Abstract
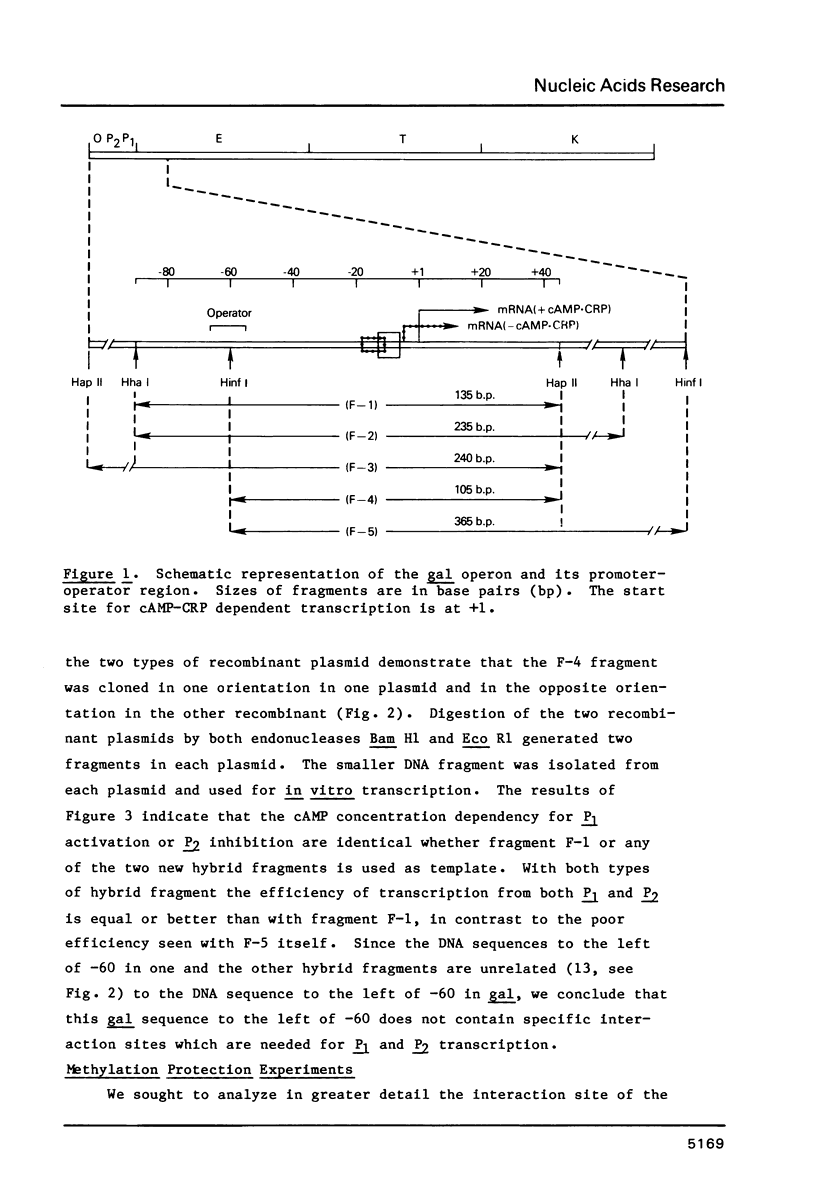
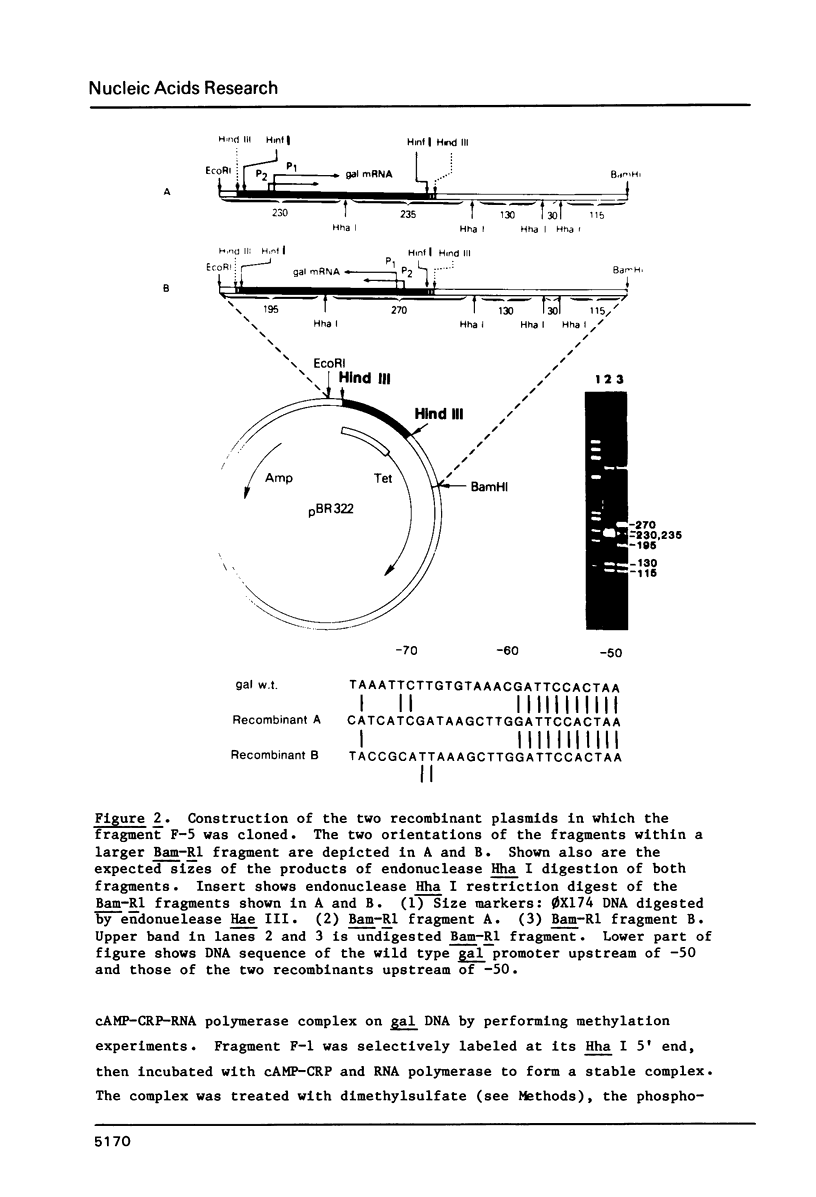
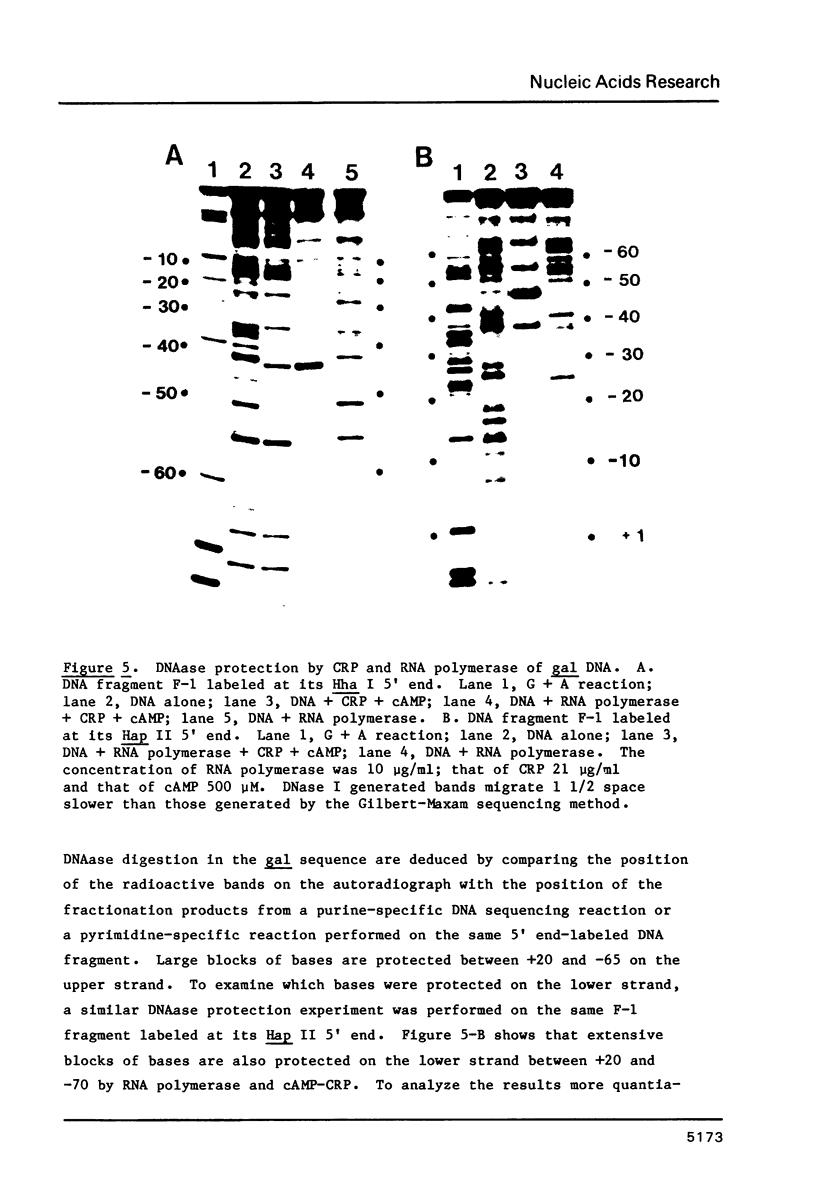
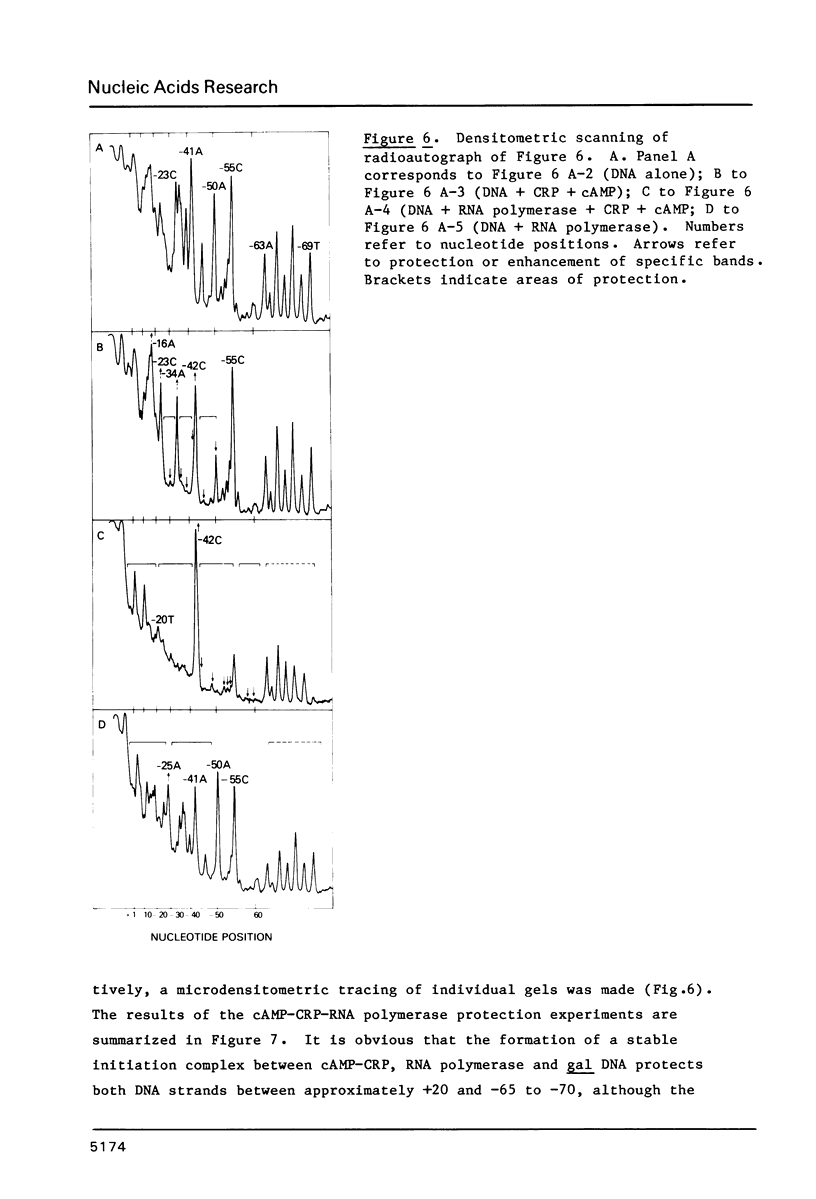
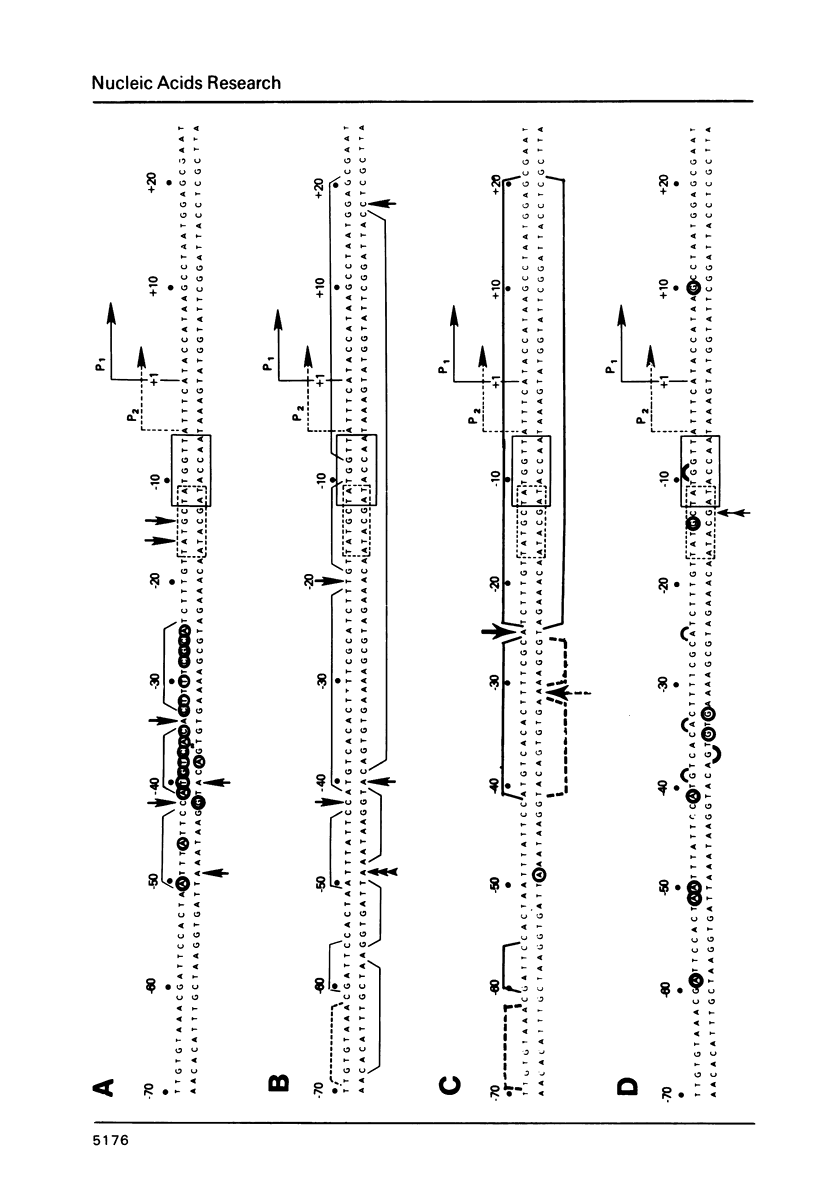
We have examined the interaction site on gal DNA for the cyclic AMP receptor protein and RNA polymerase when both are present together to form a stable initiation complex at the P1 gal promoter. Substitution of the bases to the left of -60 by unrelated DNA sequences does not change the cyclic AMP concentration dependency for in vitro transcription at P1 and inhibition of P2. Although the presence of some DNA to the left of -60 appears to be needed for efficient in vitro transcription at P1, the gal sequence to the left of -60 does not provide any specific interactions for transcription initiation at P1. Similarly, efficient in vitro transcription from P2 also requires non-specific DNA sequences to the left of -60. We have also examined which bases were protected by RNA polymerase and CRP together from the action of DNAase and dimethylsulfate. Some of the interactions that take place when cAMP-CRP alone interacts with gal DNA appear to be preserved in the cAMP-CRP-RNA polymerase-gal DNA complex, suggesting that CRP occupies the same site in the DNA when it is alone or together with RNA polymerase. Our results suggest that the formation of an open complex at different promoters can result from different interaction patterns between RNA polymerase and promoter DNA.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Busby S., Aiba H., de Crombrugghe B. Mutations in the Escherichia coli operon that define two promoters and the binding site of the cyclic AMP receptor protein. J Mol Biol. 1982 Jan 15;154(2):211–227. doi: 10.1016/0022-2836(82)90061-4. [DOI] [PubMed] [Google Scholar]
- DiLauro R., Taniguchi T., Musso R., de Crombrugghe B. Unusual location and function of the operator in the Escherichia coli galactose operon. Nature. 1979 Jun 7;279(5713):494–500. doi: 10.1038/279494a0. [DOI] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsrud L. Contacts between Escherichia coli RNA polymerase and a lac operon promoter. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5314–5318. doi: 10.1073/pnas.75.11.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kolb A., Buc H. Is DNA unwound by the cyclic AMP receptor protein? Nucleic Acids Res. 1982 Jan 22;10(2):473–485. doi: 10.1093/nar/10.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb A., Busby S., Herbert M., Kotlarz D., Buc H. Comparison of the binding sites for the Escherichia coli cAMP receptor protein at the lactose and galactose promoters. EMBO J. 1983;2(2):217–222. doi: 10.1002/j.1460-2075.1983.tb01408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure W. R. Rate-limiting steps in RNA chain initiation. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5634–5638. doi: 10.1073/pnas.77.10.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso R. E., Di Lauro R., Adhya S., de Crombrugghe B. Dual control for transcription of the galactose operon by cyclic AMP and its receptor protein at two interspersed promoters. Cell. 1977 Nov;12(3):847–854. doi: 10.1016/0092-8674(77)90283-5. [DOI] [PubMed] [Google Scholar]
- Saxe S. A., Revzin A. Cooperative binding to DNA of catabolite activator protein of Escherichia coli. Biochemistry. 1979 Jan 23;18(2):255–263. doi: 10.1021/bi00569a003. [DOI] [PubMed] [Google Scholar]
- Shanblatt S. H., Revzin A. Two catabolite activator protein molecules bind to the galactose promoter region of Escherichia coli in the presence of RNA polymerase. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1594–1598. doi: 10.1073/pnas.80.6.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Simpson R. B. Contacts between Escherichia coli RNA polymerase and thymines in the lac UV5 promoter. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3233–3237. doi: 10.1073/pnas.76.7.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., O'Neill M., de Crombrugghe B. Interaction site of Escherichia coli cyclic AMP receptor protein on DNA of galactose operon promoters. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5090–5094. doi: 10.1073/pnas.76.10.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Sobel M. E., Adams S. L., Avvedimento V. E., DiLauro R., Pastan I., de Crombrugghe B., Showalter A., Pesciotta D., Fietzek P. Construction of a recombinant bacterial plasmid containing pro-alpha 1(I) collagen DNA sequences. J Biol Chem. 1980 Mar 25;255(6):2612–2615. [PubMed] [Google Scholar]