Abstract
Aims
Uncertainty exists over the importance of device-detected short-duration atrial arrhythmias. Continuous atrial diagnostics, through home monitoring (HM) technology (BIOTRONIK, Berlin, Germany), provides a unique opportunity to assess frequency and quantity of atrial fibrillation (AF) episodes defined as atrial high-rate events (AHRE).
Methods and results
Prospective data from 560 heart failure (HF) patients (age 67 ± 10 years, median ejection fraction 27%) patients with a cardiac resynchronization therapy (CRT) device capable of HM from two multi-centre studies were analysed. Atrial high-rate events burden was defined as the duration of mode switch in a 24-h period with atrial rates of >180 beats for at least 1% or total of 14 min per day. The primary endpoint was incidence of a thromboembolic (TE) event. Secondary endpoints were cardiovascular death, hospitalization because of AF, or worsening HF. Over a median 370-day follow-up AHRE occurred in 40% of patients with 11 (2%) patients developing TE complications and mortality rate of 4.3% (24 deaths, 16 with cardiovascular aetiology). Compared with patients without detected AHRE, patients with detected AHRE>3.8 h over a day were nine times more likely to develop TE complications (P= 0.006). The majority of patients (73%) did not show a temporal association with the detected atrial episode and their adverse event, with a mean interval of 46.7 ± 71.9 days (range 0–194) before the TE complication.
Conclusion
In a high-risk cohort of HF patients, device-detected atrial arrhythmias are associated with an increased incidence of TE events. A cut-off point of 3.8 h over 24 h was associated with significant increase in the event rate. Routine assessment of AHRE should be considered with other data when assessing stroke risk and considering anti-coagulation initiation and should also prompt the optimization of cardioprotective HF therapy in CRT patients.
Keywords: Heart failure, Atrial fibrillation, Home monitoring, Thromboembolism, Anticoagulation, Cardiac resynchronization therapy
Introduction
The morbidity and mortality of atrial fibrillation (AF) have a major impact on health-care expenditure, largely as a consequence of thromboembolic (TE) complications.1
A significant proportion of AF is asymptomatic but carries important prognostic implications, as emphasized in the AFFIRM study.2 Patients with undetected, ‘unprotected’ paroxysms of AF remain at a continued risk of TE. Conventional detection methods, such as Holter monitoring, are ineffective at detecting asymptomatic paroxysmal atrial fibrillation (PAF).3 Although data monitoring of modern implantable pacemakers has enhanced diagnostic capability,4 recognition of relevant data generally remains unchecked until the scheduled device review with consequent delay in therapeutic intervention. Implantable devices with remote monitoring offer a solution to this problem. Continuous atrial diagnostic data provide a unique opportunity to assess the frequency and quantity of AF episodes defined as atrial high-rate events (AHRE). The home monitoring (HM) technology (BIOTRONIK, Berlin, Germany), provides both daily and instantaneous transmission on event detection. This does not require patient involvement, is validated, and is a safe and reliable automatic remote system.5
However, interpretation of the comprehensive data provided by these devices increasingly falls outside of conventional guidelines. There are limited data available as to what level of atrial high-rate burden is clinically relevant and when thromboprophylaxis should be employed.
Therefore, we proceeded to evaluate the clinical impact of high atrial arrhythmic burden in a heart failure (HF) population receiving cardiac resynchronization therapy (CRT) with remote monitoring technology. Data were pooled from two international prospective multi-centre studies.
Methods
Patient population
Data were considered from all patients enrolled in the international prospective multi-centre observational Home CARE (clinicaltrials.gov code NCT00376116) and everesT trials (Evaluation of the new Biotronik Resynchronization+ICD System) (Appendix 1). All patients had HF and a CRT device capable of continuous heart rhythm monitoring via HM. Patients in sinus rhythm (including patients with a prior history of AF) with >70% HM transmissions in the follow-up period and >3 months of HM follow-up were included in the evaluation. All trials complied with the Declaration of Helsinki and were approved by the locally appointed ethics committee of each participating centre. Informed consent had been obtained from all the patients.
Device and Home Monitoring characteristics
Patients had received CRT devices with or without a defibrillator (Stratos LV-T, Kronos LV-T, or Lumax HF-T from BIOTRONIK) with HM turned on. This wireless, mobile remote monitoring system continuously records details of relevant clinical events (e.g. rhythm disturbances and delivered therapies). However, as this was an observational study, no interventions were undertaken when high atrial arrhythmia burden was detected. Also for the purposes of study analysis we were unable to verify the detected atrial arrhythmias with the available intracardiac electrograms (IEGM Online HD®).
Detection of atrial burden
We defined AHRE as the duration of mode-switch in a 24 h period. Mode-switching in Stratos and Kronos devices occurs when five of eight consecutive atrial beats are >180 bpm and continues until five of eight are below 180. In Lumax devices the criteria for onset are 36 out of 48 atrial cycles with a rate >180 bpm and termination occurs when 20 out of 24 atrial beats are at a slower rate than a programmed value. Prior studies using similar detection algorithms have shown >95% sensitivity and specificity for the detection of atrial tachycardia/atrial fibrillation (AT/AF) episodes and measurements of AT/AF burden.6 Patients were included in the analysis if the total detected AHRE burden in a 24 h period exceeded an accumulative total of 14 min. This 14 min limit is due to the definition of AF burden in the HM system in per cent per day. So 1% (= 14.4 min) is the minimal amount detected per day.
Outcome events
The primary study endpoint was the incidence of (TE) event, including stroke, transient ischaemic attack (TIA), and peripheral arterial embolism (PAE). The secondary endpoints were cardiovascular death, hospitalization because of AF, or worsening of HF. Ischaemic stroke was defined as an acute onset neurological deficit due to focal cerebrovascular inhibition of flow, persisting for >24 h.
Data analysis
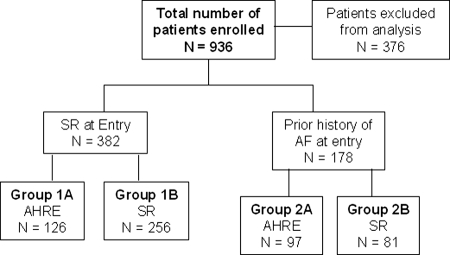
Baseline demographic characteristics were collated including individual CHADS2 score.7 The patients analysed in this study were divided into the following groups : (Figure 1):
- Group 1A. Sinus rhythm at enrollment, no prior history of AF but HM detected AHRE over the follow-up period.
- Group 1B. Sinus rhythm at enrollment and no prior history of AF with no detected AHRE.
- Group 2A. Prior history of AF with detected AHRE over the follow-up period.
- Group 2B. Prior history of AF with no detected AHRE over the follow-up period.
Figure 1.
Study flow chart. AHRE, atrial high rate events; SR, sinus rhythm.
Patients were included in Group 1A or 2A, if they experienced ≥1 AHRE event during the follow-up period. A prior history of AF was defined as attacks of AF lasting from 2 min to <7 days (PAF) or AF >7 days but <1 year (persistent AF).8 A diagnosis of prior AF required documentation by EKG or Holter monitoring. All patients were in SR at enrollment.
Statistical analysis
Normally distributed continuous variables were calculated using mean and standard deviation. Categorical data are presented as number and percentage. Distributions were tested for normality using Shapiro−Wilkinson statistics. Non-normally distributed variables were represented as median with 25th–75th interquartile range (IQR).
Comparison of normally distributed continuous variables was performed using Student's t-test for paired and unpaired data. Non-normally distributed variables were compared using Mann–Whitney Rank Sum tests and Kruskal–Wallis tests. Comparison of categorical data was performed using χ2 and Fisher's Exact tests where appropriate. A global P value was calculated when comparing all four groups. Statistical significance was established as P< 0.05.
Differences in event rates over time were analysed by the Kaplan–Meier analysis with Gehan–Breslow test for each outcome in the four study groups. Multiple comparisons for the four different groups were performed with the Holm–Sidak method. The Cox regression model was used to analyse the effect of daily AHRE on TE and other composite endpoints [(TE/cardiovascular death) and (AF and HF admissions/TE/cardiovascular death)]. Thereby, the categorization of no AF, daily AHRE> median (3.8 h), and daily AHRE ≥ median was used. SPSS software (version 16 statistical package, SPSS Inc., Chicago, IL, USA) was used for all the statistical analyses.
Results
Study population
Between August 2004 to August 2008, 936 HF/CRT patients were enrolled from 77 European cardiac centres in the two trials. Of the 376 patients excluded from analysis, pre-enrollment AF status was missing in 87 (9%) patients, 75 (8%) had <70% HM data available for analysis, 56 (6%) had <90 days surveillance via HM, and 158 (17%) dropped out of the study. Analysis was undertaken on prospective data from 560 patients, over a median follow-up period of 370 days (IQR from 253 to 390 days). See Table 1 for study population demographics.
Table 1.
Patient Characteristics for all 560 patients including subgroup demographics
| Variable baseline patient characteristics | Total study population | AHRE Group 1A | No AHRE Group 1B | Prior history of AF pre entry | Prior history of AF pre entry | Global P value |
|---|---|---|---|---|---|---|
| (n = 560) | (n = 126) | (n = 256) | Group 2A AHRE | Group 2B no AHRE | ||
| (n = 97 | (n = 81) | |||||
| Male | 434 (77.4%) | 107 (84.9%) | 189 (73.8%) | 73 (75.3%) | 65 (80.2%) | 0.086 |
| Age (years) | 66 ± 10 | 65 ± 10 | 67 ± 10 | 68 ± 10 | 69 ± 9 | 0.07 |
| QRS (ms) | 160 (140–178) | 165 (140–180) | 160 (132–173) | 160 (140–180) | 160 (140–173) | 0.14 |
| Non-ischaemic | 258 (46.1%) | 48 (38.1%) | 126 (49.2%) | 46 (47.4%) | 38 (46.1%) | 0.23 |
| NYHA I | 7 (1.25%) | 1 (0.8%) | 4 (1.6%) | 0 (0%) | 2 (2.5%) | |
| NYHA II | 108 (19.3%) | 24 (19.0%) | 51 (19.9%) | 17 (17.5%) | 16 (19.8%) | 0.81 |
| NYHA III | 401 (71.6%) | 94 (74.6% | 178 (69.5%) | 74 (76.3%) | 55 (67.9%) | |
| NYHA IV | 36 (6.43%) | 5 (4.0%) | 19 (7.4%) | 6 (6.2%) | 6 (7.4%) | |
| EF (%) | 25 (20–30) | 27 (20–30) | 25 (20–30) | 28 (20–32) | 25 (20–28) | 0.14 |
| Diabetes | 179 (32%) | 37 (29.4%) | 82 (32.0%) | 31 (32%) | 29 (35.8%) | 0.82 |
| Hypertension | 210 (37.5%) | 45 (35.7%) | 95 (37.1%) | 35 (36.1%) | 35 (43.2%) | 0.71 |
| Pre-implant Stroke | 1 (0.18%) | 1 (0.79%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0.33 |
| CHADS2 Score 1 | 202 (36.1%) | 49 (38.9%) | 95 (37.1%) | 34 (35.1%) | 24 (29.6%) | |
| CHADS2 Score 2 | 216 (38.6%) | 48 (38.1%) | 95 (37.1%) | 36 (37.1%) | 37 (45.7%) | 0.47 |
| CHADS2 Score 3 | 128 (22.9%) | 29 (23.0%) | 56 (21.9%) | 24 (24.7%) | 19 (23.5%) | |
| CHADS2 Score 4 | 14 (2.5%) | 0 (0.0%) | 10 (3.9%) | 3 (3.1%) | 1 (1.2%) | |
| ACEI | 354 (63.2%) | 89 (70.6%) | 165 (64.5%) | 55 (56.7%) | 45 (55.6%) | 0.07 |
| Beta-blockers | 353 (63%) | 91 (72.2%) | 166 (64.8)% | 50 (51.5%) | 46 (56.8%) | 0.008 |
| Amiodarone | 103 (18.4%) | 17 (13.5%) | 36 (14.1%) | 22 (22.7%) | 28 (34.6%) | 0.0001 |
| Other anti-arrythmics | 110 (19.6%) | 18 (14.3%) | 38 (14.8%) | 24 (24.7%) | 30 (37.0%) | 0.0004 |
| Anti-platelets | 239 (42.7%) | 64 (50.8%) | 119 (46.5%) | 28 (28.9%) | 28 (34.6%) | 0.002 |
| Warfarin | 67 (12.0%) | 9 (7.14%) | 22 (8.59%) | 19 (19.6%) | 17 (21.0%) | 0.0007 |
| CRT (%) bivent pacing | 98 (95–99) | 98 (95–99) | 98 (95–99) | 98 (95–99) | 99 (95–99) | 0.07 |
| HM performance % | 93 (87–97) | 94 (89–97) | 93 (87–97) | 92 (84–96) | 94 (86–97) | 0.31 |
Data are presented as the mean value ± SD or median value (25th–75th percentile) for continuous variables and number and percentage of patients for categorical data.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; AHRE, atrial high-rate events; BiV biventricular pacing; EF, ejection fraction; HM, Home Monitoring; NYHA, New York Heart Association; other anti-arrythmics include Sotolol and Class 1 anti-arrythmics.
All four groups had similar baseline characteristic, although there were significantly more patients with ischaemic HF in Group 1A compared with the other three groups. Accordingly, the uptake of cardioprotective medication including beta-blockers, angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARBs) and anti-platelets was increased in this group. Anticoagulation use among patients with known prior AF (Group 2A 19.6% and Group 2B 21%) was significantly higher than in Group 1A (7%) or Group 1B (8%). The zero atrial burden noted in Group 2B may have been explained by the significantly increased usage of anti-arrhythmic medication, including amiodarone, observed in this group. Of further relevance to our CRT cohort, the median frequency of biventricular pacing over the total follow-up period was >98% (IQR 95–99) for all four groups.
Quantification of atrial tachyarrhythmic burden
At least, 40% of the study population had at least 1 day where they had atrial tachyarrhythmias totalling >14 min (1% of day). Furthermore, in the sinus rhythm group with no prior history of AF, the annual incidence of a device detected atrial episode was 33%. Despite the prior history of AF only 64% of patients in Group 2 demonstrated a monitored atrial event. However, the median daily AHRE was substantially greater in Group 2A (647 min IQR 84–700) compared with Group 1A (44 min IQR 11–179; P= 0.0001).
Clinical events
See Table 2, for summary of clinical event rates in all four groups.
Table 2.
Adverse event rates for all four study groups
| Clinical event | Total events | Group 1A | Group 1B | Group 2A | Group 2B | Global P value |
|---|---|---|---|---|---|---|
| n= 126 | n= 256 | n= 97 | n= 81 | |||
| TE | 11 (2.0%) | 5 (4.0%) | 0 (0%) | 4 (4.1%) | 2 (2.5%) | 0.02 |
| All-cause mortality | 24 (4.3%) | 7 (5.6%) | 8 (3.1%) | 4 (4.1%) | 5 (6.2%) | 0.56 |
| CVS mortality | 16 (2.9%) | 4 (3.2%) | 4 (1.6%) | 4 (4.1%) | 4 (4.9%) | 0.33 |
| HF admission | 52 (9.3%) | 10 (7.9%) | 13 (5.1%) | 16 (16.5%) | 13 (16%) | 0.001 |
Data are presented as number and percentage.
Arterial thromboembolic complications
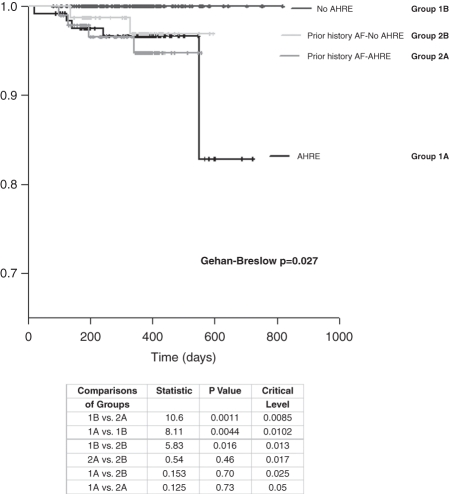
Over a median follow-up period of 370 days TE complications occurred in 11 patients (2.0%). In particular, five patients suffered a PAE, four ischaemic strokes, and two TIA. There were no associated TE events in those patients with no prior history of AF and zero atrial burden over the follow-up period. However, two PAE events (18.1%) did occur in Group 2B. Consequently, if a patient developed new atrial episodes or had a prior history of AF, they were more likely to develop a TE complication compared with patients in Group 1B. Furthermore, there was no statistical difference in the cumulative probability of developing a TE complication between Group 1A and 2A (P= 0.124). Despite there being no detected atrial arrhythmia in Group 2B, the TE outcomes were similar to those patients experiencing AHRE (Group 2B vs. 1A P= 0.15 and Group 2B vs. Group 2A, P= 0.5) (Figure 2).
Figure 2.
Kaplan−Meier cumulative survival from thromboembolic (TE) events for all four groups of patients. Multiple comparisons of the groups by the Holm−Sidak method are presented.
Mortality
There were 24 (4.3%) deaths in total, with 16 (67%) due to a cardiovascular aetiology. There was no difference in all-cause mortality between the four study groups.
Heart failure admissions
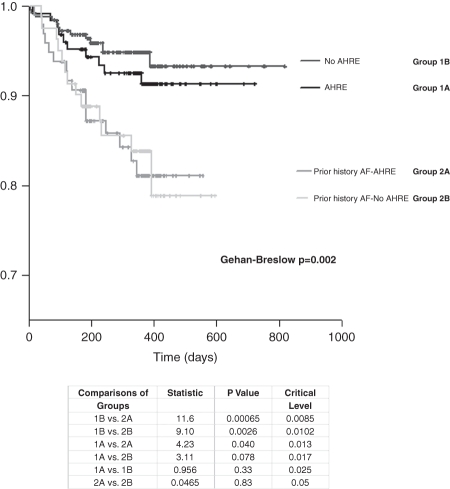
A total of 52 (9.3%) admissions for HF were observed in the study population. Patients with a prior history of AF, whether or not they experienced a period of atrial burden, were more likely to be admitted with decompensated HF [7.9% (Group 1A), 5.1% (Group 1B) vs. 16.5% in Group 2A and 16% in Group 2B; P= 0.001] (Figure 3).
Figure 3.
Kaplan−Meier cumulative survival from heart failure (HF) admissions for all four groups of patients. Multiple comparisons of the groups by the Holm−Sidak method are presented.
The temporal association between atrial arrhythmias and clinical events
Arterial thromboembolic complications
The last detected episode of AHRE was 46.7 ± 71.9 days (range 0–194) before the TE event. Of those with detected atrial burden, who suffered a TE complication, three (27.3%) were in an atrial arrhythmia at the time of diagnosis. Interestingly, four patients (36.4%) who developed TE events had no prior atrial arrhythmia recorded, including two patients from Group 2B.
Heart failure admissions
As for TE complications, there was a similar period of delay between the admission for HF and the detected atrial event (mean 47.4 ± 88.6 days, range 0–339). Moreover, 63.5% (n= 33/52) of patients had no atrial trigger recorded prior to their admission. Only seven (13.4%) patients were in an AHRE at the time of admission.
An atrial burden cut-off
Based on published analytical strategy,9 a cut-off was defined before data analysis as the observed median value of daily AHRE burden. Among those with detected AHRE the median value was 16% per day or 3.8 h and the study population was divided into three subsets: zero AHRE, low AHRE burden if below the median and high AHRE burden if above.
After adjusting for TE risk factors, Cox multivariate analysis demonstrated that a maximum AHRE longer than the median 3.8 h over a 24 h period was independently associated with TE events compared with patients with zero AHRE (HR 9.4; CI 1.8–47.0 P= 0.006). However, when comparing patients with a high AHRE burden (≥3.8 h) to those with a low burden (<3.8 h), a composite endpoint of admissions for AF or HF, or TE event or cardiovascular death was highly predictive for an AHRE burden cut-off of 3.8 h (HR 3.9 CI 1.9–7.9, P< 0.0001). (Table 3).
Table 3.
Results of hazard ratios comparing atrial high-rate events variables for each clinical outcome based on Cox regression model
| Clinical outcome | AHRE variable |
||
|---|---|---|---|
| Low AHRE vs. zero AHRE | High AHRE vs. zero AHRE | High AHRE vs. low AHRE | |
| TE | HR 4.3 | HR 9.4 | HR 2.4 |
| (CI 0.73–26.2) | (CI 1.8–47.0) | CI (0.58–9.8) | |
| P= 0.11 | P= 0.006 | P= 0.23 | |
| TE + cardiovascular death | HR 2.1 | HR 4.0 | HR 2.0 |
| (CI 0.72–6.0) | (CI 1.5–10.1) | (CI 0.73- 5.6) | |
| P= 0.17 | P= 0.004 | P= 0.18 | |
| TE + AFa+ HFb+ cardiovascular death | HR 1.0 | HR 3.8 | HR 3.9 |
| (CI 0.49–2.1) | (CI 2.3–6.3) | (CI 1.9–7.9) | |
| P= 1.0 | P< 0.0001 | P< 0.0001 | |
Low AHRE corresponds to a burden of 14 min to <3.8 h in 24 h monitoring period, high AHRE corresponds to a burden >3.8 h in a 24 h monitoring period.
aAF admissions.
bHeart failure admissions.
Discussion
Using data from two international multi-centre studies, this study has shown that the detection of atrial events by remote device monitoring is associated with TE complications in sinus rhythm patients with HF and CRT; moreover, they also have a similar TE event rate to those patients with a prior history of AF.
Compared with patients with no detected AHRE, patients with device-detected AHRE burden >3.8 h in 1 day were nine times more likely to develop TE complications and four times more likely to die from a cardiovascular cause.
In addition, those patients with 3.8 h or more of AHRE were at significantly higher risk of being admitted with AF or decompensated HF, to develop a TE complication or die from a cardiac cause when compared with patients with an AHRE burden <3.8 h. Interestingly, the majority of patients (73%) did not show a temporal association with the detected atrial episode and their adverse event, with a mean lag period of 46.7 ± 71.9 days before the TE complication.
Atrial burden studies to date
In the few studies that have assessed the clinical impact of device-detected AHRE9–16 only three have been in patients with HF and CRT, although none assessed the impact on TE event rates.10–12 Two of the studies relied on routine device interrogation.10,11 In a retrospective analysis of their single-centre experience of 161 patients with CRT devices, Caldwell et al. did not demonstrate significant difference in outcomes between patients in sinus rhythm or newly detected PAF.10 In a subsequent study by Borleff et al. 11 there was a significant difference in outcomes between the AHRE group and the sinus rhythm group, using a composite endpoint of all cardiac hospitalizations and (appropriate or inappropriate) shock therapy.
However, Santini et al.12 have recently demonstrated similar adverse outcome data to our study with the use of continuous device diagnostics in 1193 patients in sinus rhythm with CRT. They clearly showed a higher incidence of the composite endpoint of death and HF hospitalizations among patients with AT/AF during follow-up when compared with patients without AT/AF. However, no data were reported on the TE event rates.
The Mode Selection Trial (MOST) substudy13 showed that almost 50% of patients with bradycardia pacing devices and sinus node disease had at least one atrial high-rate event lasting a minimum of 5 min, and this was an independent predictor for the combined endpoint of death or non-fatal stroke. Subsequently, Glotzer et al.9 attempted to define a threshold for stroke risk in device-detected AF. They demonstrated that patients, with moderate stroke-risk factors, evidence of atrial arrhythmias >5.5 h/day in a 30-day period had a TE event rate of 2.4% per year. Although the hazard ratio of 2.2 suggested that this cut-off doubled the risk of TE event compared to the low-burden group, the confidence intervals were wide (0.96–5.05). Capucci et al. 14 reported that patients with bradycardia pacing indications and atrial events >24 h were three times more likely, after adjustment for risk factors, to have TE complications than those who had atrial arrhythmias <24 h. Similarly, in the recent ASSERT Trial (Asymptomatic AF and Stroke Evaluation in Pacemaker Patients and AF Reduction Atrial Pacing Trial), Healey et al. were able to demonstrate, in hypertensive patients receiving a dual-chamber pacemaker with no prior history of PAF, more than a doubling of embolic risk with the presence of atrial arrhythmias.15
Clinical relevance of device-detected atrial burden
Although it is accepted that the stroke risk in PAF, persistent and permanent AF17 is similar, uncertainty exists over the importance of short-duration atrial arrhythmias. While prothrombogenic electrical remodelling of the human atria may occur with AF durations >20 min18 few would consider anti-coagulation at this level. Our study supports the findings in the MOST study14 suggesting that device-detected atrial events of short duration may be as relevant as having a clinical history of PAF. Certainly, our study shows that the incidence of TE complications was similar in the group with a pre-existing AF compared with the sinus rhythm group with newly detected atrial arrhythmias.
However, the difficulty encountered for predicting a clinically relevant AHRE burden threshold, is the low TE event rate in the atrial burden studies. In a total of five atrial burden studies9,13,14,16 (including our data) totalling 4651 followed up over a median period of 18 months, there were only 51 TE events (1.1% incidence). This low event rate may be a consequence of a short follow-up period or reflect under-reporting of embolic events. Interestingly, in the relatively longer ASSERT study15 (mean 3-year follow-up) the overall rate of the TE complications was 0.72% per year.
The presence of device-detected AHRE: is it a surrogate marker of disease severity
Although, a causal link between the onset of AHRE and TE complications can be inferred in (3 of 11) 27% of patients in our study, the majority did not demonstrate a direct temporal link with both TE complications and HF admissions. Our findings correlate with the recently reported 40-patient subgroup analysis of patients with TE complications from the TRENDS study. Daoud et al.19 were able to show that in their 40 patients, who demonstrated atrial episodes prior to a TE event, 29 (73%) did not have an atrial arrhythmia in the 30 days prior to a TE event; a similar incidence as shown in our study. They go on to postulate that there may be other mechanisms, such as vascular disease risk factors, other than cardioembolism, which may come into play. Others have suggested that patients with device-detected atrial arrhythmias have more ‘severe underlying heart disease’ and hence higher mortality;13 while it is also well known that the development of AF is a marker of increased mortality in patients with underlying heart disease.20
Heart failure admissions
The significantly increased admissions for decompensated HF observed in Groups 1A and 2 compared with those with no prior history of AF who remained in sinus rhythm throughout the follow-up period (Group 1B) may be explained by Borleffs et al.11 observation that patients with newly detected AF post-CRT-D, had less reverse LV remodelling and no improvement in MR severity or LA size. Although our results do not demonstrate a causal relationship between the development of AF and worsening HF, our study highlights that in CRT HF patients the device detection of AHRE may, in fact, represent a marker for worsening HF or HF hospitalization. Furthermore, the excellent biventricular pacing rate, observed in all four study groups, would further reiterate that the presence of AHRE may represent a marker of increased disease state. This would counter the findings shown by Santini et al.12 who had linked the deterioration in HF with the impact of reduced biventricular pacing, due to an overriding atrial rate.
The role of anti-coagulation for device-detected atrial arrhythmias
Although our study does not define a level of atrial burden at which anti-coagulation is required it does support prior studies in considering lower levels of atrial fibrillation to be clinically significant.9–15
Following an interesting study correlating stroke risk and device-detected AHRE, Botto et al.,16 recommended anti-coagulation if patients developed 5 min or more of AF and had a CHADS2 score of >2. They also suggested that if patients experienced 24 h or more of AF despite a CHADS2 score of <1, they also should be considered for therapy. We eagerly await the results of the ongoing interventional prospective multi-centre study of remote monitoring of AF, which should provide the strongest evidence for the role of anticoagulation.21 In the worldwide survey, Lazarus retrospectively analysed 3 million transmissions in over 11 000 patients with brady pacing, implantable cardioverter defibrillator (ICD) and CRT-D devices. He reported that events were detected 2–5 months earlier than allowed by standard care and was associated with expedited anti-coagulation in 30% of patients and modification of anti-arrhythmic therapy in 42%.22
Study limitations
The major limitations were that atrial events were not verified by evaluating the electrograms and that two different AF burden-detection methods were employed. Although, all atrial rates <180 beats per minute were excluded to minimize the false-positive detection of arrhythmias, patients were included if they had an accumulative total of (1%) or 14 min of data detected per day, which would allow shorter episodes (<5 min) to be included and increase the chance of oversensing. Previously published data suggest that excluding episodes <5 min reduces the chance of oversensing.23 Also, the inclusion requirement of >70% HM data transmission and the minimum of 90 days of follow-up over the total study period may have contributed to some patients having limited amount of data for analysis.
The low frequency of TE complications introduces room for statistical error, especially when assessing for predictors of TE events and obtaining an AHRE burden cut-off. Another limitation is that we are unable to truly verify whether patients in Group 1 did in fact have previous asymptomatic AF. Furthermore, our study was not designed to assess the clinical response to CRT and this could have had an impact on the incidence of atrial arrhythmia burden. However, in the larger randomized trials CRT has not been shown to decrease the incidence of AF.24,25
Conclusions
Remote monitoring of implantable cardiac devices is a feasible and effective means of arrhythmia surveillance in HF patients. We have demonstrated that atrial arrhythmias detected by implanted devices are associated with an increased incidence of TE events, with a cut-off point of 3.8 h being associated with a significant increase in event rate. Device-detected atrial episodes may also represent a marker of disease severity or risk and hence the routine assessment of AHRE data, in patients with HF, should be considered with other diagnostic information when assessing stroke risk and considering anti-coagulation initiation. Furthermore, it should also prompt the optimization of cardioprotective HF therapy and arrhythmia management in CRT patients.
Funding
Home CARE and everesT trials were supported by BIOTRONIK SE & Co. KG (Woermannkehre 1, D-12359 Berlin, Germany), who also provided funding for the Open Access publication charges.
Acknowledgements
The authors thank Bernd Brüsehaber for his expert assistance in statistical analysis.
Conflict of interest: N.S. has received grant support from Medtronic and St Jude Medical. A.B. and J.P. are employees of Biotronik. P.O. and O.V. have no conflicts of interest. S.K.G.M. has received speaker honoraria/grant support from Biotronik, Medtronic and St Jude Medical. W.R.B. is an advisor for Biotronik. V.P. has received speaker fees and fees for proctoring from Biotronik. S.S. receives grant support and is an advisor for Biotronik.
Appendix 1
everesT Study
Evaluation of the new BIOTRONIK Resynchronization + ICD System
Objective
The international multi-centre prospective study ‘everesT’ is designed to evaluate safety and efficacy of BIOTRONIK's CRT-D device: Lumax HF-T 300 and 340, and BIOTRONIK's bipolar LV lead: Corox OTW BP Steroid and Corox OTW-S BP Steroid. This study documents first clinical experience with the handling and function of these products during the intra- and post-operative course.
Patient selection
Inclusion criteria
All the following criteria must be fulfilled to include the patient in this study:
Patient is willing and able to comply with the protocol and has provided written informed consent.
Indication for CRT
Indication for implantation of an ICD
Stable residence anticipated for 6 months after enrollment
Exclusion criteria
The patient is not eligible to enter this study, if one of the below listed exclusion criteria is fulfilled:
Planned cardiac surgical procedures within 6 months after enrollment
Life expectancy ≤ 6 months
Pregnant and breast-feeding women
Age < 18 years or otherwise missing complete contractually capability
Participation in another clinical study
For part A only: patient inclusion stopped by sponsor
For part B only: Corox OTW(-S) BP not yet available on the market.
For part B only: LV lead implanted or any failed attempt to implant an LV lead prior to enrolment
Primary endpoints
Safety and efficacy of Lumax 300/340 HF-T - Part A
Safety and efficacy of Corox OTW(-S) BP - Part B
Secondary endpoints
Observations and complications related to Lumax 300/340 HF-T (A)
Rate of inappropriate ICD therapies (A)
Shock impedance (A)
VF detection time (A)
Observations and complications related to Corox OTW(-S) BP (B)
Lead parameters (B)
Patient deaths (A/B)
Publication
Results of the study have not been published yet. But the data received and analysed during the everesT clinical study demonstrated the safety and efficacy of the Lumax HF-T CRT-D device and Corox OTW(-S) BP Steroid LV leads and supported the FDA approval of Corox BP lead.
References
- 1.National Collaborating Centre for Chronic conditions. 2006. Atrial fibrillation: national clinical guideline for the management in primary and secondary care Royal College of Physicians. Ref Type: Report.
- 2.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 3.Schaer BA, Zellweger MJ, Cron TA, Kaiser CA, Osswald S. Value of routine Holter monitoring for the detection of paroxysmal atrial fibrillation in patients with cerebral ischemic events. Stroke. 2004;35:e68–70. doi: 10.1161/01.STR.0000117568.07678.4B. [DOI] [PubMed] [Google Scholar]
- 4.Israel CW, Gronefeld G, Ehrlich JR, Li YG, Hohnloser SH. Long-term risk of recurrent atrial fibrillation as documented by an implantable monitoring device: implications for optimal patient care. J Am Coll Cardiol. 2004;43:47–52. doi: 10.1016/j.jacc.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Varma N, Epstein AE, Irimpen A, Schweikert R, Love C TRUST Investigators. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation. 2010;122:325–32. doi: 10.1161/CIRCULATIONAHA.110.937409. [DOI] [PubMed] [Google Scholar]
- 6.Purerfellner H, Gillis AM, Holbrook R, Hettrick DA. Accuracy of atrial tachyarrhythmia detection in implantable devices with arrhythmia therapies. Pacing Clin Electrophysiol. 2004;27:983–92. doi: 10.1111/j.1540-8159.2004.00569.x. [DOI] [PubMed] [Google Scholar]
- 7.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 8.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 9.Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2:474–80. doi: 10.1161/CIRCEP.109.849638. [DOI] [PubMed] [Google Scholar]
- 10.Caldwell JC, Contractor H, Petkar S, Ali R, Clarke B, Garratt CJ, et al. Atrial fibrillation is under-recognized in chronic heart failure: insights from a heart failure cohort treated with cardiac resynchronization therapy. Europace. 2009;11:1295–300. doi: 10.1093/europace/eup201. [DOI] [PubMed] [Google Scholar]
- 11.Borleffs CJ, Ypenburg C, van Bommel RJ, Delgado V, van Erven L, Schalij MJ, et al. Clinical importance of new-onset atrial fibrillation after cardiac resynchronization therapy. Heart Rhythm. 2009;6:305–10. doi: 10.1016/j.hrthm.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Santini M, Gasparini M, Landolina M, Lunati M, Proclemer A, Padeletti L, et al. Device-detected atrial tachyarrhythmias predict adverse outcome in real-world patients with implantable biventricular defibrillators. J Am Coll Cardiol. 2011;57:167–72. doi: 10.1016/j.jacc.2010.08.624. [DOI] [PubMed] [Google Scholar]
- 13.Glotzer TV, Hellkamp AS, Zimmerman J, Sweeney MO, Yee R, Marinchak R, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST) Circulation. 2003;107:1614–9. doi: 10.1161/01.CIR.0000057981.70380.45. [DOI] [PubMed] [Google Scholar]
- 14.Capucci A, Santini M, Padeletti L, Gulizia M, Botto G, Boriani G, et al. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol. 2005;46:1913–20. doi: 10.1016/j.jacc.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 15.Healey JS, Connolly SJ, Gold MR, Capucci A, Israel C, van Gelder IC, et al. The relationship between atrial high-rate episodes and stroke: the Asymptomatic Stroke and Atrial Fibrillation Evaluation in Pacemaker Patients (ASSERT) trial. Circulation. 2010;122:2220–1. 23-11-2010. Abstract 21838. [Google Scholar]
- 16.Botto GL, Padeletti L, Santini M, Capucci A, Gulizia M, Zolezzi F, et al. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol. 2009;20:241–8. doi: 10.1111/j.1540-8167.2008.01320.x. [DOI] [PubMed] [Google Scholar]
- 17.Hart RG, Pearce LA, Rothbart RM, McAnulty JH, Asinger RW, Halperin JL. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. Stroke prevention in atrial fibrillation investigators. J Am Coll Cardiol. 2000;35:183–7. doi: 10.1016/s0735-1097(99)00489-1. [DOI] [PubMed] [Google Scholar]
- 18.Sparks PB, Jayaprakash S, Mond HG, Vohra JK, Grigg LE, Kalman JM. Left atrial mechanical function after brief duration atrial fibrillation. J Am Coll Cardiol. 1999;33:342–9. doi: 10.1016/s0735-1097(98)00585-3. [DOI] [PubMed] [Google Scholar]
- 19.Daoud EG, Glotzer TV, Wyse DG, Ezekowitz MD, Hilker C, Koehler J, et al. TRENDS Investigators. The temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: a subgroup analysis of TRENDS. Heart Rhythm. 2011;8:1416–23. doi: 10.1016/j.hrthm.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 20.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 21.Ip J, Waldo AL, Lip GY, Rothwell PM, Martin DT, Bersohn MM, et al. Multicenter randomized study of anticoagulation guided by remote rhythm monitoring in patients with implantable cardioverter-defibrillator and CRT-D devices: rationale, design, and clinical characteristics of the initially enrolled cohort The IMPACT study. Am Heart J. 2009;158:364–70. doi: 10.1016/j.ahj.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Lazarus A. Remote, wireless, ambulatory monitoring of implantable pacemakers, cardioverter defibrillators, and cardiac resynchronization therapy systems: analysis of a worldwide database. Pacing Clin Electrophysiol. 2007;30(Suppl 1):S2–12. doi: 10.1111/j.1540-8159.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- 23.Pollak WM, Simmons JD, Interian A, Jr., Atapattu SA, Castellanos A, Myerburg RJ, et al. Clinical utility of intraatrial pacemaker stored electrograms to diagnose atrial fibrillation and flutter. Pacing Clin Electrophysiol. 2001;24(4 Pt 1):424–9. doi: 10.1046/j.1460-9592.2001.00424.x. [DOI] [PubMed] [Google Scholar]
- 24.Hoppe UC, Casares JM, Eiskjaer H, Hagemann A, Cleland JG, Freemantle N, et al. Effect of cardiac resynchronization on the incidence of atrial fibrillation in patients with severe heart failure. Circulation. 2006;114:18–25. doi: 10.1161/CIRCULATIONAHA.106.614560. [DOI] [PubMed] [Google Scholar]
- 25.Saxon LA. Does cardiac resynchronization therapy reduce the incidence of atrial fibrillation, and does atrial fibrillation compromise the cardiac resynchronization therapy effect? Heart Rhythm. 2007;4(3 Suppl):S31–3. doi: 10.1016/j.hrthm.2006.12.026. [DOI] [PubMed] [Google Scholar]