Abstract
The mechanisms underlying the increased risk of cardiovascular disease associated with diabetes mellitus (DM) are not fully defined. Insulin resistance in human metabolic syndrome patients is associated with decreased expression of the insulin receptor substrate-2- (Irs2-) AKT2 axis in mononuclear leukocytes (MLs). Moreover, acute coronary syndrome (ACS) has been linked through genome-wide association studies to the 2q36-q37.3 locus, which contains the Irs1 gene. Here, we investigated the expression of insulin-signaling pathway genes in MLs from patients with DM, ACS, and ACS plus DM. Quantitative real-time PCR expression studies showed no differences in the mRNA levels of Irs2, Akt2, and Akt1 among all patients. However, Irs1 mRNA expression was significantly increased in patients with ACS—diabetics and nondiabetics—compared with diabetic patients without ACS (P < .02 and P < .005, resp.). The present study reveals for the first time an association between increased Irs1 mRNA levels in MLs of patients with ACS which is not related to DM.
1. Introduction
The risk of cardiovascular disease (CVD) and atherosclerosis is 2–5 times higher in patients with diabetes mellitus (DM). Indeed, most DM patients die from CVD complications, mainly myocardial infarction and stroke [1–3]. The prevalence of DM is expected to increase significantly in the next 40 years as a consequence of population aging and the adoption of sedentary lifestyle patterns and is thus set to become a social and health care burden [4]. It is therefore important to understand the molecular mechanisms underlying the acceleration of CVD in DM, in order to identify novel markers and develop preventive and therapeutic strategies.
Insulin resistance (IR) in patients with metabolic syndrome (MetS), who are at high cardiovascular risk, is associated with impaired insulin signaling via the insulin receptor substrate-2- (Irs2-) AKT2 pathway in mononuclear leukocytes (MLs) [5]. In mice, deficiency for the Irs2 gene produces MetS [6–8], and partial or total inactivation of Irs2 aggravates atheroma development in the apolipoprotein E-null (apoE−/−) mouse model of atherosclerosis [5, 9, 10]. Consistent with the human studies, partial ablation of Irs2 in apoE−/− mice also reduces IRS2/AKT2 and Ras/ERK1/2-dependent signaling [5], suggesting a causal link between decreased insulin signaling and accelerated atherosclerosis.
Genome-wide studies suggest a link between acute coronary syndrome (ACS) and the 2q36-q37.3 locus, which encompasses the insulin receptor substrate-1 (Irs1) gene [11]. Here, we analyzed the expression of Irs2, Irs1, Akt1, and Akt2 genes in MLs from patients with DM (ACS−DM+), ACS (ACS+DM−), and ACS plus DM (ACS+DM+).
2. Materials and Methods
2.1. Study Population
Patients aged 40 to 85 years old admitted to Hospital General Universitario Gregorio Marañon (Madrid, Spain) and Hospital Universitario Virgen de la Victoria (Málaga, Spain) between 2007 and 2008 were studied. The cases (DM+ACS+) and ACS controls (DM−ACS+) were patients admitted to the departments of cardiology of the participant hospitals for an ACS. Non-ACS diabetic controls (DM+ACS−) were obtained from patients admitted to internal medicine departments or visited outpatient offices for non-CVD-related causes.
The patients were included in a prospective, hospital-based, case-control study to compare patient differences in the genetic expression of IRS-AKT-dependent signaling. DM patients with ACS (ACS+DM+) were compared with age- and sex-matched patients with DM hospitalized for a non-CVD, who had neither prior known CVD nor evidence of CVD according to the physician interview and physical examination plus no evidence of abnormal electrocardiogram and/or ventricular dysfunction on echocardiogram (ACS−DM+) or with ACS without DM (ACS+DM−). DM was defined as fasting plasma glucose >126 mg/dL or previously treated with insulin and/or antidiabetic drugs. ACS was defined as the clinical condition with a written diagnosis in the clinical record of ACS, unstable angina, or acute myocardial infarction, which prompted urgent hospitalization, and was characterized by the following criteria: (1) chest pain or symptom compatible with myocardial ischemia associated with at least one of the following: (a) rise in biomarkers of myocardial necrosis, (b) acute repolarization changes in the ECG, or (c) angiographic evidence of coronary artery disease and (2) absence of extreme pathophysiological condition as a trigger, such as (a) tachycardia of any cause >120 beats per minute, (b) severe hypotension < 80 mmHg, (c) hypertensive emergency (systolic blood pressure > 200 mmHg), and (d) severe anaemia (haemoglobin < 8 gr/dL). The study was approved by the local ethical committees in the two hospitals, and patients were enrolled after providing written informed consent.
2.2. Gene Expression Analysis by Quantitative Real-Time PCR (qPCR)
Total RNA from human MLs was isolated from 3 mL of frozen blood by addition of 7.5 mL RNAlater (Ambion) and extraction with the RiboPure-Blood kit (Ambion, AM1928). RNA purity and concentration were determined from the A260/280 ratio. 0.5–1 μg RNA was reverse-transcribed using the Superscript III First Strand Synthesis Supermix (Invitrogen), and cDNA was PCR amplified with the Platinum Quantitative PCR Supermix-UDG kit and ROX dye (Invitrogen). qPCR reactions were run on a 7500 Fast System thermal cycler (Applied Biosystems), and results were analyzed with the software provided by the manufacturer. The following primers (Forward: Fw; Reverse: Rv) were designed with the Primer Express program (Applied Biosystems): Irs1: Fw-5′-CGGAGAGCGATGGCTTCTC-3′; Rv 5′-GTTTGTGCATGCTCTTGGGTTT-3′; Irs2: Fw-5′-CCGACGCCAAGCACAAGTA-3′; Rv 5′-GGCCACGGCGAAGTA-3′; Akt1: Fw 5′-CCGACGCCAAGCACAAGTA-3′; Rv 5′-CGGCCACGGCGAAGTA-3′; Akt2: Fw 5′-CAAGGATGAAGTCGCTCACACA-3′; Rv 5′-GAACGGGTGCCTGGTGTTC-3′; Gapdh: Fw 5′-ACCACAGTCCATGCCATCAC-3′; Rv 5′-TCCACCACCCTGTTGCTGTA-3′.
2.3. Statistical Analysis
qPCR data were analyzed by one-way ANOVA. Differences in demographic and clinical characteristics were analyzed by one-way ANOVA for quantitative parameters and Chi-square test for qualitative variants, using the SPSS program (Statistical Package for Social Sciences) version 13.0 for Windows (SPSS Inc., Chicago, IU, USA). Outliers were identified by Grubb's test and eliminated. Quantitative variables appear as means ± standard deviation and qualitative variables as percentages. Statistical significance was assigned at P < .05.
3. Results
The study groups consisted of 35 cases (ACS+DM+), 45 nondiabetic ACS controls (ACS+DM−), and 31 diabetic controls without CVD (ACS−DM+). Table 1 shows the baseline characteristics according to the study groups.
Table 1.
Baseline characteristics according to study group.
| ACS−DM+ | ACS+DM− | ACS+DM+ | P value | |
|---|---|---|---|---|
| n = 31 | n = 45 | n = 35 | ||
| Age in years (average ± SEM) | 62.7 ± 2.0 | 65.1 ± 2.5 | 65.1 ± 1.9 | .36 |
| Sex (% male) | 68.8 | 81.1 | 68.3 | .36 |
| Body weight Kg (average ± SEM) | 72.8 ± 2.0 | 80.0 ± 2.9 | 78.1 ± 2.6 | .15 |
| Heigh cm (average ± SEM) | 164.8 ± 1.1 | 165.6 ± 1.7 | 165.8 ± 1.5 | .85 |
| Arterial hypertension (%) | 62.5 | 54.1 | 73.2 | .21 |
| Dyslipidemia (%) | 56.3 | 29.7 | 63.4 | .007 |
| Smoking (%) | 57.4 | 64.9 | 58.5 | .77 |
Dyslipidemia was more frequent in ACS+DM+ than in ACS−DM+ and ACS+DM− patients (P < .007). No differences were detected in arterial hypertension, age, sex, body weight, or smoking among the three groups.
Previous studies showed that patients with type 2 DM plus IR or IR alone have reduced expression of insulin receptor (Ins-r), Irs2 and Akt2 in pancreatic β-cells, and MLs [5, 12]. Total RNA was prepared from MLs from patients with ACS−DM+, ACS+DM−, or ACS+DM+, and the expression of insulin signaling genes was analyzed by qPCR. The expression of Akt1, Irs2, and Akt2 mRNA did not vary between the three patient groups (Figure 1). In contrast, Irs1 transcript levels were significantly higher in MLs from ACS+DM− (P < .005) or ACS+DM+ (P < .02) patients than in cells from ACS−DM+ patients. No differences in mRNA expression were observed between ACS+DM− and ACS+DM+ patients, suggesting that increased expression of Irs1 is associated with ACS independently of DM.
Figure 1.
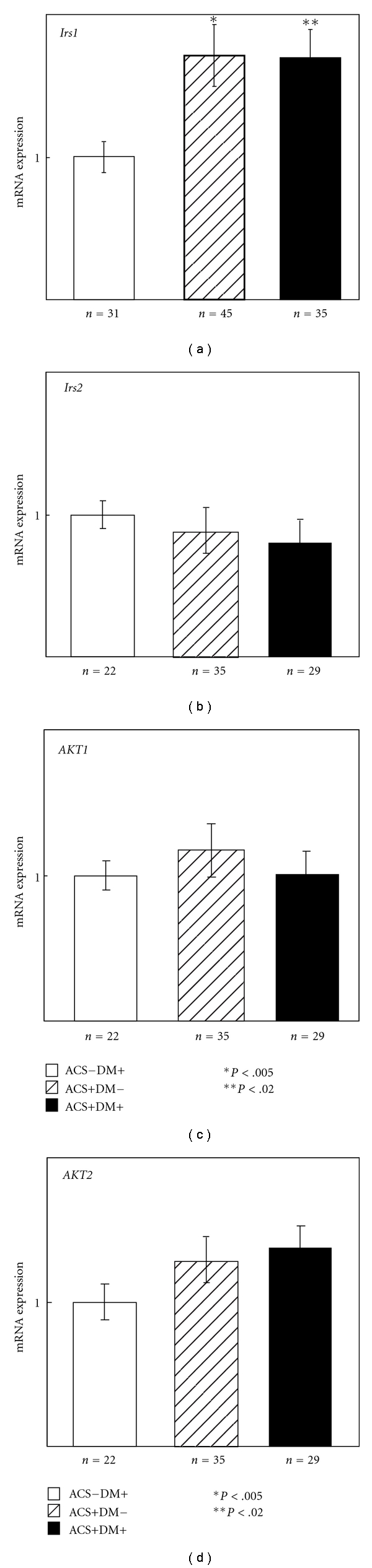
mRNA expression of insulin signaling genes in MLs from patients with ACS+DM−, ACS+DM+, and ACS−DM+. Expression of Irs1, Irs2, Akt1, and Akt2 in human WMBCs was analyzed by qPCR. mRNA amounts were normalized to the expression of Gapdh, which did not vary between groups, and expression levels are presented relative to the values in patients with DM without ACS (=1).
4. Discussion
Our results show that Irs1 mRNA expression in MLs from ACS+DM− and ACS+DM+ patients is higher than in cells from ACS−DM+ patients. Previous studies in our laboratory showed elevated Irs1 mRNA expression in MLs from prediabetic IR-MetS patients, who also exhibited increased CVD risk [5]. These findings are consistent with genome-wide studies that suggest linkage between ACS and the 2q36-q37.3 locus, which contains the Irs1 gene [11]. More specifically, the IRS1 972Arg variant has also been associated with a 3-year post-ACS mortality [13], suggesting a role of IRS1 in ACS complications. In the present study, Irs1 mRNA levels in ACS+DM− and ACS+DM+ patients were similar, suggesting that the presence of DM does not have an effect on Irs1 expression.
Interestingly, myocardial infarction induced by artery ligation in nondiabetic rats causes a partial impairment of insulin response that is associated with cardiac contractile dysfunction [14]. ACS in human patients might produce a similar defect in insulin-mediated signaling, resulting in a compensatory increase in Irs1 expression. The present findings suggest that Irs1 expression levels might be a marker of ACS; however, further studies are required to assess the importance of increased Irs1 expression in ACS and to define the underlying molecular mechanisms.
5. Conclusion
In the present study, we demonstrate an association between increased Irs1 mRNA levels in human ML of patients with ACS which is not related to DM and suggest that Irs1 levels might be a potential marker for ACS.
Conflicts of Interests
The authors declared that there is no conflicts of interests.
Authors' Contributions
M. F. Jiménez-Navarro participated in the design, acquisition of human samples, and data and contributed to the writing of paper; H. Bueno participated in the design and acquisition of human samples and contributed to the writing of paper; L. Alvarez-Sala participated in the design of the study and revised critically the paper; N. Rodriguez-Losada participated in the acquisition of human samples and revised critically the paper; V. Andrés participated in the design of the study and wrote the paper; H. González-Navarro participated in the design of the study, performed expression studies and analysis of data, and wrote the paper.
Acknowledgments
The authors thank Simon Bartlett for English editing. This study was supported by grants from the Spanish Ministry of Science and Innovation (MICINN), the European Regional Development Fund (SAF2007-62110), and the Instituto de Salud Carlos III (Grant RD06/0014/0021, RECAVA). The CNIC is supported by Fundación Pro-CNIC. H. González-Navarro was supported by the CSIC JAE-Doctor programme and the Miguel Servet programme (Instituto de Salud Carlos III CP10/00555).
Abbreviations
- ACS:
Acute coronary syndrome
- DM:
Diabetes mellitus
- IRS:
Insulin receptor substrate
- MetS:
Metabolic syndrome
- ML:
Mononuclear leukocytes
- CVD:
Cardiovascular disease
- qPCR:
Quantitative real-time PCR.
References
- 1.Moreno PR, Fuster V. New aspects in the pathogenesis of diabetic atherothrombosis. Journal of the American College of Cardiology. 2004;44(12):2293–2300. doi: 10.1016/j.jacc.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 2.Murcia AM, Hennekens CH, Lamas GA, et al. Impact of diabetes on mortality in patients with myocardial infarction and left ventricular dysfunction. Archives of Internal Medicine. 2004;164(20):2273–2279. doi: 10.1001/archinte.164.20.2273. [DOI] [PubMed] [Google Scholar]
- 3.Hasin T, Hochadel M, Gitt AK, Behar S, Bueno H, Hasin Y. Comparison of treatment and outcome of acute coronary syndrome in patients with versus patients without diabetes mellitus. The American Journal of Cardiology. 2009;103(6):772–778. doi: 10.1016/j.amjcard.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 4.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis epidemiology, pathophysiology, and management. Journal of the American Medical Association. 2002;287(19):2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Navarro H, Vinue A, Vila-Caballer M, et al. Molecular mechanisms of atherosclerosis in metabolic syndrome: role of reduced IRS2-dependent signaling. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(12):2187–2194. doi: 10.1161/ATVBAHA.108.175299. [DOI] [PubMed] [Google Scholar]
- 6.Burks DJ, De Mora JF, Schubert M, et al. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature. 2000;407(6802):377–382. doi: 10.1038/35030105. [DOI] [PubMed] [Google Scholar]
- 7.Kubota N, Tobe K, Terauchi Y, et al. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory β-cell hyperplasia. Diabetes. 2000;49(11):1880–1889. doi: 10.2337/diabetes.49.11.1880. [DOI] [PubMed] [Google Scholar]
- 8.Withers DJ, Gutierrez JS, Towery H, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391(6670):900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- 9.Baumgartl J, Baudler S, Scherner M, et al. Myeloid lineage cell-restricted insulin resistance protects apolipoproteinE-deficient mice against atherosclerosis. Cell Metabolism. 2006;3(4):247–256. doi: 10.1016/j.cmet.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Navarro H, Vila-Caballer M, Pastor MF, et al. Plasma insulin levels predict the development of atherosclerosis when IRS2 deficiency is combined with severe hypercholesterolemia in apolipoprotein E-null mice. Frontiers in Bioscience. 2007;12:2291–2298. doi: 10.2741/2231. [DOI] [PubMed] [Google Scholar]
- 11.Harrap SB, Zammit KS, Wong ZYH, et al. Genome-wide linkage analysis of the acute coronary syndrome suggests a locus on chromosome 2. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22(5):874–878. doi: 10.1161/01.atv.0000016258.40568.f1. [DOI] [PubMed] [Google Scholar]
- 12.Gunton JE, Kulkarni RN, Yim S, et al. Loss of ARNT/HIF1β mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell. 2005;122(3):337–349. doi: 10.1016/j.cell.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 13.Morgan TM, Xiao L, Lyons P, Kassebaum B, Krumholz HM, Spertus JA. Investigation of 89 candidate gene variants for effects on all-cause mortality following acute coronary syndrome. BMC Medical Genetics. 2008;9, article 66 doi: 10.1186/1471-2350-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amorim PA, Nguyen TD, Shingu Y, et al. Myocardial infarction in rats causes partial impairment in insulin response associated with reduced fatty acid oxidation and mitochondrial gene expression. The Journal of Thoracic and Cardiovascular Surgery. 2010;140(5):1160–1166. doi: 10.1016/j.jtcvs.2010.08.003. [DOI] [PubMed] [Google Scholar]


