Abstract
The relationship between blood viscosity, hematocrit (Hct), and mean arterial blood pressure (MAP) was studied in a healthy population of 91 men and 66 women with an average age of 30.6 ± 8.0 years, from the city of Victoria de Durango (1800 m elevation). In women and men, Hct values were 42.4% ± 2.9% and 47.2% ± 2.3%, blood viscosities were 4.5 ± 0.7 and 6.1 ± 1.0 cP, and MAP was 83.0 ± 6.8 and 88.0 ± 6.1 mmHg, respectively. The correlation between blood viscosity and Hct was linear and positive (r2 = 0.48) and identical to that of previous studies reported in the literature when men and women are taken as a single group. Separating the data by gender yielded positive, linear correlations (r2 = 0.18 and 0.10, respectively) with identical slopes, however blood viscosity for men was 1.2 cP greater than in women (P = 0.02). MAP and blood viscosity (and Hct) were not statistically associated when men and women were analyzed separately and were weakly positively correlated (r2 = 0.08, P < 0.02) when treated as a group. The present results suggest that studies that show a positive correlation between MAP and blood viscosity (and Hct) do not differentiate data according to gender, or involve populations that do not compensate for increased blood viscosity and potentially increased shear stress.
Keywords: blood pressure, blood viscosity, hematocrit, gender, endothelial dysfunction
Introduction
The perception that blood pressure is universally positively correlated with blood viscosity is embodied in the statement: “In conclusion, our study provides good evidence of a strong association between blood viscosity and arterial pressure, independently of many possible confounding factors,” summarizing the findings of the Edinburgh Artery Study, which evaluated the blood pressure/viscosity association in 1592 men and women aged 55–74 years.1 This same conclusion emerges from studies on the relationship between hypertension and blood rheological changes.2 However, it is possible that in these studies the cardiovascular system presents some form of regulatory impairment, due to either age or incipient disease, which hinders its blood pressure regulatory ability vis-a-vis changes in blood viscosity.
Although it is not entirely satisfactory to compare animal and human physiology, normal healthy awake hamsters respond paradoxically to acute changes in hematocrit (Hct) and therefore blood viscosity.3 In these studies, small increases in Hct lower blood pressure and vice versa due to the effect of blood viscosity and shear stress on the management of nitric oxide (NO) production by the endothelium.4 Although it is commonly accepted that sustained hyperviscosity can decrease perfusion and increase blood pressure, it should be noted that increased blood viscosity has two effects in the cardiovascular system: it may act to increase shear stress on the endothelium and increase NO release, promoting vasodilation as well as an increase in the viscous component of vascular resistance. Thus increased blood viscosity can cause vasodilation, which has a large non-linear effect in lowering peripheral vascular resistance that counteracts the increase due to viscosity.5
The relationship between blood pressure and blood viscosity can be explored by performing transversal population studies in healthy subjects, taking advantage of the naturally occurring variability in Hct and therefore blood viscosity. The variability in Hct arises from genetic factors, gender, diet, environmental conditions, exercise, season, time of the year, and age and leads to corresponding variability in blood viscosity, as reported by Kameneva et al,6 who also measured the dependence of blood viscosity on Hct for a population at sea level.
The present study was undertaken to test the hypothesis that blood pressure is independent of blood viscosity and Hct in a population presumed healthy according to medical history, anthropometric measurements, and blood parameters. Blood viscosity is a parameter which is technically difficult to measure: however it is a direct and strong function of Hct, which is relatively easy to determine, therefore we also analyzed the association between blood viscosity and Hct, to relate our findings to studies that investigated the blood pressure/Hct association. The study was conducted in the population of the city Victoria de Durango, in Durango, Mexico, which has fairly uniform ethnic characteristics, eating habits, and lifestyle. The city is at 1800 m elevation.
Materials and methods
A transversal study was carried out to determine the distribution of Hct levels in healthy females and males in the city of Victoria de Durango.
The protocol was approved by the Local Ethics and Investigation Committee of the Medical Faculty (UJED). Inclusion of individuals required their agreement to participate in the study and completion of a signed consent form. Individuals were excluded from the study by the following criteria: having been previously diagnosed with a blood disorder ischemic cardiovascular disease, respiratory diseases, neoplasia, renal or hepatic disease, or rheumatic diseases; alcohol consumption (defined as ≥30 g per week); pregnancy; lactation; smoking; or having had major surgery or a blood transfusion within the past 6 months.
The health status of each individual was determined following evaluation of his or her medical history. Anthropometric measurements taken were: weight ( participant in light clothing); height; waist circumference (tape placed at level of the navel and at the level of iliac crests); and blood pressure (according to the standards and recommendations of the Joint National Committee 1997). Mean arterial pressure (MAP) was determined using the relation:
Blood samples (12 mL) were obtained from the antecubital vein, after 8–10 hours of fasting, and collected in 3 mL EDTA anticoagulated tubes. Hematocrit was measured by microhematocrit centrifuge (3000 rpm, 20°C, Sun-Bat Centrifuge M-600, Readacrit L-10; BD Clay Adams, Franklin Lakes, NJ).
Five milliliter blood samples were taken in tubes without anticoagulants for glucose, cholesterol, triglycerides, high density lipoprotein (HDL), and low density lipoprotein (LDL) measurements.
Blood and plasma viscosities were determined with a cone and plate Brookfield viscometer (Model Dv-II; Brookfield Engineering Laboratories, Middleboro, MA) at a shear rate of 160 s−1 at 37°C.
Statistical analysis
Results are presented as mean ± SD unless otherwise noted. Data within each group were analyzed using ANOVA. Changes were considered statistically significant if P < 0.05. The data was fitted to a straight line. Results were compared by means of the F-test, and considered to be different if the F-test indicated a significantly smaller sum of squares for the deviations in each individual fit compared to the deviation in the fit to the pooled data.7 Analysis was made using Prism (GraphPad Software Inc, La Jolla, CA).
Results
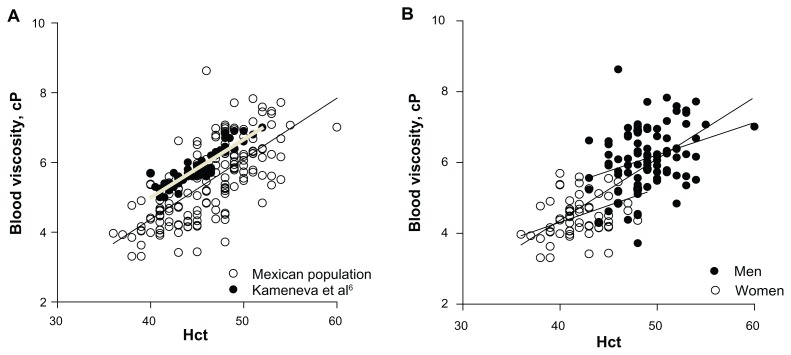
A total of 157 individuals (66 women and 91 men) completed the study. Anthropometric data and blood biochemistry are shown in Tables 1 and 2. The data on blood viscosity was linearly correlated with Hct (r2 = 0.50, P < 0.0001) when men and women were treated as a single group. The trend found in the present study had a slope of 0.199 ± 0.014 and was practically identical to that found by Kameneva et al6 (slope 0.166 ± 0.010), but our results were consistently lower by approximately 1 cP as shown in Figure 1A.
Table 1.
Anthropometric data
| Women | Men | |
|---|---|---|
| N | 66 | 91 |
| Age (years) | 30.6 ± 7.7 | 30.3 ± 7.6 |
| Weight (kg)* | 61.2 ± 8.3 | 83.1 ± 6.8 |
| Height (m)* | 1.50 ± 0.60 | 1.75 ± 0.05 |
| BMI* | 24.0 ± 2.8 | 27.0 ± 2.1 |
| MAP (mmHg)* | 83.0 ± 6.8 | 88.0 ± 6.1 |
Note: Statistically significantly different, P < 0.05.
Abbreviations: BMI, body mass index; MAP, mean arterial pressure.
Table 2.
Blood parameters
| Women | Men | |
|---|---|---|
| Hematocrit (%)* | 42.4 ± 2.9 | 49.0 ± 3.0 |
| Glucose (mg/dL) | 85.0 ± 7.5 | 89.7 ± 9.1 |
| Cholesterol (mg/dL) | 171 ± 33 | 181 ± 39 |
| Triglycerides (mg/dL) | 118 ± 58 | 167 ± 102 |
| HDL (mg/dL) | 52 ± 16 | 45 ± 11 |
| LDL (mg/dL) | 101 ± 27 | 109 ± 32 |
| Blood viscosity (cP)* | 4.50 ± 0.65 | 6.11 ± 0.95 |
| Plasma viscosity (cP) | 1.70 ± 0.36 | 1.67 ± 0.29 |
Note: Statistically significantly different, P < 0.05.
Abbreviations: HDL, high density lipoprotein; LDL, low density lipoprotein.
Figure 1.
(A) Blood viscosity versus Hct for men and women in the tested population. Comparison with the data of Kameneva et al.6 The two data sets are statistically significantly correlated and the slopes of the regression lines are statistically identical. (B) Blood viscosity versus Hct for the population of this study according to gender. Comparison with (A). Upper line corresponds to men, and the lower line to women. The regression lines for men and women have statistically identical slopes.
Abbreviation: Hct, hematocrit.
Analyzing men and women separately also showed a strong correlation between blood pressure and blood viscosity with both groups exhibiting essentially identical trends (slopes 0.091 ± 0.029, P < 0.002 vs 0.095 ± 0.025, P < 0.0004 respectively), which were statistically significantly different from the slope of the trend for both men and women treated as a group as shown in Figure 1B.
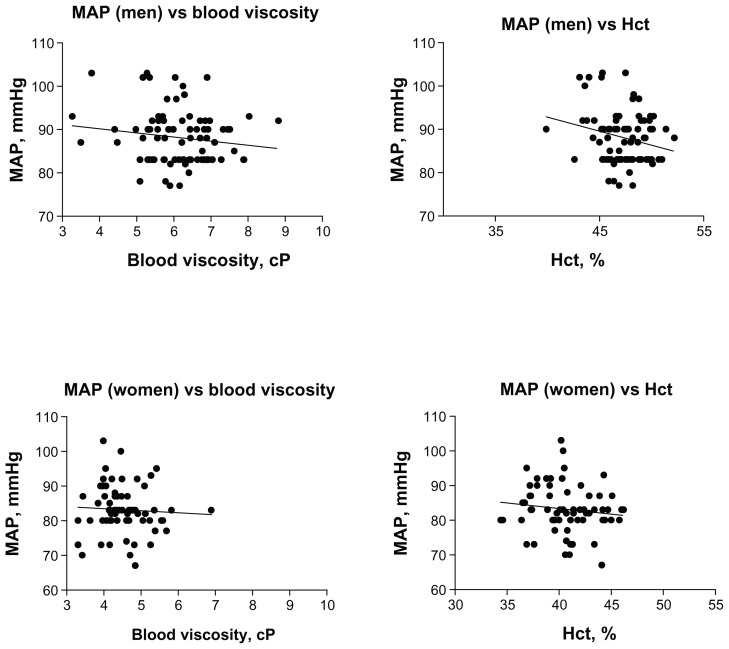
MAP was uncorrelated to blood viscosity for both men and women studied separately, as shown in Figure 2. In all groupings the trends were negative (lowering blood pressure with increasing blood viscosity). MAP and blood viscosity were weakly correlated (r2 = 0.06, P < 0.02) when men and women were treated as a group.
Figure 2.
Trends in the relationship between mean arterial blood pressure and blood viscosity and Hct.
Note: All trends are negative, however none are statistically significant, with the exception of the trend of MAP versus Hct for men, which shows a weak negative linear correlation (r2 = 0.06, P = 0.03).
Abbreviations: Hct, hematocrit; MAP, mean arterial pressure.
Blood viscosity in women was uncorrelated to plasma viscosity (P = 0.75), while these two parameters were positively and weakly correlated for men (r2 = 0.19, P < 0.0001).
None of the blood biochemistry parameters were statistically correlated to MAP.
Discussion
The principal finding of this study is that MAP is independent of blood viscosity in a population of healthy individuals segregated by gender, the data showing a non-statistically significant negative trend. When both sets of data are pooled, the trend becomes positive since women have a statistically lower Hct (blood viscosity) and MAP than men. This result is consistent with the findings of de Simone et al8 who found a negative correlation between the Hct and blood viscosity and pulse pressure in a population of American Indians 62 ± 7 years of age without prevalent cardiovascular disease or use of antihypertensive medications, digoxin, or aspirin. In a study of 128 normotensive members of a large employed population in New York City (51 ± 10 years) there was a significant independent relation between MAP and blood viscosity,9 and a study of 628 individuals in the population of Victoria de Durango with men and women pooled data showed a weak positive correlation between MAP and Hct.10 Our findings and some11,12 but not all results in the literature13,14 support the contention that Hct and blood viscosity are not a factor in regulating blood pressure in the healthy normal population within limits that include the systematic elevation of Hct due to adaptation to moderate altitude.
Men and women showed a different BMI (P < 0.05), with a tendency for men to be overweight. This could explain the differences in trends in the MAP/Hct association, although notably this trend is never positive.
The present study shows that the relationship between blood viscosity and Hct of men and women taken as a group is virtually identical to that found in a previous study6 in a population of similar age but different ethnic makeup and environmental conditions, measured with different methodology. This result confirms the strong association between blood viscosity and Hct for the population as a whole. The consistent shift between the two sets of data amounting to about 1 cP suggests a difference due to instrumentation and calibration procedures. The study of Kameneva et al6 was performed with a capillary viscometer (Cannon Instruments Co, State College, PA) at a shear rate of approximately 500 s−1 while our study was performed using the Brookfield cone and plate viscometer, at a shear rate of 167 s−1.
The finding that the relationship between blood viscosity and Hct for men is shifted by a constant increment of blood viscosity relative to women has not been previously reported and suggests a consistent difference inherent in the red blood cells rather than the plasma composition.
The effect of altitude was evident in the increased Hct in men (47.2% ± 2.3% vs 45.8% ± 2.3% reported for sea level).6 This effect was also present in women whose Hct was 42.4% ± 2.9% versus 40.8% ± 2.4% for sea level individuals. All changes were statistically significant (P < 0.05). This environmental bias increases Hct by approximately 5% relative to conditions at sea level and may be a limiting factor in this study. However, consistent physiological changes due to adaptation to altitude resulting in pathological conditions such as Monge’s disease are reported for adaptation to elevation that increase Hct >20%.15
The results of this study, those from experimental studies16 and results in children12 and premenopausal women11 indicate that blood pressure is not correlated with blood viscosity. Therefore a population presenting a positive correlation between blood pressure and blood viscosity (or Hct) is probably affected by endothelial dysfunction and/or pathologies that reduce or abolish the endothelial response to shear stress.17 In this context, the finding that Hct is increased in a population that subsequently develops hypertension18 may be in part explained by the presence of incipient, subclinical endothelial dysfunction.
The finding that blood pressure is independent of blood viscosity in healthy individuals could be of clinical interest since the population shows variability in these parameters. In particular, individuals with subclinical elevations of Hct and blood pressure could be signaling a state of endothelial dysfunction that may progress to the clinical condition of hypertension. In a different context these studies suggest that moderate elevation of blood viscosity is not necessarily a health hazard, and in some circumstances may be beneficial, an effect shown experimentally for the elevation of plasma viscosity in hemorrhage resuscitation19 and cardiovascular effects due to the small acute elevation of Hct.
In conclusion, blood pressure and blood viscosity are not related in healthy individuals, an effect probably due to shear stress regulation of blood flow.20 This conclusion is derived solely on the basis of the analysis of effects due to the normal variability of blood viscosity and Hct on blood pressure in the population. This variability is small, and its acute consequences have only been recently tested.3,4 The long-term effects of this variability are not well known. In view of the close relationship between blood viscosity and Hct, the latter can in general be used as a substitute for measurements of blood viscosity. Given these findings it could be of clinical importance to investigate individuals with subclinical increases in both blood pressure and Hct. This association could be of interest because it is related to parameters that, analyzed independently, do not constitute signals for clinical attention.
Acknowledgment
Research supported in part by Fondo Mixto CONACYTGobierno del Estado de Durango, Dgo, Mexico, Grant No. Dgo-2008-C0-93400.
Footnotes
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Fowkes FGR, Lowe GDO, Rumley A, Lennie SE, Smith FB, Donnan PT. The relationship between blood viscosity and blood pressure in a random sample of the population aged 55 to 74 years. Eur Heart J. 1993;14(5):597–601. doi: 10.1093/eurheartj/14.5.597. [DOI] [PubMed] [Google Scholar]
- 2.Devereux RB, Case DB, Alderman MH, Pickering TG, Chien S, Laragh JH. Possible role of increased blood viscosity in the hemodynamics of systemic hypertension. Am J Cardiol. 2000;85(10):1265–1268. doi: 10.1016/s0002-9149(00)00744-x. [DOI] [PubMed] [Google Scholar]
- 3.Salazar Vázquez BY, Martini J, Tsai AG, Johnson PC, Cabrales P, Intaglietta M. The variability of blood pressure due to small changes of hematocrit. Am J Physiol Heart Circ Physiol. 2010;299(3):H863–867. doi: 10.1152/ajpheart.00496.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martini J, Carpentier B, Chávez Negrete A, Frangos JA, Intaglietta M. Paradoxical hypotension following increased hematocrit and blood viscosity. Am J Physiol Heart Circ Physiol. 2005;289(5):H2136–2143. doi: 10.1152/ajpheart.00490.2005. [DOI] [PubMed] [Google Scholar]
- 5.Melkumyants AM, Balashov SA, Khayutin VM. Endothelium dependent control of arterial diameter by blood viscosity. Cardiovasc Res. 1989;23(9):741–747. doi: 10.1093/cvr/23.9.741. [DOI] [PubMed] [Google Scholar]
- 6.Kameneva MV, Watach MJ, Borovetz HS. Gender difference in rheologic properties of blood and risk of cardiovascular diseases. Clin Hemorheol Microcirc. 1999;21(3–4):357–363. [PubMed] [Google Scholar]
- 7.Motulsky HJ, Ransnas LA. Fitting curves to data using nonlinear regression: a practical and nonmathematical review. FASEB J. 1987;1(5):365–374. [PubMed] [Google Scholar]
- 8.de Simone G, Devereux RB, Chinali M, Best LG, Lee ET, Welty TK. Association of blood pressure with blood viscosity in American Indians. The strong heart study. Hypertension. 2005;45(4):625–630. doi: 10.1161/01.HYP.0000157526.07977.ec. [DOI] [PubMed] [Google Scholar]
- 9.de Simone G, Devereux RB, Chien S, Alderman MH, Atlas SA, Laragh JH. Relation of blood viscosity to demographic and physiologic variables and to cardiovascular risk factors in apparently normal adults. Circulation. 1990;81(1):107–117. doi: 10.1161/01.cir.81.1.107. [DOI] [PubMed] [Google Scholar]
- 10.Salazar Vázquez BY, Martini J, Chávez Negrete A, et al. Cardiovascular benefits in moderate increases of blood and plasma viscosity surpass those associated with lowering viscosity: Experimental and clinical evidence. Clin Hemorheol Microcirc. 2010;44(2):75–85. doi: 10.3233/CH-2010-1261. [DOI] [PubMed] [Google Scholar]
- 11.Salazar Vázquez BY, Salazar Vázquez MA, Cabrales P, de Faire U, Fagrell B, Intaglietta M. Hematocrit and mean arterial blood pressure in pre and postmenopause women. Vasc Health Risk Manag. 2009;5(2):483–488. doi: 10.2147/vhrm.s5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salazar Vázquez BY, Salazar Vázquez MA, Jáquez MG, Bracho Huemoeller AH, Intaglietta M, Cabrales P. Blood pressure directly correlates with blood viscosity in diabetes type 1 children but not in normals. Clin Hemorheol Microcirc. 2010;44(1):55–61. doi: 10.3233/CH-2010-1252. [DOI] [PubMed] [Google Scholar]
- 13.Fossum E, Hoieggen A, Moan A, Nordby G, Velund TL, Kjeldsen SE. Whole blood viscosity, blood pressure and cardiovascular risk factors in healthy blood donors. Blood Press. 1997;6(3):161–165. doi: 10.3109/08037059709061932. [DOI] [PubMed] [Google Scholar]
- 14.Letcher RL, Chien S, Pickering TG, Sealey JE, Laragh JH. Direct relationship between blood pressure and blood viscosity in normal and hypertensive subjects. Role of fibrinogen and concentration. Am J Med. 1981;70(6):1195–1202. doi: 10.1016/0002-9343(81)90827-5. [DOI] [PubMed] [Google Scholar]
- 15.Jefferson JA, Escudero E, Hurtado ME, et al. Excessive erythrocytosis, chronic mountain sickness, and serum cobalt levels. Lancet. 2002;359(9304):407–408. doi: 10.1016/s0140-6736(02)07594-3. [DOI] [PubMed] [Google Scholar]
- 16.Martini J, Tsai AG, Cabrales P, Johnson PC, Intaglietta M. Increased cardiac output and microvascular blood flow during mild hemoconcentration in hamster window model. Am J Physiol Heart Circ Physiol. 2006;291(1):H310–317. doi: 10.1152/ajpheart.01218.2005. [DOI] [PubMed] [Google Scholar]
- 17.Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108(17):2054–2059. doi: 10.1161/01.CIR.0000089191.72957.ED. [DOI] [PubMed] [Google Scholar]
- 18.Strand A, Gudmundsdottir H, Høieggen A, et al. Increased hematocrit before blood pressure in men who develop hypertension over 20 years. J Am Soc Hypertens. 2007;1(6):400–406. doi: 10.1016/j.jash.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Cabrales P, Tsai AG, Intaglietta M. Is resuscitation from hemorrhagic shock limited by blood oxygen-carrying capacity or blood viscosity? Shock. 2007;27(4):380–389. doi: 10.1097/01.shk.0000239782.71516.ba. [DOI] [PubMed] [Google Scholar]
- 20.Smieško V, Johnson PC. The arterial lumen is controlled by flow related shear stress. News Physiol Sci. 1993;8:34–38. [Google Scholar]