The inhibitory and enhancing effects of γδ T cells on immune responses are convertible and are mediated by γδ T cell expansion and activation.
Abstract
Purpose.
To determine how the activation of γδ T cells affects the generation of uveitogenic αβ T cells and the development of experimental autoimmune uveitis (EAU).
Methods.
γδ T cells were isolated from B6 mice immunized with the uveitogenic peptide IRBP1–20 and αβ T cells from immunized TCR-δ−/− mice. Resting γδ T cells were prepared by culture of separated γδ T cells in cytokine-free medium for 3 to 5 days, when they showed downregulation of CD69 expression. Activated γδ T cells were prepared by incubating resting γδ T cells with anti-γδ TCR (GL3) for 2 days. Responder αβ T cells were cocultured with immunizing antigen and antigen-presenting cells. The numbers of antigen-specific T cells expressing IL-17 or IFN-γ were determined by intracellular staining followed by FACS analysis after stimulation, with or without the addition of purified γδ T cells. The cytokines in the culture medium were measured by ELISA.
Results.
Highly enriched γδ T cells exert widely different effects on autoreactive αβ T cells in EAU, depending on the activation status of the γδ T cells. Whereas nonactivated γδ T cells had little effect on the activation of interphotoreceptor retinoid-binding protein–specific αβ T cells in vitro and in vivo, activated γδ T cells promoted the generation of uveitogenic T cells and exacerbated the development of EAU.
Conclusions.
The functional ability of γδ T cells is greatly influenced by their activation status. Activated γδ T cells exacerbate EAU through increased activation of uveitogenic T cells.
The γδ T cells play an active role in the regulation and resolution of inflammatory processes associated with infectious disease and autoimmunity and accumulate in the inflammatory lesions associated with experimental models of autoimmune diseases.1–4 Studies have shown that γδ T cells can have either an upregulation5–8 or a downregulation9–11 effect on adaptive immune responses. This functional diversity has been previously attributed to different γδ T cell subsets expressing distinct T cell receptors (TCRs).12–19 In addition, the immunosuppressive activity of human γδ T cells has been shown to be reversed by exposure to Toll ligand20 or mycobacteria.17 Although clinical approaches have been developed to expand the number or function, or both, of human γδ T cells as a potential therapeutic modality for cancers21 and infections,22,23 knowledge of the mechanism by which these cells exert their regulatory functions is still limited. This seriously hampers their therapeutic use.
We have previously reported that γδ T cells isolated from mice with experimental autoimmune uveitis (EAU) can either promote or inhibit the activation of IL-17+ autoreactive T cells. Using an EAU model, we have further shown that the relative frequency of γδ T cells and αβ T cells among the responding T cell population determines the outcomes—fewer γδ T cells enhance the αβ T cell response whereas higher numbers of γδ T cells inhibit it.24,25 In the present study, we showed that the effect of γδ T cells critically depends on their state of activation. In examining the generation and activation of uveitogenic αβ T cells, we found that nonactivated γδ T cells have little effect on αβ cells, whereas activated γδ T cells promote the activation of uveitogenic αβ T cells and enhance EAU development.
Materials and Methods
Animals and Reagents
Pathogen-free female C57BL/6 (B6) and γδ TCR-δ−/− mice (12–14 weeks old) were purchased from Jackson Laboratory (Bar Harbor, ME) and were housed and maintained in the animal facilities of the University of Southern California. Institutional approval was obtained, and institutional guidelines regarding animal experimentation were followed. Recombinant murine IL-2 and IL-23 were purchased from R&D Systems (Minneapolis, MN). The human interphotoreceptor retinoid-binding protein peptide IRBP1–20 was synthesized by Sigma (St. Louis, MO), and complete Freund's adjuvant (CFA) was obtained from the same source. Fluorescein isothiocyanate (FITC)-conjugated anti-mouse IL-17 antibodies and FITC-anti-mouse IFN-γ, PE-anti-mouse CD69, and PE-anti-mouse CD62L antibodies were purchased from BioLegend (San Diego, CA).
EAU Model
The method for induction of EAU and the scoring of clinical symptoms has been described previously.24,26,27
Preparation of Resting and Activated γδ T Cells
γδ T cells were purified from IRBP1–20 immunized B6 mice.24,25,28 The newly isolated γδ T cells expressed modest levels of CD69 and were partially activated. Resting cells were harvested from this isolate after culture in cytokine-free medium for 3 to 5 days, when they showed downregulation of CD69 expression. Activated γδ T cells were prepared by incubating the resting γδ T cells with anti-γδTCR (GL3) and anti-CD28 antibodies (2 μg/mL) for 2 days. Resting γδ T cells do no produce IL-17 and express low levels of CD69 but high levels of CD62L, whereas activated γδ T cells produce IL-17 and express CD69 at increased and CD62L at decreased levels. Highly activated cells also downregulated TCR expression and expressed major histocompatibility complex (MHC) class II molecules, as we previously reported.28
Preparation of αβ IRBP1–20-Specific Responder T Cells
γδ-free IRBP1–20-specific responder T cells were obtained from IRBP1–20-immunized TCR-δ−/− mice. Briefly, at 13 days after immunization, T cells were isolated from lymph node cells and spleen cells by passage through a nylon wool column and stimulated in vitro with the immunizing antigen under either Th17 (culture medium containing IL-23) or nonpolarizing conditions.
Assessment of Antigen-Specific T Cell Responses
Responder αβ T cells (1 × 106/well) were cocultured with IRBP1–20 (10 μg/mL) and antigen-presenting cells (APCs) (irradiated spleen cells) in a 24-well plate, with or without the addition of 2% (2 × 104/well) purified γδ T cells. Then cytokines in the culture medium were measured by ELISA after 2 days of stimulation, and the numbers of antigen-specific T cells expressing IL-17 or IFN-γ were determined by intracellular staining followed by FACS analysis after 5 days of stimulation.
Immunofluorescence Flow Cytometry
Aliquots of 2 × 105 cells were double-stained with combinations of FITC- or PE-conjugated monoclonal antibodies. Data collection and analysis were performed on a flow cytometer (FACScalibur; BD Biosciences, Franklin Lakes, NJ) using acquisition software (CellQuest; BD Biosciences). To assess intracellular cytokine expression, unfractionated IRBP1–20-specific T cells from immunized B6 mice were stimulated in vitro for 4 hours with 50 ng/mL phorbol myristate acetate, 1 μg/mL ionomycin, and 1 μg/mL brefeldin A (Sigma) and were permeabilized overnight with buffer (Cytofix/Cytoperm; eBioscience, San Diego, CA).
ELISA
IL-17 and IFN-γ were measured using commercially available ELISA kits (R&D Systems).
Statistical Analysis
The data are expressed as mean ± SD for the results from at least three separate experiments. Significant differences are indicated by asterisks. Differences were considered significant when P ≤ 0.05 and very significant when P ≤ 0.01.
Results
Activated γδ T Cells Promote the αβ T Cell Response to IRBP1–20
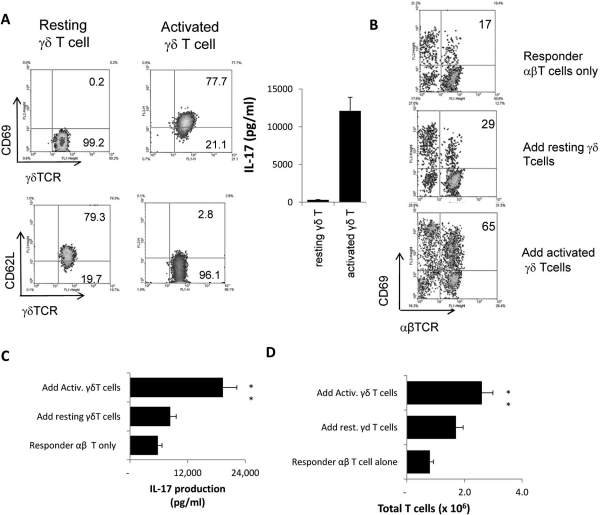
To investigate the effect of γδ T cells on αβ T cell activation, we separated γδ T cells from immunized B6 mice using a separator column (autoMACS; Miltenyi Biotec, Auburn, CA).24 Resting and activated γδ T cells were prepared as detailed in Materials and Methods. Responder αβ T cells were separated from the spleens and draining lymph nodes of TCR-δ−/− mice immunized with the uveitogenic peptide IRBP1–20. Figure 1A shows that the activated γδ T cells expressed increased levels of CD69 and decreased levels of CD62L compared with nonactivated cells, and activated γδ T cells produced a substantial amount of IL-17.
Figure 1.
Activated γδ T cells promote αβ T cell response. (A) Phenotypes of resting and activated γδ T cells, showing that activated γδ T cells expressed increased levels of CD69 and decreased levels of CD62L. Activated, but not resting, γδ T cells produced significant amounts of IL-17. (B) In vivo–primed IRBP-specific responder αβ T cells expressed increased levels of CD69 during in vitro stimulation in the presence of activated, but not nonactivated, γδ T cells. Responder αβ T cells (1 × 106/well) from immunized TCR-δ−/− mice were subjected to antigenic stimulation for 2 days by exposure to immunizing antigen and APCs, with or without the addition of 2% (2 × 104/well) of activated or resting γδ T cells. Then activated T cells were separated on Ficoll and stained for the expression of CD69 and either αβTCR or γδTCR. The numbers indicated in the upper right quadrants are calculated percentage values of CD69+ cells among the αβTCR+ cells. (C) ELISA assay for cytokine production after 2 days of in vitro stimulation. IL-17 in the culture supernatants were assessed by ELISA. (D) Assessment of total number of T cells after 5 days of in vitro stimulation. The results shown are representative of those from multiple (>10) experiments. **P ≤ 0.01; differences were considered very significant.
When the in vivo primed αβ T cells were stimulated in vitro with immunizing peptide and APCs with or without the addition of a small number (2%) of γδ T cells, rapid activation of the αβ T cells was observed in cultures with added activated γδ T cells. As shown in Figure 1B, FACS analysis of the αβ T cells 2 days after in vitro stimulation with immunizing antigen and APCs showed that only 17% expressed the early activation marker CD69 in the absence of γδ T cells, whereas 65% expressed CD69 when 2% of activated γδ T cells were added and 29% expressed CD69 when resting γδ T cells were added. Consistently, IL-17 production was much increased in these cultures (Fig. 1C), and total T cells increased significantly when activated γδ T cells were added (Fig. 1D). In contrast, nonactivated γδ T cells had smaller effects.
Activated γδ T Cells Promote Development of IL-17+ Uveitogenic αβ T Cells
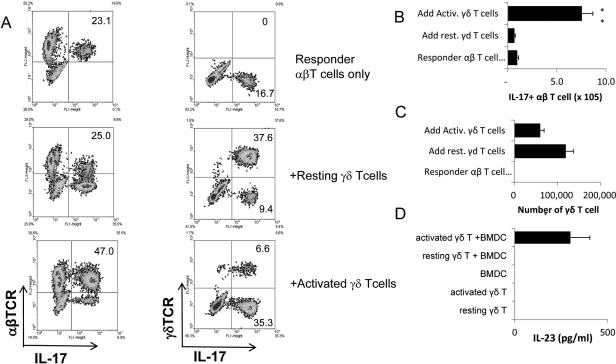
We compared the effects of resting and activated γδ T cells on the activation of in vivo primed IL-17+ IRBP-specific T cells. Enriched αβ responder T cells prepared from IRBP1–20-immunized TCR-δ−/− mice were stimulated for 5 days with immunizing antigen and APCs in vitro under Th17-polarizing conditions (culture medium supplemented with 10 ng/mL IL-23), either alone or in the presence of resting or preactivated γδ T cells. Subsequently, absolute numbers and relative frequencies of cytokine-producing αβ T cells were determined by intracellular staining followed by FACS analysis. Numbers and frequencies of cytokine-producing γδ T cells were also determined.
As shown in Figure 2A, in cultures containing αβ T cells only, 23.1% of the αβ T cells expressed IL-17. This relative frequency did not change substantially when 2% of resting γδ T cells were added, whereas adding 2% of activated γδ T cells increased the relative frequency of IL-17+ αβT cells approximately twofold. In the presence of the activated γδ T cells, the IL-17+ αβ T cells also expanded so that their absolute number increased sevenfold to eightfold while remaining essentially unchanged in the presence of resting γδ T cells (Fig. 2B). However, in the cultures with added resting γδ T cells (Fig. 2C), their absolute numbers increased more than in the cultures with added activated γδ T cells, and the relative frequency of IL-17+ γδ T cells was also higher (Fig. 2A). To investigate the mechanism by which activated γδ T cells gain an increased ability to promote the activation of IL-17+ αβT cells, we also assessed the IL-23 production by bone marrow dendritic cells (BMDCs). As demonstrated in Figure 2D, cultured BMDCs do not produce IL-23. After culture with activated, but not resting, γδ T cells, a significant amount of IL-23 was detected in the culture supernatants.
Figure 2.
Activated γδ T cells promote, whereas nonactivated γδ T cells inhibit, the activation of IRBP-specific IL-17+ αβ T cells. (A) Intracellular staining for IL-17+ αβ and γδ T cells. Responder αβ T cells (1 × 106/well) prepared from IRBP1–20 immunized TCR-δ−/− mice on day 5 after immunization were stimulated for 2 days with immunizing antigen and APCs under Th17-polarized conditions, with or without the addition of 2% (2 × 104/well) of resting or activated γδ T cells. Numbers indicated in the upper right quadrants are calculated percentage values of IL-17+ cells among the αβTCR+ (left) and γδTCR+ (right) cells. (B, C) Total number of IL-17+ αβ T cells and γδ T cells among the responder T cells in (A). (D) BMDCs (5 × 105/well) were cocultured with resting or activated γδ T cells (1 × 105) for 48 hours. Culture supernatants were tested by ELISA. The results shown are representative of those from five experiments. *P ≤ 0.05; differences were considered significant. **P ≤ 0.01; differences were considered very significant.
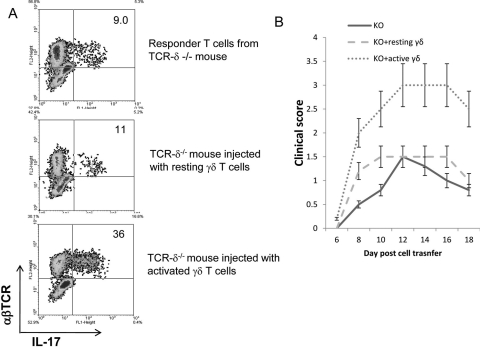
To determine whether a similar effect of γδ T cells might be seen in vivo, we injected TCR-δ−/− mice intraperitoneally with a small number (2 × 105 mouse) of activated or nonactivated γδ T cells prepared from IRBP1–20 immunized B6 mice before immunizing them with a pathogenic dose of IRBP1–20. The T cells from the immunized mice were then stimulated in vitro with immunizing antigen and APCs under Th17-polarized conditions, and the activated T cells were separated on Ficoll and subjected to intracellular staining to assess the percentage of αβ T cells expressing IL-17. As shown in Figure 3A, TCR-δ−/− mice injected with activated γδ T cells generated an approximately fourfold higher percentage of IL-17+ IRBP-specific αβ T cells compared with mice that received no injection or resting γδ T cells. In these mice, IL-17–producing γδ T cells were not seen (Fig. 3A). Importantly, when adoptively transferred to naive recipients, IRBP-specific T cells from mice injected with activated γδ T cells but not resting γδ T cells induced more severe EAU (Fig. 3B).
Figure 3.
Injection of TCR-δ−/− mice with activated, but not nonactivated, γδ T cells, before IRBP1–20 immunization increases the generation of IL-17+ IRBP-specific T cells. (A) Groups (n = 6) of TCR-δ−/− mice with or without injection of activated or resting γδ T cells (2 × 105/mouse) were immunized 1 day later with a pathogenic dose of IRBP1–20, then the Th1 and Th17 responses were assessed 5 days after in vitro stimulation, as described in the legend to Figure 2. The numbers indicated in the upper right quadrants are calculated percentage values of IL-17+ cells among the αβTCR+ cells. (B) IRBP-specific T cells (3 × 106) isolated from donor mice with or without injection of activated or resting γδ T cells were adoptively transferred to naive B6 mice after in vitro stimulation with IRBP1–20 for 2 days. Clinical scores were determined by funduscopy and pathologic examination.24,43
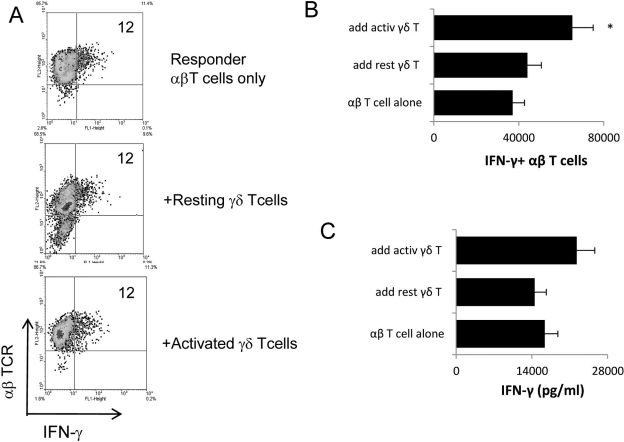
Regulatory Effect of γδ T Cells on IFN-γ+ (Th1-type) Uveitogenic T Cells
To determine whether activated γδ T cells also enhance the development of IFN-γ+ uveitogenic αβ T cells, in vivo–primed IRBP-specific T cells from TCR-δ−/− mice were stimulated with immunizing antigen under nonpolarizing conditions in the absence or presence of added γδ T cells. Intracellular staining showed that neither resting nor activated γδ T cells substantially changed the relative frequency of IFN-γ+ IRBP-specific αβ T cells (Fig. 4A). However, in cultures containing activated γδ T cells, the total number of IFN-γ+ IRBP-specific αβ T cells increased significantly (Fig. B), and the amount of produced IFN-γ increased marginally (Fig. 4C). Thus, under Th17-polarizing conditions, activated γδ T cells increased relative frequencies of IL-17+ αβ T cells, their total numbers, and their cytokine production, whereas under nonpolarizing conditions, activated γδ T cells increased total numbers of IFN-γ+ αβ T cells and the amount of IFNγ production, but not the relative frequency of IFN-γ+ αβ T cells.
Figure 4.
Effect of γδ T cells on the generation of IFN-γ+ IRBP-specific T cells. (A) Responder αβ T cells (1 × 106/well) from immunized TCR-δ−/− mice were stimulated with immunizing antigen and APCs under nonpolarized conditions, with or without the addition of γδ T cells (2 × 104/well, or 2%) for 2 days. (B, C) Assessment of the total number of IFN-γ+ αβ T cells among the responder T cells and the production of IFN-γ. Procedures used were the same as those described in the legend to Figure 2. *P ≤ 0.05; differences were considered significant.
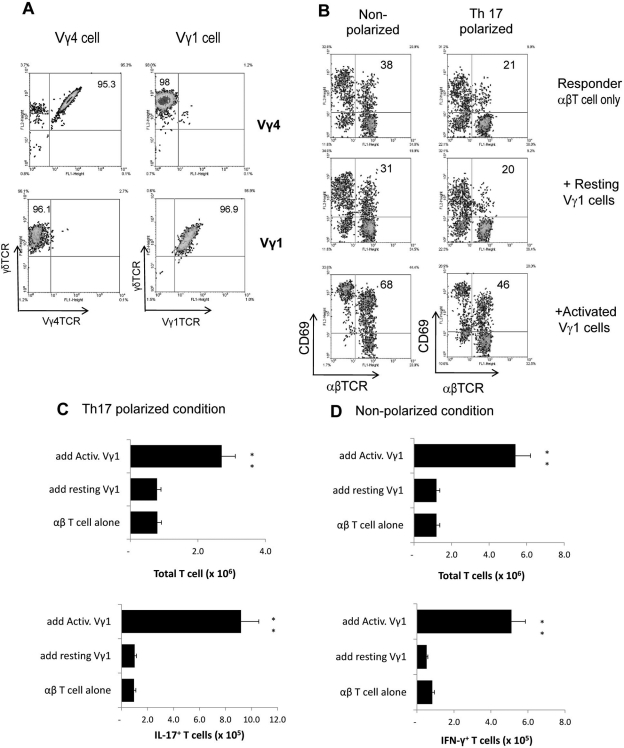
Activated Vγ1+ γδ T Cells also Promote the αβ T Cell Response to IRBP1–20
We previously showed that γδ T cells isolated from the IRBP1–20 immunized mice dominantly express Vγ4+ TCR segments.24 We therefore asked whether the functions of γδ T cells expressing different TCR segments might be modulated similarly by activation process. We isolated Vγ1+ T cells from mice expressing a Vγ1 TCR transgene14 and prepared resting and activated Vγ1+ T cells as before (Fig. 5A). As shown in Figure 5, highly enriched γδ T cells expressing Vγ1+ TCRs also promoted the responses of both IL-17+ and IFN-γ+ autoreactive αβ T cells, as was evident by increased frequencies of CD69+ αβ T cells when they were present (Fig. 5B). Again, this effect required activated γδ T cells. Moreover, the activated TCR-transgenic γδ T cells also enhanced the generation of IL-17+ and IFN-γ+ autoreactive αβ T cells under either Th17-polarizing (Fig. 5C) or nonpolarizing (Fig. 5D) conditions, whereas nonactivated γδ T cells had no substantial effect.
Figure 5.
Vγ1+ γδ T cells acquire upregulatory activity as well after in vitro activation. (A) Preparation of highly enriched γδ T cells expressing Vγ1 or Vγ4 TCR segments. Vγ1+ T cells were isolated from mice expressing a Vγ1 TCR transgene,14 and unseparated γδT cells isolated from immunized B6 mice dominantly express Vγ4+.24 (B) Intracellular staining assay. Activated and resting Vγ4+ or Vγ1+ γδ T cells were prepared as detailed in Materials and Methods. Experimental procedures were the same as those described in the legend to Figure 2. (C, D) Assessment of total T cell numbers and IL-17+ (Th17 polarized conditions) or IFN-γ+ (nonpolarized conditions) T cells after 5 days of in vitro stimulation. These studies were repeated four times. **P ≤ 0.01; differences were considered very significant.
Discussion
A prompt innate immune response not only fills the gap in immunologic defense before fully effective adaptive responses are ready but also regulates the intensity and pattern of the adaptive response.29,30 Thus, a better understanding of the cellular and molecular mechanisms of the interactions between innate and adaptive immunity should allow us to manipulate the specific adaptive immune response more effectively.
Manipulation of γδ T cell activity has shown beneficial effect on correcting immune defects.21–23,31,32 Nevertheless, the complexity of γδ T cell function has been realized, and inherent risks exist that may offset the therapeutic attempts. For example, it was reported that the functions of γδ T cells changed as the immune response progressed.33 When γδ T cells were depleted with mAbs specific for the γδ T-cell receptor, different results were obtained, depending on the time of antibody administration.25,34 Therefore, clarification of the cellular and molecular mechanisms underlying γδ T cell-mediated immune regulation should provide the information needed to understand the flexibility of γδ T cell function, which will ultimately improve the therapeutic approaches for γδ-based immunotherapy.
Studies in our laboratory have established reproducible in vitro and in vivo assay systems for assessing the regulatory effect of γδ T cells in a well-established murine model of EAU.24,25,28 In previous studies, we reported that the initiation and progression of EAU are closely associated with increased activation of γδ T cells, even though the expanded γδ T cells do not directly respond to the immunizing antigen.24,25 In addition, the relative ratio between γδ and αβ T cells has a major effect on the induced immune response. Although a small percentage of γδ T cells among the responder T cells promotes the response, a high percentage has the opposite effect.25 The activated γδ T cells enhanced the development of both IL-17– and IFN-γ–producing αβ T cells, but the effect on IL-17+ cells was stronger. Moreover, the activated cells had this effect on αβ T cells regardless of whether they predominantly expressed TCR-Vγ4 or transgenic TCR-Vγ1, suggesting that at this level, γδ TCR no longer plays a decisive role in determining γδ T cell immune-regulatory function.
The functional heterogeneity of γδ T cells has been largely attributed to the existence of γδ T cell subsets expressing different TCR segments.12–19 Very little information is available regarding how activation of γδ T cells alters their regulatory influence on αβ T cells. The present study shows that the ability of γδ T cells to promote the development of uveitogenic αβ T cells, and EAU, is strongly dependent on their state of activation. Given that γδ T cells can be readily activated by multiple pathways, it is important to note that the functional diversity of γδ T cells is closely related to their activation status.
The mechanism by which activated γδ T cells, which regulate autoimmune responses, gain an enhanced ability to promote the autoreactive T cell response requires further scrutiny. Given that γδ T cells can be readily activated by multiple pathways, such cells must be compared for their functional capabilities. In a previous report we showed that activated, but not resting, γδ T cells express MHC class II molecules and can act as APCs.28 In the present study, we show that activated γδ T cells gain greater ability promoting the generation of uveitogenic T cells, both in vitro and in vivo. We also show that activated γδ T cells gain increased ability to induce DCs to produce IL-23, which is required for the activation and expansion of Th17-type autoreactive T cells. Petermann et al.35 have recently reported that the IL-23–activated γδ T cells rendered αβ effector T cells refractory to the suppressive activity of Tregs. It appears that IL-23 treatment tips the balance between Treg and effector T cells toward the net effect of enhancement, whereas the outcome of the enhancement may involve augmented effector cell activity, weakened Treg activity, or both. In our recent studies, we were able to show that IL-23 is one of several major cytokines that can induce γδ activation and expansion. Given that IL-23 is not the only cytokine capable of activating γδ T cells, that combinations of two or more cytokines render a higher degree of γδ T cell activation, and that synergism is also seen when γδ T cells are activated by different pathways, such as via TLR ligands, cytokines, and activated macrophages (unpublished observation), we predict that in an inflammatory environment the mechanisms leading to altered regulatory functions of γδ T cells can be complex.
γδ T cells normally constitute only less than 1% of the total lymphocytes in the peripheral lymphoid organs, but, during infection, their number can expand to more than 50% of all circulating T cells within a few days.36 Given that γδ T cells can be activated by multiple pathways, not necessarily involving ligation of the γδ TCR,37–40 and that γδ T cell activation and expansion can be dissociated,41,42 we predicted that factors that affect γδ T cell activation and expansion have impact on the regulatory effect of γδ T cells on autoimmune responses. In the current and previous studies,24,25 we repeatedly observed that γδ T cells have a stronger effect on Th17-type than Th1-type autoreactive T cells. However, it remains to be determined whether γδ T cells activated to various degrees or by different means have different effects on the immune responses.
In summary, γδ T cells actively participate in the pathogenic process in EAU development and have a strong regulatory effect on the disease, and activation of γδ T cells plays a major role in shaping their regulatory potential.
Footnotes
Supported in part by National Institutes of Health Grants EY018827, EY017373, and EY003040.
Disclosure: H. Nian, None; H. Shao, None; R.L. O'Brien, None; W.K. Born, None; H.J. Kaplan, None; D. Sun, None
References
- 1. Selmaj K, Brosnan CF, Raine CS. Colocalization of lymphocytes bearing γδ T-cell receptor and heat shock protein hsp65+ oligodendrocytes in multiple sclerosis. Proc Natl Acad Sci USA. 1991;88:6452–6456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wucherpfennig KW, Newcombe J, Li H, et al. γδ T-cell receptor repertoire in acute multiple sclerosis lesions. Proc Natl Acad Sci USA. 1992;89:4588–4592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peng SL, Madaio MP, Hayday AC, Craft J. Propagation and regulation of systemic autoimmunity by γδ T cells. J Immunol. 1996;157:5689–5698 [PubMed] [Google Scholar]
- 4. Mukasa A, Lahn M, Pflum EK, et al. Evidence that the same γδ T cells respond during infection- induced and autoimmune inflammation. J Immunol. 1997;159:5787–5794 [PubMed] [Google Scholar]
- 5. Rajan AJ, Asensio VC, Campbell IL, Brosnan CF. Experimental autoimmune encephalomyelitis on the SJL mouse: effect of γδ T cell depletion on chemokine and chemokine receptor expression in the central nervous system. J Immunol. 2000;164:2120–2130 [DOI] [PubMed] [Google Scholar]
- 6. Spahn TW, Issazadah S, Salvin AJ, Weiner HL. Decreased severity of myelin oligodendrocyte glycoprotein peptide 33–35-induced experimental autoimmune encephalomyelitis in mice with a disrupted TCR δ chain gene. Eur J Immunol. 1999;29:4060–4071 [DOI] [PubMed] [Google Scholar]
- 7. Odyniec A, Szczepanik M, Mycko MP, et al. γδ T cells enhance the expression of experimental autoimmune encephalomyelitis by promoting antigen presentation and IL-12 production. J Immunol. 2004;173:682–694 [DOI] [PubMed] [Google Scholar]
- 8. Tagawa T, Nishimura H, Yajima T, et al. Vδ1+ γδ T cells producing CC chemokines may bridge a gap between neutrophils and macrophages in innate immunity during Escherichia coli infection in mice. J Immunol. 2004;173:5156–5164 [DOI] [PubMed] [Google Scholar]
- 9. Tsuchiya T, Fukuda S, Hamada H, et al. Role of γδ T cells in the inflammatory response of experimental colitis mice. J Immunol. 2003;171:5507–5513 [DOI] [PubMed] [Google Scholar]
- 10. Uezu K, Kawakami K, Miyagi K, et al. Accumulation of γδ T cells in the lungs and their regulatory roles in Th1 response and host defense against pulmonary infection with Cryptococcus neoformans. J Immunol. 2004;172:7629–7634 [DOI] [PubMed] [Google Scholar]
- 11. D'Souza CD, Cooper AM, Frank AA, et al. An anti-inflammatory role for γδ T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–1221 [PubMed] [Google Scholar]
- 12. Dalton JE, Pearson J, Scott P, Carding SR. The Interaction of γδ T cells with activated macrophages is a property of the Vγ1 subset. J Immunol. 2003;171:6488–6494 [DOI] [PubMed] [Google Scholar]
- 13. Glatzel A, Wesch D, Schiemann F, et al. Patterns of chemokine receptor expression on peripheral blood γδ T lymphocytes: strong expression of CCR5 is a selective feature of Vδ2/Vγ9 γδ T cells. J Immunol. 2002;168:4920–4929 [DOI] [PubMed] [Google Scholar]
- 14. Hahn YS, Taube C, Jin N, et al. Different potentials of γδ T cell subsets in regulating airway responsiveness: Vγ1+ cells, but not Vγ4+ cells, promote airway hyperreactivity, Th2 cytokines, and airway inflammation. J Immunol. 2004;172:2894–2902 [DOI] [PubMed] [Google Scholar]
- 15. Huber SA, Graveline D, Newell MK, et al. Vγ1+ T cells suppress and Vγ4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J Immunol. 2000;165:4174–4181 [DOI] [PubMed] [Google Scholar]
- 16. Meissner N, Radke J, Hedges JF, et al. Serial analysis of gene expression in circulating γδ T cell subsets defines distinct immunoregulatory phenotypes and unexpected gene expression profiles. J Immunol. 2003;170:356–364 [DOI] [PubMed] [Google Scholar]
- 17. Spencer CT, Abate G, Blazevic A, Hoft DF. Only a subset of phosphoantigen-responsive γ9δ2 T cells mediate protective tuberculosis immunity. J Immunol. 2008;181:4471–4484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van der Heyde HC, Elloso MM, Chang WL, et al. Expansion of the γδ T cell subset in vivo during bloodstage malaria in B cell-deficient mice. J Leukoc Biol. 1996;60:221–229 [DOI] [PubMed] [Google Scholar]
- 19. O'Brien RL, Yin X, Huber SA, et al. Depletion of a γδ T cell subset can increase host resistance to a bacterial infection. J Immunol. 2000;165:6472–6479 [DOI] [PubMed] [Google Scholar]
- 20. Peng G, Wang HY, Peng W, et al. Tumor-infiltrating γδ T cells suppress T and dendritic cell function via mechanisms controlled by a unique Toll-like receptor signaling pathway. Immunity. 2007;27:334–348 [DOI] [PubMed] [Google Scholar]
- 21. Kabelitz D, Wesch D, He W. Perspectives of γδ T cells in tumor immunology. Cancer Res. 2007;67:5–8 [DOI] [PubMed] [Google Scholar]
- 22. Girardi M. Immunosurveillance and immunoregulation by γδ T cells. J Invest Dermatol. 2006;126:25–31 [DOI] [PubMed] [Google Scholar]
- 23. Poccia F, Agrati C, Martini F, et al. Vγ9Vδ2 T cell-mediated non-cytolytic antiviral mechanisms and their potential for cell-based therapy. Immunol Lett. 2005;100:14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cui Y, Shao H, Lan C, et al. Major role of γδ T cells in the generation of IL-17+ uveitogenic T cells. J Immunol. 2009;183:560–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nian H, Shao H, Zhang G, et al. Regulatory effect of T cells on IL-17+ uveitogenic T cells. Invest Ophthalmol Vis Sci. 2010;51:4661–4667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peng Y, Han G, Shao H, et al. Characterization of IL-17+ interphotoreceptor retinoid-binding protein-specific T cells in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2007;48:4153–4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peng Y, Shao H, Ke Y, et al. Minimally activated CD8 autoreactive T cells specific for IRBP express a high level of Foxp3 and are functionally suppressive. Invest Ophthalmol Vis Sci. 2007;48:2178–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheng L, Cui Y, Shao H, et al. Mouse γδ T cells are capable of expressing MHC class II molecules, and of functioning as antigen-presenting cells. J Neuroimmunol. 2008;203:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Medzhitov R, Janeway CA. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298. [DOI] [PubMed] [Google Scholar]
- 30. Carroll M. Innate immunity in the etiopathology of autoimmunity. Nat Immunol. 2001;2:1089–1090 [DOI] [PubMed] [Google Scholar]
- 31. Sicard H, Ingoure S, Luciani B, et al. In vivo immunomanipulation of Vγ9Vδ2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J Immunol. 2005;175:5471–5480 [DOI] [PubMed] [Google Scholar]
- 32. Wilhelm M, Kunzmann V, Eckstein S, et al. γδ T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–206 [DOI] [PubMed] [Google Scholar]
- 33. Carding SR, Egan PJ. γδ T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345 [DOI] [PubMed] [Google Scholar]
- 34. Peterman GM, Spencer C, Sperling AI, Bluestone JA. Role of γδ T cells in murine collagen-induced arthritis. J Immunol. 1993;151:6546–6558 [PubMed] [Google Scholar]
- 35. Petermann F, Rothhammer V, Claussen MC, et al. γδ T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33:351–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eberl M, Moser B. Monocytes and γδ T cells: close encounters in microbial infection. Trends Immunol. 2009;30:562–568 [DOI] [PubMed] [Google Scholar]
- 37. Aydintug MK, Roark CL, Chain JL, et al. Macrophages express multiple ligands for γδ TCRs. Mol Immunol. 2008;45:3253–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Havlir DV, Ellner JJ, Chervenak KA, Boom WH. Selective expansion of human γδ T cells by monocytes infected with live Mycobacterium tuberculosis. J Clin Invest. 1991;87:729–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rincon-Orozco B, Kunzmann V, Wrobel P, et al. Activation of Vγ9Vδ2 T cells by NKG2D. J Immunol. 2005;175:2144–2151 [DOI] [PubMed] [Google Scholar]
- 40. Wesch D, Beetz S, Oberg HH, et al. Direct costimulatory effect of TLR3 ligand poly(I:C) on human γδ T lymphocytes. J Immunol. 2006;176:1348–1354 [DOI] [PubMed] [Google Scholar]
- 41. Hanrahan CF, Kimpton WG, Howard CJ, et al. Cellular requirements for the activation and proliferation of ruminant γδ T cells. J Immunol. 1997;159:4287–4294 [PubMed] [Google Scholar]
- 42. Wesch D, Marx S, Kabelitz D. Comparative analysis of and T cell activation by Mycobacterium tuberculosis and isopentenyl pyrophosphate. Eur J Immunol. 1997;27:952–956 [DOI] [PubMed] [Google Scholar]
- 43. Cui Y, Shao H, Sun D, Kaplan HJ. Regulation of interphotoreceptor retinoid-binding protein (IRBP)-specific Th1 and Th17 cells in anterior chamber-associated immune deviation (ACAID). Invest Ophthalmol Vis Sci. 2009;50:5811–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]