nooten G, Clement DL, Jordaens L. Variations of autonomic tone preceding onset of atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 1998;82:22–25. doi: 10.1016/s0002-9149(98)00231-8. [DOI] [PubMed] [Google Scholar]
- 102.Amar D, Zhang H, Miodownik S, Kadish AH. Competing autonomic mechanisms precedethe onset of postoperative atrial fibrillation. J Am Coll Cardiol. 2003;42:1262–68. doi: 10.1016/s0735-1097(03)00955-0. [DOI] [PubMed] [Google Scholar]
- 103.Hogue CW, Jr, Domitrovich PP, Stein PK, Despotis GD, Re L, Schuessler RB, et al. RR interval dynamics before atrial fibrillation in patients after coronary artery bypass graft surgery. Circulation. 1998;98:429–34. doi: 10.1161/01.cir.98.5.429. [DOI] [PubMed] [Google Scholar]
- 104.Waldo AL. Mechanisms of atrial fibrillation, atrial flutter, and ectopic atrial tachycardia—a brief review. Circulation. 1987;75:III37–40. [PubMed] [Google Scholar]
- 105.Hogue CW, Jr., Hyder ML. Atrial fibrillation after cardiac operation: risks, mechanisms, and treatment. Ann Thorac Surg. 2000;69:300–6. doi: 10.1016/s0003-4975(99)01267-9. [DOI] [PubMed] [Google Scholar]
- 106.Melo J, Voigt P, Sonmez B, Ferreira M, Abecasis M, Rebocho M, et al. Ventral cardiac denervation reduces the incidence of atrial fibrillation after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2004;127:511–16. doi: 10.1016/s0022-5223(03)01283-2. [DOI] [PubMed] [Google Scholar]
- 107.Alex J, Guvendik L. Evaluation of ventral cardiac denervation as a prophylaxis against atrial fibrillation after coronary artery bypass grafting. Ann Thorac Surg. 2005;79:517–20. doi: 10.1016/j.athoracsur.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 108.Cummings JE, Gill I, Akhrass R, Dery M, Biblo LA, Quan KJ. Preservation of the anterior fat pad paradoxically decreases the incidence of postoperative atrial fibrillation in humans. J Am Coll Cardiol. 2004;43:994–1000. doi: 10.1016/j.jacc.2003.07.055. [DOI] [PubMed] [Google Scholar]
- 109.Omran AS, Karimi A, Ahmadi H, Yazdanifard P, Sheikh Fahtollahi M, Tazik M. Prophylactic ventral cardiac denervation: Does it reduce incidence of atrial fibrillation after coronary artery bypass grafting? J Thorac Cardiovasc Surg. 2010;140:1036–39. doi: 10.1016/j.jtcvs.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 110.Levy MN. Brief reviews: sympathetic-parasympathetic interactions in the heart. Circ Res. 1971;29:437–45. doi: 10.1161/01.res.29.5.437. [DOI] [PubMed] [Google Scholar]
- 111.Fleming GA, Murray KT, Yu C, Byrne JG, Greelish JP, Petracek MR, et al. Milrinone use is associated with postoperative atrial fibrillation after cardiac surgery. Circulation. 2008;118:1619–25. doi: 10.1161/CIRCULATIONAHA.108.790162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Feneck RO, Sherry KM, Withington PS, Oduro-Dominah A. Comparison of the hemodynamic effects of milrinone with dobutamine in patients after cardiac surgery. J Cardiothorac Vasc Anesth. 2001;15:306–15. doi: 10.1053/jcan.2001.23274. [DOI] [PubMed] [Google Scholar]
- 113.Argalious M, Motta P, Khandwala F, Samuel S, Koch CG, Gillinov AM, et al. ‘Renal dose’ dopamine is associated with the risk of new-onset atrial fibrillation after cardiac surgery. Crit Care Med. 2005;33:1327–32. doi: 10.1097/01.ccm.0000166876.41694.ca. [DOI] [PubMed] [Google Scholar]
- 114.Sampson KJ, Terrenoire C, Cervantes DO, Kaba RA, Peters NS, Kass RS. Adrenergic regulation of a key cardiac potassium channel can contribute to atrial fibrillation: evidence from an IKs transgenic mouse. J Physiol. 2008;586:627–37. doi: 10.1113/jphysiol.2007.141333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.El-Armouche A, Boknik P, Eschenhagen T, Carrier L, Knaut M, Ravens U, et al. Molecular determinants of altered Ca2+ handling in human chronic atrial fibrillation. Circulation. 2006;114:670–80. doi: 10.1161/CIRCULATIONAHA.106.636845. [DOI] [PubMed] [Google Scholar]
- 116.Patterson E, Yu X, Huang S, Garrett M, Kem DC. Suppression of autonomic-mediated triggered firing in pulmonary vein preparations, 24 hours postcoronary artery ligation in dogs. J Cardiovasc Electrophysiol. 2006;17:763–70. doi: 10.1111/j.1540-8167.2006.00507.x. [DOI] [PubMed] [Google Scholar]
- 117.Lamb RK, Prabhakar G, Thorpe JAC, Smith S, Norton R, Dyde JA. The use of atenolol in the prevention of supraventricular arrhythmias following coronary artery surgery. Eur Heart J. 1988;9:32–36. [PubMed] [Google Scholar]
- 118.White HD, Antman EM, Glynn MA, Collins JJ, Cohn LH, Shemin RJ, et al. Efficacy and safety of timolol for prevention of supraventricular tachyarrhythmias after coronary artery bypass surgery. Circulation. 1984;70:479–84. doi: 10.1161/01.cir.70.3.479. [DOI] [PubMed] [Google Scholar]
- 119.Ali IM, Sanalla AA, Clark V. Beta-blocker effects on postoperative atrial fibrillation. Eur J Cardio-Thorac Surg. 1997;11:1154–57. doi: 10.1016/s1010-7940(97)01215-3. [DOI] [PubMed] [Google Scholar]
- 120.Workman AJ, Kane KA, Russell JA, Norrie J, Rankin AC. Chronic beta-adrenoceptor blockade and human atrial cell electrophysiology: evidence of pharmacological remodelling. Cardiovasc Res. 2003;58:518–25. doi: 10.1016/s0008-6363(03)00263-3. [DOI] [PubMed] [Google Scholar]
- 121.Kailasam R, Palin CA, Hogue CW., Jr. Atrial fibrillation after cardiac surgery: an evidence-based approach to prevention. Semin Cardiothorac Vasc Anesth. 2005;9:77–85. doi: 10.1177/108925320500900108. [DOI] [PubMed] [Google Scholar]
- 122.Epstein FH, McCord JM. Oxygen-derived free radicals in postischemic tissue injury. New Engl J Med. 1985;312:159–63. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 123.Carmeliet E. Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev. 1999;79:917–1017. doi: 10.1152/physrev.1999.79.3.917. [DOI] [PubMed] [Google Scholar]
- 124.Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, et al. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–8. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- 125.Shiroshita-Takeshita A, Schram G, Lavoie J, Nattel S. Effect of simvastatin and antioxidant vitamins on atrial fibrillation promotion by atrial-tachycardia remodeling in dogs. Circulation. 2004;110:2313–9. doi: 10.1161/01.CIR.0000145163.56529.D1. [DOI] [PubMed] [Google Scholar]
- 126.Shishehbor MH, Brennan M-L, Aviles RJ, Fu X, Penn MS, Sprecher DL, et al. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation. 2003;108:426–31. doi: 10.1161/01.CIR.0000080895.05158.8B. [DOI] [PubMed] [Google Scholar]
- 127.Mihm MJ, Yu F, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, et al. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–80. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- 128.Kim YM, Guzik TJ, Zhang YH, Zhang MH, Kattach H, Ratnatunga C, et al. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ Res. 2005;97:629–36. doi: 10.1161/01.RES.0000183735.09871.61. [DOI] [PubMed] [Google Scholar]
- 129.Ferrari R, Alfieri O, Curello S, Ceconi C, Cargnoni A, Marzollo P, et al. Occurrence of oxidative stress during reperfusion of the human heart. Circulation. 1990;81:201–11. doi: 10.1161/01.cir.81.1.201. [DOI] [PubMed] [Google Scholar]
- 130.De Vecchi E, Pala MG, Di Credico G, Agape V, Paolini G, Bonini PA, et al. Relation between left ventricular function and oxidative stress in patients undergoing bypass surgery. Heart. 1998;79:242–47. doi: 10.1136/hrt.79.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ramlawi B, Otu H, Mieno S, Boodhwani M, Sodha NR, Clements RT, et al. Oxidative stress and atrial fibrillation after cardiac surgery: a case-control study. Ann Thorac Surg. 2007;84:1166–73. doi: 10.1016/j.athoracsur.2007.04.126. [DOI] [PubMed] [Google Scholar]
- 132.Kim YM, Kattach H, Ratnatunga C, Pillai R, Channon KM, Casadei B. Association of atrial nicotinamide adenine dinucleotide phosphate oxidase activity with the development of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51:68–74. doi: 10.1016/j.jacc.2007.07.085. [DOI] [PubMed] [Google Scholar]
- 133.Clermont G, Vergely C, Jazayeri S, Lahet JJ, Goudeau JJ, Lecour S, et al. Systemic free radical activation is a major event involved in myocardial oxidative stress related to cardiopulmonary bypass. Anesthesiology. 2002;96:80–7. doi: 10.1097/00000542-200201000-00019. [DOI] [PubMed] [Google Scholar]
- 134.Eslami M, Badkoubeh RS, Mousavi M, Radmehr H, Salehi M, Tavakoli N, et al. Oral ascorbic acid in combination with beta-blockers is more effective than beta-blockers alone in the prevention of atrial fibrillation after coronary artery bypass grafting. Tex Heart Inst J. 2007;34:268–74. [PMC free article] [PubMed] [Google Scholar]
- 135.Ozaydin M, Peker O, Erdogan D, Kapan S, Turker Y, Varol E, et al. N-acetylcysteine for the prevention of postoperative atrial fibrillation: a prospective, randomized, placebo-controlled pilot study. Eur Heart J. 2008;29:625–31. doi: 10.1093/eurheartj/ehn011. [DOI] [PubMed] [Google Scholar]
- 136.Tossios P, Bloch W, Huebner A, Raji MR, Dodos F, Klass O, et al. N-acetylcysteine prevents reactive oxygen species-mediated myocardial stress in patients undergoing cardiac surgery: results of a randomized, double-blind, placebo-controlled clinical trial. J Thoracic Cardiovasc Surg. 2003;126:1513–20. doi: 10.1016/s0022-5223(03)00968-1. [DOI] [PubMed] [Google Scholar]
- 137.Elahi MM, Worner M, Khan JS, Matata BM. Inspired nitric oxide and modulation of oxidative stress during cardiac surgery. Curr Drug Saf. 2009;4:188–98. doi: 10.2174/157488609789006958. [DOI] [PubMed] [Google Scholar]
- 138.Cavolli R, Kaya K, Aslan A, Emiroglu O, Erturk S, Korkmaz O, et al. Does sodium nitroprusside decrease the incidence of atrial fibrillation after myocardial revascularization?: a pilot study. Circulation. 2008;118:476–81. doi: 10.1161/CIRCULATIONAHA.107.719377. [DOI] [PubMed] [Google Scholar]
- 139.Lesnefsky EJ, Lundergan CF, Hodgson JM, Nair R, Reiner JS, Greenhouse SW, et al. Increased left ventricular dysfunction in elderly patients despite successful thrombolysis: the GUSTO-I angiographic experience. J Am Coll Cardiol. 1996;28:331–37. doi: 10.1016/0735-1097(96)00148-9. [DOI] [PubMed] [Google Scholar]
- 140.Fontaine D, Pradier O, Hacquebard M, Stefanidis C, Carpentier Y, de Canniere D, et al. Oxidative stress produced by circulating microparticles in on-pump but not in off-pump coronary surgery. Acta Cardiol. 2009;64:715–22. doi: 10.2143/AC.64.6.2044733. [DOI] [PubMed] [Google Scholar]
- 141.Matata BM, Sosnowski AW, Galiñanes M. Off-pump bypass graft operation significantly reduces oxidative stress and inflammation. Ann Thorac Surg. 2000;69:785–91. doi: 10.1016/s0003-4975(99)01420-4. [DOI] [PubMed] [Google Scholar]
- 142.Orhan G, Sargin M, Senay S, Yuksel M, Kurc E, Tasdemir M, et al. Systemic and myocardial inflammation in traditional and off-pump cardiac surgery. Tex Heart Inst J. 2007;34:160–5. [PMC free article] [PubMed] [Google Scholar]
- 143.Yue L, Feng J, Gaspo R, Li G-R, Wang Z, Nattel S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res. 1997;81:512–25. doi: 10.1161/01.res.81.4.512. [DOI] [PubMed] [Google Scholar]
- 144.Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999;85:428–36. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- 145.Brandt MC, Priebe L, Böhle T, Südkamp M, Beuckelmann DJ. The ultrarapid and the transient outward K+ current in human atrial fibrillation. their possible role in postoperative atrial fibrillation. J Mol Cell Cardiol. 2000;32:1885–96. doi: 10.1006/jmcc.2000.1221. [DOI] [PubMed] [Google Scholar]
- 146.Dobrev D, Friedrich A, Voigt N, Jost N, Wettwer E, Christ T, et al. The G protein-gated Potassium current IK,ACh is constitutively active in patients with chronic atrial fibrillation. Circulation. 2005;112:3697–706. doi: 10.1161/CIRCULATIONAHA.105.575332. [DOI] [PubMed] [Google Scholar]
- 147.Dobrev D, Wettwer E, Kortner A, Knaut M, Schuler S, Ravens U. Human inward rectifier potassium channels in chronic and postoperative atrial fibrillation. Cardiovasc Res. 2002;54:397–404. doi: 10.1016/s0008-6363(01)00555-7. [DOI] [PubMed] [Google Scholar]
- 148.Swartz MF, Fink GW, Lutz CJ, Taffet SM, Berenfeld O, Vikstrom KL, et al. Left versus right atrial difference in dominant frequency, K(+) channel transcripts, and fibrosis in patients developing atrial fibrillation after cardiac surgery. Heart Rhythm. 2009;6:1415–22. doi: 10.1016/j.hrthm.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Spach MS, Dolber PC. Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle. Evidence for electrical uncoupling of side-to-side fiber connections with increasing age. Circ Res. 1986;58:356–71. doi: 10.1161/01.res.58.3.356. [DOI] [PubMed] [Google Scholar]
- 150.Ad N, Snir E, Vidne BA, Golomb E. Histologic atrial myolysis is associated with atrial fibrillation after cardiac operation. Ann Thorac Surg. 2001;72:688–93. doi: 10.1016/s0003-4975(01)02882-x. [DOI] [PubMed] [Google Scholar]
- 151.Mariscalco G, Engström KG, Ferrarese S, Cozzi G, Bruno VD, Sessa F, et al. Relationship between atrial histopathology and atrial fibrillation after coronary bypass surgery. J Thorac Cardiovasc Surg. 2006;131:1364–72. doi: 10.1016/j.jtcvs.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 152.Ak K, Akgun S, Tecimer T, Isbir CS, Civelek A, Tekeli A, et al. Determination of Histopathologic risk factors for postoperative atrial fibrillation in cardiac surgery. Ann Thorac Surg. 2005;79:1970–75. doi: 10.1016/j.athoracsur.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 153.Goette A, Juenemann G, Peters B, Klein HU, Roessner A, Huth C, et al. Determinants and consequences of atrial fibrosis in patients undergoing open heart surgery. Cardiovasc Res. 2002;54:390–6. doi: 10.1016/s0008-6363(02)00251-1. [DOI] [PubMed] [Google Scholar]
- 154.Cosgrave J, Foley JB, Flavin R, O'Briain DS, Fitzpatrick E, Bennett K, et al. Preoperative atrial histological changes are not associated with postoperative atrial fibrillation. Cardiovasc Pathol. 2006;15:213–17. doi: 10.1016/j.carpath.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 155.Anné W, Willems R, Roskams T, Sergeant P, Herijgers P, Holemans P, et al. Matrix metalloproteinases and atrial remodeling in patients with mitral valve disease and atrial fibrillation. Cardiovasc Res. 2005;67:655–66. doi: 10.1016/j.cardiores.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 156.Asher CR, Miller DP, Grimm RA, CosgroveIii DM, Chung MK. Analysis of risk factors for development of atrial fibrillation early after cardiac valvular surgery. Am J Cardiol. 1998;82:892–95. doi: 10.1016/s0002-9149(98)00498-6. [DOI] [PubMed] [Google Scholar]
- 157.Kanagaratnam P, Kojodjojo P, Peters NS. Electrophysiological abnormalities occur prior to the development of clinical episodes of atrial fibrillation: observations from human epicardial mapping. Pacing Clin Electrophysiol. 2008;31:443–53. doi: 10.1111/j.1540-8159.2008.01014.x. [DOI] [PubMed] [Google Scholar]
- 158.Dupont E, Ko Y, Rothery S, Coppen SR, Baghai M, Haw M, et al. The gap-junctional protein connexin40 is elevated in patients susceptible to postoperative atrial fibrillation. Circulation. 2001;103:842–9. doi: 10.1161/01.cir.103.6.842. [DOI] [PubMed] [Google Scholar]
- 159.Steinberg JS, Zelenkofske S, Wong SC, Gelernt M, Sciacca R, Menchavez E. Value of the P-wave signal-averaged ECG for predicting atrial fibrillation after cardiac surgery. Circulation. 1993;88:2618–22. doi: 10.1161/01.cir.88.6.2618. [DOI] [PubMed] [Google Scholar]
- 160.Chandy J, Nakai T, Lee RJ, Bellows WH, Dzankic S, Leung JM. Increases in P-wave dispersion predict postoperative atrial fibrillation after coronary artery bypass graft surgery. Anesthesia Analgesia. 2004;98:303–10. doi: 10.1213/01.ANE.0000096195.47734.2F. [DOI] [PubMed] [Google Scholar]
- 161.Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91:265–325. doi: 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- 162.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort: the framingham heart study. JAMA. 1994;271:840–44. [PubMed] [Google Scholar]
- 163.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB, Sr., et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–45. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nakai T, Chandy J, Nakai K, Bellows WH, Flachsbart K, Lee RJ, et al. Histologic assessment of right atrial appendage myocardium in patients with atrial fibrillation after coronary artery bypass graft surgery. Cardiology. 2007;108:90–96. doi: 10.1159/000095936. [DOI] [PubMed] [Google Scholar]
- 165.Kojodjojo P, Kanagaratnam P, Markides V, Davies DW, Peters N. Age-related changes in human left and right atrial conduction. J Cardiovasc Electrophysiol. 2006;17:120–27. doi: 10.1111/j.1540-8167.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 166.Brembilla-Perrot B, Burger G, Beurrier D, Houriez P, Nippert M, Miljoen H, et al. Influence of age on atrial fibrillation inducibility. Pacing Clin Electrophysiol. 2004;27:287–92. doi: 10.1111/j.1540-8159.2004.00429.x. [DOI] [PubMed] [Google Scholar]
- 167.Rensma PL, Allessie MA, Lammers WJ, Bonke FI, Schalij MJ. Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circ Res. 1988;62:395–410. doi: 10.1161/01.res.62.2.395. [DOI] [PubMed] [Google Scholar]
- 168.Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724–30. doi: 10.1161/01.cir.89.2.724. [DOI] [PubMed] [Google Scholar]
- 169.Roberts-Thomson KC, Stevenson I, Kistler PM, Haqqani HM, Spence SJ, Goldblatt JC, et al. The role of chronic atrial stretch and atrial fibrillation on posterior left atrial wall conduction. Heart Rhythm. 2009;6:1109–17. doi: 10.1016/j.hrthm.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 170.Roberts-Thomson KC, Stevenson IH, Kistler PM, Haqqani HM, Goldblatt JC, Sanders P, et al. Anatomically determined functional conduction delay in the posterior left atrium: relationship to structural heart disease. J Am Coll Cardiol. 2008;51:856–62. doi: 10.1016/j.jacc.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 171.Verheule S, Wilson E, Everett TIV, Shanbhag S, Golden C, Olgin J. Alterations in atrial electrophysiology and tissue structure in a canine model of chronic atrial dilatation due to mitral regurgitation. Circulation. 2003;107:2615–22. doi: 10.1161/01.CIR.0000066915.15187.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Barasch E, Gottdiener JS, Aurigemma G, Kitzman DW, Han J, Kop WJ, et al. Association between elevated fibrosis markers and heart failure in the elderly/clinical perspective. Circulation: Heart Fail. 2009;2:303–10. doi: 10.1161/CIRCHEARTFAILURE.108.828343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 174.L'Allier PL, Ducharme A, Keller P-F, Yu H, Guertin M-C, Tardif J-C. Angiotensin-converting enzyme inhibition in hypertensive patients is associated with a reduction in the occurrence of atrial fibrillation. J Am Coll Cardiol. 2004;44:159–64. doi: 10.1016/j.jacc.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 175.Shariff N, Zelenkofske S, Eid S, Weiss M, Mohammed M. Demographic determinants and effect of pre-operative angiotensin converting enzyme inhibitors and angiotensin receptor blockers on the occurrence of atrial fibrillation after CABG surgery. BMC Cardiovasc Disorders. 2010;10:7. doi: 10.1186/1471-2261-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.White CM, Kluger J, Lertsburapa K, Faheem O, Coleman CI. Effect of preoperative angiotensin converting enzyme inhibitor or angiotensin receptor blocker use on the frequency of atrial fibrillation after cardiac surgery: a cohort study from the atrial fibrillation suppression trials II and III. Eur J Cardio-Thorac Surg. 2007;31:817–20. doi: 10.1016/j.ejcts.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 177.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 178.Buch P, Friberg J, Scharling H, Lange P, Prescott E. Reduced lung function and risk of atrial fibrillation in The Copenhagen City Heart Study. Eur Respir J. 2003;21:1012–16. doi: 10.1183/09031936.03.00051502. [DOI] [PubMed] [Google Scholar]
- 179.Zhang X, Wu Z, Peng X, Wu A, Yue Y, Martin J, et al. Prognosis of diabetic patients undergoing coronary artery bypass surgery compared with nondiabetics: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2010;25:288–98. doi: 10.1053/j.jvca.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 180.de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen R-JS, et al. Progression from paroxysmal to persistent atrial fibrillation: clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725–31. doi: 10.1016/j.jacc.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 181.Lopez CM, House-Fancher MA. Management of atrial fibrillation in patients with chronic obstructive pulmonary disease. J Cardiovasc Nurs. 2005;20:133–40. doi: 10.1097/00005082-200503000-00009. [DOI] [PubMed] [Google Scholar]