Abstract
Animals produce a variety of structures to modify their environments adaptively. Such structures represent extended phenotypes whose development is rarely studied. To begin to rectify this, we used micro-computed tomography (CT) scanning and time-series experiments to obtain the first high-resolution dataset on the four-dimensional growth of ant nests. We show that extrinsic features within the environment, such as the presence of planes between layers of sediment, influence the architecture of Lasius flavus nests, with ants excavating horizontal tunnels along such planes. Intrinsically, the dimensions of the tunnels are associated with individual colonies, the dynamics of excavation can be explained by negative feedback and the angular distribution of tunnels is probably a result of local competition among tunnels for miners. The architecture and dynamics of ant nest excavation therefore result from local interactions of ants with one another and templates inherent in the environment. The influence of the environment on the form of structures has been documented across both biotic and abiotic domains. Our study opens up the utility of CT scanning as a technique for observing the morphogenesis of such structures.
Keywords: self-organization, social insects, building behaviour, computed tomography scanning, Lasius flavus, growth
1. Introduction
Many animals create structures but perhaps the most impressive are those of the social insects, the relative scale of some of which are rivalled only by structures produced by humans [1–3]. The social insects are an incredibly successful and ecologically important group of animals, dominating many terrestrial ecosystems [4]. The nests of social insects have undoubtedly contributed to their success and can be considered as examples of extended phenotypes [5], providing benefits to survival in terms of shelter, defence, establishing suitable microclimates for colony growth, organizing colony functions or indeed in some cases for farming fungi [6–12]. The expression of phenotypes in traditional developmental biology is typically concerned with relating anatomical characters [13], and more rarely behaviour [14], to the underlying genotype. To date, there has been little quantitative research into how extended phenotypes are expressed over time and to what extent they are influenced by the nature of the animals themselves and the environments within which they occur.
Ant nests provide an excellent model system for studying the development of extended phenotypes because ant colonies can be deconstructed and experimentally manipulated in order to tease out their salient features. Ant nests have varied and seemingly complex architectures that may serve, or are the outcome of, important colony functions [12,15–18]. A conspicuous feature of many such ant nests is the presence of horizontal chambers that can be at regular or more irregular intervals along a vertical tunnel. Of further fascination is that ant nests are produced without any knowledge at the individual level of the overall plan; rather, nest excavation and structure is an emergent property of individuals acting on local information through interactions with other individuals and the environment [19–25]. In this sense, while there may be no centralized control in the form of an architect, the architecture and dynamics of nest excavation could be the result of a combination of self-organization and the presence of a template inherent in the environment upon which the local activities of the ants can act. Rather than the need for specific behavioural mechanisms to explain ants switching from vertical to horizontal excavation and their ability to distribute chambers along a tunnel, these phenomena could be explained by a combination of self-organization and responses to heterogeneities in the layering and structure of the sediment within which the nests are being excavated. Preliminary support for this comes from the observation of chambers at equal depths in nests with multiple vertical tunnels (see figs in Tschinkel [16,17]). As in termite pillar formation [1,26], self-organization would also be expected to result in an even angular separation of tunnels and the efficient use of space owing to local competition between tunnels for miners.
Examples of the influence of the environment on the form of structures occur across the biotic and abiotic domains. The distribution of food within the environment has been shown to affect the outcomes of decentralized behaviour in both army ant swarm raids and slime mould networks [27,28]. Also, in the purely physical domain, pH gradients can allow ‘maze solving’ by chemotactic droplets [29] and changes from vertical to horizontal propagation of magmatic intrusions within the Earth's crust can be affected by the physical properties of the layers of rock [30]. Social insect nests also provide a model system for investigating network formation [22,31–34] and, together with insights from studies of other biological networks [28,35–37], can inform artificial network design.
Previous studies of ant nest architecture have largely involved making casts of nests [12,15–18]. Such methods are inherently destructive and only ever represent a snapshot of a nest in time. X-ray computed tomography, or CT scanning, is best known in its medical application where it is used to visualize internal structures of tissues in two and three dimensions. CT scanning has been used previously to investigate static three-dimensional network architecture in termite mounds [33,34]. However, despite wide potential applications for studying time series, CT scanning has only been used once previously in a coarse study of the dynamics of ant nest excavation [38]. In this study, we combined micro-CT scanning and time-series experiments to provide the first high-resolution four-dimensional reconstructions of individual ant nests. Micro-CT represents a relatively new technological development, with high-resolution imaging capabilities. In contrast to traditional CT, micro-CT scanners employ a focused cone X-ray beam and an areal detector, potentially producing three-dimensional voxel sizes of less than 50 µm. This study provides (i) a validation of micro-CT scanning as a novel method for elucidating the expression of extended phenotypes. More specifically, we employ this technique to investigate (ii) the dynamics of nest growth; (iii) that the structure of the sediment can act as a template for nest architecture; (iv) whether there is intercolony variation in tunnel dimensions; and (v) the angular separation among tunnels.
2. Methods
2.1. Study species
Six ‘sub-colonies’ of the yellow meadow ant, Lasius flavus, were collected from Wiltshire, UK, between January and March 2010. Each sub-colony was from a different donor colony and consisted of over 1000 workers and associated brood. L. flavus is common throughout Europe and is typically found on chalk grassland, where it produces subterranean nests with mounds above ground. It is a largely subterranean species, rarely seen above ground, and is therefore ideal for studying excavation. The natural nest architecture of L. flavus has not been studied in detail. Observations made during collection revealed that the superficial mounds consisted of a network of interconnected tunnels. Prior to experiments, colonies were housed in covered test tubes in large square Petri dishes (220 × 220 mm) with Fluon-coated walls and fed with water, honey solution and Drosophila melanogaster.
2.2. Experimental protocol
Ants excavated nests in sediment-filled containers (63 mm internal diameter and 68 mm deep) under three different treatments: one 50 mm deep layer of sediment with no planes (0P); three 16.7 mm deep layers of sediment creating two equidistant planes (2P); and five 10 mm deep layers of sediment creating four equidistant planes (4P). Perfectly spherical and rounded 45–150 µm diameter glass balls of Ballotini (Potters Ballotini Ltd, Yorkshire, UK) were used as the sediment. Layered deposits were produced by adding 12.5 per cent by weight of water to pre-weighed samples of sediment and compacting them to the required depth. A plastic disc, 2 mm thick and with a 5 mm diameter hole in the centre, was placed on top of the sediment. A 7 mm long tube was inserted into the hole. This ensured that initial excavation was central and oriented downwards. Our experiments did not attempt to replicate the natural nests of L. flavus, but were designed to use controlled conditions to test the hypothesis that features within the sediment could act as a template for nest architecture and to investigate the general mechanisms by which ant nests are excavated.
One hundred workers with 25 brood items were selected randomly from a sub-colony to excavate each nest. They were introduced onto the plastic discs on top of the sediment. A small square Petri dish cover (105 × 105 mm) was placed over the container to prevent escape while permitting air transfer. The Petri dish lid was replaced with the airtight container lid when in the micro-CT scanner. The gap between the top of the plastic disc and the top of the container meant that ants could excavate while the container was covered.
Each experimental run involved a different sub-colony to control for any colony-level differences. A run consisted of one replicate of a nest excavated under each of the three experimental treatments. Each of these nests was scanned on repeated occasions at 3, 6, 9, 12, 18, 24, 36 and 48 h after excavation began. Scans were conducted in a SkyScan 1172 micro-CT scanner (SkyScan, Kontich, Belgium). Nests were scanned at 100 kV, 36 µA and with a 0.5 mm thick aluminium filter. The object stage rotated through 180° in increments of 1.5°. The scans were conducted with a resolution of 35 µm cubic voxel size and obtained with a four-part oversize scan with camera offset. The total scan time under these parameters was 36 min. This represents a trade-off between maximizing resolution and minimizing scan time. Six runs were conducted in total. Controls to test for any adverse effects of repeated exposure to radiation were conducted in parallel to the experimental treatments within each run. Control nests were excavated under the same conditions as the nests excavated in sediment with one 50 mm deep layer of sediment and no planes; the difference being that a control nest was scanned only once with seven control nests corresponding to each of the repeated scan times. The total radiation doses received by the ants in our study are over an order of magnitude less than those that have been shown to result in reduced complexity of nest excavation [39].
2.3. Data analysis
The raw micro-CT data were reconstructed using NRecon (NRecon, v. 1.6.1.5, SkyScan, Kontich, Belgium) on a cluster of four networked PCs. The resulting two-dimensional images were processed using CTAn (CTAn, v. 1.9.2.5, SkyScan, Kontich, Belgium) in order to segment the nest from the sediment and air spaces in between (see electronic supplementary material, S1) and to create three-dimensional renderings of the nests that were then visualized using CTVol (CTVol, v. 2.2.0.0, SkyScan, Kontich, Belgium). Statistical analyses were conducted using R (R, v. 2.10.0, The R Foundation for Statistical Computing, http://www.r-project.org/foundation/).
The volumes of the nests of the different colonies and treatments at each time interval were obtained using the 3D morphometric analysis tool in CTAn. The volumetric growth of the nests was modelled using the recovery exponential equation. In the recovery exponential model,
| 2.1 |
where Vt is the volume of the nest at time t, K is the maximum volume of the nest estimated from the experimental data, A is the growth rate estimated by the gradient in
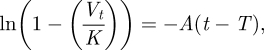 |
2.2 |
and T is the time that excavation begins.
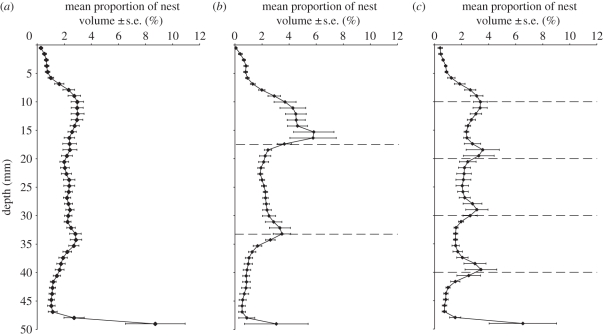
The distribution of the proportion of volume with depth was obtained by splitting the nests formed at 48 h after excavation began into sections 1.054 mm thick (15 scan slices). The volume found in each section was then calculated as a percentage of the total for the nest. The proportions of nest volume at the positions of the planes were calculated by totalling the percentage volumes found in 3 mm thick sections located 1.5 mm above and below the positions of the planes. These were compared with the percentage volumes from the corresponding positions in nests with no planes. Sections that were 3 mm thick were taken to allow for small variations in the positions of the planes across nests and because this thickness is greater than the average tunnel diameters within nests. This allowed for the identification of horizontal tunnels following the planes.
The percentage frequency distributions of tunnel diameters in the nests formed at 48 h after excavation began were also obtained using the three-dimensional morphometric analysis tool in CTAn. The mid-points of the size classes were replicated by their respective percentages to permit statistical comparison.
Static plan view images of the reconstructed nests were exported from CTVol and the angles between tunnels measured using ImageJ (ImageJ, v. 1.42, 2009, Research Services Branch, National Institute of Mental Health, Bethesda, MD, USA). The resultant vectors formed by the angles between initial tunnels depending on their number was calculated using the following equation:
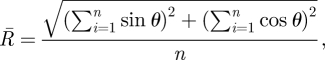 |
2.3 |
where θ is the angle of separation between tunnels and n is the sample size. The sample mean angle of separation between tunnels is calculated using the following equation:
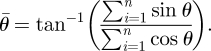 |
2.4 |
Whether the predicted optimized means fall within the 95% confidence limits of the samples is calculated using the following equation:
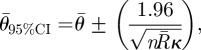 |
2.5 |
where κ is the concentration parameter for the value of 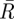 , for each of the scenarios with different numbers of initial tunnels and the latter part is in radians.
, for each of the scenarios with different numbers of initial tunnels and the latter part is in radians.
3. Results
3.1. Effect of radiation
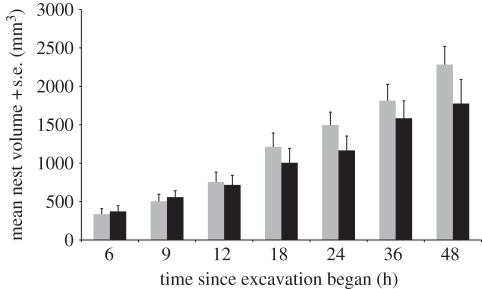
There was no adverse effect of repeated exposure to X-rays on the excavation activity of the ants over the timescale that the experiments were conducted (figure 1). The volumes of nests that were excavated in sediment with no planes and scanned on multiple occasions were compared with those from the control group using a two-way ANOVA. Treatment, time and the interaction between treatment and time were included as the factors. Donor colony identity was a random factor. As expected, there was a significant overall effect of time on volume (two-way ANOVA: F6,30 = 44.58, p < 0.001). However, there were no significant effects of the treatment of repeated exposure to X-rays on volume (two-way ANOVA: F1,30 = 4.9512, p = 0.076) or of an interaction between treatment and time (two-way ANOVA: F6,30 = 1.8629, p = 0.120).
Figure 1.
Effects of exposure to X-ray radiation measured as the comparison of nest volume at each time interval between nests excavated under the 0P experimental treatment (grey) and control treatment nests (black). The 0P treatment consisted of six individual replicates excavated by different donor colonies that were all scanned at each of the time intervals while the control treatment consisted of six replicates of seven nests excavated by ants from the same donor colonies where each nest was only scanned once at one of the corresponding time intervals.
3.2. Volumetric growth of nests
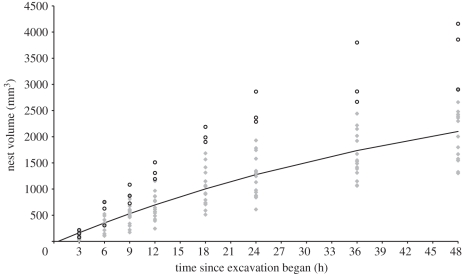
The volumes of the nests excavated under the experimental treatments with different numbers of planes were compared using a two-way ANOVA. Treatment, time and the interaction between treatment and time were included as the factors. Donor colony identity was a random factor. There were no significant effects of the particular treatment on volume (two-way ANOVA: F2,70 = 2.9552, p = 0.098) or of an interaction between treatment and time (two-way ANOVA: F14,70 = 0.9149, p = 0.547), although there was a significant overall effect of time on volume (two-way ANOVA: F7,70 = 43.022, p < 0.001). When colony identity and treatment and an interaction between the two are used as the factors, there is a significant effect of colony identity on volume (two-way ANOVA: F5,126 = 5.5317, p < 0.001). Tukey's post hoc tests reveal that this is due to differences between colony 6 and all but colony 1. The data from colony 6 were therefore removed and the remainder pooled within each time interval for the purposes of model fitting. The maximum rate of excavation occurs between 3 and 6 h; however, a recovery exponential model provides the best overall fit for the growth of the nests in terms of the percentage variation explained (figure 2). The recovery exponential equation also described well the dynamics of growth for all individual nests (all r2 > 0.85 and the majority > 0.95).
Figure 2.
Volumetric growth of ant nests over time. The data represent a total of 144 scans from the growth of 18 nests. The growth data from colony 6 were excluded from the analysis (open circles; see text). The recovery exponential model was fitted to the raw data using the parameters: K = 3500 mm3, T = 0.5 and assuming V0.5 = 1% V3 for each nest. This yields A = 0.019 and r2 = 0.74.
3.3. Effect of the presence of planes between layers of sediment
Reconstructions of the nests demonstrate the influence of planes between layers of sediment on the architecture of ant nests, whereby tunnels switch from vertical or diagonal to horizontal progression upon encountering planes (figures 3 and 4). In some nests, the response is observed at each plane; whereas in other nests there is only a strong response at the first plane to be encountered; and in yet others still, some planes are by-passed with minor deviations in the tunnel orientation before one of the deeper planes is followed (figure 4). This is recorded by the proportion of nest volume at 48 h after excavation began varying with depth among treatments and donor colony (see electronic supplementary material, S2). For nests excavated in sediment with no planes, the plots of mean nest volume with depth reveal no overall peaks in nest volume other than where the ants have reached the bottom of the container (figure 5a). In comparison, nests excavated in sediment with two planes between layers of sediment demonstrate two peaks in the proportion of volume corresponding to the positions of the planes (figure 5b), and those excavated in sediment with four planes reveal four peaks corresponding to the positions of the planes (figure 5c). The total percentage nest volume 1.5 mm above and below the positions of the planes in the nests excavated in sediment with two planes is statistically significantly different from that for the corresponding positions in the nests excavated in sediment with no planes (two-tailed paired t-test, t = 2.6556, d.f. = 5, p = 0.046). The equivalent test for the nests excavated in sediment with four planes also reveals that the total percentage nest volume at the positions of the planes is statistically significantly different from that for the corresponding positions in the nests excavated in sediment with no planes (two-tailed paired t-test, t = 2.9299, d.f. = 5, p = 0.032).
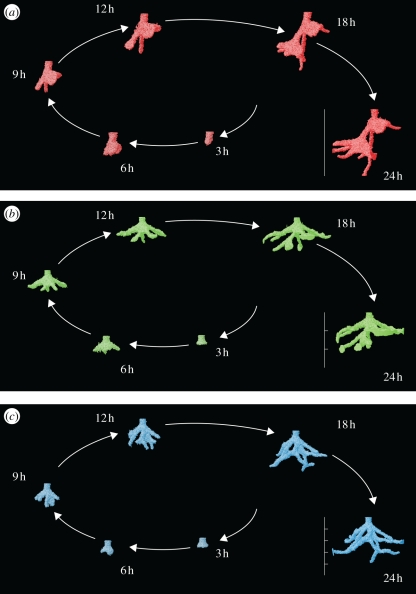
Figure 3.
Four-dimensional ants nests from the same donor colony under the different experimental treatments. (a) Colony 6 under the 0P treatment. The nest has reached the edges and bottom of the experimental container by 24 h. (b) Colony 6 under the 2P treatment. The nest has reached the edges of the experimental container by 18 h. (c) Colony 6 under the 4P treatment. The nest has reached the edges of the experimental container by 24 h. Scale bars, 50 mm in total for the final nests and divisions show the position of the planes between layers. (Online version in colour.)
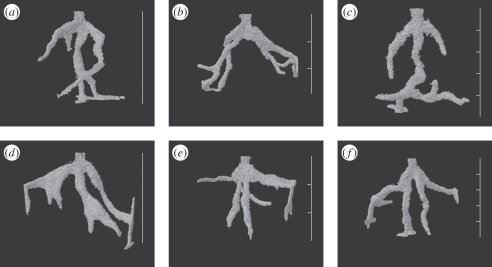
Figure 4.
Selected three-dimensional reconstructions of ant nests illustrating changes in tunnel direction at the positions of planes between layers of sediment in the 2P and 4P treatments and comparison with those from the same donor colony under the 0P treatment. (a) Colony 4 under the 0P treatment at 48 h. The nest has reached the bottom of the experimental container. (b) Colony 4 under the 2P treatment at 48 h. The nest has reached the edges and bottom of the experimental container. (c) Colony 4 under the 4P treatment at 48 h. The nest has reached the bottom of the experimental container. (d) Colony 5 under the 0P treatment at 48 h. The nest has reached the edges and bottom of the experimental container. (e) Colony 5 under the 2P treatment at 36 h. The nest has reached the edges of the experimental container. (f) Colony 5 under the 4P treatment at 48 h. The nest has reached the edges and bottom of the experimental container. Scale bars, 50 mm in total and divisions show the positions of the planes between layers.
Figure 5.
Mean proportion of nest volume with depth at 48 h after excavation began. (a) Nests excavated under the 0P treatment. (b) Nests excavated under the 2P treatment. (c) Nests excavated under the 4P treatment. Dashed lines indicate the positions of the planes between layers of sediment. Each treatment consisted of six replicates with the same six donor colonies used across treatments in a repeated measures design.
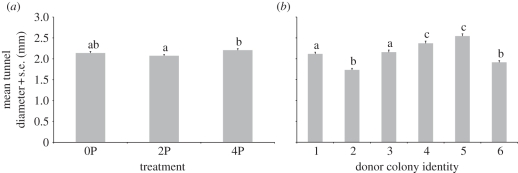
3.4. Tunnel diameters
Average tunnel diameters across nests range from 1.65 to 2.57 mm and the overall mean tunnel diameter across all nests is 2.14 mm. Comparison of tunnel diameters across treatments and donor colonies reveals that there are significant effects of both the treatment (two-way ANOVA, F2,1789 = 4.2208, p = 0.015) and donor colony identity (two-way ANOVA, F5,1789 = 40.8084, p < 0.001) and a significant interaction between the treatment and donor colony identity (two-way ANOVA, F10,1789 = 3.0808, p < 0.001). Tukey's post hoc tests reveal that there is a significant difference in tunnel diameters for nests excavated in sediment with two planes compared with those in sediment with four planes but not in any of the other pairwise treatment comparisons. Tukey's post hoc tests of tunnel diameters between colonies also reveal significant differences in all cases except for colonies 1 and 3, colonies 2 and 6 and colonies 4 and 5 (figure 6).
Figure 6.
Tunnel diameters in nests at 48 h after excavation began. (a) Comparison of tunnel diameters across treatments. (b) Comparison of tunnel diameters across donor colonies. Plots and analysis are based on expanded percentage frequency distributions using the mid-points of the size classes and integer values for the percentages. Groups sharing the same letters are not significantly different under Tukey's multiple comparisons.
3.5. Angles of separation between tunnels
The angles of separation, in the horizontal plane, between the first tunnels to be excavated are optimized depending on the number of initial tunnels. Analysis of the resultant vectors formed by the angles between initial tunnels depending on their number reveals that there is a preferred trend in the degree of separation between tunnels (table 1). The sample mean angle of separation between tunnels also falls within the 95% confidence limits of the samples and is therefore consistent with the tunnels having optimal angles of separation (table 1).
Table 1.
Results of the angles of separation between initial tunnels. n, sample size; 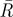 , mean resultant vector length, asterisks indicate p < 0.05;
, mean resultant vector length, asterisks indicate p < 0.05; 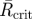 , critical value for
, critical value for 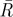 depending on the relevant sample size at a significance level of 0.05;
depending on the relevant sample size at a significance level of 0.05; 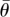 , mean sample direction;
, mean sample direction; 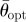 , hypothetical optimal angle of separation between tunnels; κ, concentration parameter for the value of
, hypothetical optimal angle of separation between tunnels; κ, concentration parameter for the value of 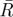 ;
; 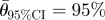 confidence interval for the mean sample direction.
confidence interval for the mean sample direction.
| initial number of tunnels | n | 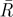 |
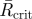 |
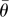 |
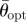 |
κ | 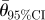 |
|---|---|---|---|---|---|---|---|
| 2 | 14 | 0.801* | 0.46 | 180.0° | 180° | 2.871 | 160.2–199.8° |
| 3 | 24 | 0.855* | 0.35 | 119.8° | 120° | 3.680 | 106.8–132.7° |
| 4 | 12 | 0.933* | 0.49 | 89.6° | 90° | 7.426 | 77.2–101.9° |
4. Discussion
This study demonstrates that both the environment and the nature of the animals themselves influence the expression of extended phenotypes. Our experiments show that features within the environment, such as the presence of planes between layers of sediment, can influence the architecture of an ant nest; while the dimensions of the tunnels are associated with individual colonies; and the dynamics of excavation and angles of tunnel separation are consistent with processes that are self-organized.
The location of horizontal tunnels and the concentration of nest volume along the planes between layers of sediment suggest that these phenomena are influenced by the environment. Accordingly, there may be no need for the ants to have specific behavioural mechanisms to switch from vertical to horizontal excavation or to distribute chambers along a tunnel. We deliberately used layers composed of the same material, rather than layers of different types of sediment or grain size, so that the sediment appears uniform with the only variation being the presence of planes of weakness between the layers of sediment. Our experiments indicate that the ants do not always respond to the presence of planes between layers of sediment and that some responses are stronger than others. In some cases, tunnels proceed horizontally along planes, while, in others, the directions of the tunnels can be deflected upon encountering planes (figure 4c). Rather than the presence of the planes themselves, the ants are probably responding to local heterogeneities in the compaction of the sediment at the positions of the planes, with less compacted sediment above and more compacted sediment below the planes. The less compacted sediment can be more readily excavated and therefore may act as a point of nucleation for a new tunnel or a change in tunnel direction. The greatest amounts of excavated volume with depth are frequently observed in the sediment just above the plane with the more compacted sediment below forming the base of the tunnel (figures 3–5). Other potential templates that could influence ant nest architecture include carbon dioxide, temperature and moisture gradients [17,40].
The dynamics of the volumetric growth of the nests is indicative of a self-organized phenomenon with feedback loops. Previous studies of excavation dynamics, involving indirect measures of three-dimensional nest growth [20] or two-dimensional scenarios, identified logistic dynamics with an initial exponential growth phase followed by a plateau [22–25]. Such studies suggested that excavation could be mediated by recruitment with ants being stimulated to excavate by pheromones, carbon dioxide concentrations or physical contacts among ants [20,22,23,41]. Alternatively, excavation could be mediated simply by the creation of space to accommodate more excavating ants at successful loci [42]. In our experiments, the increase in nest volume over time is best explained by a recovery exponential model. Such a model indicates that there is an ever-decreasing rate of excavation over time that is probably caused by the decreasing density of the ants as their efforts increase the nest volume. However, the maximum rate of excavation occurs at between 3 and 6 h, indicating that there is a weak initial exponential growth phase. This may be due to the ants being highly motivated to excavate near the beginning of the experiment and so there is little requirement or opportunity for recruitment and positive feedback to develop before the controlling factor becomes the density of ants within the nest and nest volume. The mode of life of L. flavus is consistent with being highly motivated to excavate. It is a largely subterranean species, farming root aphids within their nests, and is rarely seen above ground.
The degree of separation between initial tunnels is spatially optimized depending on their number. This suggests that there is competition for workers at digging sites with individuals being attracted to successful sites. Such attraction probably causes local inhibition of other digging sites that are too close. A similar scenario has been suggested for termite pillar building. Mathematical modelling of this phenomenon with pheromone-mediated recruitment [1,26] shows that recruitment of workers to certain pillars is amplified and putative pillars that are too close to one another compete for workers. This results in evenly spaced pillars through the inhibition of pillars that are too close to one another [1,26]. In our ant nests, competition for workers among loci of excavation could inhibit sites that are too close to one another and facilitate the emergence of tunnels with an optimal spatial arrangement.
Specific nest architectures have been identified for different species of ant [12,15–18]; however, our results suggest that some of this variation may in part be associated with the different environments that species encounter when they excavate their nests. Indeed, this is similar to the form of army ant swarm raids and slime mould networks being affected by the distribution of food [27,28]. Furthermore, this is also similar to some phenomena in the purely physical domain; for example, pH gradients can allow ‘maze solving’ by chemotactic droplets [29] and physical properties of layers of rock in the Earth's crust can affect the propagation of magmatic intrusions [30]. The structure of the sediment should therefore be recorded in detail, through either sedimentary logging or geophysical techniques, alongside the architecture of any ant nest. The finding of colony-specific variation in tunnel diameters also suggests that there is control through the ants themselves, possibly because of intercolony variation in the sizes of ants and the miners using their bodies as templates for the dimensions of the structures that they are producing. Workers of L. flavus generally have head widths between 0.5 and 1.0 mm [43] and so the observed tunnel diameters of approximately 2.0 mm permit two-way traffic between the sites of excavation.
Our results demonstrate that CT scanning provides an avenue for beginning to investigate the dynamics of network formation in a three-dimensional scenario. Previous studies of network topology in social insect nests have looked at dynamics in unnatural two-dimensional set-ups in which multiple networks coalesce [22,31,32] or have used CT scanning to elucidate static three-dimensional topology [33,34]. The nests excavated in our experiments have a dendritic topology. Following excavation of the entrance tunnel, the nests branch into two to four separate tunnels. Excavation then proceeds exclusively at the front of the propagating tunnels with periodic bi- or trifurcations. Infilling of existing tunnels was not observed. This differs from many biological foraging networks that undergo an initial exploratory phase and then a consolidation phase whereby certain links are removed [27,28,35,37]. This may be because a tunnel is a long-term structure compared, for instance, with an ant foraging trail that requires maintenance and reinforcement to survive [27,37]. Therefore, once a tunnel has been excavated, the cost has been expended and infilling would require additional expenditure. With time, however, tunnels may be abandoned and might collapse. Static CT scans of termite mounds reveal disconnected tunnels, although the mechanism of abandonment is unclear [33,34].
The initial form of our ant nest networks probably arises from minimizing the distances between the nest entrance and multiple excavation fronts, thereby reducing transport costs for removing excavated material, at the same time as having some internal connectivity among sites of excavation beyond the initial branching point. Unlike previous topological studies of ant and termite nests [22,31–34] our ants did not excavate nests with a meshed network topology other than when tunnels were following the edges of the experimental container. However, observations made during collection of L. flavus revealed that the superficial mounds of the nests consist of a meshed network of tunnels. Future work with the ability to carry out micro-CT scan of larger nests and with shorter throughput times will enable the full elucidation of network topology and the acquisition of probabilistic values for the rules of excavation that can be parametrized into a mathematical model of biological network formation.
An animal's phenotype is not limited to its morphology, but also includes its behaviour and those functional extensions resulting from its behaviour that modify its environment. Developmental biology commonly only investigates the link between genotype and phenotype in terms of the expression of genes and morphological characters [13]. Less common is the study of the developmental genetics of behaviour [14] and even rarer are investigations of the developmental biology of extended phenotypes. Our results on the dynamics of excavation and architecture of ant nests over time provide a validation and open up the utility of CT scanning as a technique for observing the developmental expression of extended phenotypes.
Acknowledgements
We thank Ana Sendova-Franks for assistance with statistics and members of the University of Bristol Ant Laboratory for helpful discussions. The White Horse Equestrian and Trekking Centre provided access to land for collecting the study species. This research was funded by a Leverhulme Trust Research Grant (awarded to K.A.R and N.R.F). The comments of three anonymous reviewers helped to improve this manuscript.
References
- 1.Bonabeau E., Theraulaz G., Deneubourg J.-L., Franks N. R., Rafelsberger O., Joly J. L., Blanco S. 1998. A model for the emergence of pillars, walls and royal chambers in termite nests. Phil. Trans. R. Soc. Lond. B 353, 1561–1576 10.1098/rstb.1998.0310 (doi:10.1098/rstb.1998.0310) [DOI] [Google Scholar]
- 2.Camazine S., Deneubourg J.-L., Franks N. R., Sneyd J., Theraulaz G., Bonabeau E. 2001. Self-organization in biological systems. Princeton, NJ: Princeton University Press [Google Scholar]
- 3.Hansell M. H. 2005. Animal architecture. Oxford, UK: Oxford University Press [Google Scholar]
- 4.Wilson E. O. 1971. The insect societies. Cambridge, MA: Harvard University Press [Google Scholar]
- 5.Dawkins R. 1999. The extended phenotype: the long reach of the gene. Oxford, UK: Oxford University Press [Google Scholar]
- 6.Brian M. V. 1956. Group form and causes of working inefficiency in the ant Myrmica rubra. Physiol. Zool. 29, 173–194 [Google Scholar]
- 7.Porter S. D., Tschinkel W. R. 1985. Fire ant polymorphism: the ergonomics of brood production. Behav. Ecol. Sociobiol. 16, 323–336 10.1007/BF00295545 (doi:10.1007/BF00295545) [DOI] [Google Scholar]
- 8.Sudd J. H., Franks N. R. 1987. The behavioural ecology of ants. Glasgow, UK: Blackie [Google Scholar]
- 9.Porter S. D., Tschinkel W. R. 1993. Fire ant thermal preferences: behavioral control of growth and metabolism. Behav. Ecol. Sociobiol. 32, 321–329 10.1007/BF00183787 (doi:10.1007/BF00183787) [DOI] [Google Scholar]
- 10.Kleineidam C., Roces F. 2000. Carbon dioxide concentrations and nest ventilation in nests of the leaf-cutting ant Atta vollenweideri. Insect. Soc. 47, 241–248 10.1007/PL00001710 (doi:10.1007/PL00001710) [DOI] [Google Scholar]
- 11.Turner J. S. 2001. On the mound of Macrotermes michaelseni as an organ of respiratory gas exchange. Physiol. Biochem. Zool. 74, 798–822 10.1086/323990 (doi:10.1086/323990) [DOI] [PubMed] [Google Scholar]
- 12.Cassill D. L., Tschinkel W. R., Vinson S. B. 2002. Nest complexity, group size and brood rearing in the fire ant, Solenopsis invicta. Insect. Soc. 49, 158–163 10.1007/s00040-002-8296-9 (doi:10.1007/s00040-002-8296-9) [DOI] [Google Scholar]
- 13.Lewis E. B. 1978. A gene complex controlling segmentation in Drosophila. Nature 276, 565–570 10.1038/276565a0 (doi:10.1038/276565a0) [DOI] [PubMed] [Google Scholar]
- 14.Wu Q., Wen T., Lee G., Park J. H., Cai H. N., Shen P. 2003. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron 39, 147–161 10.1016/S0896-6273(03)00396-9 (doi:10.1016/S0896-6273(03)00396-9) [DOI] [PubMed] [Google Scholar]
- 15.Tschinkel W. R. 1999. Sociometry and sociogenesis of colonies of the harvester ant, Pogonomyrmex badius: distribution of workers, brood and seeds within the nest in relation to colony size and season. Ecol. Entomol. 24, 222–237 10.1046/j.1365-2311.1999.00184.x (doi:10.1046/j.1365-2311.1999.00184.x) [DOI] [Google Scholar]
- 16.Tschinkel W. R. 2003. Subterranean ant nests: trace fossils past and future? Palaeogeogr. Palaeoclimatol. Palaeoecol. 192, 321–333 10.1016/S0031-0182(02)00690-9 (doi:10.1016/S0031-0182(02)00690-9) [DOI] [Google Scholar]
- 17.Tschinkel W. R. 2004. The nest architecture of the Florida harvester ant, Pogonomyrmex badius. J. Insect Sci. 4, 1–19 10.1672/1536-2442(2004)004[0001:TNAOTF]2.0.CO;2 (doi:10.1672/1536-2442(2004)004[0001:TNAOTF]2.0.CO;2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tschinkel W. R. 2005. The nest architecture of the ant, Camponotus socius. J. Insect Sci. 5, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deneubourg J.-L., Franks N. R. 1995. Collective control without explicit coding: the case of communal nest excavation. J. Insect Behav. 8, 417–432 10.1007/BF01995316 (doi:10.1007/BF01995316) [DOI] [Google Scholar]
- 20.Rasse P., Deneubourg J.-L. 2001. Dynamics of nest excavation and nest size regulation of Lasius niger (Hymenoptera: Formicidae). J. Insect Behav. 14, 433–449 10.1023/A:1011163804217 (doi:10.1023/A:1011163804217) [DOI] [Google Scholar]
- 21.Theraulaz G., Gautrais J., Camazine S., Deneubourg J.-L. 2003. The formation of spatial patterns in social insects: from simple behaviours to complex structures. Phil. Trans. R. Soc. Lond. A 361, 1263–1282 10.1098/rsta.2003.1198 (doi:10.1098/rsta.2003.1198) [DOI] [PubMed] [Google Scholar]
- 22.Buhl J., Gautrais J., Deneubourg J.-L., Theraulaz G. 2004. Nest excavation in ants: group size effect on the size and structure of tunneling networks. Naturwissenschaften 91, 602–604 10.1007/s00114-004-0577-x (doi:10.1007/s00114-004-0577-x) [DOI] [PubMed] [Google Scholar]
- 23.Buhl J., Deneubourg J.-L., Grimal A., Theraulaz G. 2005. Self-organized digging activity in ant colonies. Behav. Ecol. Sociobiol. 58, 9–17 10.1007/s00265-004-0906-2 (doi:10.1007/s00265-004-0906-2) [DOI] [Google Scholar]
- 24.Toffin E., Di Paolo D., Campo A., Detrain C., Deneubourg J.-L. 2009. Shape transition during nest digging in ants. Proc. Natl Acad. Sci. USA 106, 18 616–18 620 10.1073/pnas.0902685106 (doi:10.1073/pnas.0902685106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toffin E., Kindekens J., Deneubourg J.-L. 2010. Excavated substrate modulates growth instability during nest building in ants. Proc. R. Soc. B 277, 2617–2625 10.1098/rspb.2010.0176 (doi:10.1098/rspb.2010.0176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ladley D., Bullock S. 2005. The role of logistic constraints in termite construction of chambers and tunnels. J. Theor. Biol. 234, 551–564 10.1016/j.jtbi.2004.12.012 (doi:10.1016/j.jtbi.2004.12.012) [DOI] [PubMed] [Google Scholar]
- 27.Franks N. R., Gomez N., Goss S., Deneubourg J.-L. 1991. The blind leading the blind in army ant raid patterns: testing a model of self-organization (Hymenoptera: Formicidae). J. Insect Behav. 4, 583–607 10.1007/BF01048072 (doi:10.1007/BF01048072) [DOI] [Google Scholar]
- 28.Tero A., Takagi S., Saigusa T., Ito K., Bebber D. P., Fricker M. D., Yumiki K., Kobayashi R., Nakagaki T. 2010. Rules for biologically inspired adaptive network design. Science 327, 439–442 10.1126/science.1177894 (doi:10.1126/science.1177894) [DOI] [PubMed] [Google Scholar]
- 29.Lagzi I., Siowling S., Wesson P. J., Browne K. P., Grzybowski B. A. 2010. Maze solving by chemotactic droplets. J. Am. Chem. Soc. 132, 1198–1199 10.1021/ja9076793 (doi:10.1021/ja9076793) [DOI] [PubMed] [Google Scholar]
- 30.Kavanagh J. L., Menand T., Sparks R. S. J. 2006. An experimental investigation of sill formation and propagation in layered elastic media. Earth Planet Sci. Lett. 245, 799–813 10.1016/j.epsl.2006.03.025 (doi:10.1016/j.epsl.2006.03.025) [DOI] [Google Scholar]
- 31.Buhl J., Gautrais J., Solé R. V., Kuntz P., Valverde S., Deneubourg J.-L., Theraulaz G. 2004. Efficiency and robustness in ant networks of galleries. Eur. Phys. J. B 42, 123–129 10.1140/epjb/e2004-00364-9 (doi:10.1140/epjb/e2004-00364-9) [DOI] [Google Scholar]
- 32.Buhl J., Gautrais J., Deneubourg J.-L., Kuntz P., Theraulaz G. 2006. The growth and form of tunnelling networks in ants. J. Theor. Biol. 243, 287–298 10.1016/j.jtbi.2006.06.018 (doi:10.1016/j.jtbi.2006.06.018) [DOI] [PubMed] [Google Scholar]
- 33.Perna A., Jost C., Couturier E., Valverde S., Douady S., Theraulaz G. 2008. The structure of gallery networks in the nests of termite Cubitermes spp. revealed by X-ray tomography. Naturwissenschaften 95, 877–884 10.1007/s00114-008-0388-6 (doi:10.1007/s00114-008-0388-6) [DOI] [PubMed] [Google Scholar]
- 34.Perna A., Valverde S., Gautrais J., Jost C., Solé R., Kuntz P., Theraulaz G. 2008. Topological efficiency in three-dimensional gallery networks of termite nests. Phys. A 387, 6235–6244 10.1016/j.physa.2008.07.019 (doi:10.1016/j.physa.2008.07.019) [DOI] [Google Scholar]
- 35.Bebber D. P., Hynes J., Darrah P. R., Boddy L., Fricker M. D. 2007. Biological solutions to transport network design. Proc. R. Soc. B 274, 2307–2315 10.1098/rspb.2007.0459 (doi:10.1098/rspb.2007.0459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buhl J., Hicks K., Miller E. R., Persey S., Alinvi O., Sumpter D. J. T. 2009. Shape and efficiency of wood ant foraging networks. Behav. Ecol. Sociobiol. 63, 451–460 10.1007/s00265-008-0680-7 (doi:10.1007/s00265-008-0680-7) [DOI] [Google Scholar]
- 37.Latty T., Ramsch K., Ito K., Nakagaki T., Sumpter D. J. T., Middendorf M., Beekman M. 2011. Structure and formation of ant transportation networks. J. R. Soc. Interface 8, 1298–1306 10.1098/rsif.2010.0612 (doi:10.1098/rsif.2010.0612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halley J. D., Burd M., Wells P. 2005. Excavation and nest architecture of Argentine ant nests. Insect. Soc. 52, 350–356 10.1007/s00040-005-0818-9 (doi:10.1007/s00040-005-0818-9) [DOI] [Google Scholar]
- 39.Krebs A., Benson B. 1966. Effects of 60Co-gamma radiation on natural digging and tunneling behavior of the ant Pogonomyrmex californicus. Naturwissenschaften 53, 131. 10.1007/BF00643344 (doi:10.1007/BF00643344) [DOI] [PubMed] [Google Scholar]
- 40.Mikheyev A. S., Tschinkel W. R. 2004. Nest architecture of the ant Formica pallidefulva: structure, costs and rules of excavation. Insect. Soc. 51, 30–36 10.1007/s00040-003-0703-3 (doi:10.1007/s00040-003-0703-3) [DOI] [Google Scholar]
- 41.Gordon D. M., Paul R. E., Thorpe K. 1993. What is the function of encounter patterns in ant colonies? Anim. Behav. 45, 1083–1100 10.1006/anbe.1993.1134 (doi:10.1006/anbe.1993.1134) [DOI] [Google Scholar]
- 42.Aleksiev A. S., Longdon B., Christmas M. J., Sendova-Franks A. B., Franks N. R. 2008. Individual and collective choice: parallel prospecting and mining in ants. Naturwissenschaften 95, 301–305 10.1007/s00114-007-0329-9 (doi:10.1007/s00114-007-0329-9) [DOI] [PubMed] [Google Scholar]
- 43.Franks N. R., Healey K. J., Byrom L. 1991. Studies on the relationship between the ant ectoparasite Antennophorus grandis (Acarina: Antennophoridae) and its host Lasius flavus (Hymenoptera: Formicidae). J. Zool. 225, 59–70 10.1111/j.1469-7998.1991.tb03801.x (doi:10.1111/j.1469-7998.1991.tb03801.x) [DOI] [Google Scholar]