Abstract
The hydrolysis of α-chloro-N-methyl-4-pyridone was found to be more than five times faster than that of α-chloro-N-methyl-2-pyridone. Structural studies of 2- and 4-pyridones have revealed the higher polarity and greater extent of zwitterionic content in 4-pyridone. The results are thus consistent with the hypothesis that polarization and higher zwitterionic content in the heterocyclic structures enhances the rate of hydrolysis in α-substituted pyridone and uracil derivatives.
Keywords: 2-pyridone, 4-pyridone, dipole moment, polarization, zwitterions, ODCase
1. Introduction
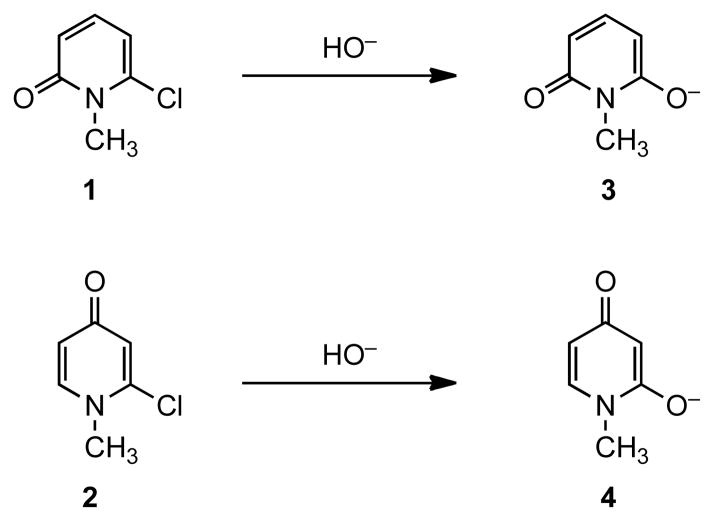
We have recently investigated the hydrolysis of α-halo and α-cyanopyridinium compounds as a model for the conversion of 6-cyanouridine 5′-monophosphate (6-cyanoUMP) to β-D-ribofuranosylbarbiturate 5′-monophosphate catalyzed by orotidine 5′-monophosphate decarboxylase (ODCase).1,2 The positively charged α-substituted pyridinium derivatives were found to undergo hydrolysis with a rate of up to 105-fold greater than those of neutral species. We have hypothesized that ODCase catalyzes the hydrolysis of 6-cyanoUMP by binding the substrate in the polar zwitterionic form and thus increasing the electrophilicity of the heterocyclic structure. According to this hypothesis, substrates with more polar structures and thus more zwitterionic character should be more reactive towards nucleophilic attack. In this Letter, we compare the respective transformation of α-chloro-substituted 2- and 4-pyridones (1 and 2) to 3 and 4 and report that the hydrolysis of 4-pyridone 2 is facilitated as a result of greater contribution of the zwitterionic resonance form to its structure.
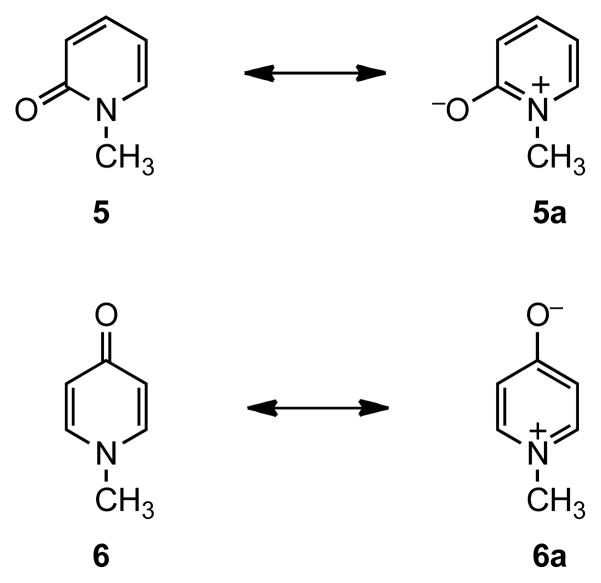
It has been reported that 4-pyridone is much more polar than 2-pyridone due to the greater contribution from its polar and zwitterionic resonance structures.3 The structures of N-methyl-2-pyridone 5 and N-methyl-4-pyridone 6 and their corresponding zwitterions 5a and 6a are shown in Figure 2. The different degrees of contribution from zwitterionic structures 5a and 6a to the overall structure of 2- and 4-pyridone are consistent with the differences in their molecular properties shown in Table 1. The carbonyl oxygen in 4-pyridone is much more basic than that in 2-pyridone. For example, the conjugate acid of the non-methylated analogue 4-pyridone has a higher pKa than that of the corresponding 2-pyridone.4–6 The gas-phase proton affinity of the carbonyl oxygen in 4-pyridone 6 is also higher than that in 2-pyridone 5.7 Furthermore, the dipole moment of 4-pyridone 6 is found to be larger than that of 2-pyridone 5.8,9 Infrared (IR) and Raman spectroscopic studies of 2- and 4-pyridones and their analogues have indicated that the structure of 2-pyridone is consistent with the lactam structure in 5 whereas the structure of 4-pyridone contains up to 10–15% contribution from the dipolar zwitterionic structure such as 6a.10,11
Figure 2.
Resonance Structures of 2- and 4-Pyridones
Table 1.
Kinetic and Thermodynamic Parameters for the Hydrolysis of α-Chloropyridones and Molecular Properties of Pyridones
| Pyridones | 1 | 2 |
|---|---|---|
| kOD at 1.0 M NaOD at 50 °C (s−1) | 1.4 x 10−5 | 7.2 x 10−5 |
| ΔG‡ (kcal/mol) | 25.9 | 24.8 |
| ΔH‡ (kcal/mol) | 22.6 | 21.2 |
| ΔS‡ (cal/mol/K) | −11.0 | −12.1 |
| pKa of conjugate acida | 0.7; 1.25 | 3.27 |
| Proton affinity (kcal/mol)b | 222.3; 223.0 | 233.1; 234.2 |
| Dipole moment in benzene (D)b | 4.15; 4.04 | 6.9 |
The pKa values are those reported for the conjugate acids of non-methylated parent compounds, 2-pyridone and 4-pyridone. The references are given in the main text.
These molecular properties refer to those of parent pyridones 5 and 6. The references are given in the main text.
The correlation between the greater rate of decarboxylation of N-Methyl-4-pyridone-2-carboxylic acid as compared to N-methyl-2-pyridone-6-carboxylic acid and the relative stability of the zwitterionic structures derived from 4-pyridone has been well documented.12,13 The enhanced contribution of the zwitterionic intermediate has been shown to be the key factor in the facile decarboxylation of the 4-pyridone-derived carboxylic acid.12 In order to understand how the structural difference in 2- and 4-pyridones affect their reactivity in the hydrolysis reaction and to test our hypothesis, we decided to investigate the hydrolysis of 6-chloro-N-methyl-2-pyridone (1) and 2-chloro-N-methyl-4-pyridone (2). According to our hypothesis, the more polarized structure of 4-pyridone should lend 4-pyridone 2 more reactivity than 2-pyridone 1. These two pyridone derivatives were synthesized as reported.14 The hydrolysis reactions were carried out in 1.0 M NaOD in D2O. The reactions were monitored using NMR spectroscopy to follow the disappearance of the substrates through integration of the pyridine proton absorptions. Sodium terephthalate and sodium maleate were added as respective external standards in the hydrolysis of 1 and 2. The pseudo first-order rate constants, kOD, for pyridones 1 and 2 thus measured in a 1.0 M solution of NaOD in D2O are 1.4 x 10−5 and 7.2 x 10−5 s−1, respectively, as shown in Table 1.
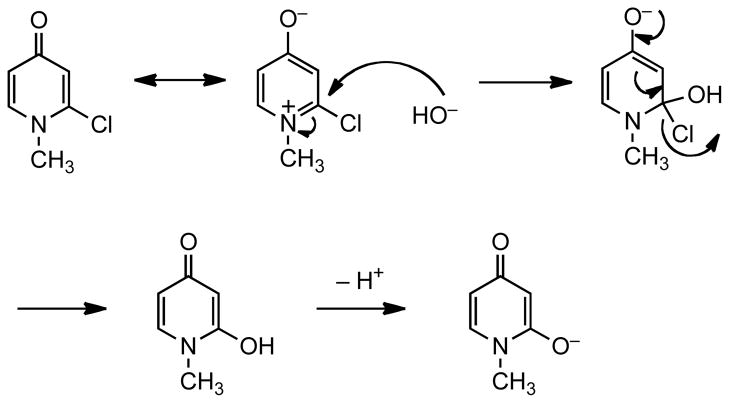
From the comparison of the rate constants, it is clear that the greater zwitterionic contribution to the structure of 4-pyridone enhances the reaction rates by more than five-fold. The proposed mechanism for the reaction of 4-pyridone 2 is shown in Figure 3. An analogous mechanism can be drawn for the reaction of 2-pyridone 1. The mechanism involves nucleophilic attack on the α-carbon to form the tetrahedral intermediate followed by the departure of the leaving group. Our earlier study has shown that the first step is likely to be the rate-determining step and that the electron density on the heterocyclic structure affect the rate of hydrolysis of α-substituted pyridinium compounds.1 The thermodynamic parameters of the reactions were determined as shown in Table 1. The nearly identical ΔS‡ means that the two reactions are likely to have similar reaction steps and thus similar mechanisms. It can be argued that the difference in ΔG‡ is mainly a result of the difference in ΔH‡, although the small difference in these parameters makes it difficult to compare the energetics of the reactions.
Figure 3.
Proposed mechanisms for the base-catalyzed hydrolysis of α-Chloro-N-methyl-4-Pyridone
The results obtained are consistent with the hypothesis that greater contribution from zwitterionic resonance forms will enhance hydrolysis of α-substituted pyridone derivatives. If the substrate 6-cyanoUMP is bound to ODCase in the zwitterionic form, its accelerated hydrolysis can be accounted for.1 The formation of a zwitterionic intermediate can also explain the decarboxylation of orotidine 5′-monophosphate (OMP) catalyzed by ODCase, although proton transfer is clearly not involved as originally proposed.12,13 However, the zwitterionic structure can be stabilized through dipole interactions by a pre-organized polar environment at the active site due to its charge distribution.1,15–18 We are currently investigating the polarity of the active site and its effect on the structure of substrate analogues.
Figure 1.
Hydrolysis of α-Chloro-Substituted 2-Pyridone and 4-Pyridone
Acknowledgments
This investigation was supported by the National Institutes of Health, MBRS SCORE Program – Grant S06 GM52588. The NMR facility was funded by the National Science Foundation (DUE-9451624 and DBI 0521342). RCT is supported by a RISE scholarship. In addition, we are indebted to Professor James Keeffe at SFSU for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Footnotes
- 1.Huang S, Wong FM, Gassner GT, Wu W. Tetrahedron Lett. 2011;52:3960–3962. doi: 10.1016/j.tetlet.2011.05.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujihashi M, Bello AM, Poduch E, Wei L, Annedi SC, Pai EF, Kotra LP. J Am Chem Soc. 2005;127:15048–15050. doi: 10.1021/ja054865u. [DOI] [PubMed] [Google Scholar]
- 3.Tieckelmann H. In: Pyridine and Its Derivatives. Supplement Part 3. Abramovitch RA, editor. Vol. 14. Wiley; New York: 1974. pp. 731–744. and references cited therein. [Google Scholar]
- 4.Christensen JJ, Hansen LD, Izatt RM. Hanbook of Proton Ionization Heats and Related Thermodynamic Quatities. Wiley; New York: 1976. pp. 176–203. [Google Scholar]
- 5.Albert A. In: Physical Methods in Heterocyyclic Chemistry. Katritzky AR, editor. Academic; New York: 1963. pp. 79–89. [Google Scholar]
- 6.Jencks WP, Reglenstein J. In: Handbook of Biochemistry and Molecular Biology. Fasman G, editor. CRC; Cleveland: 1976. p. 338. [Google Scholar]
- 7.Gronert S, Feng WY, Chew F, Wu W. Int J Mass Spectrom. 2000;195/196:251–258. [Google Scholar]
- 8.Albert A, Philips JN. J Chem Soc. 1956:1294–1304. [Google Scholar]
- 9.Krackov MH, Lee CM, Mautner HG. J Am Chem Soc. 1965;87:892–896. [Google Scholar]
- 10.Keller GH, Bauer L, Bell CL. Can J Chem. 1968;46:2475–2479. [Google Scholar]
- 11.Batts BD, Spinner E. Aust J Chem. 1969;22:2581–2593. [Google Scholar]
- 12.Feng WY, Austin TJ, Chew F, Gronert S, Wu W. Biochemistry. 2000;39:1778–1783. doi: 10.1021/bi992553w. [DOI] [PubMed] [Google Scholar]
- 13.Beak P, Siegel B. J Am Chem Soc. 1976;98:3601–3606. doi: 10.1021/ja00428a035. [DOI] [PubMed] [Google Scholar]
- 14.Katricky AR, Rowe JD, Roy SK. J Chem Soc (B) 1967:758–761. [Google Scholar]
- 15.Wu N, Pai EF. J Biol Chem. 2002;277:28080–28087. doi: 10.1074/jbc.M202362200. [DOI] [PubMed] [Google Scholar]
- 16.Warshel A, Strajbl M, Villa J, Florian J. Biochemistry. 2000;39:14728–14738. doi: 10.1021/bi000987h. [DOI] [PubMed] [Google Scholar]
- 17.Wong FW, Capule CC, Wu W. Org Lett. 2006;8:6019–6022. doi: 10.1021/ol0624981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang S, Gronert S, Wu W. Bioorg Med Chem Lett. 2011;21:6341–6342. doi: 10.1016/j.bmcl.2011.08.109. [DOI] [PMC free article] [PubMed] [Google Scholar]