Abstract
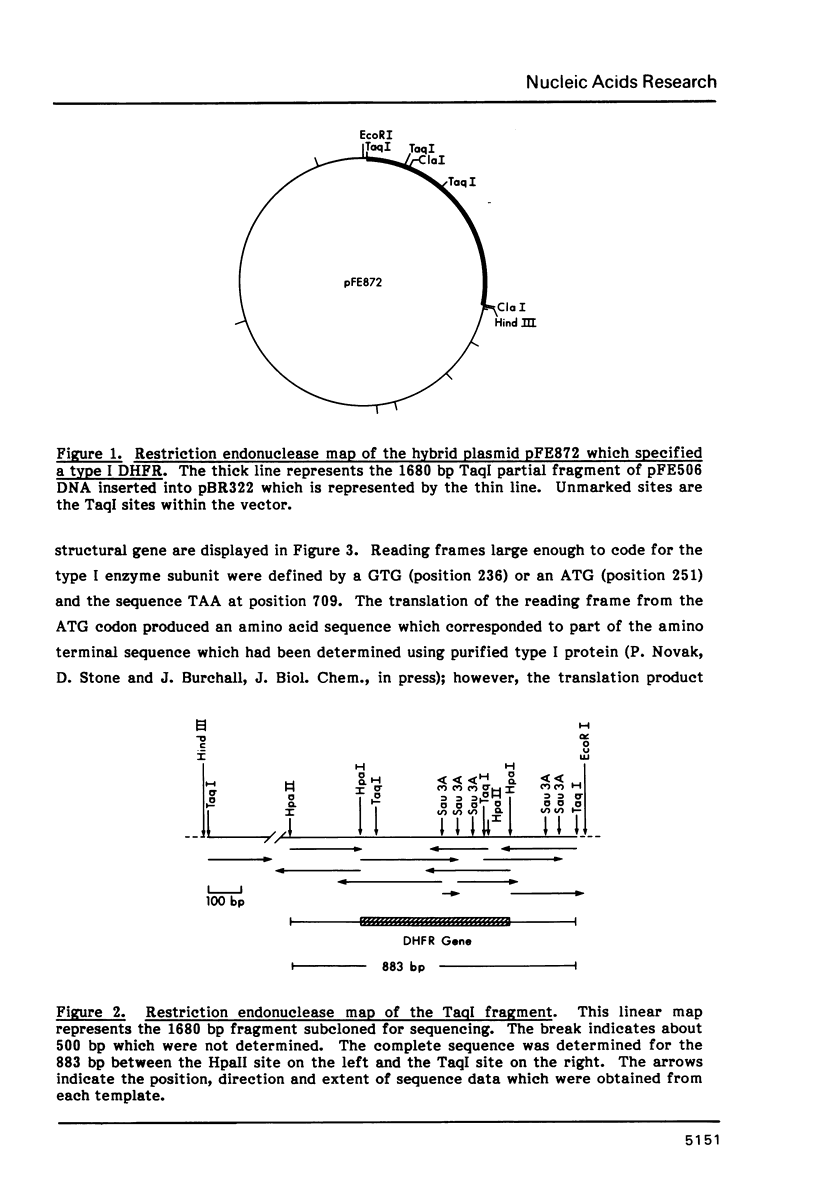
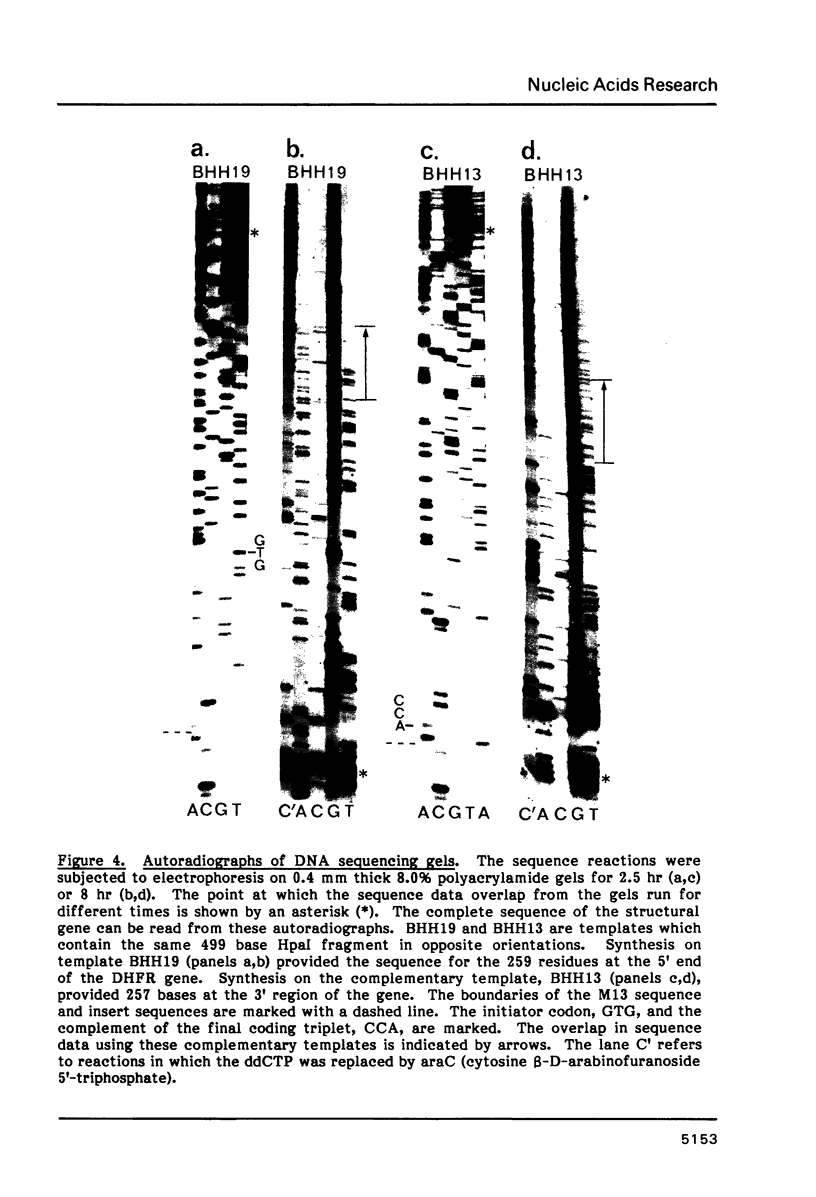
The complete nucleotide sequence of the type I dihydrofolate reductase gene from Tn7 was determined. The structural gene coded for a polypeptide of 157 amino acid residues. The polypeptide deduced from the DNA sequence had a molecular weight of 17,577 which was in good agreement with that estimated by mobility in SDS-polyacrylamide gels. Sequences were identified proximal to the coding region which were similar to those found in the consensus E. coli promoter region and for the initiation of protein synthesis. Features consistent with the termination of RNA transcription were present distal to the structural gene. No homology was apparent when the DNA sequence of the type I gene was compared to the sequence of the type II plasmid DHFR genes, but sequence homology was evident when the type I and E. coli chromosomal enzymes were compared. Homology was greatest in the regions coding for amino acids which in the E. coli chromosomal enzyme are associated with substrate, cofactor and inhibitor binding.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth P. T., Datta N., Hedges R. W., Grinter N. J. Transposition of a deoxyribonucleic acid sequence encoding trimethoprim and streptomycin resistances from R483 to other replicons. J Bacteriol. 1976 Mar;125(3):800–810. doi: 10.1128/jb.125.3.800-810.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitar K. G., Blankenship D. T., Walsh K. A., Dunlap R. B., Reddy A. V., Freisheim J. H. Amino acid sequence of dihydrofolate reductase from an amethopterin-resistant strain of Lactobacillus casei. FEBS Lett. 1977 Aug 1;80(1):119–122. doi: 10.1016/0014-5793(77)80420-1. [DOI] [PubMed] [Google Scholar]
- Blumenthal R. M., Rice P. J., Roberts R. J. Computer programs for nucleic acid sequence manipulation. Nucleic Acids Res. 1982 Jan 11;10(1):91–101. doi: 10.1093/nar/10.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Datta N., Dacey S., Hughes V., Knight S., Richards H., Williams G., Casewell M., Shannon K. P. Distribution of genes for trimethoprim and gentamicin resistance in bacteria and their plasmids in a general hospital. J Gen Microbiol. 1980 Jun;118(2):495–508. doi: 10.1099/00221287-118-2-495. [DOI] [PubMed] [Google Scholar]
- Datta N., Nugent M., Richards H. Transposons encoding trimethoprim or gentamicin resistance in medically important bacteria. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):45–51. doi: 10.1101/sqb.1981.045.01.009. [DOI] [PubMed] [Google Scholar]
- Fling M. E., Elwell L. P. Protein expression in Escherichia coli minicells containing recombinant plasmids specifying trimethoprim-resistant dihydrofolate reductases. J Bacteriol. 1980 Feb;141(2):779–785. doi: 10.1128/jb.141.2.779-785.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling M. E., Walton L., Elwell L. P. Monitoring of plasmid-encoded, trimethoprim-resistant dihydrofolate reductase genes: detection of a new resistant enzyme. Antimicrob Agents Chemother. 1982 Nov;22(5):882–888. doi: 10.1128/aac.22.5.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey D., Hamilton-Miller J. M., Brumfitt W. Incidence and mechanisms of resistance to trimethoprim in clinically isolated gram-negative bacteria. Chemotherapy. 1979;25(3):147–156. doi: 10.1159/000237834. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A. A., Blankenship D. T., Kaufman B. T., Freisheim J. H. Primary structure of chicken liver dihydrofolate reductase. Biochemistry. 1980 Feb 19;19(4):667–678. doi: 10.1021/bi00545a010. [DOI] [PubMed] [Google Scholar]
- Lau P. C., Spencer J. H. An efficient synthetic primer for the M13 cloning dideoxy sequencing system. Biosci Rep. 1982 Sep;2(9):687–696. doi: 10.1007/BF01114830. [DOI] [PubMed] [Google Scholar]
- Lichtenstein C., Brenner S. Site-specific properties of Tn7 transposition into the E. coli chromosome. Mol Gen Genet. 1981;183(2):380–387. doi: 10.1007/BF00270644. [DOI] [PubMed] [Google Scholar]
- Matthews D. A., Alden R. A., Bolin J. T., Filman D. J., Freer S. T., Hamlin R., Hol W. G., Kisliuk R. L., Pastore E. J., Plante L. T. Dihydrofolate reductase from Lactobacillus casei. X-ray structure of the enzyme methotrexate.NADPH complex. J Biol Chem. 1978 Oct 10;253(19):6946–6954. [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Weber H., Meyer F., Weissmann C. Site-directed mutagenesis in DNA: generation of point mutations in cloned beta globin complementary dna at the positions corresponding to amino acids 121 to 123. J Mol Biol. 1978 Sep 15;124(2):343–358. doi: 10.1016/0022-2836(78)90303-0. [DOI] [PubMed] [Google Scholar]
- Pattishall K. H., Acar J., Burchall J. J., Goldstein F. W., Harvey R. J. Two distinct types of trimethoprim-resistant dihydrofolate reductase specified by R-plasmids of different compatibility groups. J Biol Chem. 1977 Apr 10;252(7):2319–2323. [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier P. H., Cortese R. A fast and simple method for sequencing DNA cloned in the single-stranded bacteriophage M13. J Mol Biol. 1979 Mar 25;129(1):169–172. doi: 10.1016/0022-2836(79)90068-8. [DOI] [PubMed] [Google Scholar]
- Sheldon R., Brenner S. Regulatory mutants of dihydrofolate reductase in Escherichia coli K12. Mol Gen Genet. 1976 Aug 10;147(1):91–97. doi: 10.1007/BF00337941. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sköld O., Widh A. A new dihydrofolate reductase with low trimethoprim sensitivity induced by an R factor mediating high resistance to trimethoprim. J Biol Chem. 1974 Jul 10;249(13):4324–4325. [PubMed] [Google Scholar]
- Smith A. J. DNA sequence analysis by primed synthesis. Methods Enzymol. 1980;65(1):560–580. doi: 10.1016/s0076-6879(80)65060-5. [DOI] [PubMed] [Google Scholar]
- Smith D. R., Calvo J. M. Nucleotide sequence of the E coli gene coding for dihydrofolate reductase. Nucleic Acids Res. 1980 May 24;8(10):2255–2274. doi: 10.1093/nar/8.10.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone D., Smith S. L. The amino acid sequence of the trimethoprim-resistant dihydrofolate reductase specified in Escherichia coli by R-plasmid R67. J Biol Chem. 1979 Nov 10;254(21):10857–10861. [PubMed] [Google Scholar]
- Swift G., McCarthy B. J., Heffron F. DNA sequence of a plasmid-encoded dihydrofolate reductase. Mol Gen Genet. 1981;181(4):441–447. doi: 10.1007/BF00428733. [DOI] [PubMed] [Google Scholar]
- Towner K. J. A clinical isolate of Escherichia coli owing its trimethoprim resistance to a chromosomally-located trimethoprim transposon. J Antimicrob Chemother. 1981 Feb;7(2):157–162. doi: 10.1093/jac/7.2.157. [DOI] [PubMed] [Google Scholar]
- Towner K. J., Venning B. M., Pinn P. A. Occurrence of transposable trimethoprim resistance in clinical isolates of Escherichia coli devoid of self-transmissible resistance plasmids. Antimicrob Agents Chemother. 1982 Feb;21(2):336–338. doi: 10.1128/aac.21.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Volz K. W., Matthews D. A., Alden R. A., Freer S. T., Hansch C., Kaufman B. T., Kraut J. Crystal structure of avian dihydrofolate reductase containing phenyltriazine and NADPH. J Biol Chem. 1982 Mar 10;257(5):2528–2536. [PubMed] [Google Scholar]
- Zolg J. W., Hänggi U. J. Characterization of a R plasmid-associated, trimethoprim-resistant dihydrofolate reductase and determination of the nucleotide sequence of the reductase gene. Nucleic Acids Res. 1981 Feb 11;9(3):697–710. doi: 10.1093/nar/9.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]




