Abstract
Objective:
Subthalamic nucleus deep brain stimulation (STN-DBS) is an effective treatment for advanced Parkinson disease (PD). Following STN-DBS, speech intelligibility can deteriorate, limiting its beneficial effect. Here we prospectively examined the short- and long-term speech response to STN-DBS in a consecutive series of patients to identify clinical and surgical factors associated with speech change.
Methods:
Thirty-two consecutive patients were assessed before surgery, then 1 month, 6 months, and 1 year after STN-DBS in 4 conditions on- and off-medication with on- and off-stimulation using established and validated speech and movement scales. Fifteen of these patients were followed up for 3 years. A control group of 12 patients with PD were followed up for 1 year.
Results:
Within the surgical group, speech intelligibility significantly deteriorated by an average of 14.2% ± 20.15% off-medication and 16.9% ± 21.8% on-medication 1 year after STN-DBS. The medical group deteriorated by 3.6% ± 5.5% and 4.5% ± 8.8%, respectively. Seven patients showed speech amelioration after surgery. Loudness increased significantly in all tasks with stimulation. A less severe preoperative on-medication motor score was associated with a more favorable speech response to STN-DBS after 1 year. Medially located electrodes on the left STN were associated with a significantly higher risk of speech deterioration than electrodes within the nucleus. There was a strong relationship between high voltage in the left electrode and poor speech outcome at 1 year.
Conclusion:
The effect of STN-DBS on speech is variable and multifactorial, with most patients exhibiting decline of speech intelligibility. Both medical and surgical issues contribute to deterioration of speech in STN-DBS patients.
Classification of evidence:
This study provides Class III evidence that STN-DBS for PD results in deterioration in speech intelligibility in all combinations of medication and stimulation states at 1 month, 6 months, and 1 year compared to baseline and to control subjects treated with best medical therapy.
Speech is affected in the majority of patients with Parkinson disease (PD) at some stage of the disease1 with a variable presentation of weak voice, monotony of pitch and loudness, short rushes of speech, and imprecise consonants. Deep brain stimulation in the subthalamic nucleus (STN-DBS) is an effective treatment for patients with advanced PD who develop motor fluctuations but its impact on speech can be variable.2,3 Speech deterioration can become a barrier toward fully utilizing the motor benefits of the procedure.4
In recent reports, using speech item 18 of the motor part of the Unified Parkinson's Disease Rating Scale (UPDRS-III),5 dysarthria has been reported with a variable prevalence from 1% at 6 months6 to 56% at 1 year7,8 and 69.7% at 3 years follow-up,9 with an average of 9.3%, according to a meta-analysis of 34 articles.10 Improvement of nonspeech articulatory and phonatory measures has also been reported.11 These conflicting results are described among selected patients and without preoperative data.
Speech motor outcome following STN-DBS could depend on clinical and surgical characteristics specific to individuals. Spread of current to adjacent structures has also been implicated.12,13 The site of stimulation within the STN area and its relationship to speech motor control has not been systematically discussed. Clinical factors, such as age, stage of disease, severity of dysarthria before surgery, and speech response to medication, have not been examined for their relationship with speech changes after surgery. This study aims to examine the short- and long-term speech response to STN-DBS prospectively in a consecutive series of patients to identify clinical and surgical factors associated with speech change.
METHODS
Patients.
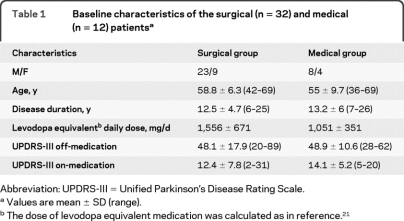
Thirty-two consecutive patients, implanted with bilateral STN-DBS electrodes between 2005 and 2006, were included in the study (henceforth “surgical group”). All 32 were followed up for 1 year, and 15 were followed up for 3 years. Twelve patient candidates for DBS, who had been randomized to 1-year medical treatment as part of a separate study, were used as a control group (henceforth “medical group”) (table 1).
Table 1.
Baseline characteristics of the surgical (n = 32) and medical (n = 12) patientsa
Abbreviation: UPDRS-III = Unified Parkinson's Disease Rating Scale.
Values are mean ± SD (range).
The dose of levodopa equivalent medication was calculated as in reference.21
Standard protocol approvals, registrations, and patient consents.
The study was approved by the National Hospital for Neurology and Neurosurgery and the Institute of Neurology Joint Research Ethics Committee (ref. number: 03/N138). Informed consent was received by all patients participating in the study.
Surgical procedure and contact localization.
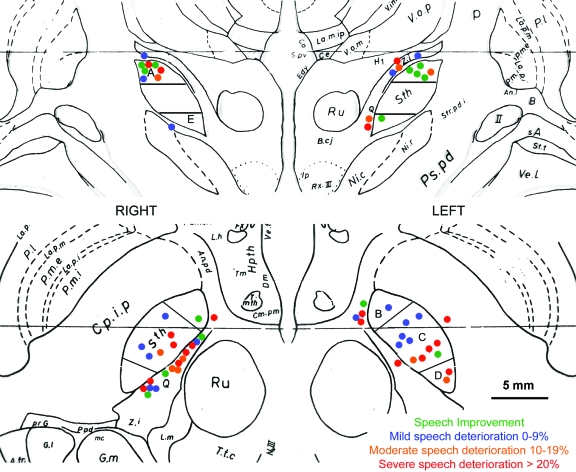
Surgery was performed as previously described.14,15 Preoperative stereotactic MRI using T2-weighted, fast-acquisition sequences were obtained in all patients.14 The subthalamic target was visualized on MRI and directly targeted using planning software (FrameLink4TM, Version 2003, Medtronic®, Minneapolis, MN). Dynamic impedance monitoring and electrical stimulation combined with clinical assessment were used to provide an indication of the target. Patients were thus implanted bilaterally with a quadripolar DBS electrode (Model 3389 DBS lead, Medtronic). Postoperative stereotactic MRI were imported into the planning software allowing 3-dimensional reconstruction of the images along the electrode trajectory (Framelink, Medtronic). Stereotactic localization of the 4 electrode contacts was performed using a template superimposed on the electrode artifact.15 The coordinates of each contact were transposed onto the preoperative stereotactic MRI.14 The targeting accuracy, defined as the perpendicular distance from the electrode trajectory to the intended target coordinates, was calculated geometrically from the stereotactic images. Three neurosurgeons (L.Z., E.H., E.P.), blinded to the results of STN-DBS on speech, independently assessed and agreed on the anatomic position of each contact in relation to the visualized STN in the axial and coronal planes. The visualized STN was subdivided into 5 segments: superior (A), anterior-medial (B), central (C), posterolateral (D), and inferior (E). Each contact was also classified as being inside, superior, medial, inferior, or lateral to the STN and its surrounding structures (figure 1).
Figure 1. Location of the active contacts at 1 year post bilateral subthalamic nucleus deep brain stimulation (STN-DBS).
Location of the active contacts in 31 patients at 1 year post bilateral STN-DBS as transposed onto the Schaltenbrand atlas adopting the radiologic imaging convention (right STN on the left side of the image). Top: coronal view adapted from plate 27, f.p. 3.0. Contacts related to the superior (A) and inferior (E) segment of the STN are shown. The middle section of the STN in coronal view is further subdivided into 3 segments in the axial plane shown below. Therefore, the coronal and axial views show different contacts. Bottom: axial view adapted from plate 55, H.v. 4.5. Contact location is shown in relation to the anteromedial (B), central (C), and posterolateral (D) segments of the STN. Selected abbreviations: Ru = red nucleus; Sth = subthalamic nucleus; Z.i. = zona incerta. (Scaltenbrand G, Wahren W. Atlas for Stereotaxy of the Human Brain, 2nd ed. New York: Thieme; 1977. Plates 27;55. Reprinted by permission.)
Patient evaluation.
Patients were assessed after overnight withdrawal of medication at baseline and post bilateral STN-DBS at 1 month, 6 months, 1 year (n = 32), and 3 years (n = 15) in all 4 medication and stimulation conditions. Evaluations were carried out on the same day for each patient and in the same order. The on-medication/on-stimulation assessment took place 1 hour after the administration of a suprathreshold dose of levodopa. One patient could not be assessed without medication at 1 year, and 3 patients were not taking any medication at 1 year.
Speech assessment consisted of sustained vowel phonation /a:/ for 3 repetitions, a 60-second monologue, and the Assessment of Intelligibility for Dysarthric Speech.16 The Assessment of Intelligibility for Dysarthric Speech is the most widely used standardized test for measuring speech intelligibility and requires the patient to read 22 sentences, each of 5 to 15 words in length, for a total of 220 words. The intelligibility score is the percentage of words correctly understood by a native English speaker, blinded to the conditions. The Computerized Speech Lab (4150, Kay Pentax) was used for recording and analysis of all samples. Movement was assessed using the UPDRS-III.
Data analysis.
Acoustic recordings and analysis were performed as previously described.17,18 The files from the sentence intelligibility test for both groups were rated blindly (E.F.), and a percentage of speech intelligibility was derived by scoring the number of words correctly understood. For the acoustical analysis of intensity of sustained phonation, reading, and monologue, the mean vocal sound pressure level (SPL dB) measure was calculated from the speech recording of each condition.17,18 We also calculated the long-term average spectrum (LTAS), a fast-Fourier transform–generated power spectrum of frequencies represented in the acoustic voice signal. By averaging across all speech sounds, the LTAS provides insights into the function of the voice and the movement of the articulators in connected speech.19,20 For the LTAS analysis, the window length was 8,192 points and the statistical moments were calculated automatically by Computerized Speech Lab for the entire frequency range of 0 to 22 KHz.
UPDRS-III subscores were divided as follows: rigidity (item 22, range 0–20), tremor (items 20–21, range 0–28), axial symptoms (items 27–30, range 0–16), speech (item 18, range 0–4), and akinesia (items 23–26, range 0–32).
Statistical analysis.
The primary outcome was the change of speech intelligibility from baseline to 12 months in the surgical group. In addition, a linear regression mixed effects model was used to assess the overall effect of time on the measurements. For this analysis, patients were declared as random effects, with time as the fixed effect to be estimated. Independent t tests were used to compare the change in speech intelligibility between the surgical and medical groups. Acoustic data and motor scores were the secondary outcomes. Continuous variables are presented using mean ± SD. Linear regression analysis was used to explore the relationship between preoperative clinical factors and speech outcome with the outcome for the regression analysis being the change in speech intelligibility over 1 year of STN-DBS (one year off-medication/on-stimulation minus preoperative off-medication, negative score denotes deterioration). Univariate analysis of variance (with 2 factors: anatomic description and STN segment) was used to explore the impact of contact location on speech outcome. Statistical analysis was performed on SPSS-16 for Mac (SPSS, Chicago, IL) and Prism 5 for Mac (GraphPad software, Inc.) and was supervised by a statistician (M.R.). This study provides Class III evidence that speech intelligibility deteriorates significantly following 1 year of bilateral STN-DBS.
RESULTS
Surgical group: Speech and motor function at 1 year.
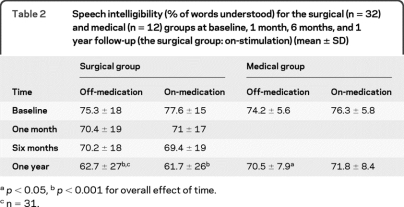
Speech intelligibility (using the Assessment of Intelligibility for Dysarthric Speech) deteriorated on average by 14.2% ± 20.1% (p < 0.001) after 1 year of STN stimulation when the patients were off-medication/on-stimulation compared to off-medication state preoperatively, and by 16.9% ± 21.8% (p < 0.01) when the patients were on-medication/on-stimulation compared to on-medication state preoperatively (table 2). There was substantial variability. Speech intelligibility deteriorated in 25 patients (range −77% to −3%) and improved in 7 patients (range 2% to 17%). UPDRS-III speech item 18 identified only 12 patients with speech deterioration. There was a significant change of speech intelligibility between 6 months and 1 year but not between baseline and 6 months. When off-medication/off-stimulation, speech intelligibility deteriorated on average by 12.6% ± 16.6% (p < 0.001) compared to off-medication preoperatively. Switching the stimulation off improved speech intelligibility by 1.5% compared to the off-medication/on-stimulation condition.
Table 2.
Speech intelligibility (% of words understood) for the surgical (n = 32) and medical (n = 12) groups at baseline, 1 month, 6 months, and 1 year follow-up (the surgical group: on-stimulation) (mean ± SD)
p < 0.05,
p < 0.001 for overall effect of time.
n = 31.
In terms of speech subsystems, loudness (SPL dB) increased by 7.4 dB for read sentences (p < 0.0001), by 7.2 dB for monologue (p < 0.0001), and by 9.9 dB for phonation (p < 0.0001) in the off-medication/on-stimulation condition at 1 year (table e-1 on the Neurology® Web site at www.neurology.org). There was an increase of the LTAS means for reading (p < 0.05) and phonation (p < 0.001) for the 1-year off-medication/on-stimulation condition (table e-2).
Mean off-medication motor score as measured by the UPDRS-III improved with STN stimulation from 47.3 ± 17.8 before surgery to 20.5 ± 11.19 at 6 months (p < 0.0001) (56.6% improvement) and 23.3 ± 11.6 at 1 year (p < 0.0001) (50.7% improvement). On-medication scores did not significantly change over 1 year. UPDRS-III score was 12.4 ± 7.8 preoperatively, 10.1 ± 7.4 at 6 months, and 13.9 ± 9.6 at 1 year. The levodopa equivalent daily dose (LEDD) was reduced over 1 year from 1,556 ± 671 mg to 744 ± 494 mg (52%, p < 0.0001). The mean (SD) targeting electrode accuracy was 1.3 (0.6) mm. The mean amplitude of stimulation at 6 months was 2.9 ± 0.7 V for the left electrode and 3.0 ± 0.6 V for the right electrode with mean frequency 133 Hz and mean pulse width 61 μs. The mean amplitude at 1 year was 3.1 ± 0.8 V for the left electrode and 3.2 ± 0.5 V for the right electrode with the same frequency and pulse width. There was no significant difference between right and left electrode settings at 6 months and 1 year.
Medical group: Speech function at 1 year.
Speech intelligibility in the medical group (n = 12) declined by 3.6% (p < 0.05) in the off-medication condition and 4.5% in the on-medication condition (table 2). This decline was not smaller than the one of the surgical group (p = 0.06 for the change on-medication and 0.08 for off-medication). Eight out of 12 patients (66%) had some degree of speech worsening (range −13% to −2%). For the medical group, loudness did not change significantly at 1 year with or without medication in any of the speech tasks. Similarly, LTAS means did not significantly increase over 1 year apart from the off-medication reading task.
Surgical group: Speech function at 3 years.
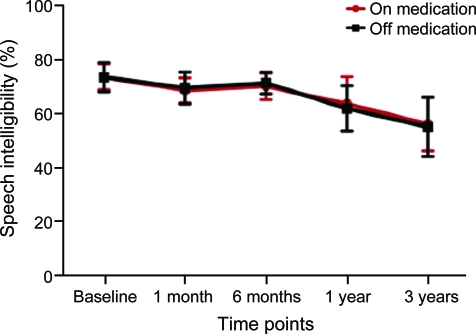
Fifteen patients out of the 32 initial patients were followed up for 3 years (table e-3). Out of these, one patient could not be assessed without medication and one patient did not take any medication at 3 years (figure 2). The average deterioration in the off-medication/on-stimulation condition from year 1 (69.07% ± 26.1%) to year 3 (59.5% ± 36.8%) was 10%. The amplitude of stimulation at 3 years (3.2 V left, 3.5 V right) was not significantly different from year 1.
Figure 2. Speech intelligibility at 3 years.
Speech intelligibility (% of words understood) for the surgical group (n = 15) at baseline, 1 month, 6 months, 1 year, and 3 years post bilateral subthalamic nucleus deep brain stimulation (on-stimulation) (mean ± SEM).
Factors associated with speech deterioration following 1 year of STN-DBS.
There was an association between the UPDRS-III on-medication preoperative motor score and speech response to STN-DBS at 1 year (coefficient −0.97, p = 0.03). Patients with a better on-medication motor score had better speech intelligibility 1 year after STN-DBS in the off-medication/on-stimulation condition, but there was no association with specific UPDRS-III subscores (i.e., tremor, akinesia, rigidity, axial symptoms). No association was found between age, sex, and time since diagnosis with speech response either. Analysis of the acoustical data showed that higher LTAS means in the on-medication condition, before surgery, for the read sentences (coefficient 0.07, p = 0.04) and monologue (coefficient 0.07, p = 0.03) were associated with better speech 1 year after STN-DBS.
Univariate analysis of variance showed that contacts positioned inside the left STN (n = 19) had less detrimental effect on speech intelligibility (mean speech change −7.89% ± 16.1) than those positioned medial to it (n = 11) (mean change −22.4% ± 22.4, p = 0.016). Comparison of the different stimulated segments inside the STN showed that speech was on average improved for the contacts in the superior part of the STN (+6.6% ± 11.2) rather than the posterolateral part (−31% ± 22.3, p = 0.014). For the right electrode, there was no significant difference between STN segment or anatomic localization and speech change (table e-4).
There was a strong relationship between the amplitude of stimulation in the left brain and speech intelligibility in the off-medication/on-stimulation condition at 1 year (coefficient −16.1, 95% confidence interval [CI] −26.8 to −5.4, p = 0.007, R2 = 0.24), as well as the mean speech change over 1 year of stimulation (coefficient −11.3, 95% CI −19.3 to −3.2, p = 0.007, R2 = 0.22). The higher the voltage needed in the left brain the worse speech was at 1 year and the worse the speech change over that year. Similarly, higher voltage was associated with worse speech in the on-medication/on-stimulation condition (p = 0.02, R2 = 0.1871 for the speech change over 1 year and p = 0.0076, R2 = 0.2521 for speech at 1 year). There was a less strong relationship between the amplitude of the right electrode and speech at 1 year in the off-medication/on-stimulation condition (−18.7, 95% CI −36.7 to −0.6, p = 0.042, R2 = 0.13) and no relationship with speech change over a year. Contacts medial to the left STN required significantly more voltage than the contacts inside the left STN. There was no such difference in voltage amplitude for the contacts inside or medial to the right STN.
Reduction in levodopa (LEDD at 1 year − LEDD preoperatively) and the amount of levodopa at 1 year was not associated with speech outcome.
DISCUSSION
Speech intelligibility deteriorated 1 year after STN-DBS in 78% of patients in contrast with the marked 50.7% improvement in parkinsonian motor symptoms. This percentage is higher than most clinical series in the literature. Nevertheless, most series have focused on the motor benefit and speech has mostly been assessed by item 18 of the UPDRS, which shows poor sensitivity to detecting speech problems22 and indeed identified only 12 patients (38%) with speech deterioration in our sample. Disease progression in the medical group accounted for only a 3.6% deterioration of speech intelligibility off-medication and 4.5% on-medication over 1 year, as reported before.23 The majority of the speech deterioration in the surgical group occurred between 6 months and 1 year. This was not alleviated by switching the stimulation off. Also, voltage was not significantly increased between 6 months and 1 year. At 3 years, 53% (95% CI 25 to 81) of patients showed speech deterioration in the off-medication/on-stimulation condition and 73% (95% CI 42 to 92) of patients in the on-medication/on-stimulation condition.
Of the clinical factors studied, only the preoperative on-medication UPDRS-III motor score was associated with speech change at 1 year of STN-DBS. This is consistent with the studies on predictive factors for movement improvement after STN-DBS.10,24 The fact that the severity of the residual parkinsonian motor score in the on-medication condition was predictive of a poor speech postoperative outcome is probably explained by the presence of nondopaminergic pathology25 and it is corroborated by the limited effect of levodopa on speech.26,27 Speech response was not improved by administration of levodopa before or after STN-DBS. Indeed, for some patients, speech was worse on-medication/on-stimulation, as reported earlier.8,12 The nature of this worsening was not linked to increased dyskinesias and would require further investigation. Postoperatively, the amount of reduction of levodopa was not associated with speech deterioration either.
Speech outcome was not linked to either age or disease duration, unlike motor outcome.24,28,29 Indeed, speech problems may arise at any stage of the disease process and are not necessarily related to the degree of motor disability.30
Interestingly, speech intelligibility before surgery, off- or on-medication, was not a predictive factor of speech intelligibility at 1 year after surgery. Higher LTAS means in both reading and monologue when on-medication was a predictive factor of good speech outcome. Dromey19 examined the use of a number of acoustical variables to describe PD speech and concluded that lower LTAS means was the variable that differentiated PD speech most from that of normal controls. However, the relationship between these acoustic measures and perceptual judgments is still not clear.31
Systematic evaluation of the anatomic location of the electrode contact and its effects on speech showed that electrodes placed medial to the left STN were worse for speech intelligibility than electrodes inside the STN, confirming results from other studies.32–34 Equally, information on the particular STN segment in which the active contact is located and speech outcome is scarce. In our study, stimulation in the left superior segment of the STN improved speech by 6.6% over a year compared to a deterioration of 31% from stimulation in the left posterolateral segment (figure 1). Despite the different methodology of electrode localization, stimulation of this same superior segment (sometimes referred to as “dorsal STN” in the literature) is reported to be more effective for limb motor control.35,36 Improvement in both speech and motor control from stimulation of this segment compared to improvement predominantly in motor control with stimulation of the posterolateral segment may have implications for surgeons when targeting the STN. However, the number of electrodes in each of the 5 segments inside the STN was too limited to make firm conclusions about their effects on speech. Higher voltage on the left STN at 1 year was also associated with speech deterioration. The worsening effect of higher voltage has been described before.12,17 Some studies have attributed this deterioration to the spread of current to the internal capsule.12,13 In our study, the strong association of a medial contact and higher voltage with poor speech outcome suggests that speech deterioration may be due to current spread to the cerebellothalamic tract.17 It also points to the preponderance of good contact localization for both speech and motor outcome: medially placed contacts needed higher voltage for control of movement, which in turn affects speech negatively. The stronger association of the left STN contact with speech response also conforms to the findings from other studies.32
There was a discrepancy between deterioration in speech intelligibility and improvement in loudness; this is contrary to other studies in PD dysarthria, where increased loudness is associated with increased speech intelligibility.37 Our finding supports similar results from the limb motor literature on the effects of STN-DBS, which show an increase in force production but deterioration on more complex movement.38 So far, evidence of impaired performance following STN-DBS has been limited to selected cognitive tasks and complex manual tasks.39,40 Speech is a uniquely human complex ability requiring fast and precise movement under constantly changing circumstances.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Laura Strong and Mark Rachovides for comments on the manuscript.
- CI
- confidence interval
- LEDD
- levodopa equivalent daily dose
- LTAS
- long-term average spectrum
- PD
- Parkinson disease
- SPL
- sound pressure level
- STN-DBS
- subthalamic nucleus deep brain stimulation
- UPDRS-III
- Unified Parkinson's Disease Rating Scale
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Michael Roughton.
DISCLOSURE
E. Tripoliti has received research support from Medtronic, Inc. and the Parkinson's Disease Society. Dr. Zrinzo has received funding for travel and speaker honoraria from Medtronic, Inc. and St. Jude Medical and has received research support from Parkinson's Appeal. Dr. Martinez-Torres has received research support from the Fondo de Inversion Sanitaria (FIS), Health Institute Carlos III, Spanish Department of Science and Innovation (FI08/00108), and the Fundacion Caja Madrid. E. Frost reports no disclosures. Dr. Pinto receives research support from Fondation Fyssen (France). Dr. Foltynie has served on a scientific advisory board for Solvay Pharmaceuticals, Inc.; has received funding for travel from Teva Pharmaceutical Industries Ltd.; has received speaker honoraria from Teva Pharmaceutical Industries Ltd., GlaxoSmithKline, and Orion Corporation; and has received research support from the Parkinson's Disease Society and the Brain Research Trust. Dr. Holl has received funding for travel and speaker honoraria from Medtronic, Inc. Dr. Petersen and M. Roughton report no disclosures. Prof. Hariz has received funding for travel and speaker honoraria from Medtronic, Inc. Dr. Limousin served on the editorial board of Movement Disorders.
REFERENCES
- 1. Logemann JA, Fisher HB, Boshes B, Blonsky ER. Frequency and cooccurrence of vocal tract dysfunctions in the speech of a large sample of Parkinson patients. J Speech Hear Disord 1978;43:47–57 [DOI] [PubMed] [Google Scholar]
- 2. Limousin P, Krack P, Pollak P, et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med 1998;339:1105–1111 [DOI] [PubMed] [Google Scholar]
- 3. Deuschl G, Herzog J, Kleiner-Fisman G, et al. Deep brain stimulation: postoperative issues. Mov Disord 2006;21:S219–S237 [DOI] [PubMed] [Google Scholar]
- 4. Hariz MI, Johansson F, Shamsgovara P, Johansson E, Hariz GM, Fagerlund M. Bilateral subthalamic nucleus stimulation in a parkinsonian patient with preoperative deficits in speech and cognition: persistent improvement in mobility but increased dependency: a case study. Mov Disord 2000;15:136–139 [DOI] [PubMed] [Google Scholar]
- 5. Fahn S, Elton RL, UPDRS Program Members Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, eds. Recent Developments in Parkinson's Disease, vol. 2. Florham Park, NJ: Macmillan Healthcare Information; 1987:153–163, 293–304 [Google Scholar]
- 6. The Deep Brain Stimulation For Parkinson's Disease Study Group Deep brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease. N Engl J Med 2001;345:956–963 [DOI] [PubMed] [Google Scholar]
- 7. Thobois S, Mertens P, Guenot M, et al. Subthalamic nucleus stimulation in Parkinson's disease: clinical evaluation of 18 patients. J Neurol 2002;249:529–534 [DOI] [PubMed] [Google Scholar]
- 8. Volkmann J, Allert N, Voges J, Weiss PH, Freund HJ, Sturm V. Safety and efficacy of pallidal or subthalamic nucleus stimulation in advanced PD. Neurology 2001;56:548–551 [DOI] [PubMed] [Google Scholar]
- 9. Piboolnurak P, Lang AE, Lozano AM, et al. Levodopa response in long-term bilateral subthalamic stimulation for Parkinson's disease. Mov Disord 2007;22:990–997 [DOI] [PubMed] [Google Scholar]
- 10. Kleiner-Fisman G, Herzog J, Fisman DN, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord 2006;21(suppl 14):S290–S304 [DOI] [PubMed] [Google Scholar]
- 11. Klostermann F, Ehlen F, Vesper J, et al. Effects of subthalamic deep brain stimulation on dysarthrophonia in Parkinson's disease. J Neurol Neurosurg Psychiatry 2008;79:522–529 [DOI] [PubMed] [Google Scholar]
- 12. Krack P, Batir A, Van BN, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med 2003;349:1925–1934 [DOI] [PubMed] [Google Scholar]
- 13. Tommasi G, Krack P, Fraix V, et al. Pyramidal tract side effects induced by deep brain stimulation of the subthalamic nucleus. J Neurol Neurosurg Psychiatry 2008;79:813–819 [DOI] [PubMed] [Google Scholar]
- 14. Hariz MI, Krack P, Melvill R, et al. A quick and universal method for stereotactic visualization of the subthalamic nucleus before and after implantation of deep brain stimulation electrodes. Stereotact Funct Neurosurg 2003;80:96–101 [DOI] [PubMed] [Google Scholar]
- 15. Yelnik J, Bardinet E, Dormont D, et al. A three-dimensional, histological and deformable atlas of the human basal ganglia: I: atlas construction based on immunohistochemical and MRI data. Neuroimage 2007;34:618–638 [DOI] [PubMed] [Google Scholar]
- 16. Yorkston K, Beukelman D. Assessment of Intelligibility of Dysarthric Speech. Austin: Pro-ed, CC Publications; 1984 [Google Scholar]
- 17. Tripoliti E, Zrinzo L, Martinez-Torres I, et al. Effects of contact location and voltage amplitude on speech and movement in bilateral subthalamic nucleus deep brain stimulation. Mov Disord 2008;23:2377–2383 [DOI] [PubMed] [Google Scholar]
- 18. Tripoliti E, Limousin P, Tisch S, Borrell E, Hariz M. Speech in Parkinson's disease following subthalamic nucleus deep brain stimulation: preliminary results. J Med Speech Lang Pathol 2006;14:309–315 [Google Scholar]
- 19. Dromey C. Spectral measures and perceptual ratings of hypokinetic dysarthria. J Med Speech Lang Pathol 2003;11:85–94 [Google Scholar]
- 20. Duffy J. Motor Speech Disorders: Substrates, Differential Diagnosis, and Management, 2nd ed. Scottsdale, AZ: Elsevier Health Sciences; 2005 [Google Scholar]
- 21. Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain 2007;130:1787–1798 [DOI] [PubMed] [Google Scholar]
- 22. Richards M, Marder K, Cote L, Mayeux R. Interrater reliability of the Unified Parkinson's Disease Rating Scale motor examination. Mov Disord 1994;9:89–91 [DOI] [PubMed] [Google Scholar]
- 23. Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA 2009;301:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Welter ML, Houeto JL, Tezenas du MS, et al. Clinical predictive factors of subthalamic stimulation in Parkinson's disease. Brain 2002;125:575–583 [DOI] [PubMed] [Google Scholar]
- 25. Agid Y. Parkinson's disease: pathophysiology. Lancet 1991;337:1321–1324 [DOI] [PubMed] [Google Scholar]
- 26. Critchley EM. Speech disorders of Parkinsonism: a review. J Neurol Neurosurg Psychiatry 1981;44:751–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Letter M, Santens P, Van Borsel J. The effects of levodopa on tongue strength and endurance in patients with Parkinson's disease. Acta Neurol Belg 2003;103:35–38 [PubMed] [Google Scholar]
- 28. Charles PD, Van BN, Krack P, et al. Predictors of effective bilateral subthalamic nucleus stimulation for PD. Neurology 2002;59:932–934 [DOI] [PubMed] [Google Scholar]
- 29. Guehl D, Cuny E, Benazzouz A, et al. Side-effects of subthalamic stimulation in Parkinson's disease: clinical evolution and predictive factors. Eur J Neurol 2006;13:963–971 [DOI] [PubMed] [Google Scholar]
- 30. Metter EJ, Hanson WR. Clinical and acoustical variability in hypokinetic dysarthria. J Commun Disord 1986;19:347–366 [DOI] [PubMed] [Google Scholar]
- 31. Tanner K, Roy N, Ash A, Buder EH. Spectral moments of the long-term average spectrum: sensitive indices of voice change after therapy? J Voice 2005;19:211–222 [DOI] [PubMed] [Google Scholar]
- 32. Santens P, De Letter M, Van Borsel J, De Reuck J, Caemaert J. Laterised effects of subthalamic nucleus stimulation on different aspects of speech in Parkinson's disease. Brain Lang 2003;87:253–258 [DOI] [PubMed] [Google Scholar]
- 33. Plaha P, Ben-Shlomo Y, Patel NK, Gill SS. Stimulation of the caudal zona incerta is superior to stimulation of the subthalamic nucleus in improving contralateral parkinsonism. Brain 2006;129:1732–1747 [DOI] [PubMed] [Google Scholar]
- 34. Paek SH, Han JH, Lee JY, Kim C, Jeon BS, Kim DG. Electrode position determined by fused images of preoperative and postoperative magnetic resonance imaging and surgical outcome after subthalamic nucleus deep brain stimulation. Neurosurgery 2008;63:925–936 [DOI] [PubMed] [Google Scholar]
- 35. Hamel W, Fietzek U, Morsnowski A, et al. Subthalamic nucleus stimulation in Parkinson's disease: correlation of active electrode contacts with intraoperative microrecordings. Stereotact Funct Neurosurg 2003;80:37–42 [DOI] [PubMed] [Google Scholar]
- 36. Yokoyama T, Ando N, Sugiyama K, Akamine S, Namba H. Relationship of stimulation site location within the subthalamic nucleus region to clinical effects on parkinsonian symptoms. Stereotact Funct Neurosurg 2006;84:170–175 [DOI] [PubMed] [Google Scholar]
- 37. Neel A. Effects of loud and amplified speech on sentence and word intelligibility in Parkinson disease. J Speech Lang Hear Res 2009;52:1021–1033 [DOI] [PubMed] [Google Scholar]
- 38. Brown P, Eusebio A. Paradoxes of functional neurosurgery: clues from basal ganglia recordings. Mov Disord 2008;23:12–20 [DOI] [PubMed] [Google Scholar]
- 39. Brown P, Chen CC, Wang S, et al. Involvement of human basal ganglia in offline feedback control of voluntary movement. Curr Biol 2006;16:2129–2134 [DOI] [PubMed] [Google Scholar]
- 40. Jahanshahi M, Ardouin CM, Brown RG, et al. The impact of deep brain stimulation on executive function in Parkinson's disease. Brain 2000;123:1142–1154 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.