Abstract
Aims
Depression is a mortality risk marker for acute coronary syndrome (ACS) patients. We hypothesized that the QT interval, a predictor for risk of sudden cardiac death, was related to depressive symptoms in ACS.
Methods and results
We performed an analysis of admission electrocardiograms from hospitalized patients with unstable angina or non-ST elevation myocardial infarction from two prospective observational studies of depression in ACS. Depressive symptoms were assessed with the Beck Depression Inventory (BDI), and depression was defined as BDI score ≥10, compared with <5. Patients with QRS duration ≥120 ms and/or who were prescribed antidepressants were excluded. QT intervals were adjusted for heart rate by two methods. Our analyses included 243 men (40.0% with BDI ≥10) and 139 women (62.0% with BDI ≥ 10). Among women, average QT corrected by Fridericia's method (QTcF) was 435.4 ± 26.6 ms in the depressed group, vs. 408.6 ± 24.3 ms in the non-depressed group (P< 0.01). However, among men, average QTcF was not significantly different between the depressed and non-depressed groups (415.4 ± 23.6 vs. 412.0 ± 25.8 ms, P= 0.29). In multivariable analyses that included hypertension, diabetes, ACS type, left ventricular ejection fraction <0.40, and use of QT-prolonging medication, there was a statistically significant interaction between depressive symptoms and gender (P< 0.001).
Conclusions
In this ACS sample, prolongation of the QT interval was associated with depressive symptoms in women, but not in men. Further investigation of the mechanism of the relationship between depression and abnormal cardiac repolarization, particularly in women, is warranted to develop treatment strategies.
Keywords: Depression, QT interval, Sudden cardiac death, Acute coronary syndrome
Introduction
Depression is a predictor of early mortality in patients after acute coronary syndrome (ACS), despite adjustment for coronary artery disease (CAD) severity.1,2 Potential mechanisms for this mortality risk include worse medical adherence, proinflammatory cytokine activation, and reduced heart rate variability.3 Furthermore, prospective studies of individuals ranging from those without known heart disease to those with implantable cardioverter–defibrillators have suggested that depression is associated with sudden cardiac death (SCD) and potentially fatal ventricular arrhythmia.4–7 More than 40% of all coronary heart disease-related mortality can be attributed to SCD,8 with arrhythmias such as sustained ventricular tachycardia or ventricular fibrillation often the suspected cause. Therefore, ventricular arrhythmia may contribute substantially to the association of mortality with depression in ACS.
Prolonged QT interval, indicative of abnormal ventricular repolarization, has been documented to be a strong predictor of SCD in recent large studies of unrelated individuals.9–11 In a case–control study of SCD in CAD, Chugh et al.11 found that prolonged QT conferred a five-fold risk of SCD among individuals without diabetes and who were not on QT-prolonging medications. The relationship between QT prolongation and SCD raises the question of what factors contribute to QT prolongation in individuals without known genetic syndromes. Interestingly, several small studies have suggested that psychosocial factors may be related to abnormalities in cardiac repolarization.12–15 To our knowledge, however, no prior study has evaluated the relationship between depression and QT prolongation after ACS.
In this study, we hypothesized that QT prolongation is related to depressive symptoms in individuals after ACS. Given the known differences in QT intervals between men and women,10 we tested whether the association was different for men vs. women.
Methods
Institutional Review Board approval for this study was obtained at each of the participating institutions. We performed an analysis of admission electrocardiograms (ECGs) among patients admitted to hospital with a diagnosis of unstable angina (UA) or non-ST elevation myocardial infarction (MI), using data from two studies that used the same ACS inclusion criteria and that assessed depressive symptoms with the identical instruments. The first study was the Coronary Psychosocial Evaluation Studies (COPES), a multi-site, observational cohort study designed to investigate the aetiology and natural course of depressive symptoms after an ACS event.16,17 Coronary Psychosocial Evaluation Studies participants were recruited from among patients admitted to three university hospitals (Mount Sinai Hospital, New York, New York, and Yale–New Haven Hospital and Hospital of St Raphael, New Haven, Connecticut) between May 2003 and June 2005. The second sample was from the first 500 patients enrolled in an ongoing prospective cohort study of depression after ACS [Prescription Use, Lifestyle, and Stress Evaluation (PULSE), NIH/NHBLI, P01 HL088117, PI Karina Davidson]. Participants of this study were recruited from among patients admitted to Columbia University Medical Center between February 2009 and June 2010.
For both studies, ACS events were defined according to American Heart Association/American College of Cardiology criteria18 as either acute MI or UA. All patients had symptoms consistent with acute myocardial ischaemia and at least one of the following: ischaemic electrocardiographic changes (i.e. ST depression and/or T wave abnormalities), an angiogram indicative of CAD on current admission, and/or documented history of CAD. Patients who presented with an acute rise in serum cardiac enzyme levels were categorized as MI. A study cardiologist confirmed ACS eligibility for all patients.
The Beck Depression Inventory (BDI),19 a 21-item self-report measure of depressive symptom severity, was administered within 1 week after the index ACS event. The BDI has repeatedly been shown to be associated with long-term mortality after ACS.2,17,20,21 Patients who scored <5 (indicative of no depressive symptoms) or ≥10 (indicative of significant depressive symptoms) were included in the study. Patients with BDI scores from five to nine were excluded to delineate more clearly depressed and non-depressed groups at baseline.22 Depressive symptoms were categorized according to BDI score: ≥10, vs. <5. In order to minimize possible confounding associated with antidepressant medication use, for our analyses we excluded participants who were prescribed antidepressant medications at hospital admission or on discharge.
Admission ECGs were analyzed by researchers and then over-read by a single cardiologist (W.W.), each of whom was blinded to depression status. For this study, we excluded patients with QRS duration ≥120 ms. ECG measurements included heart rate, PR interval, presence of Q waves, and ST depression ≥1 mm in two or more contiguous leads. Left ventricular hypertrophy (LVH) was defined according to Cornell voltage criteria23 (S in V3 + R in aVL≥28 mm in men and S in V3 + R in aVL ≥ 20 mm in women) and/or Solkolow-Lyon criteria24 (S in V1 + R in V5 or V6 ≥ 35 mm and R in aVL ≥ 11 mm). The QT interval was measured from the onset of the QRS complex to the end of the T wave, defined as the point of return of the T wave to the isoelectric line or to the nadir between the T and U waves in cases where a U wave was present.25 QT intervals were corrected for heart rate in two different ways: according to Fridericia's method (QTcF = QT/RR1/3), and using the nomogram-based QT correction (QTNc) proposed by Karjalainen et al.26
Left ventricular ejection fraction (LVEF) was measured quantitatively by left ventriculogram during cardiac catheterization, echocardiogram, or radionuclide study. If multiple measures were available, the value from the ventriculogram was used first, followed by the value from the echocardiogram. Left ventricular ejection fraction was then classified as normal-to-mild dysfunction (LVEF ≥ 0.40) and moderate-to-severe dysfunction (LVEF <0.40). Medication use was collected at hospital admission and discharge, and each participant's medication list was compared with an online list of possible or known QT-prolonging medications maintained by the Arizona Center for Education and Research on Therapeutics (www.qtdrugs.org).27 Prescription of QT-prolonging medications was included as an indicator variable in our analyses.
Statistical analyses
Fisher exact χ2 tests and two-sample t-tests were used to compare categorical and continuous measurements between the depressed (BDI ≥ 10) and non-depressed (BDI < 5) groups. ECG measures were compared between the depressed and non-depressed groups, separately in men and women. Average QT interval was compared with correction for heart rate in three different ways, as noted above. Linear regression models of the QT interval were estimated with inclusion of variables for the main effects of depressive symptoms and gender, as well as a multiplicative interaction term. Other variables included in the analyses were age, body mass index ≥25 kg/m2, non-White race, hypertension, diabetes, type of ACS event, LVEF <0.40, heart rate, electrocardiographic LVH, and prescription of QT-prolonging medication. In addition, multivariable models of the QT interval were estimated separately for men and women. All analyses were performed using SPSS, version 18 (SPSS Inc, Chicago, IL, USA).
Results
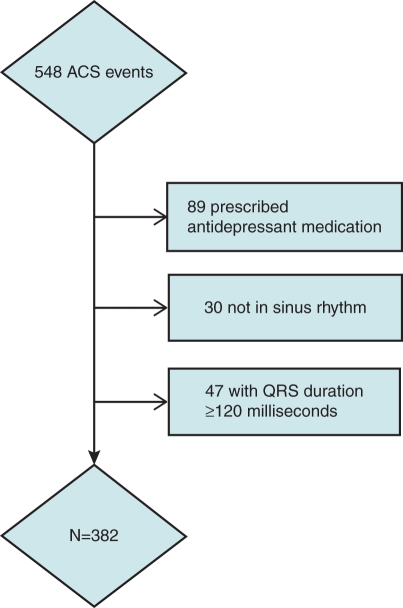
A consort diagram describing the cohort, we studied is provided in Figure 1. From a total of 548 COPES and PULSE participants with ACS and technically adequate admission ECGs, we excluded 89 patients who were prescribed antidepressant medications at ACS admission or discharge. Among this group, 30 patients who were not in sinus rhythm, and 47 who had QRS duration ≥120 ms were also removed from the analysis. This left a sample of 382 patients with UA/non-ST elevation ACS.
Figure 1.
Consort diagram of ECG analysis.
In our sample, 85 of 139 women (62.0%) and 97 of 243 men (40.0%) had significant depressive symptoms (BDI score ≥10). Table 1 shows the distribution of clinical characteristics by depressive symptom category. Depressed patients were more likely to be female (46.7 vs. 27.0%) and non-white (44.0 vs. 25.0%). The proportion with body mass index ≥25 kg/m2, hypertension, diabetes, presentation with non-STEMI (ST elevation myocardial infarction), and LVEF <0.40 was similar between the depressed and non-depressed patients. Overall, QT-prolonging medications were prescribed in 10.5% of patients (anti-arrhythmic medications in 5%, antibiotics in 3.4%, other QT-prolonging medications in 2.1%), and a similar proportion of depressed and non-depressed patients were prescribed such medications.
Table 1.
Demographic and clinical characteristics of acute coronary syndrome patients by depressive symptom status
| BDI < 5 (n= 200), % | BDI ≥ 10 (n= 182), % | P value | |
|---|---|---|---|
| Age > 65 | 36.0 | 34.6 | 0.83 |
| Female | 27.0 | 46.7 | <0.01 |
| Non-White | 25.0 | 44.0 | <0.01 |
| BMI ≥ 25 kg/m2 | 64.5 | 58.2 | 0.25 |
| Hypertension | 63.0 | 69.2 | 0.23 |
| Diabetes | 26.0 | 33.0 | 0.15 |
| QT-prolonging medication | 9.5 | 11.5 | 0.61 |
| Non-STEMI | 33.0 | 34.1 | 0.83 |
| LVEF <0.40 | 8.5 | 6.0 | 0.43 |
BDI, Beck Depression Inventory; BMI, body mass index; STEMI, ST elevation myocardial infarction; LVEF, left ventricular ejection fraction.
Table 2 shows unadjusted values for ECG indices by depression status, in men and women. Average heart rate, PR interval, proportion of patients with Q waves, and proportion with ST depression >1 mm were similar between the depressed and non-depressed patients for both men and women. However, average QT interval was longer in the depressed group among women, but not in men. For instance, using Fridericia's correction for heart rate (QTcF), among women average QTcF was 435.4 ± 26.6 ms in the depressed group, vs. 408.6 ± 24.3 ms in the non-depressed group (P< 0.01). However, among men average QTcF was not significantly different between the depressed and non-depressed groups (415.4 ± 23.6 vs. 412.0 ± 25.8 ms, P= 0.29). Also, among women, more patients in the depressed group had LVH by voltage criteria (23.8 vs. 7.7%, P= 0.02).
Table 2.
Baseline electrocardiographic characteristics of acute coronary syndrome patients according to depressive symptoms by Beck Depression Inventory score
| BDI < 5 | BDI ≥ 10 | P value | |
|---|---|---|---|
| Women (n= 139) | |||
| Heart rate (bpm) | 68.0 (11.2) | 70.2 (12.0) | 0.29 |
| PR interval (ms) | 168.0 (23.7) | 160.8 (18.8) | 0.06 |
| QTcF (ms) | 408.6 (24.3) | 435.4 (26.6) | <0.01 |
| QTNc (ms) | 410.0 (23.3) | 434.4 (28.9) | <0.01 |
| ECG-LVH | 7.7 % | 23.8 % | 0.02 |
| Q waves | 14.8 % | 18.8 % | 0.65 |
| ST depression ≥1 mm | 11.1 % | 16.5 % | 0.46 |
| Men (n= 243) | |||
| Heart rate (bpm) | 68.9 (13.7) | 66.5 (12.4) | 0.15 |
| PR interval (ms) | 171.8 (33.1) | 172.0 (29.1) | 0.97 |
| QTcF (ms) | 412.0 (25.8) | 415.4 (23.6) | 0.29 |
| QTNc (ms) | 414.0 (25.6) | 417.2 (29.0) | 0.38 |
| ECG-LVH | 11.0 % | 13.5 % | 0.55 |
| Q waves | 23.3 % | 15.5 % | 0.15 |
| ST depression ≥1 mm | 11.6 % | 14.4 % | 0.56 |
Numbers in parentheses represent standard deviation for continuous measures.
bpm, beats per minute; ms, milliseconds; LVH, left ventricular hypertrophy; QTcF, QT corrected for heart rate using Fridericia's method; QTNc, QT corrected for heart rate using the nomogram-based method.
We estimated the relationship between QT interval and depressive symptoms (BDI score ≥10) in analyses that included both men and women, in multivariable linear regression models that adjusted for gender, gender−depression interaction, age, hypertension, diabetes, body mass index ≥25 kg/m2, type of ACS event, LVEF <0.40, heart rate, electrocardiographic LVH, and prescription of QT-prolonging medication (Table 3). The interaction term between gender and depressive symptoms was statistically significant (for QTcF beta coefficient 22.9, 95% CI 12.3–33.5, P< 0.001), indicating that the relationship between depressive symptoms and prolonged QT interval was stronger in women. This finding remained consistent regardless of the method used to correct QT interval for heart rate. Next, we estimated separate multivariable models of the QT interval for men and women. In women, there was a statistically significant relationship between depressive symptoms and QT interval. For QTcF, this corresponded to a 24.5 ms difference for depressed patients compared with non-depressed patients (95% CI 15.4–33.7, P< 0.001), and results were similar using the nomogram-based method (beta coefficient 22.1, 95% CI 12.8–31.3, P< 0.001). In men, however, the relationship between depressive symptoms and QT was not statistically significant (for models of QTcF , beta coefficient 3.5, 95% CI −2.9 to 9.8, P= 0.29). When depressive symptom severity was treated as a continuous measure in multivariable analyses using score on the BDI, the relationship between QT and depression symptom severity was statistically significant in women (for QTcF beta coefficient 0.85 per 1 point increment in BDI, 95% CI 0.36–1.35, P= 0.001), but not in men (beta coefficient 0.24, 95% CI −0.24 to 0.68, P= 0.35).
Table 3.
Multivariable linear regression analyses of QT interval in milliseconds, with correction for heart rate by two different methods
| QTcF |
QTNc |
|||
|---|---|---|---|---|
| Beta coefficient (95% CI) | P value | Beta coefficient (95% CI) | P value | |
| BDI ≥ 10 | 3.3 (−3.1, 9.6) | 0.31 | 2.5 (−4.2, 9.2) | 0.47 |
| Female | −3.4 (−11.1, 4.4) | 0.39 | −4.1 (−12.2, 4.1) | 0.33 |
| Female×BDI ≥ 10 | 22.9 (12.3, 33.5) | <0.001 | 20.9 (9.8, 32.0) | <0.001 |
Results are from models that also adjusted for age, hypertension, diabetes, body mass index ≥25 kg/m2, type of acute coronary syndrome event, left ventricular ejection fraction <0.40, heart rate, electrocardiographic left ventricular hypertrophy, and prescription of QT-prolonging medication. Female× BDI ≥ 10 refers to the interaction between female gender and BDI score category.
QTcF, QT corrected for heart rate using Fridericia's method; QTNc, QT corrected for heart rate using the nomogram-based method; BDI, Beck Depression Inventory.
Discussion
In this study of patients admitted to hospital with UA/non-ST elevation MI, we found that depressive symptoms measured by BDI score were associated with increased QT interval in women, but not in men. The incremental difference in QT interval, we found in women with significant depressive symptoms, 24.5 ms for QTcF in adjusted analyses compared with women without significant depressive symptoms, was large and likely to be clinically significant. Prolonged QT has been associated with a three to five-fold increased risk of SCD in recent studies involving adults 55 years or older10 and individuals with CAD.11 We made efforts not to include patients who might have prolonged QT due to factors other than depression. For instance, we excluded those who were prescribed antidepressant medications, some of which are known to prolong the QT interval. In addition, we excluded patients with prolonged QRS duration to prevent inclusion of patients with left or right bundle branch block. Also, our results remained consistent with use of two different methods for correction for heart rate, and we adjusted for use of QT-prolonging medications. The results of our analysis suggest that arrhythmia may play an important role in the poorer prognosis after ACS associated with depression, particularly in women.
Our findings are consistent with prior studies that have noted differences in cardiac repolarization associated with depression and other affective states. For instance, a study by Takimoto et al.12 reported a positive correlation between QT interval corrected by Bazett's formula (QTC) and self-reported depression in patients with bulimia nervosa, while in a comparison of 20 post-MI patients with major depression and 20 post-MI patients without depression, Carney et al.13 found that QT variability was significantly higher in the depressed group. Studies involving patients with congenital long QT syndrome have shown increases in QT interval associated with ‘low-arousal’ emotional states,15 as well as a correlation between reported happiness and reduced risk of arrhythmic events.28 In a laboratory setting, Critchley et al.14 documented asymmetric right midbrain activity by positron emission tomography scan during stress, and correlated these changes with cardiac repolarization abnormalities by ECG.
To our knowledge, ours is the first study that has noted a unique relationship between depressive symptoms and repolarization abnormalities in women, and this observation has some correlates to other findings in the literature. Depression is known to be more prevalent in women, particularly after myocardial infarction.2,29,30 Still, compared with women without depression, those with elevated depressive symptoms have higher mortality post-MI30 and in suspected CAD.21 Women are also known to be at greater risk for drug-induced torsades de pointes compared with men,31 and women have greater response in their QT intervals to isoproterenol compared with men.32 Previous hypothesized mechanisms of the association between depression and prognosis in cardiac disease have often invoked abnormal cardiac sympathetic activity.33,34 The concept of ‘repolarization reserve’ has been developed where given the different possible mechanisms that contribute to cardiac repolarization, more than one insult may be necessary to influence arrhythmia risk significantly.35 Sympathetic activation and differences in repolarization reserve may help explain the differential relationship between depressive symptoms and QT interval seen in our study. In addition, there has been speculation that depression may make patients more susceptible to stress cardiomyopathy,36 a condition consequent to emotion-induced catecholamine surges that is often accompanied by pronounced repolarization abnormalities by ECG37 and that is much more frequent in women. Further study of the mechanisms underlying the depression−gender interaction in cardiac repolarization may help clarify potential therapies to address the mortality risk associated with depression in ACS.
Our analysis has several limitations. We cannot rule out the possibility of reverse causality, in which more severe cardiac disease could result in worse depressive symptoms and a perceived association both with our ECG markers and with mortality. The depressed and non-depressed groups in our sample were similar in terms of type of ACS event, proportion with Q waves or ST depression on their ECG, and proportion with reduced LVEF, but there may be other unmeasured confounders for which we have not accounted. Also, our study involved secondary analysis of two separately collected samples of patients, neither of which was designed in advance to evaluate gender differences. Finally, we did not have serial ECG collections, and so we cannot estimate for any changes in repolarization associated with changes in depressive symptoms.
In summary, we found in this ACS sample that QT interval prolongation was associated with depressive symptoms in women, but not in men. Abnormal cardiac repolarization may be an important mediator of the poor prognosis associated with depression, and further investigation into the mechanism of this relationship, particularly in women, is warranted to develop possible treatment strategies.
Conflict of interest: none declared.
Funding
This work was supported by grants HC-25197, HL-076857, HL-088117, and HL-084034 from the National Institutes of Health, and by a Scientist Development Grant to W.W. from the American Heart Association Founders Affiliate.
References
- 1.van Melle JP, de Jonge P, Spijkerman TA, Tijssen JG, Ormel J, van Veldhuisen DJ, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med. 2004;66:814–22. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- 2.Frasure-Smith N, Lesperance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995;91:999–1005. doi: 10.1161/01.cir.91.4.999. [DOI] [PubMed] [Google Scholar]
- 3.Carney RM, Freedland KE, Miller GE, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res. 2002;53:897–902. doi: 10.1016/s0022-3999(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 4.Empana JP, Jouven X, Lemaitre RN, Sotoodehnia N, Rea T, Raghunathan TE, et al. Clinical depression and risk of out-of-hospital cardiac arrest. Arch Intern Med. 2006;166:195–200. doi: 10.1001/archinte.166.2.195. [DOI] [PubMed] [Google Scholar]
- 5.Whang W, Albert CM, Sears SF, Jr, Lampert R, Conti JB, Wang PJ, et al. Depression as a predictor for appropriate shocks among patients with implantable cardioverter–defibrillators: results from the Triggers of Ventricular Arrhythmias (TOVA) study. J Am Coll Cardiol. 2005;45:1090–5. doi: 10.1016/j.jacc.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 6.Whang W, Kubzansky LD, Kawachi I, Rexrode KM, Kroenke CH, Glynn RJ, et al. Depression and risk of sudden cardiac death and coronary heart disease in women: results from the Nurses' Health Study. J Am Coll Cardiol. 2009;53:950–8. doi: 10.1016/j.jacc.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luukinen H, Laippala P, Huikuri HV. Depressive symptoms and the risk of sudden cardiac death among the elderly. Eur Heart J. 2003;24:2021–6. doi: 10.1016/j.ehj.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Fox CS, Evans JC, Larson MG, Kannel WB, Levy D. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: the Framingham Heart Study. Circulation. 2004;110:522–7. doi: 10.1161/01.CIR.0000136993.34344.41. [DOI] [PubMed] [Google Scholar]
- 9.Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83:1888–94. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- 10.Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–7. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 11.Chugh SS, Reinier K, Singh T, Uy-Evanado A, Socoteanu C, Peters D, et al. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation. 2009;119:663–70. doi: 10.1161/CIRCULATIONAHA.108.797035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takimoto Y, Yoshiuchi K, Akabayashi A. Effect of mood states on QT interval and QT dispersion in eating disorder patients. Psychiatry Clin Neurosci. 2008;62:185–9. doi: 10.1111/j.1440-1819.2008.01753.x. [DOI] [PubMed] [Google Scholar]
- 13.Carney RM, Freedland KE, Stein PK, Watkins LL, Catellier D, Jaffe AS, et al. Effects of depression on QT interval variability after myocardial infarction. Psychosom Med. 2003;65:177–80. doi: 10.1097/01.psy.0000033129.21715.4b. [DOI] [PubMed] [Google Scholar]
- 14.Critchley HD, Taggart P, Sutton PM, Holdright DR, Batchvarov V, Hnatkova K, et al. Mental stress and sudden cardiac death: asymmetric midbrain activity as a linking mechanism. Brain. 2005;128:75–85. doi: 10.1093/brain/awh324. [DOI] [PubMed] [Google Scholar]
- 15.Lane RD, Zareba W, Reis HT, Peterson DR, Moss AJ. Changes in ventricular repolarization duration during typical daily emotion in patients with Long QT syndrome. Psychosom Med. 2011;73:98–105. doi: 10.1097/PSY.0b013e318203310a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimbo D, Rieckmann N, Paulino R, Davidson KW. Relation between C reactive protein and depression remission status in patients presenting with acute coronary syndrome. Heart. 2006;92:1316–8. doi: 10.1136/hrt.2005.075861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whang W, Shimbo D, Kronish IM, Duvall WL, Julien H, Iyer P, et al. Depressive symptoms and all-cause mortality in unstable angina pectoris (from the Coronary Psychosocial Evaluation Studies [COPES]) Am J Cardiol. 2010;106:1104–7. doi: 10.1016/j.amjcard.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, et al. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. J Am Coll Cardiol. 2001;38:2114–30. doi: 10.1016/s0735-1097(01)01702-8. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee) [DOI] [PubMed] [Google Scholar]
- 19.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 20.Lesperance F, Frasure-Smith N, Juneau M, Theroux P. Depression and 1-year prognosis in unstable angina. Arch Intern Med. 2000;160:1354–60. doi: 10.1001/archinte.160.9.1354. [DOI] [PubMed] [Google Scholar]
- 21.Rutledge T, Reis SE, Olson M, Owens J, Kelsey SF, Pepine CJ, et al. Depression is associated with cardiac symptoms, mortality risk, and hospitalization among women with suspected coronary disease: the NHLBI-sponsored WISE study. Psychosom Med. 2006;68:217–23. doi: 10.1097/01.psy.0000195751.94998.e3. [DOI] [PubMed] [Google Scholar]
- 22.Davidson KW, Rieckmann N, Rapp MA. Definitions and distinctions among depressive syndromes and symptoms: implications for a better understanding of the depression-cardiovascular disease association. Psychosom Med. 2005;67(Suppl 1):S6–9. doi: 10.1097/01.psy.0000162257.19266.fc. [DOI] [PubMed] [Google Scholar]
- 23.Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation. 1987;75:565–72. doi: 10.1161/01.cir.75.3.565. [DOI] [PubMed] [Google Scholar]
- 24.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161–86. doi: 10.1016/0002-8703(49)90562-1. [DOI] [PubMed] [Google Scholar]
- 25.Lepeschkin E, Surawicz B. The measurement of the Q–T interval of the electrocardiogram. Circulation. 1952;6:378–88. doi: 10.1161/01.cir.6.3.378. [DOI] [PubMed] [Google Scholar]
- 26.Karjalainen J, Viitasalo M, Manttari M, Manninen V. Relation between QT intervals and heart rates from 40 to 120 beats/min in rest electrocardiograms of men and a simple method to adjust QT interval values. J Am Coll Cardiol. 1994;23:1547–53. doi: 10.1016/0735-1097(94)90654-8. [DOI] [PubMed] [Google Scholar]
- 27. Drugs that prolong the QT interval and/or induce torsades de pointes http://www.qtdrugs.org/ (30 May 2011, date last accessed)
- 28.Lane RD, Reis HT, Peterson DR, Zareba W, Moss AJ. Happiness and stress alter susceptibility to cardiac events in Long QT Syndrome. Ann Noninvasive Electrocardiol. 2009;14:193–200. doi: 10.1111/j.1542-474X.2009.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naqvi TZ, Naqvi SS, Merz CN. Gender differences in the link between depression and cardiovascular disease. Psychosom Med. 2005;67(Suppl 1):S15–8. doi: 10.1097/01.psy.0000164013.55453.05. [DOI] [PubMed] [Google Scholar]
- 30.Frasure-Smith N, Lesperance F, Juneau M, Talajic M, Bourassa MG. Gender, depression, and one-year prognosis after myocardial infarction. Psychosom Med. 1999;61:26–37. doi: 10.1097/00006842-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA. 1993;270:2590–7. doi: 10.1001/jama.270.21.2590. [DOI] [PubMed] [Google Scholar]
- 32.Nakagawa M, Ooie T, Ou B, Ichinose M, Takahashi N, Hara M, et al. Gender differences in autonomic modulation of ventricular repolarization in humans. J Cardiovasc Electrophysiol. 2005;16:278–84. doi: 10.1046/j.1540-8167.2005.40455.x. [DOI] [PubMed] [Google Scholar]
- 33.Hemingway H, Malik M, Marmot M. Social and psychosocial influences on sudden cardiac death, ventricular arrhythmia and cardiac autonomic function. Eur Heart J. 2001;22:1082–101. doi: 10.1053/euhj.2000.2534. [DOI] [PubMed] [Google Scholar]
- 34.Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosom Med. 2005;67:S29–33. doi: 10.1097/01.psy.0000162254.61556.d5. [DOI] [PubMed] [Google Scholar]
- 35.Roden DM. Taking the ‘idio’ out of ‘idiosyncratic’: predicting torsades de pointes. Pacing Clin Electrophysiol. 1998;21:1029–34. doi: 10.1111/j.1540-8159.1998.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 36.Ziegelstein RC. Depression and tako-tsubo cardiomyopathy. Am J Cardiol. 2010;105:281–2. doi: 10.1016/j.amjcard.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 37.von Korn H, Yu J, Lotze U, Ohlow MA, Huegl B, Schulte W, et al. Tako-Tsubo-like cardiomyopathy: specific ECG findings, characterization and clinical findings in a European single center. Cardiology. 2009;112:42–8. doi: 10.1159/000137698. [DOI] [PubMed] [Google Scholar]