Abstract
Although it is well known that damage to neurons results in release of substances that inhibit axonal growth, release of chemical signals from damaged axons that attract axon growth cones has not been observed. In this study, a 532 nm 12 ns laser was focused to a diffraction-limited spot to produce site-specific damage to single goldfish axons in vitro. The axons underwent a localized decrease in thickness (‘thinning’) within seconds. Analysis by fluorescence and transmission electron microscopy indicated that there was no gross rupture of the cell membrane. Mitochondrial transport along the axonal cytoskeleton immediately stopped at the damage site, but recovered over several minutes. Within seconds of damage nearby growth cones extended filopodia towards the injury and were often observed to contact the damaged site. Turning of the growth cone towards the injured axon also was observed. Repair of the laser-induced damage was evidenced by recovery of the axon thickness as well as restoration of mitochondrial movement. We describe a new process of growth cone response to damaged axons. This has been possible through the interface of optics (laser subcellular surgery), fluorescence and electron microscopy, and a goldfish retinal ganglion cell culture model.
Keywords: laser ablation, growth cone, axon, axonal injury, repair, guidance cue
1. Introduction
Using lasers, whole cells and intracellular organelles can be altered with spatial and temporal precision at the micro, nano and femtosecond scale levels. This is possible because of the spectral purity and short exposure time of a laser beam that is focused to a diffraction-limited spot by a high numerical aperture (NA = 1.3–1.4) microscope objective [1–3]. Such systems have been used to perform nanosurgery on neurons in organisms such as Caenorhabditis elegans [4,5] and cricket larvae [6], as well as in individual neurons growing in culture [7,8]. Through precise control of the laser parameters, it is possible to cause either complete transection of neurites extending from neurons in culture or localized sub-axotomy damage from which the neurites recover [8]. In a recent study, axons from embryonic rat cortical neurons were grown as oriented bundles using a patterned substrate and were completely severed using a 532 nm 180 ps laser microbeam [9]. Subsequent dieback and regeneration of these axons were studied. However, in that study, only complete transection was performed and the damage could not be resolved on the level of single axons. In the study reported here, we (i) produce highly localized sub-axotomy damage to single axons, (ii) analyse the damage and recovery by fluorescence microscopy and transmission electron microscopy (TEM), and (iii) demonstrate that growth cones respond by turning towards the damage site and that they often extend filopodia towards that site often contacting it directly. The ability to study the response of growth cones to localized damage on a single axon is novel and opens up opportunities not only for basic research on individual axons, but also for studies on repair and regeneration that will impact our understanding of traumatic brain and spinal cord injury.
Traumatic brain injury (TBI), stroke and other insults to the central nervous system (CNS) result in the rapid release of excitotoxins and other substances from neurons and glia that can kill or damage neurons [10–12]. With time, astroglial cells produce an assortment of substances that affect neuronal survival and growth [13]. Though many of these, such as chrondroitin sulphate proteoglycans, are inhibitory to axonal growth and prevent brain repair, others like neuronotrophic factors are beneficial and promote neuronal survival and recovery [14–16]. Since axonal growth has been shown to be directed by many guidance molecules such as netrins, slits, ephrins, semaphorins, Wnts and Shh, some of which are diffusible [17–19], it is not unreasonable to suppose that molecules could be released following neuronal damage that could direct axonal growth although this has not been previously reported. These or other growth-directing molecules might be responsible for the growth cone responses observed in the studies we report here. The interface of laser microsurgery, fluorescence and TEM with an in vitro nerve culture system to study reversible axonal damage and the response of growth cones to this damage is an interdisciplinary approach to probe important questions relating to nerve cell damage and repair.
2. Material and methods
2.1. Primary culture
Retinal explants of the common goldfish, Carassius auratus, were cultured under conditions that support the selective outgrowth of retinal ganglion cell axons onto the substrate as previously described [20,21]. In brief, adult fish received a priming lesion of the optic nerve 7–9 days before retinal removal. Retinas were removed from eyes immediately after sacrifice and cut into 400 µm square explants on a McIlwain tissue chopper. For ease of observation of growth cones, only a few (approx. 5) explants were transferred into each sterile glass-bottomed 35 mm Petri dish (Mat-Tek, WPI), which were previously coated with 0.75 mg per dish poly-d-lysine (Sigma-Aldrich) and 5 µg per dish of laminin (Collaborative Research). Explants were oriented with the retinal ganglion cell layer towards the substrate. The explants were incubated at room air and temperature in Leibovitz's L15 medium (Invitrogen) supplemented with 10 per cent foetal bovine serum (FBS, Invitrogen) and 50 µg ml−1 gentamicin (Sigma-Aldrich).
2.2. Laser ablation system
The laser ablation system employed a 12 ns-pulsed 532 nm second harmonic Nd:YVO4 laser (Prisma, Coherent) that was coupled to the microscope through the epi-fluorescence port. The beam was steered using a fast steering mirror (FSM, Newport) and a dichroic mirror and was expanded to fill the back aperture of a 63x phase Zeiss microscope objective (NA = 1.4). It was focused to a diffraction-limited spot of approximately 0.5 µm in the focal plane. The laser was operated at 20 KHz frequency, and a mechanical shutter with a 30 ms open time was used to control the number of the pulses entering the microscope and ultimately the specimen. In this study, the 30 ms macropulse contained 600 12 ns micropulses. The laser power was gradually increased until a decrease in thickness (‘thinning’) of the axon at the irradiated site was consistently observed. A micropulse energy of 100 nJ (peak irradiance of 4.9 × 109 W cm−2) or total macropulse energy of 60 µJ was determined to be optimal for the desired damage without inducing a complete transection of the axon. This energy and irradiance were typically used except in one experiment, where progressive increments in laser dose from 100 to 400 nJ were needed to elicit a growth cone response. All experiments were performed at room temperature (20°C–23°C). Phase contrast images and fluorescence images were captured before and after laser irradiation.
2.3. Transmission electron microscopy and fluorescence live cell staining
Laser-irradiated axons were fixed in Karnovsky solution consisting of 2 per cent paraformaldehyde and 3 per cent glutaraldehyde in sodium cacodylate buffer at different time points (30 s to 10 min) post-laser. Single irradiated axons were serial thin-sectioned and relocated under the TEM using established single-cell TEM methods described previously [22]. The 60 nm serial sections were stained and examined with a Philips transmission electron microscope (Tecnai 12, Philips).
For live fluorescence analysis of membrane structure, axons were pre-loaded with calcein AM (10 µM, molecular weight = 994.87, Invitrogen) for 30 min and washed three times in fresh culture medium prior to laser exposure. In order to observe mitochondrial movement (transport) within the axon, cells were incubated in rhodamine 123 (100 nM, Invitrogen) for 30 min and washed with fresh culture medium three times prior to experiments. Fluorescence imaging was achieved using Orca R2 charged coupled device (CCD) camera (Hamamatsu) and a standard Zeiss green fluorescent protein (GFP) excitation/emission filter set.
2.4. Statistical analysis
The Irwin–Fisher exact test was used to determine statistical significance of the observed frequency of the response of growth cone filopodia to laser irradiation of a nearby axon (experimental group) compared with laser irradiation of the culture media (control group). The software used to calculate the p-value for the Irwin–Fisher exact test was written by Howard Tucker, PhD (Department of Mathematics, University of California, Irvine).
3. Results
3.1. Nerve response
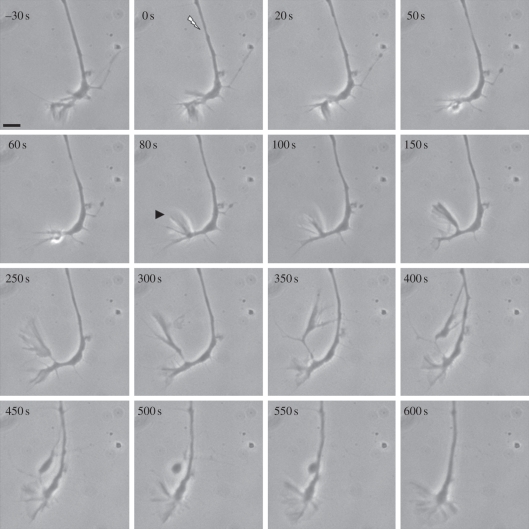
The original intent of this study was to explore the response of regenerating axons to different levels of damage at different locations along the axonal shaft. Unexpectedly, when a sub-axotomy lesion was placed in the axonal shaft 10–20 µm behind the growth cone, the growth cone responded by extending a filopodium in a retrograde direction towards the injury site (figure 1). Immediately after lesion induction (0 s), a slight thinning of the axon occurred at the irradiation site. The thinning increased by 20 s, but resolved by 150 s restoring the axon approximately to pre-irradiation thickness. More specifically, the growth cone responded by ceasing its forward progression, and at 80 s (prior to the restoration of normal axonal thickness), it extended a large lateral filopodium (figure 1, arrowhead). The filopodium progressively lengthened to about 15 µm by 300 s. By 350 s, it turned sharply posterior towards the lesion site and appeared to contact the axonal shaft at the damage site. Contact increased and was followed by collapse of the filopodium onto the damaged axon at 400 s, resulting in a large varicosity by 450–500 s. Filopodial contact with the axonal shaft was maintained during this time period. By 600 s, the filopodium, including the varicosity, appeared to fuse with the axonal shaft, and axonal growth resumed in the original pre-irradiation direction. The results of the retrograde extension of growth cone filopodia towards the injury sites on the same axons are summarized in table 1. Two responses were observed, (i) filopodia coming in contact with damage site and (ii) filopodia extending towards the damage site but not actually contacting it. This occurred in 48 per cent (10/21) of the irradiated cells.
Figure 1.
Directed filopodial extension in response to a laser injury 20 µm behind a growth cone. Following irradiation at the site indicated by the arrow (0 s), thinning of the axon was observed beginning at 20 s followed by restoration of thickness by 150 s. At 80 s, a large filopodium indicated by the arrowhead has formed nearest the lesion site. By 300–350 s, it has extended and contacted the axonal shaft near the lesion site. The filopodium regresses forming a large vesicle at 450–550 s. At 600 s, the growth cone resumes growing in the pre-irradiation direction. Scale bar, 5 µm.
Table 1.
Responses of growth cone to the laser-induced injury on the same axon.
| filopodia responses |
number | percentage (%) | |
|---|---|---|---|
| response 1 | filopodia contact the injured axon | 5 | 24 |
| response 2 | filopodia extend towards but do not contact the injured axon | 5 | 24 |
| response 3 | no response | 11 | 52 |
| total | — | 21 | 100 |
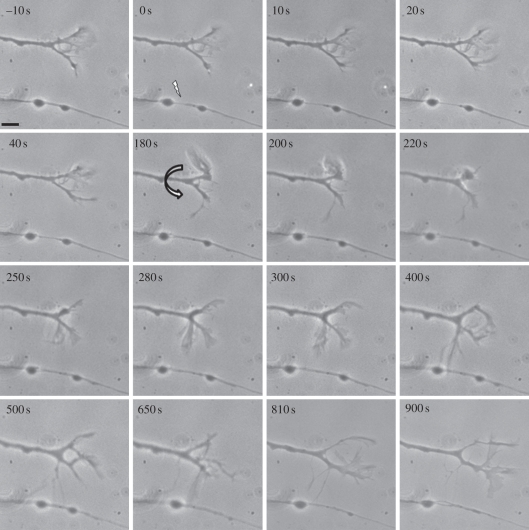
This rapid and directed extension of a filopodium from the growth cone towards the site of the lesion suggests that the damaged axon is releasing a chemical (or chemicals) from the lesion site that is capable of modulating the direction and growth of the filopodial extension as well as the direction of the growth cone and ultimately the entire nerve. However, it could be argued that the laser lesion caused an intracellular damage response that resulted in the observed behaviour. This possibility was addressed by observing the behaviour of growth cones on neurons adjacent to the damaged axons (summary of results, table 2). Extensions of filopodia from the growth cones on adjacent axons were directed towards the injury sites in 56 per cent (28/50) of the cells irradiated in this experimental series. We observed axons 3–5 min prior to laser irradiation of a nearby axon to ensure that the ‘responding’ axon was growing in a straight line as indicated by the lack of a bend in the axonal shaft just behind the growth cone. In addition, axons were only chosen that were over 60 µm long and that had been growing in a continual straight line. This insured that they were growing in a straight line for at least 1 h prior to irradiation of the adjacent axon. A change in direction of the growth cone soon after laser irradiation of the adjacent axon was, therefore, likely a direct result to the irradiation event. An example of this response is shown in figure 2 (also see electronic supplementary material, movie S1), where an axonal shaft paralleled the path taken by a growth cone of an adjacent neuron 16 µm away. Damage to the lower axon was produced by focusing the laser 16 µm from the base of the growth cone (0 s, figure 2). The laser was focused in between two ‘beaded’ varicosities, common to goldfish retinal ganglion cell axons [23]. An immediate thinning of the axon followed by the recovery was observed (0–180 s). At 180 s, the adjacent growth cone initiated an extension of a filopodium towards the lesion site (curved arrow). The filopodium rapidly enlarged by 300 s and appeared to contact the axon at the site of injury at 400 s. A second filopodium extended towards the same spot at 650 s. By 810 s, the extended filopodia had largely withdrawn, and the adjacent non-irradiated axon resumed growing in its original direction.
Table 2.
Responses of growth cone to nearby damaged axon.
| filopodia responses |
growth cone re-direction | number | subtotal | percentage (%) | |
|---|---|---|---|---|---|
| response 1 | filopodia contact the injured axon | direction changes towards the injured axon | 5 | 13 | 26 |
| growth along the injured axon | 3 | ||||
| no change of direction | 5 | ||||
| response 2 | filopodia extend towards but do not contact the injured axon | no change of direction | 15 | 15 | 30 |
| response 3 | no response | no change of direction | 22 | 22 | 44 |
| total | — | — | — | 50 | 100 |
Figure 2.
Directed filopodial extension in response to laser injury on a parallel growing axon. Irradiation site is indicated by the arrow. Irradiation at 0 s leads to thinning of the axon shaft. At 180 s, the non-irradiated growth cone extends a filopodium towards the injury site, makes apparent contact at 300–400 s, and subsequently withdraws. The growth cone continues moving in its original direction. Scale bar, 5 µm.
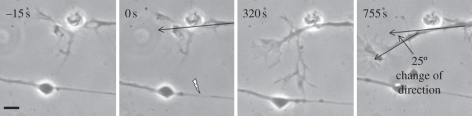
As in most studies of growth cone behaviour [24], there was some variation in the responses. In our study, 50 axons were irradiated and subsequently analysed (table 2). In 13 cases (26%), the response was even more dramatic in that the filopodial extensions contacting the damage sites were much larger than the example presented in figure 2. In five of these cases, the growth cones changed direction and migrated towards the injured axons. The growth cones and their associated axons continued migrating in the new direction even after withdrawing their filopodial contacts from the injured axons (figure 3). In the example shown, the neuron continued to grow at a 25° angle from the original direction. Furthermore, in three of these cases, the extending filopodia appeared to adhere to and grow along the damaged axon. The change in growth direction of the growth cones and the associated neurons appeared to be a sustained rather than a transient response.
Figure 3.
The response of a growth cone to laser irradiation of a nearby axon. Filopodia extended towards the lesion as shown before, but, in addition, the entire growth cone turned towards the injured axon beginning at 320 s. Scale bar, 5 µm.
In 15 cases (table 2, 30%), the filopodia extended towards the injury zone, but did not appear to contact the axon. Thirty control experiments were conducted in which the medium approximately 15 µm from the growth cones was irradiated with the experimental laser parameters. No turning of the growth cones or extension of filopodia towards the irradiated sites was observed. In summary, directed filopodia extension and/or growth cone turning was observed in 28 out of 50 (56%) experiments involving focal laser damage to an axon. This is a statistically significant difference from ‘no response’ in the 30 control irradiations of the adjacent media (p-value < 1 × 10−5, Irwin–Fisher exact test).
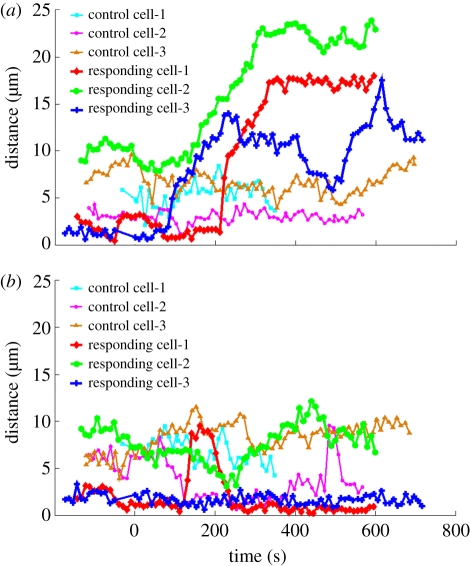
To better characterize the growth cone response behaviour and further quantitatively clarify whether the filopodium was responding to a true signal from the damaged axon or a possible laser effect on the culture medium rather than the axon, the filopodial distribution around the growth cone was analysed and compared between laser-irradiated axons and the irradiation of the culture medium. We examined the change in length over time of the longest single filopodium on both sides of the growth cone—relative to the nearby damaged axon. The magnitude of the filopodial response to the injury site was plotted as a function of time and is defined as the longest filopodium measured from the apex of the triangle at the centre of the axon, where the responding filopodium grows out, to the tip of the extending filopodium (electronic supplementary material, figure S1). For the ‘toward zone,’ one geometric base of the triangle is on the damaged axon. The length of the base is about 10 µm with the lesion in the centre. The ‘away zone’ triangle is a mirror image of the ‘toward zone’ triangle with respect to the growing direction of the whole axon (electronic supplementary material, figure S1). A plot of the response of the growth cone filopodium shown in figure 2 is presented in figure 4a (‘responding’ cell 1 in the ‘response 1’ category in table 2, diamond symbols). Filopodial extensions from two additional responding cells (‘responding’ cells 2, and 3 in the ‘response 1’ category in table 2; figure 4a) are also presented. These plots reveal the dramatic nature of the damage-initiated response characterized by a sudden long filopodial extension towards the lesion site in the ‘toward zone’ when compared with control cells that are not near a damaged axon and which do not exhibit any filopodial responses (figure 4a, control cells 1, 2 and 3). In addition, the extension of filopodia from the sides of ‘responding’ growth cones that were facing away from the damaged axon in the ‘away zone’ (figure 4b, ‘responding’ cells 1, 2, and 3) as well as from control cells not near any damaged axons (figure 4b, control cells 1, 2, and 3) were analysed. Filopodia facing away from the damaged axons were unaffected, further suggesting that the observed filopodial extensions and/or change in growth cone direction are responses to specific molecules and/or chemicals released from the damaged site.
Figure 4.
Plots of the longest filopodium extending (a) towards the damaged axon or (b) away from the axon as a function of time. (a) Filopodia were measured in the ‘toward zone’ extending from the growth cone and subtending a 10 µm region centred along the injured axon. (b) As a comparison, filopodia were measured in the mirror image of the ‘toward zone’ triangle reflected across the growth cone in the ‘away zone’. (Online version in colour.)
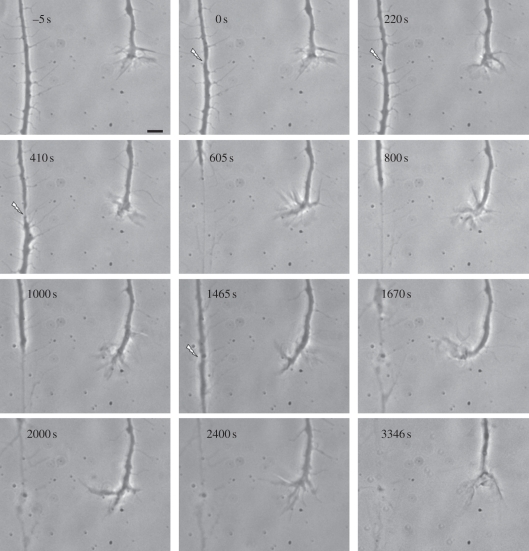
It is also possible that the extent of cellular damage and subsequent release of damage-related material might vary as a function of the individual axon: its physiological state, age, thickness and absorbed laser energy. To address the possible variation caused by axonal thickness, an experiment was conducted on a relatively thick axon of 2 µm, when compared with 0.5 to 1 µm thickness axons, which were generally chosen for experimentation (tables 1 and 2). In this case, the adjacent growth cone did not respond to the initial axonal injury using the standard 100 nJ exposure (figure 5, 0 s). When the same axon was re-irradiated using progressively higher laser doses (figure 5, 200 nJ at 220 s and 300 nJ at 410 s), the non-responding adjacent growth cone first extended filopodia towards the damaged axon, and eventually the growth cone turned and grew towards the damage site (figure 5, 605–1465 s). At 1465 s (figure 5), an even higher energy (400 nJ) was used to completely truncate the axon. The damaged axon was followed for an additional 1881 s. During this 31 min period, the adjacent growth cone retracted from the damaged axon and continued to grow in its pre-irradiation direction (figure 5, 1465–3346 s). This result suggests that the severely damaged axon did not release chemicals at the levels necessary to induce an attraction response in the adjacent growth cone, and in fact, may have released growth-inhibiting substances reported by others [10–13].
Figure 5.
The effect of sequential irradiation of one position of an axon at increasing laser power: 0 s = 100 nJ, 220 s = 200 nJ, 410 s = 300 nJ and 1465 s = 400 nJ. The adjacent growth cone showed no obvious response to 100 and 200 nJ, but at 300 nJ, it turned towards the injured axon. After completely truncating the axon, the responding growth cone retracted to its original direction instead of turning more toward the highly damaged axon. Scale bar, 5 µm.
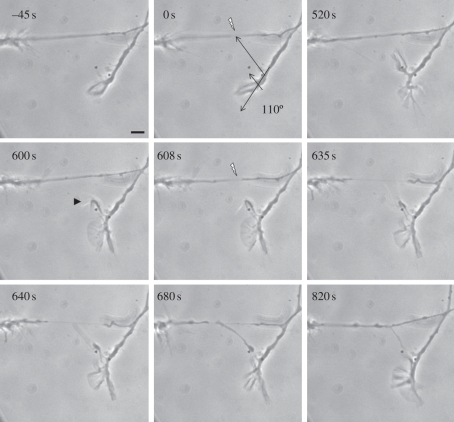
In the studies summarized in tables 1 and 2, the extension of a filopodium and/or the turning of a growth cone took between 2 and 20 min (figures 1–3). Retraction of the filopodium was observed either before or after it contacted the damaged axon. A possible explanation of this reversible response is that the signal generated by axonal injury is transient. The maximal morphological change to the axonal shaft induced by laser irradiation was typically observed between 20 s and a minute, and was followed by a return to normal morphology within 3 min of the injury, depending on the axon size. Alternatively, the response of the growth cone could be inherently transient as might occur with desensitization [25]. Experiments to examine this possibility were conducted. In three replicate experiments, an initial directed filopodial response was elicited. When the filopodia stopped responding, a second filopodial response was elicited following a second laser exposure. Results from one of these experiments are shown in figure 6. In this case, the initial filopodial response was elicited by damaging an axon shaft that was growing at an angle of 110° with respect to the growth cone (figure 6, 0–520 s). At 520 s, when the filopodium stopped growing towards the damaged site and began to retract (figure 6, 600 s, arrowhead), the axon was laser-irradiated a second time at the same site causing more severe damage but not enough to completely sever the axon (figure 6, 608 s). A second filopodial response involving extension of the filopodium and contact with the damaged axon were observed (see the period of 635–820 s in figure 6).
Figure 6.
Repeated filopodial response to two sequential axonal lesions at the same laser power. After irradiation at 0 s, a directed filopodial response was seen at 520 s. A second lesion at 608 s induced a second robust response, resulting in filopodial extension and contact with the injured axon that lasted until 820 s. Scale bar, 5 µm.
3.2. Membrane permeability studies
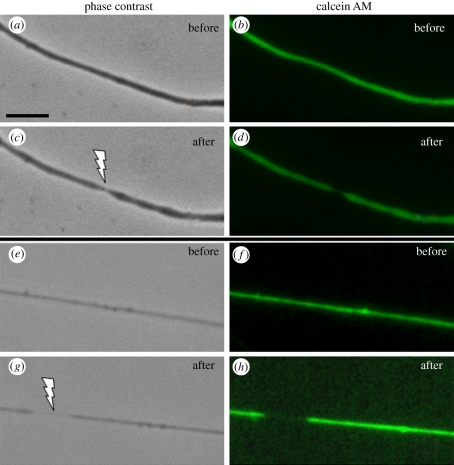
Previous studies have shown that the plasma membrane can be permeabilized by different amounts and types of laser energy in order to facilitate DNA transfection [26,27]. In addition, high irradiance laser energy has been used to rupture single cells in order to release their contents for subsequent analysis by micro-capillary electrophoresis [28], and a bursting of GFP-expressing axoplasm has been observed after laser incision of motor neurons in C. elegans [5]. To test the possibility that laser microirradiation is damaging the cell membrane causing the axon to release some or all of its contents into the culture medium, cells were pre-loaded with calcein AM and subsequently laser microirradiated to produce sub-axotomy localized damage (figure 7). No fluorescence was detected outside the laser-damaged sites even though the axon thinning was highly visible and a high light-sensitive digital imaging camera was used. Photobleaching should not be a significant factor in this experiment. The absorption maximum of calcein AM at 494 nm is well away from 532 nm laser so that photobleaching would be highly unlikely. More importantly, the laser was focused to a diffraction-limited spot of approximately 0.5 µm directed transversely across the thin axon, so the volume of cytoplasm subjected to photobleaching would be quite small. Since calcein AM is small and readily diffusible, it should quickly enter any area where photobleaching may have occurred.
Figure 7.
Calcein AM staining of two different axons pre-and post-laser irradiation: (a,c) phase contrast images pre- and post-laser; (b,d) live fluorescent images pre- and post-laser showing ‘thinning’ in the irradiated region. (e,g) phase contrast images pre- and post-laser of a second axon; (f,h) live fluorescent images pre- and post-laser showing ‘thinning’. Arrows indicate laser damage region following exposure. No extrusion of dye from the damage region is evident. (a) Scale bar, 5 µm. (Online version in colour.)
3.3. Structural analysis (electron microscopy)
Since the fluorescence dye studies do not provide definitive structural information on the nature of the laser damage, laser microirradiated axons were analysed using TEM.
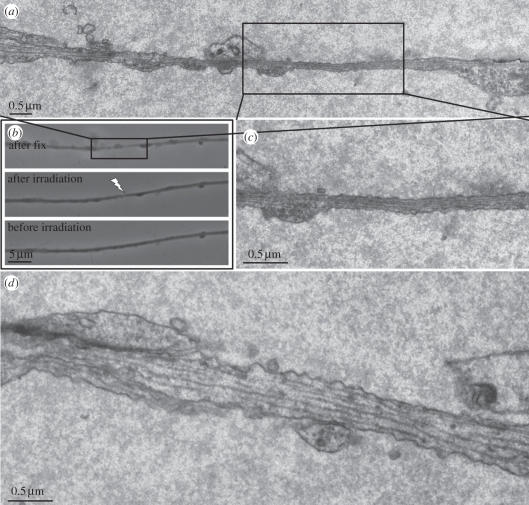
Light and electron microscope images of a single irradiated axon fixed 30 s post-laser are shown in figure 8. The pre- and post-irradiation, and post-fixation images (figure 8b) are precisely aligned with the TEM images. The arrow in figure 8b indicates the point of laser exposure. The TEM images demonstrate the following: (i) the thickness of the axon in the irradiated region has thinned considerably and is similar to the thinning observed by live cell light microscopy at 30 s post-laser exposure (figures 1 and 2), (ii) the outer cell membrane is structurally intact (not ruptured), and (iii) microtubules can be seen extending through the irradiated zone (figure 8c).
Figure 8.
TEM and phase contrast images of damaged axon: (a) reconstructed collage of multiple TEM images of an axon fixed 30 s after laser irradiation. (b) Live phase contrast images taken before (bottom) and after irradiation (middle) and after fixation (top). Images are matched with the electron microscope images in (a). (c) Electron micrograph of the region in the centre of the ‘thinned’ zone. Note the intact cell membrane and the presence of contiguous microtubules. (d) Non-irradiated region 36 µm away from the laser-irradiated region.
3.4. Axonal transport (mitochondrial migration)
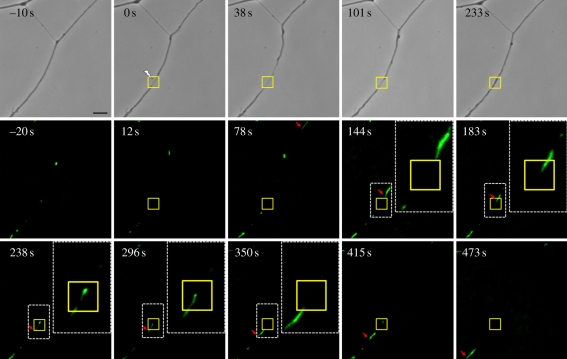
To examine the possibility that the laser damage affects axonal transport and specifically mitochondrial movement along the cytoskeleton, cells were incubated in the mitochondria-specific fluorescence probe, rhodamine 123 prior to irradiation. Time-lapse fluorescence images pre- and post-laser irradiation following one specific mitochondrion show that it is unable to move through the damage zone (square box in figure 9) immediately following laser irradiation, but it resumes moving through the irradiated region after several minutes, but at a slower rate (figure 9 and electronic supplementary material, movie S2). Specifically, the mitochondrion tracked in figure 9 (electronic supplementary material, movie S2) took 66 s to move 26.9 µm (velocity = 24.3 µm min−1) from the un-irradiated area (arrow, 78 s) to the damage site in the irradiated region of the axon (arrow, 144 s) where it began to significantly reduce its rate of movement. After 271 s, the same mitochondrion was only able to progress 9.2 µm through the irradiated zone at an overall rate of 2.0 µm min−1 (arrow, 415 s). This is 1/12 the velocity through the normal un-irradiated region of the axon. Additionally, from fluorescence images at times of 238 s and 296 s, it appears that the same mitochondrion stops moving for a period of about 1 min. During this time, the damaged axon shaft appears to have regained its pre-irradiation thickness as can be seen in the phase contrast image at 233 s (figure 9). After passing through the laser-irradiated zone, the velocity of the mitochondrial movement increased to 10.2 µm min−1, approximately 50 per cent of the pre-irradiation velocity. In this case, it took 58 s to travel 9.9 µm to the position indicated by the arrow in the frame at 473 s (figure 9). However, when three other mitochondria were followed post-laser for periods up to 16 min (at 774, 906 and 966 s, electronic supplementary material, movie S2), mitochondrial velocity through the irradiated zone was 32.2 ± 6.0 µm min−1, which was equivalent to the pre-irradiation rate of 30.5 ± 2.9 µm min−1. The pre-irradiation rate was obtained by measuring the velocities of two mitochondria before the laser irradiation at times of −129 s and −23 s (electronic supplementary material, movie S2). In summary, the axonal transport rates of six mitochondria were analysed: (i) three ‘control’ mitochondria after recovery from laser damage, (ii) two prior to laser damage, and (iii) the one mitochondrion that stopped and then moved at a greatly reduced rate through the damage zone (figure 9).
Figure 9.
Mitochondrial transport study using rhodamine 123 staining of a live axon subjected to laser irradiation. Phase contrast images (top row) before and immediately after laser damage, and time-lapse fluorescence images (middle and bottom rows) of rhodamine 123 staining of mitochondria at different time points. A mitochondrion was tracked (additional mitochondria in electronic supplementary material, movie S2, were tracked and analysed) to calculate the velocity of mitochondrial movement through the damage zone (square box). Arrows in the images at time 78–473 s indicate the position of the mitochondrion. A white box encloses the tracked mitochondrion near the laser-irradiated zone (square box) and a magnified view (inset) of the area containing the tracked mitochondrion are shown in the images at 144 s, 183 s, 238 s, 296 s and 350 s. From fluorescence images at time of 238 s and 296 s, it appears that the same mitochondrion stops moving in the damage zone within a period of about 1 min. However, from the phase contrast image at 233 s, the damage axon shaft appears to have regained its pre-irradiation thickness. Scale bar, 5 µm. (Online version in colour.)
4. Discussion and conclusions
An axon that sustains limited transient damage can induce a response from its own growth cone or a growth cone from a nearby undamaged axon. The response involves the extension of filopodia, which may result in turning of the growth cone and the subsequent growth of the axon towards the damaged site. These responses suggest that the injured axons release a substance or substances (chemoattractants) that elicit a response from nearby growth cones. This is exemplified by the extension of filopodia towards, and frequently touching, the damaged site on the axonal shaft, and/or an actual turning of the growth cone towards the site of damage. These responses have been shown to be highly significant at p < 1 × 10−5. Additionally, in the experiments examining the rate of filopodia growth towards the damaged axon, there was no stimulation of filopodial extensions from the side of the ‘responding’ growth cone that was facing away from the damaged axon (figure 4b), as opposed to the side that was facing towards the damaged axon (figure 4a) that was presumably detecting the chemical signals released from the damaged site (figure 4). Control experiments in which the culture medium alone was exposed to laser doses equivalent to those used to damage the axon did not result in any stimulated filopodial extension or turning of the growth cones (figure 4). This contrasts with other studies [29,30] in which laser energy has been shown to cause a growth response of axons when focused directly on the growth cone or in front of the axon in the culture medium. However in those studies, the laser type and dosimetry were different from the studies reported here.
Even though our results are statistically significant, the lack of response of 44 per cent of growth cones/filopodia to damage produced on the nearby axons needs to be addressed. One possible explanation could be that, in some cases, the amount of chemical(s) released from the irradiation site was insufficient or became so diluted that the amount actually reaching the growth cone was too low to elicit a response. Future studies will be conducted to determine if the response rate correlates with the distance between the irradiated axon and the growth cone. In addition, factors such as age of the culture and individual variation in the physiological state and/or thickness of different axons in the same and different culture dishes might also account for some of the variability in response. For example, as shown in figure 5, when an axon initially was irradiated with a laser dose that produced visible damage, no response was elicited from a nearby growth cone. However with repeated exposures and escalation of the laser dose, the adjacent growth cone eventually extended filopodia towards the damaged axon, turned, and then migrated towards the damaged axon. This result could be explained either by a variation in the amount of substance(s) released by the damaged axon, such that more severe damage was needed to release enough activating substance(s) to elicit a response from the adjacent growth cone, or conversely, the adjacent growth cone required more released substance to activate its response. Thus, the individual physiology of the responding growth cone as well as the amount of damage in the exposed axon may influence whether or not a growth cone responds and may account for some of the variability observed in these experiments. In addition, because there is variation in the thickness between axons, it is also possible that this variable impacts the amount of damage needed to produce a visible ‘thinning’ effect as well as the amount of substance that may be released upon irradiation and damage, e.g. thicker axons may release more substances when damaged than thinner axons. Similarly, the transient nature of the filopodial response was demonstrated in the three experimental replicates, where initial damage of an axon caused initiation of a filopodial extension towards the damaged axon but which subsequently retracted requiring a second exposure before the filopodium extended all the way to the damage site (figure 6). This result suggests that either the responding growth cone (filopodium) has a threshold for sensing the chemical gradient, or the damaged cell is not releasing enough of the chemical(s) to stimulate the filopodial response, thus requiring multiple laser exposures. Either explanation, again, suggests potential variability between individual cells. Notwithstanding, our results are consistent with studies that have shown that axonal growth and navigation can be directed by guidance molecules such as slits, ephrins and semaphorins, some of which, such as netrin, Wnt and Shh are known to diffuse [17–19]. Which of these and possibly other molecules and ions may be responsible for the response of growth cones and their associated filopodia to localized axonal damage sites remains to be determined.
The physical mechanisms of laser damage in our studies could be a combination of highly localized shock waves from a small micro-plasma with an additional rapid thermal component, similar to what has been used to disrupt microtubules in the mitotic spindle [31]. In those studies, even though a relatively high irradiance was achieved, the cells remained viable and no rupture of the cell membrane was detected by TEM. In the studies reported here, the 12 ns 100 nJ micro pulse generates a peak power density of 4.9 × 109 W cm−2 when a 63x NA 1.4 microscope objective is used to focus the beam to a near diffraction-limited spot of approximately 0.5 µm in diameter. This power density is an order of magnitude lower than the reported optical breakdown threshold in water at 7.7 × 1010 W cm−2 and the laser power of 5.1 × 1010 W cm−2 that was used for optotransfection of living cells [32–34]. Thus, it would appear that the irradiance levels used in our studies are not sufficiently high to cause a structurally catastrophic event resulting in rupture of the axon cell membrane. However, the possibility of multi-photon absorption resulting in sub-plasma UV-like alterations cannot be entirely eliminated since the effective two-photon wavelength of the 532 nm laser we used would be 266 nm, which is within the absorption band for numerous proteins.
To examine the integrity of the irradiated axon, we used transmission electron microcopy, the ‘gold standard’ for structural analysis. It is clear from the TEM analysis that there is no gross rupture of the outer cell membrane in the damaged zone. In addition, microtubules were also observed in the ‘thinned’ region. The observation that the region of thinning extends considerably beyond the actual laser focused spot may be due to axonal stretching as a result of the loss of some internal cytoarchitecture [35], possibly neurofilaments. Though no evidence of this is found in the TEM sections, future immunostaining studies could help resolve this question. Further, it is possible that the fixation process following laser exposure may have introduced artefacts such as membrane blebbing and vesicle formation that are due to a weakening of the membrane in the irradiated zone. However, fluorescence staining with calcein AM, a non-structural binding molecule, suggests maintenance of membrane integrity in the irradiated region, as no dye appears to be leaking from the cell. Though it is possible that the CCD camera is not sensitive enough to detect small amounts of the calcein AM passing through the cell membrane, these studies in combination with the TEM are strong evidence that there is no gross rupture of the cell membrane in the irradiated and subsequently ‘thinned’ region of the axon. What remains intriguing is the question of what substances and/or molecules are released from this region to attract filopodia and ultimately the growth cones to the damage site. Future molecular-based studies will examine this question.
Axonal transport also seems to be affected, at least with respect to the movement of the mitochondria (figure 9 and electronic supplementary material, movie S2). Though individual mitochondria initially stop at the lesion site, possibly because the axon has become too thin for them to pass, within several minutes post-laser exposure, the axon regains its thickness and the mitochondria regain movement through the irradiated region. They return to their pre-irradiation migration rate by 12 min post-irradiation. This demonstrates that, with respect to mitochondrial transport, the axon has returned to its pre-damage physiological sate. The ability of mitochondria to move through the damaged region is supported by the TEM studies that show contiguous microtubules in the irradiation zone within 30 s of irradiation. However, the fact that it takes such a long period of time for mitochondrial movement to fully recover even though the axon appears to return to its pre-irradiation thickness within 3 min of laser damage, may be indicative of a delayed recovery of microtubule associated proteins such as kinesins and dyneins [36,37]. Future immunostaining and possibly siRNA studies will help resolve this question.
In summary, we have interfaced laser microirradiation, analytical microscopy (fluorescence and TEM) and a neuronal cell culture model to uncover a novel process of growth cone response to damage produced at a localized site on a single axon. It is likely that this response is mediated by specific molecules released from the damaged area. Further studies to determine the nature of the chemical signals and the physiological pathways involved in the growth cone response are necessary. Understanding, and perhaps controlling, these mechanisms might facilitate the growth of axons into damaged neural circuits, thus promoting their restoration and repair.
Acknowledgements
This work was supported by grants from the US Air Force under grant AFOSR FA9550-10-0538, a grant from the Beckman Laser Institute Foundation (M.W.B.) and a School of Biological Science Grant (R.L.M.). We acknowledge Khyati Dave who contributed during the early stages of this project.
References
- 1.Berns M. W. 1974. Directed chromosome loss by laser microirradiation. Science 186, 700–705 10.1126/science.186.4165.700 (doi:10.1126/science.186.4165.700) [DOI] [PubMed] [Google Scholar]
- 2.Berns M. W., et al. 1981. Laser microsurgery in cell and developmental biology. Science 213, 505–513 10.1126/science.7017933 (doi:10.1126/science.7017933) [DOI] [PubMed] [Google Scholar]
- 3.Berns M. W. 2007. A history of laser scissors (microbeams). Method Cell Biol. 82, 3–58 10.1016/s0091-679x(06)82001-7 (doi:10.1016/s0091-679x(06)82001-7) [DOI] [PubMed] [Google Scholar]
- 4.Yanik M. F., Cinar H., Cinar H. N., Chisholm A. D., Jin Y., Ben-Yakar A. 2004. Neurosurgery: functional regeneration after laser axotomy. Nature 432, 822. 10.1038/432822a (doi:10.1038/432822a) [DOI] [PubMed] [Google Scholar]
- 5.Santos S. I. C. O., Mathew M., Loza-Alvarez P. 2010. Real time imaging of femtosecond laser induced nano-neurosurgery dynamics in C. elegans. Opt. Express 18, 364–377 10.1364/OE.18.000364 (doi:10.1364/OE.18.000364) [DOI] [PubMed] [Google Scholar]
- 6.Edwards J. S., Chen S. W., Berns M. W. 1981. Cercal sensory development following laser microlesions of embryonic apical cells in Acheta domesticus. J. Neurosci. 1, 250–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rieske E., Kreutzberg G. W. 1978. Neurite regeneration after cell surgery with laser microbeam irradiation. Brain Res. 143, 478–483 10.1016/0006-8993(78)90734-5 (doi:10.1016/0006-8993(78)90734-5) [DOI] [PubMed] [Google Scholar]
- 8.Gross G. W., Lucas J. H., Higgins L. M. 1983. Laser microbeam surgery: ultrastructural changes associated with neurite transection in culture. J. Neurosci. 3, 1979–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hellman A. N., Behrad V., Kim H. J., Mismar W., Steward O., Jeon N. L., Venugopolan V. 2010. Examination of axonal injury and regeneration in micropatterned neuronal culture using pulsed microbeam dissection. Lab Chip 10, 2083–2092 10.1039/b927153h (doi:10.1039/b927153h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bramlett H. M., Dietrich W. D. 2004. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J. Cereb. Blood Flow Metab. 24, 133–150 10.1097/01.WCB.0000111614.19196.04 (doi:10.1097/01.WCB.0000111614.19196.04) [DOI] [PubMed] [Google Scholar]
- 11.Zipfel G. J., Babcock D. J., Lee J. M., Choi D. W. 2000. Neuronal apoptosis after CNS injury: the roles of glutamate and calcium. J. Neurotrauma 17, 857–869 10.1089/neu.2000.17.857 (doi:10.1089/neu.2000.17.857) [DOI] [PubMed] [Google Scholar]
- 12.Silver J., Miller J. H. 2004. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 5, 146–156 10.1038/nrn1326 (doi:10.1038/nrn1326) [DOI] [PubMed] [Google Scholar]
- 13.Sofroniew M. W., Vinters H. V. 2010. Astrocytes: biology and pathology. Acta Neuropathol. 119, 7–35 10.1007/s00401-009-0619-8 (doi:10.1007/s00401-009-0619-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sofroniew M. V. 2009. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 32, 638–647 10.1016/j.tins.2009.08.002 (doi:10.1016/j.tins.2009.08.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rolls A., Shechter R., Schwartz M. 2009. The bright side of the glial scar in CNS repair. Nat. Rev. Neurosci. 10, 235–241 10.1038/nrn2591 (doi:10.1038/nrn2591) [DOI] [PubMed] [Google Scholar]
- 16.Yiu G., He Z. 2006. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 7, 617–627 10.1038/nrn1956 (doi:10.1038/nrn1956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanes D. H., Reh T. A., Harris W. A. 2006. Development of the nervous system, 2nd edn. Boston, MA: Elsevier [Google Scholar]
- 18.Guan K. L., Rao Y. 2003. Signalling mechanisms mediating neuronal responses to guidance cues. Nat. Rev. Neurosci. 4, 941–956 10.1038/nrn1254 (doi:10.1038/nrn1254) [DOI] [PubMed] [Google Scholar]
- 19.Tessier-Lavigne M., Goodman C. S. 1996. The molecular biology of axon guidance. Science 15, 1123–1133 10.1126/science.274.5290.1123 (doi:10.1126/science.274.5290.1123) [DOI] [PubMed] [Google Scholar]
- 20.Bates C. A., Meyer R. L. 1996. Heterotrimeric G protein activation rapidly inhibits outgrowth of optic axons from adult and embryonic mouse, and goldfish retinal explants. Brain Res. 714, 65–75 10.1016/0006-8993(95)01468-3 (doi:10.1016/0006-8993(95)01468-3) [DOI] [PubMed] [Google Scholar]
- 21.Landreth G. E., Agranoff B. W. 1979. Explant culture of adult goldfish retina: a model for the study of CNS regeneration. Brain Res. 161, 39–53 10.1016/0006-8993(79)90194-x (doi:10.1016/0006-8993(79)90194-x) [DOI] [PubMed] [Google Scholar]
- 22.Liaw L. H., Berns M. W. 1981. Electron microscope autoradiography on serial sections of preselected single living cells. J. Ultrastruct. Res. 75, 187–194 10.1016/S0022-5320(81)80134-7 (doi:10.1016/S0022-5320(81)80134-7) [DOI] [PubMed] [Google Scholar]
- 23.Koenig E., Adams P. 1982. Local protein synthesizing activity in axonal fields regenerating in vitro. J. Neurochem. 39, 386–400 10.1111/j.1471-4159.1982.tb03960.x (doi:10.1111/j.1471-4159.1982.tb03960.x) [DOI] [PubMed] [Google Scholar]
- 24.Sanford S. D., Gatlin J. C., Hokfelt T., Pfenninger K. H. 2008. Growth cone responses to growth and chemotropic factors. Eur. J. Neurosci. 28, 268–278 10.1111/j.1460-9568.2008.06327.x (doi:10.1111/j.1460-9568.2008.06327.x) [DOI] [PubMed] [Google Scholar]
- 25.Piper M., Salih S., Weinl C., Holt C. E., Harris W. A. 2005. Endocytosis-dependent desensitization and protein synthesis-dependent resensitization in retinal growth cone adaptation. Nat. Neurosci. 8, 179–186 10.1038/nn1380 (doi:10.1038/nn1380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett L. E., Sul J. Y., Takano H., Van Bockstaele E. J., Haydon P. G., Eberwine J. H. 2006. Region-directed phototransfection reveals the functional significance of a dendritically synthesized transcription factor. Nat. Meth. 3, 455–460 10.1039/b503658e (doi:10.1039/b503658e) [DOI] [PubMed] [Google Scholar]
- 27.Stevenson D. J., Gunn-Moore F. J., Campbell P., Dholakia K. 2010. Single cell optical transfection. J. R. Soc. Interface 7, 863–871 10.1098/rsif.2009.0463 (doi:10.1098/rsif.2009.0463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sims C. E., Meredith G. D., Krasieva T. B., Berns M. W., Tromberg B. J., Allbritton N. L. 1998. Laser-micropipet combination for single-cell analysis. Anal. Chem. 70, 4570–4577 10.1021/ac9802269 (doi:10.1021/ac9802269) [DOI] [PubMed] [Google Scholar]
- 29.Ehrlicher A., Betz T., Stuhrmann B., Koch D., Milner V., Raizen M. G., Kas J. 2002. Guiding neuronal growth with light. Proc. Natl Acad. Sci. USA 99, 16 024–16 028 10.1073/pnas.252631899 (doi:10.1073/pnas.252631899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathew M., Amat-Roldan I., Andrés R., Santos S. I. C. O., Artigas D., Soriano E., Loza-Alvarez P. 2010. Signalling effect of NIR pulsed lasers on axonal growth. J. Neurosci. Method 186, 196–201 10.1016/j.jneumeth.2009.11.018 (doi:10.1016/j.jneumeth.2009.11.018) [DOI] [PubMed] [Google Scholar]
- 31.Botvinick E. L., Venugopalan V., Shah J. V., Liaw L. H., Berns M. W. 2004. Controlled ablation of microtubules using a picosecond laser. Biophys. J. 87, 4203–4212 10.1529/biophysj.104.049528 (doi:10.1529/biophysj.104.049528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venugopalan V., Guerra A., III, Nahen K., Vogel A. 2002. Role of laser-induced plasma formation in pulsed cellular microsurgery and micromanipulation. Phys. Rev. Lett. 88, 078103. 10.1103/PhysRevLett.88.078103 (doi:10.1103/PhysRevLett.88.078103) [DOI] [PubMed] [Google Scholar]
- 33.Vogel A., Noack J., Huttman G., Paltauf G. 2005. Mechanisms of femtosecond laser nanosurgery of cells and tissues. Appl. Phys. B 81, 1015–1047 10.1007/s00340-005-2036-6 (doi:10.1007/s00340-005-2036-6) [DOI] [Google Scholar]
- 34.Krasieva T. B., Chapman C. F., Lamorte V. J., Venugopalan V., Berns M. W., Tromberg B. J. 1998. Cell permeabilization and molecular transport by laser microirradiation. Proc. SPIE 3260, 38–44 10.1117/12.307113 (doi:10.1117/12.307113) [DOI] [Google Scholar]
- 35.Morrison B., III, Saatman K. E., Meaney D. F., Mcintosh T. K. 1998. In vitro central nervous system models of mechanically induced trauma: a review. J. Neurotrauma 15, 911–928 10.1089/neu.1998.15.911 (doi:10.1089/neu.1998.15.911) [DOI] [PubMed] [Google Scholar]
- 36.Glater E. E., Megeath L. J., Stowers R. S., Schwarz T. L. 2006. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J. Cell Biol. 22, 545–557 10.1083/jcb.200601067 (doi:10.1083/jcb.200601067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pilling A. D., Horiuchi D., Lively C. M., Saxton W. M. 2006. Kinesin-1 and dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol. Biol. Cell 17, 2057–2068 10.1091/mbc.E05-06-0526 (doi:10.1091/mbc.E05-06-0526) [DOI] [PMC free article] [PubMed] [Google Scholar]