Abstract
Telmisartan is indicated for the prevention of cardiovascular events in high-risk patients, based on comparable efficacy to the angiotensin-converting enzyme (ACE) inhibitor, ramipril, in the ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET®) trial. However, tolerability must be considered when selecting treatments. This analysis compared the tolerability of telmisartan and ACE inhibitors using data pooled from 12 comparative, randomized studies involving 2564 telmisartan-treated patients and 2144 receiving ACE inhibitors (enalapril, lisinopril, or ramipril). Incidence rates of adverse events for the combined ACE inhibitor treatments and for telmisartan were similar (42.8% vs 43.9%, respectively) as were the rates of serious adverse events (1.8% vs 1.7% for telmisartan, respectively). Patients receiving ACE inhibitors had more cough (8.6% vs 2.6% with telmisartan, P < 0.0001). Results were similar irrespective of age, gender, or ethnicity. The adverse event of angioedema was observed in four patients (0.2%) receiving ACE inhibitors versus none with telmisartan (P = 0.043). There were small, numerical differences in serious adverse events. A total of 107 patients (5.0%) receiving ACE inhibitors and 93 patients (3.6%) receiving telmisartan discontinued treatment because of adverse events (P = 0.021); of these, 32.7% and 5.4%, respectively, were discontinuations due to cough (relative risk reduction of 88% [P < 0.0001] with telmisartan). Telmisartan and ACE inhibitors produced comparable blood pressure reductions at marketed doses. Telmisartan and ACE inhibitors are suitable for the prevention of cardiovascular events in high-risk patients, but telmisartan is better tolerated, particularly with regard to cough.
Keywords: adverse drug event, angiotensin-converting enzyme inhibitors, angiotensin II type 1 receptor blockers, cough, hypertension
Introduction
Angiotensin II receptor blockers (ARBs) inhibit the deleterious angiotensin type 1 receptor-mediated vasoconstrictor, proliferative, and atherogenic effects of angiotensin II, which play important roles in the development of hypertension and cardiovascular disease.1 Like angiotensin-converting enzyme (ACE) inhibitors, ARBs provide effective blood pressure control in hypertensive patients.2 Furthermore, the ARB, telmisartan, has been shown to reduce cardiovascular mortality and morbidity in a broad population of high-risk patients.3 Moreover, ARBs are associated with greater adherence to therapy than ACE inhibitors,4,5 possibly due to their favorable tolerability profile.6 This latter finding has important clinical implications because treatment discontinuations are a major factor contributing to poor blood pressure control7 and are associated with increased cardiovascular risk and increased health care costs.8
Telmisartan is an ARB that is indicated both for the treatment of hypertension and for the reduction of cardiovascular morbidity in patients at high risk of cardiovascular events.9 This latter indication is based on the results of the ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET®), which involved 25,620 patients with vascular disease or diabetes mellitus and end-organ damage, in which telmisartan reduced the incidence of the primary endpoint (a composite of cardiovascular death, myocardial infarction, stroke, or hospitalization for heart failure) to the same extent as the established treatment for such patients, the ACE inhibitor, ramipril.3 In this study, more patients discontinued treatment with ramipril than with telmisartan (23.7% vs 21.0%, respectively), despite the fact that patients were screened for intolerance to ACE inhibitors before enrolment, and that strenuous efforts were made to retain patients in the study. It might be expected that a larger discrepancy between patient discontinuation rates should be seen in patients who had not been screened for ACE inhibitor intolerance, because cough associated with ACE inhibitors is known to be an important factor limiting adherence with these medications.10
We earlier presented evidence from a pooled analysis showing that telmisartan has a tolerability profile resembling placebo.11 However, to date, no study has compared in detail the tolerability of ARBs and ACE inhibitors. Furthermore, a recent analysis of treatment discontinuations from antihypertensive treatment has found significant within-class differences. 12 In this analysis of 131,472 patients aged 40–80 years who lived in Lombardy, Italy, there was significant heterogeneity in treatment discontinuations among ACE inhibitors and, to a lesser extent, among ARBs. Thus, comparisons of tolerability between drugs within a class, as well as between drug classes, are important. In this study, we used pooled data from manufacturer-sponsored comparative studies of telmisartan in hypertensive patients to assess the incidence of adverse events with telmisartan compared with ACE inhibitors, as a class and individually. In contrast with previous analyses that have pooled published data from a wide variety of trials (eg, Bangalore et al10), we had access to the complete telmisartan trials database. This allowed us to analyze patient-level data, to ensure consistency in the recording of adverse events to avoid publication bias (which can introduce errors into analyses that rely on published sources).
Methods and materials
Study design
This analysis used safety data from all studies comparing telmisartan and ACE inhibitors in hypertensive patients, which were included in the Boehringer Ingelheim database and completed between 1994 and 2007. These comprised 12 randomized studies (study designations 1236.1, 502.202, 502.206, 502.210, 502.211, 502.214, 502.222, 502.223, 502.317, 502.331, 502.391, 502.392). Two of the trials (502.222 and 502.223) selected patients who had previously experienced cough on ACE inhibitors. In all studies, treatment was given once daily in the morning. The trial-specified duration of treatment ranged from 28 to 365 days. All trials were of monotherapy only and no antihypertensive treatment other than the study drug was allowed during the treatment period. The ONTARGET trial was not included because patients recruited to this study were prescreened for ACE inhibitor tolerance and the study allowed additional antihypertensive therapy. All studies were approved by local ethics committees and patients provided informed consent before enrolment.
Eight studies were double-blind, and four used a prospective randomized, open-label, blinded-endpoint design. All involved patients with mild-to-moderate hypertension, with most of the studies being defined as having diastolic blood pressure between 95 and 114 mmHg. Patients were randomized to treatment with telmisartan (at daily doses of between 20 mg and 160 mg) or ACE inhibitors; the ACE inhibitors used were enalapril 5–20 mg; lisinopril 10–40 mg; or ramipril 1.25–20 mg. In patients who had previously received antihypertensive therapy, randomized treatment was preceded by a washout period, usually of 4 weeks’ duration, during which patients received placebo. Treatment was given at a fixed dose in seven studies, and following dose titration according to blood pressure responses in five studies.
Safety evaluation
An adverse event was defined as any untoward medical occurrence that was reported by a patient or identified during clinical evaluation. Serious adverse events were defined as those that were fatal or life-threatening, or required hospitalization of the patient or extension of the period of hospitalization. All adverse events, whether reported spontaneously by the patient or detected by the investigator, that occurred during the treatment phase or within a day after discontinuation of treatment, were recorded and coded according to the Medical Dictionary for Regulatory Activities (MedDRA) v 8.1. The intensity and causality of adverse events were recorded by the investigators; drug-related adverse events were defined as events for which a causal relationship to the treatment had been suspected by the reporting or reviewing healthcare professional (usually the investigator or study monitor). Multiple occurrences of a specific adverse event in an individual patient were counted only once, whereas if a patient experienced more than one adverse event of different types, each event was included in the analysis.
Statistical analysis
Adverse event frequencies are reported as raw percentages and as occurrences per patient-year; the latter approach reflects differences in patient exposure to the drug and provides a standardized number of events observed in a patient treated for 1 year. Expressing data in terms of patient-years’ exposure enables physicians to identify long-term adverse events associated with a particular treatment, and facilitates comparisons between studies of different lengths.12 However, this concept assumes that the rate of events is constant over time, and may be misleading if this assumption is not met. If appropriate, differences in event rates were tested using the Chi-squared test and relative risk ratios were calculated. The incidence of cough over time was presented as a Kaplan–Meier curve.
Results
The 12 studies included a total of 4708 patients, of whom 2564 received telmisartan, 755 received enalapril, 220 received lisinopril, and 1169 received ramipril. The patients’ baseline characteristics are summarized in Table 1. The duration of exposure to antihypertensive medication ranged from 95.8 patient-years in patients receiving lisinopril to 698.0 patient-years in telmisartan-treated patients (Table 2).
Table 1.
Baseline demographic characteristics
| Enalapril (n = 755) | Lisinopril (n = 220) | Ramipril (n = 1169) | Combined ACE inhibitors (n = 2144) | Telmisartan (n = 2564) | |
|---|---|---|---|---|---|
| Male/female, (n, %) | 435/320 (57.6%/42.4%) | 136/84 (61.8%/38.2%) | 691/478 (59.1%/40.9%) | 1262/882 (58.9%/41.1%) | 1548/1016 (60.4%/39.6%) |
| Age, years | 60.4 (12.0) | 54.1 (10.1) | 53.7 (10.1) | 56.1 (11.3) | 55.8 (11.6) |
| Age ≥ 65 years, (n, %) | 371 (49.1%) | 38 (17.3%) | 162 (13.9%) | 571 (26.6%) | 656 (25.6%) |
| Race (n, %) | |||||
| White | 703 (93.1%) | 184 (83.6%) | 979 (83.7%) | 1866 (87.0%) | 2193 (85.5%) |
| Black | 17 (2.3%) | 33 (15.0%) | 65 (5.6%) | 115 (5.4%) | 191 (7.4%) |
| Asian | 8 (1.1%) | 3 (1.4%) | 7 (3.3%) | 51 (2.4%) | 57 (2.2%) |
| Body mass index (kg/m2) | 27.8 ± 4.3 | 30.3 ± 5.3 | 30.3 ± 5.5 | 29.4 ± 5.2 | 29.3 ± 5.2 |
| Obese (n, %) | 196 (26.0%) | 107 (48.6%) | 525 (44.9%) | 828 (38.6%) | 990 (38.6%) |
| Current smoker (n, %) | 122 (16.2%) | 37 (16.8%) | 185 (15.8%) | 344 (16.0%) | 451 (17.6%) |
| Exsmoker (n, %) | 181 (24.0%) | 85 (38.6%) | 358 (30.6%) | 624 (29.1%) | 746 (29.1%) |
| Duration of hypertension (years) | 8.5 ± 8.8 | 10.3 ± 9.4 | 7.1 ± 8.3 | 7.9 ± 8.6 | 8.2 ± 8.6 |
| SBP at baseline (mmHg) | 164.1 ± 16.5 | 152.8 ± 15.4 | 155.3 ± 12.6 | 158.2 ± 15.0 | 158.3 ± 14.9 |
| DBP at baseline (mmHg) | 99.2 ± 7.2 | 100.0 ± 4.8 | 100.5 ± 4.6 | 100.0 ± 5.7 | 100.3 ± 5.8 |
Note: Data are presented as the mean ± standard deviation, except where indicated.
Abbreviations: ACE, angiotensin-converting enzyme; DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation.
Table 2.
Duration of exposure to antihypertensive medication
| Enalapril (n = 755) | Lisinopril (n = 220) | Ramipril (n = 1169) | Combined ACE inhibitors (n = 2144) | Telmisartan (n = 2564) | |
|---|---|---|---|---|---|
| Total exposure (patient-years) | 194.9 | 95.8 | 264.7 | 555.3 | 698.0 |
| Mean exposure (days) | 94.3 | 159.0 | 82.7 | 94.6 | 99.4 |
| Exposure range (days) | 1–222 | 4–425 | 1–139 | 1–425 | 1–426 |
Abbreviation: ACE, angiotensin-converting enzyme.
The overall incidence of patients with adverse events was 37.7% (1.46 per patient-year) with enalapril, 69.1% (1.59 per patient-year) with lisinopril, 41.1% (1.82 per patient-year) with ramipril (combined ACE inhibitor incidence 42.8%, 1.65 per patient-year) and 43.9% (1.61 per patient-year) with telmisartan (Table 3). The corresponding incidences of drug-related adverse events were 15.8% (0.61), 32.7% (0.75), 10.3% (0.45) (combined ACE inhibitor incidence 14.5% [0.56]) and 10.2% (0.37), respectively. The incidences of the most common adverse events (those occurring in more than 1% of patients in either the telmisartan or ACE inhibitor groups) are summarized in Table 4. In general, the incidence per patient-year of these events was similar with both treatments.
Table 3.
Overall incidence of adverse events
| Enalapril (n = 755) | Lisinopril (n = 220) | Ramipril (n = 1169) | Combined ACE inhibitors (n = 2144) | Telmisartan (n = 2564) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||||||
| n | % | Per patient-year | n | % | Per patient-year | n | % | Per patient-year | n | % | Per patient-year | n | % | Per patient-year | |
| Patients with any adverse event | 285 | 37.7 | 1.46 | 152 | 69.1 | 1.59 | 481 | 41.1 | 1.82 | 918 | 42.8 | 1.65 | 1126 | 43.9 | 1.61 |
| Patients with drug-related adverse events | 119 | 15.8 | 0.61 | 72 | 32.7 | 0.75 | 120 | 10.3 | 0.45 | 311 | 14.5 | 0.56 | 261 | 10.2 | 0.37 |
| Patients with serious adverse events | 21 | 2.8 | 0.11 | 5 | 2.3 | 0.05 | 13 | 1.1 | 0.05 | 39 | 1.8 | 0.07 | 44 | 1.7 | 0.06 |
| Discontinued due to adverse events | 28 | 3.7 | 0.14 | 28 | 12.7 | 0.29 | 51 | 4.4 | 0.19 | 107 | 5.0 | 0.19 | 93 | 3.6 | 0.13 |
Abbreviation: ACE, angiotensin-converting enzyme.
Table 4.
Incidence of the most common adverse events (those occurring in more than 1% of patients in either the telmisartan or ACE inhibitor groups)
| Enalapril (n = 755) | Lisinopril (n = 220) | Ramipril (n = 1169) | Combined ACE inhibitors (n = 2144) | Telmisartan (n = 2564) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||||||
| n | % | Per patient-year | n | % | Per patient-year | n | % | Per patient-year | n | % | Per patient-year | n | % | Per patient-year | |
| Accident at home | 5 | 0.7 | 0.03 | 12 | 5.5 | 0.13 | 0 | 0.0 | 0.0 | 17 | 0.8 | 0.03 | 41 | 1.6 | 0.06 |
| Back pain | 6 | 0.8 | 0.03 | 8 | 3.6 | 0.08 | 13 | 1.1 | 0.05 | 27 | 1.3 | 0.05 | 63 | 2.5 | 0.09 |
| Bronchitis | 6 | 0.8 | 0.03 | 9 | 4.1 | 0.09 | 12 | 1.0 | 0.05 | 27 | 1.3 | 0.05 | 27 | 1.1 | 0.04 |
| Chest pain | 6 | 0.8 | 0.03 | 7 | 3.2 | 0.07 | 5 | 0.4 | 0.02 | 18 | 0.8 | 0.03 | 37 | 1.4 | 0.05 |
| Cough | 72 | 9.5 | 0.37 | 30 | 13.6 | 0.31 | 82 | 7.0 | 0.31 | 184 | 8.6 | 0.33 | 67 | 2.6 | 0.10 |
| Diarrhea | 14 | 1.9 | 0.07 | 4 | 3.6 | 0.08 | 20 | 1.7 | 0.08 | 42 | 2.0 | 0.08 | 65 | 2.5 | 0.09 |
| Dizziness | 15 | 2.0 | 0.08 | 13 | 5.9 | 0.14 | 22 | 1.9 | 0.08 | 50 | 2.3 | 0.09 | 75 | 2.9 | 0.11 |
| Dyspepsia | 6 | 0.8 | 0.03 | 6 | 2.7 | 0.06 | 11 | 0.9 | 0.04 | 23 | 1.1 | 0.04 | 26 | 1.0 | 0.04 |
| Fatigue | 14 | 1.9 | 0.07 | 17 | 7.7 | 0.18 | 15 | 1.3 | 0.06 | 46 | 2.1 | 0.08 | 64 | 2.5 | 0.09 |
| Headache | 53 | 7.0 | 0.27 | 30 | 13.6 | 0.31 | 67 | 5.7 | 0.25 | 150 | 7.0 | 0.27 | 203 | 7.9 | 0.29 |
| Influenza-like illness | 8 | 1.1 | 0.04 | 9 | 4.1 | 0.09 | 4 | 0.3 | 0.02 | 21 | 1.0 | 0.04 | 39 | 1.5 | 0.06 |
| Nasopharyngitis | 7 | 0.9 | 0.04 | 0 | 0.0 | 0.0 | 34 | 2.9 | 0.13 | 41 | 1.9 | 0.07 | 24 | 0.9 | 0.03 |
| Nausea | 7 | 0.9 | 0.04 | 6 | 2.7 | 0.06 | 16 | 1.4 | 0.06 | 29 | 1.4 | 0.05 | 33 | 1.3 | 0.05 |
| Peripheral edema | 4 | 0.5 | 0.02 | 7 | 3.2 | 0.07 | 1 | 0.1 | 0.0 | 12 | 0.6 | 0.02 | 31 | 1.2 | 0.04 |
| Pain | 5 | 0.7 | 0.03 | 12 | 5.5 | 0.13 | 3 | 0.3 | 0.01 | 20 | 0.9 | 0.04 | 29 | 1.1 | 0.04 |
| Sinusitis | 4 | 0.5 | 0.02 | 7 | 3.2 | 0.07 | 16 | 1.4 | 0.06 | 27 | 1.3 | 0.05 | 38 | 1.5 | 0.05 |
| Upper respiratory tract infection | 21 | 2.8 | 0.11 | 34 | 15.5 | 0.35 | 20 | 1.7 | 0.08 | 75 | 3.5 | 0.14 | 133 | 5.2 | 0.19 |
Abbreviation: ACE, angiotensin-converting enzyme.
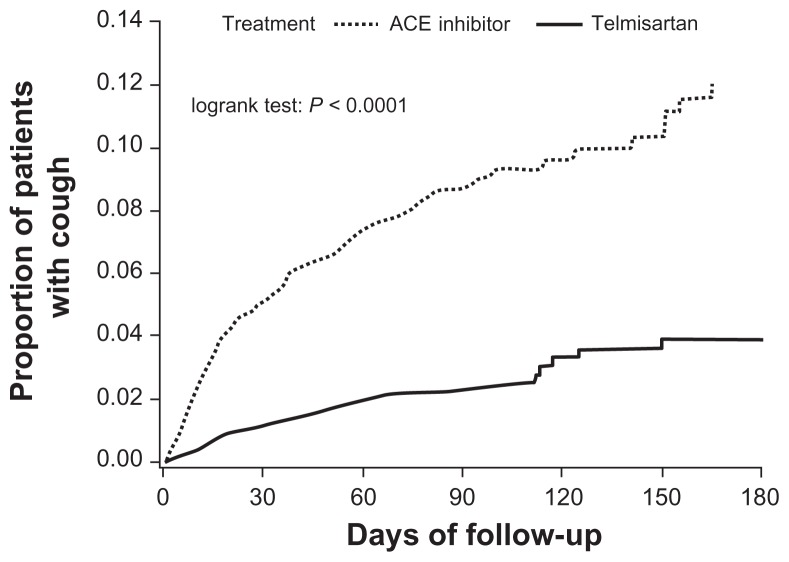
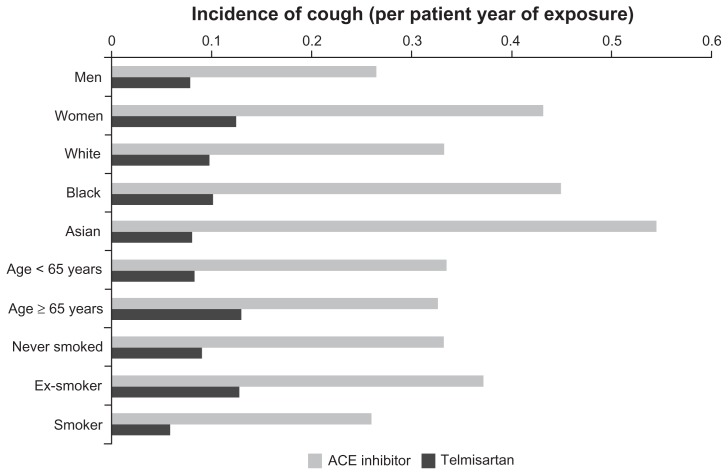
However, the incidence of cough was significantly higher in patients receiving ACE inhibitors (8.6%, 0.33 per patient-year) than in those receiving telmisartan (2.6%, 0.10 per patient-year). The incidence of cough over time is presented in Figure 1 (P < 0.0001 in log rank test). The incidence of cough in patients receiving ACE inhibitors tended to be higher in women than in men, and also in Black or Asian patients (Figure 2). Telmisartan was associated with a lower incidence of cough than ACE inhibitors in all patient subgroups studied, irrespective of age, gender, or race (Figure 2). The relative risk reduction was broadly constant across all subgroups, although it was higher among the Asian patients (85%) than Black (75%) or White (69%) patients, comparable among women (68%) and men (70%), higher among those aged <65 years (74%) than those aged ≥65 years (58%) and lower among ex-smokers (63%) than never smokers (72%) and among current smokers (77%).
Figure 1.
Proportion of patients with cough within 6 months of treatment in patients receiving ACE inhibitors or telmisartan.
Abbreviation: ACE, angiotensin-converting enzyme.
Figure 2.
Incidence of cough in patients receiving ACE inhibitors or telmisartan, in relation to age, gender, race, and smoking history.
Abbreviation: ACE, angiotensin-converting enzyme.
The incidence of angioedema (considered a nonserious adverse event) was also statistically significantly higher with ACE inhibitors than with telmisartan: four patients (0.2%) receiving ACE inhibitors developed angioedema, whereas no telmisartan-treated patient did so (P = 0.043). The incidence of upper respiratory tract infections was numerically higher with telmisartan than with ACE inhibitors, but the difference was not statistically significant (0.19 vs 0.14 per patient-year, respectively).
Adverse events considered to be drug-related were reported in 311 (14.5%) patients receiving ACE inhibitors and in 261 (10.2%) telmisartan-treated patients (P < 0.0001), giving a standardized incidence of 0.56 per patient-year for ACE inhibitors and 0.37 per patient-year for telmisartan (Table 3).
Serious adverse events were reported in 39 (1.8%) patients receiving ACE inhibitors and in 44 (1.7%) telmisartan- treated patients, giving a standardized incidence of 0.07 per patient-year for ACE inhibitors and 0.06 per patient-year for telmisartan (Table 3). There were small, numerical differences in the incidence of serious adverse events between telmisartan and ACE inhibitors, and between individual ACE inhibitors. Overall, 107 patients (5.0%) receiving ACE inhibitors discontinued treatment because of adverse events, compared with 93 patients (3.6%) receiving telmisartan; this corresponds to a relative risk reduction of 27% (P = 0.021) in the telmisartan group. Cough was an important cause of treatment discontinuation: 35 patients receiving ACE inhibitors withdrew because of cough (32.7% of all discontinuations due to adverse events), compared with only five (5.4%) telmisartan-treated patients, corresponding to a relative risk reduction of 88% (P < 0.0001) in the telmisartan group.
Although the focus of this analysis was on the safety and tolerability of telmisartan compared with ACE inhibitors, the efficacy of the two treatments was assessed by comparing the mean changes in systolic and diastolic blood pressure from baseline to endpoint. It should be noted that these data are provided for the sake of completeness, and should be treated with caution due to different study designs and small patient numbers in some groups. The blood pressure reductions achieved with telmisartan at marketed doses (40–80 mg) were comparable with those produced by ACE inhibitors (Table 5).
Table 5.
Adjusteda mean (95% confidence interval) blood pressure at baseline and change from baseline, separated for fixed dose and titration design studies (only marketed doses included)
| SBP | DBP | |||
|---|---|---|---|---|
|
|
|
|||
| Baseline | Change | Baseline | Change | |
| Fixed-dose design | ||||
| Enalapril 20 mg (n = 150) | 157.0 (154.2, 159.8) | −10.8 (−13.3, −8.3) | 100.5 (99.5, 101.6) | −9.3 (−10.8, −7.8) |
| Lisinopril 20 mg (n = 25) | 154.7 (149.0, 160.5) | −18.8 (−25.0, −12.6) | 96.7 (94.8, 98.5) | −11.0 (−14.7, −7.3) |
| Ramipril 10 mg (n = 927) | 155.3 (154.4, 156.1) | −9.3 (−10.7, −7.8) | 100.3 (100.0, 100.6) | −8.0 (−8.9, −7.2) |
| Ramipril 20 mg (n = 123) | 153.9 (151.7, 156.1) | −11.1 (13.9, −8.2) | 101.5 (100.8, 102.2) | −9.0 (−10.8, −7.3) |
| Telmisartan 40 mg (n = 112) | 155.1 (152.5, 157.8) | −13.2 (−16.1, −10.3) | 101.4 (100.6, 102.2) | −10.2 (−11.9, −8.4) |
| Telmisartan 80 mg (n = 1150) | 156.0 (155.2, 156.7) | −14.1 (−15.2, −12.9) | 100.3 (100.0, 100.6) | −10.8 (−11.5, −10.1) |
| Titration designb | ||||
| Enalapril 20 mg (n = 468) | 163.1 (161.8, 164.5) | −17.6 (−19.1, −16.1) | 98.9 (98.2, 99.6) | −12.1 (−13.1, −11.1) |
| Lisinopril 40 mg (n = 110) | 151.0 (148.3, 153.7) | −14.2 (−17.7, −10.8) | 99.9 (99.1, 100.8) | −7.4 (−9.5, −5.3) |
| Telmisartan 80 mg (n = 578) | 160.6 (159.4, 161.8) | −19.0 (−20.3, −17.7) | 99.5 (98.9, 100.0) | −13.0 (−13.8, −12.2) |
Notes: Adjusted for baseline and study;
maximum dose is given.
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure.
Discussion
This pooled analysis of 12 randomized, controlled studies with telmisartan and ACE inhibitors revealed that, although both ACE inhibitors and telmisartan are generally well tolerated (with a similar overall incidence of adverse events and serious adverse events), there were statistically significantly fewer discontinuations due to adverse events with telmisartan. A strength of our study, compared with other assessments of the tolerability of ARBs and ACE inhibitors, is that we had access to a large pool of data from prospective trials in which adverse events were carefully assessed in a standardized fashion. Thus, these data provide a robust assessment of the relative incidence of adverse events with these drugs in the clinical trial setting.
The most important finding is the precise estimate of the incidence of cough which, as expected, was significantly lower with telmisartan than with ACE inhibitors. Overall, cough occurred in 8.6% of patients treated with ACE inhibitors, which is comparable with the 10.6% reported in a recent meta-analysis by Bangalore et al.10 By contrast, only 2.6% of telmisartan-treated patients reported cough as an adverse event. Telmisartan significantly reduced the risk of cough (by approximately 70%), and reduced discontinuations due to cough, compared with ACE inhibitors.
The finding that telmisartan is associated with a lower incidence of cough than ACE inhibitors is clinically relevant because it is widely recognized that cough is an important factor limiting patient adherence to ACE inhibitor therapy.13 This is perhaps not surprising; hypertension is often asymptomatic and hence adverse effects of antihypertensive therapy may make patients unwilling to continue with a treatment that appears to deliver no tangible benefit but may markedly impair their quality of life.14 In our analysis, 1.6% of ACE inhibitor-treated patients withdrew from clinical trials because of cough, which is comparable with the rates reported in the meta-analysis of Bangalore et al.10 The rate of discontinuations due to cough with ACE inhibitors was approximately eight times higher than the respective figure for telmisartan (0.19%, a relative risk reduction of 88% [P < 0.0001] in the telmisartan group), a finding that is consistent with the experience in the ONTARGET study. In ONTARGET, discontinuations due to cough were nearly four times more frequent with ramipril than with telmisartan (4.2% vs 1.1%, respectively), despite the fact that patients in ONTARGET were prescreened for ACE inhibitor tolerance.3
The large database from the studies included in this analysis provided an opportunity to investigate the patient characteristics associated with ACE inhibitor treatment-related cough. Our results showed that ACE inhibitor-related cough tended to be more common in women, in Black or Asian patients, and in older patients, whereas smoking did not increase the incidence of cough. The latter finding differs from those of a previous study,15 which reported that smoking was an independent risk factor for ACE inhibitor-related cough. Our finding that ACE inhibitor-related cough was more common in Asian patients is consistent with previous studies;15,16 indeed, Asian ethnicity has been included as a predictive factor in algorithms for estimating the risk of ACE inhibitor-related cough.17
A recent analysis has investigated the incidence of treatment discontinuations due to adverse events in Asian and non-Asian patients in the ONTARGET study.18 Among telmisartan- treated patients, the overall incidence of discontinuations due to adverse events was significantly lower in Asian than in non-Asian patients (6.6% vs 10.3%, P = 0.0001), whereas the corresponding figures in ramipril-treated patients were similar in both groups (11.4% vs 11.8%). However, in ramipril-treated patients, discontinuations due to cough were significantly more common in Asian than in non-Asian patients (6.1% vs 3.9%, P < 0.001). Overall, telmisartan reduced the risk of discontinuation due to cough by more than 70% (relative risk: 0.26, 95% confidence interval [CI]: 0.21–0.33): similar risk reductions were seen both in Asian and non-Asian patients, although the absolute risk of cough was higher in Asian patients.
We were also able to compare the incidence of adverse events with three different ACE inhibitors. In the analysis of the 131,472-patient Lombardy database, discontinuations with ramipril were lower than with enalapril or lisinopril.12 In the current study, discontinuations with enalapril were lower than with ramipril. This likely reflects the different nature of the current analysis (which uses data from prospective, relatively short, and mostly blinded clinical trials), compared with the Lombardy study (which was observational and followed patients for up to 30 months). The relatively low rate of discontinuations from ramipril in the Lombardy study may be due to the “popularity factor”, ie, the fact that, as a result of the Heart Outcomes Prevention Evaluation trial, ramipril is widely acknowledged as an established treatment to reduce cardiovascular risk. Although the Lombardy study found relatively low discontinuations with ramipril, it should be noted that a large proportion of elderly patients may be unwilling to take medication with proven cardiovascular benefit if it is associated with even mild adverse effects. For example, a questionnaire-based study of community-living older persons found that 88% would be willing to take medication that reduced 5-year cardiovascular risk from 20% to 12%, but only 46% would still be willing if that medication was associated with daily fatigue and dizziness, even if this had no effect on function.19
Treatment adherence is critically important if the full benefits of cardiovascular risk reduction in hypertensive patients are to be attained. Among 18,806 newly diagnosed hypertensive patients treated for ≥35 years by primary care physicians in Italy, those who were most adherent (≥80% of days covered) had reduced cardiovascular risk compared with those who had low adherence (≤40% of days covered, hazard ratio: 0.62; 95% CI: 0.40–0.96; P = 0.032).20 In a cohort of nearly 60,000 patients in Québec, Canada, those with low adherence (<80% of days covered) were more likely to have coronary artery disease, cerebrovascular disease, or chronic heart failure within the 3-year follow-up period.21 Patients with low adherence were more likely to be hospitalized (odds ratio: 1.17, 95% CI: 1.12–1.22) and, among hospitalized patients, those with low adherence incurred increased costs of $3574 per person within the 3-year period. In Italy, lower adherence to diuretics versus ARBs has been estimated to result in higher overall treatment costs of around €500 per patient per year, despite lower drug acquisition cost.22 In a recent study from the United States, adherence to antihypertensive therapy was found to reduce average annual health care costs by almost US $4500 per patient.8
Better tolerability is only beneficial if combined with efficacy that is at least comparable. In this regard, telmisartan generally provides blood pressure reductions that are equal to or greater than those with ACE inhibitors. For example, telmisartan 80 reduced 24-hour ambulatory systolic/diastolic blood pressure more than ramipril 10 mg in a pooled analysis of two 6-week studies.23 Telmisartan 80 mg reduced systolic blood pressure and diastolic blood pressure more than enalapril 20 mg in a 12-week, placebo-controlled study that included 440 patients with mild-to-moderate hypertension.24 Blood pressure reductions with telmisartan 40–80 mg were similar to those with enalapril 20–40 mg in an open-label, dose-titrated study.25 Similarly, a dose-titrated comparison of telmisartan 40–160 mmHg with lisinopril 10–40 mg found comparable blood pressure reductions between the two dosage regimens.26 In this study, we pooled these and other data to provide an estimate of blood pressure reductions for each of the drugs studied. The blood pressure-lowering data presented here should be treated with caution because the data come from trials with different designs, including inclusion criteria, treatment duration, and fixed versus flexible dosing. Nevertheless, these pooled data broadly support at least comparable blood pressure-lowering efficacy with telmisartan compared with ACE inhibitors.
The efficacy of a medical treatment is the extent to which a drug has the ability to bring about its intended effect under ideal circumstances. Randomized clinical trials are typically designed to assess efficacy. However, more relevant to daily clinical practice is a drug’s effectiveness, ie, the extent to which a drug achieves its intended effect in the usual clinical setting. In the ONTARGET trial, telmisartan was found to have a similar efficacy to ramipril in preventing cardiovascular events in patients without hypertension but with additional atherothrombotic risk factors. Patients were screened for ACE inhibitor tolerance, and there were active efforts to ensure medication adherence and to retain patients on treatment. Given the differences in discontinuations seen in the current study, it is possible that, in patients not screened for ACE inhibitor tolerability and without close monitoring, the effectiveness (rather than efficacy) of telmisartan for preventing cardiovascular events may be greater than ramipril.27
In summary, this analysis has shown that telmisartan is associated with a lower incidence of cough and fewer treatment discontinuations due to cough, while having a similar or greater antihypertensive efficacy, compared with ACE inhibitors. Together with the ONTARGET study,3 which showed that telmisartan is as effective as ramipril in reducing cardiovascular mortality and morbidity in high-risk patients, these findings underline that telmisartan is suitable for the prevention of cardiovascular events in all high-risk patients, including those who are at risk of, or have a history of, ACE inhibitor-related cough.
Footnotes
Disclosures
Writing and editorial assistance was provided by Tomas Rees, of Parexel, which was contracted by Boehringer Ingelheim International GmbH for these services. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors and were fully responsible for all content and editorial decisions, and were involved at all stages of manuscript development. The authors received no compensation related to the development of the manuscript. GM has received speaker or consultancy fees from Boehringer Ingelheim, Novartis, Menarini, Recordati, Servier, Bayer, Takeda, Sankyo, Merck-Sharpe-Dohme, and AstraZeneca. HS is an employee of Boehringer Ingelheim Pharma GmbH and Co KG.
References
- 1.Wassmann S, Nickenig G. The role of the AT1 receptor in the cardiovascular continuum. Eur Heart J. 2004;6(Suppl H):H3–9. [Google Scholar]
- 2.Matchar DB, McCrory DC, Orlando LA, et al. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann Intern Med. 2008;148:16–29. doi: 10.7326/0003-4819-148-1-200801010-00189. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S, Teo KK, Pogue J, et al. on behalf of The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 4.Corrao G, Zambon A, Parodi A, et al. Discontinuation of and changes in drug therapy for hypertension among newly-treated patients: a population-based study in Italy. J Hypertens. 2008;26:819–824. doi: 10.1097/HJH.0b013e3282f4edd7. [DOI] [PubMed] [Google Scholar]
- 5.Kronish IM, Woodward M, Sergie Z, et al. Meta-analysis: impact of drug class on adherence to antihypertensives. Circulation. 2011;123:1611–1621. doi: 10.1161/CIRCULATIONAHA.110.983874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tolerability and quality of life in ARB-treated patients. Am J Manag Care. 2005;11(13 Suppl):S392–394. [No authors listed] [PubMed] [Google Scholar]
- 7.Marques-Vidal P, Tuomilehto J. Hypertension awareness, treatment and control in the community: is the ‘rule of halves’ still valid. J Hum Hypertens. 1997;11:213–220. doi: 10.1038/sj.jhh.1000426. [DOI] [PubMed] [Google Scholar]
- 8.Roebuck MC, Liberman JN, Gemmill-Toyama M, Brennan TA. Medication adherence leads to lower health care use and costs despite increased drug spending. Health Aff (Millwood) 2011;30:91–99. doi: 10.1377/hlthaff.2009.1087. [DOI] [PubMed] [Google Scholar]
- 9.European Medicines Agency. Summary of Product Characteristics. [Accessed November 3, 2011]. Available at: http://www.emea.europa.eu.
- 10.Bangalore S, Kumar S, Messerli FH. Angiotensin-converting enzyme inhibitor associated cough: deceptive information from the Physicians’ Desk Reference. Am J Med. 2010;123:1016–1030. doi: 10.1016/j.amjmed.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Schumacher H, Mancia G. The safety profile of telmisartan as monotherapy or combined with hydrochlorothiazide: a retrospective analysis of 50 studies. Blood Press. 2008;17:32–40. doi: 10.1080/08038020802144383. [DOI] [PubMed] [Google Scholar]
- 12.Mancia G, Parodi A, Merlino L, Corrao G. Heterogeneity in antihypertensive treatment discontinuation between drugs belonging to the same class. J Hypertens. 2011;29:1012–1018. doi: 10.1097/HJH.0b013e32834550d0. [DOI] [PubMed] [Google Scholar]
- 13.Squire B. Angiotensin converting enzyme inhibition in heart failure: clinical trials and clinical practice. Cardiovasc Drugs Ther. 2002;16:67–74. doi: 10.1023/a:1015375717044. [DOI] [PubMed] [Google Scholar]
- 14.Nunes MI. The relationship between quality of life and adherence to treatment. Curr Hypertens Rep. 2001;3:462–465. doi: 10.1007/s11906-001-0007-9. [DOI] [PubMed] [Google Scholar]
- 15.Morimoto T, Gandhi TK, Fiskio JM, et al. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J Eval Clin Pract. 2004;10:499–509. doi: 10.1111/j.1365-2753.2003.00484.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee YJ, Tsai JC. Angiotensin-converting enzyme gene insertion/deletion, not bradykinin B2 receptor-58T/C gene polymorphism, associated with angiotensin-converting enzyme inhibitor-related cough in Chinese female patients with non-insulin-dependent diabetes mellitus. Metabolism. 2001;50:1346–1350. doi: 10.1053/meta.2001.27212. [DOI] [PubMed] [Google Scholar]
- 17.Morimoto T, Gandhi TK, Fiskio JM, et al. Development and validation of a clinical prediction rule for angiotensin-converting enzyme inhibitor-induced cough. J Gen Intern Med. 2004;19:684–691. doi: 10.1111/j.1525-1497.2004.30016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dans AL, Teo K, Gao P, et al. In a subgroup of high-risk Asians, telmisartan was non-inferior to ramipril and better tolerated in the prevention of cardiovascular events. PLoS One. 2010;5:e13694. doi: 10.1371/journal.pone.0013694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fried TR, Tinetti ME, Towle V, O’Leary JR, Iannone L. Effects of benefits and harms on older persons’ willingness to take medication for primary cardiovascular prevention. Arch Intern Med. 2011;171:923–928. doi: 10.1001/archinternmed.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazzaglia G, Ambrosioni E, Alacqua M, et al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120:1598–1605. doi: 10.1161/CIRCULATIONAHA.108.830299. [DOI] [PubMed] [Google Scholar]
- 21.Dragomir A, Côté R, Roy L, et al. Impact of adherence to antihypertensive agents on clinical outcomes and hospitalization costs. Med Care. 2010;48:418–425. doi: 10.1097/MLR.0b013e3181d567bd. [DOI] [PubMed] [Google Scholar]
- 22.Ambrosioni E, Borghi C. Pharmacoeconomical and cost-benefit aspects. In: Mancia G, editor. ESH Manual of Hypertension. Amsterdam, The Netherlands: Elsevier; 2008. [Google Scholar]
- 23.Williams B, Lacourcière Y, Schumacher H, Gosse P, Neutel JM. Antihypertensive efficacy of telmisartan vs ramipril over the 24-h dosing period, including the critical early morning hours: a pooled analysis of the PRISMA I and II randomized trials. J Hum Hypertens. 2009;23:610–619. doi: 10.1038/jhh.2009.4. [DOI] [PubMed] [Google Scholar]
- 24.Smith DHG, Neutel JM, Morgenstern P. Adv Ther. Vol. 15. Once; 1998. -daily telmisartan compared with enalapril in the treatment of hypertension; pp. 229–240. [Google Scholar]
- 25.Neutel JM, Smith DH, Reilly PA. The efficacy and safety of telmisartan compared to enalapril in patients with severe hypertension. Int J Clin Pract. 1999;53:175–178. [PubMed] [Google Scholar]
- 26.Neutel JM, Frishman WH, Oparil S, Papademitriou V, Guthrie G. Comparison of telmisartan with lisinopril in patients with mildto- moderate hypertension. Am J Ther. 1999;6:161–166. doi: 10.1097/00045391-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Serebruany VL. Realistic assessment of drug-induced adverse events: a double-edged sword. Am J Med. 2010;123:971. doi: 10.1016/j.amjmed.2010.07.012. [DOI] [PubMed] [Google Scholar]