Abstract
Objective
To describe the clinical phenotype and genetics of equine Multiple Congenital Ocular Anomalies (MCOA) syndrome in PMEL17 (Silver) mutant ponies.
Animals studied
Five presumably unrelated ponies.
Procedures
The ponies were examined under field conditions in their barn by slit lamp biomicroscopy, indirect ophthalmoscopy, and applanation tonometry. Blood was collected and genomic DNA extracted for MCOA genotyping using the PMEL17ex11 marker.
Results
One pony solely presented with temporal ciliary body cysts, suggestive of the less severe Cyst phenotype of MCOA; the animal was heterozygous at the MCOA locus. Multiple bilateral anterior segment anomalies were identified in four ponies, consistent with the more severe MCOA phenotype characterized by cornea globosa, iris hypoplasia, encircling granula iridica along the pupillary ruff, and cataracts. These animals were homozygous for the mutant MCOA allele. Four of the ponies had a silver dapple or chocolate coat color with white or flaxen manes and tails. Silver dappling was masked by the palomino coloring of a 5th pony that was homozygous at the MCOA locus.
Conclusions
The MCOA syndrome can be seen in ponies. The results of both clinical evaluation and genotyping resembled the previously described MCOA of both Rocky Mountain and Kentucky Mountain Saddle horses.
Keywords: anterior segment dysgenesis, equine multiple congenital ocular anomalies, MCOA, PMEL17, pony, silver dapple
INTRODUCTION
The equine Multiple Congenital Ocular Anomalies (MCOA) syndrome consists of various combinations of clinical symptoms predominantly affecting the anterior segment of the eye, including cornea globosa, uveal cysts, and iris hypoplasia. The syndrome has been well described in both Rocky Mountain and Kentucky Mountain Saddle horses, where it typically affects animals with a silver coat color.1,2 Equine MCOA has also been observed in other breeds, including the Mountain Pleasure horse (derived from the same founder as Rocky Mountain and Kentucky Mountain horses), Morgan horse, ponies and miniature breeds.1 However, to the best of our knowledge, a formal description of MCOA in ponies does not exist. Ponies are defined as horses of various breeds with adults measuring <14.2 hands or 147 cm at the withers.3 Here, we describe the occurrence of MCOA in five presumably unrelated ponies, and we propose the same mode of inheritance, and association with the Silver mutation and coat color, as in the MCOA of Rocky Mountain horses.
MATERIALS AND METHODS
The five ponies were independently examined at the farms where they were stabled in the states of California (1), New Jersey (2), New York (1), and Pennsylvania (1). Anterior segments were examined with diffuse and focal illumination using portable hand-held slit-lamp biomicroscopes (Kowa SL14; Kowa Company, Tokyo, Japan, and Kowa SL15; Kowa Optimed, Torrance, CA, USA). Fundic examinations were performed with portable binocular indirect ophthalmoscopes (Keeler All Pupil II; Keeler Instruments, Broomall, PA, USA) and condensing lenses (Double Aspheric 14D and 15D, Pan Retinal 2.2D, Digital ClearField, and Digital ClearMag; Volk Optical, Mentor, OH, USA). The Schirmer tear test I (Schering-Plough Animal Health, Union, NJ, USA) was used to measure aqueous tear production in one pony. Intraocular pressures were measured by applanation tonometry (TonoPen XL; Mentor, Norwell, MA, USA, and Tono-Pen Vet; Reichert, Depew, NY, USA) following the application of ocular surface anesthetic (0.5% proparacaine HCl ophthalmic solution). One animal was examined by ocular B-scan ultrasonography with a 10-MHz probe (VuMax; Sonomed, Inc., Lake Success, NY, USA).
In addition to the ophthalmic evaluation, whole blood was collected in tubes with EDTA from each pony and genomic DNA was extracted using the QIAmp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. Previously published primers surrounding the PMEL17ex11 marker were used for PCR amplification (Table 1; Integrated DNA Technologies, Coralville, IA, USA).4 Each of the two forward primers was combined with each of the three reverse primers (total = 6 PCR products) to cover the region of interest with multiple PCR products and for DNA sequencing redundancy. The amplified products were gel purified using the QIAquick Gel Extraction Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. The products were then sequenced with both forward and reverse PCR primers at the University of Pennsylvania DNA Sequencing Facility (ABI 3730XL and ABI 3100 Genetic Analyzers; Applied Biosystems, Foster City, CA, USA). The sequences were evaluated for the presence or absence of the cytosine (C; wildtype) to thymine (T; Silver) missense mutation at the 5th base within exon 11 of the PMEL17 (Silver) gene using Sequencher 3.1.1 software (Gene Codes, Ann Arbor, MI, USA).4 This mutation is responsible for the silver dapple coat color and a marker for MCOA (PMEL17ex11).4,5
Table 1.
PCR primers used for genotyping PMEL17ex11 (4)
| Forward primers | Reverse primers |
|---|---|
| 5′-GCA GGG AAG CTT GTA GAG TGA-3′ | 5′-CTC TCA CCA AAG GGG GAA G-3′ |
| 5′-AGA GGC AGG CCT TGG GCA G-3′ | 5′-TGC TCT CAC CAA AGG GGG AAG-3′ |
| 5′-AGG GAA GAC TGG AGA CAA GA-3′ |
Nucleotides: A, adenine; T, thymine; G, guanine; C, cytosine.
RESULTS
Case 1
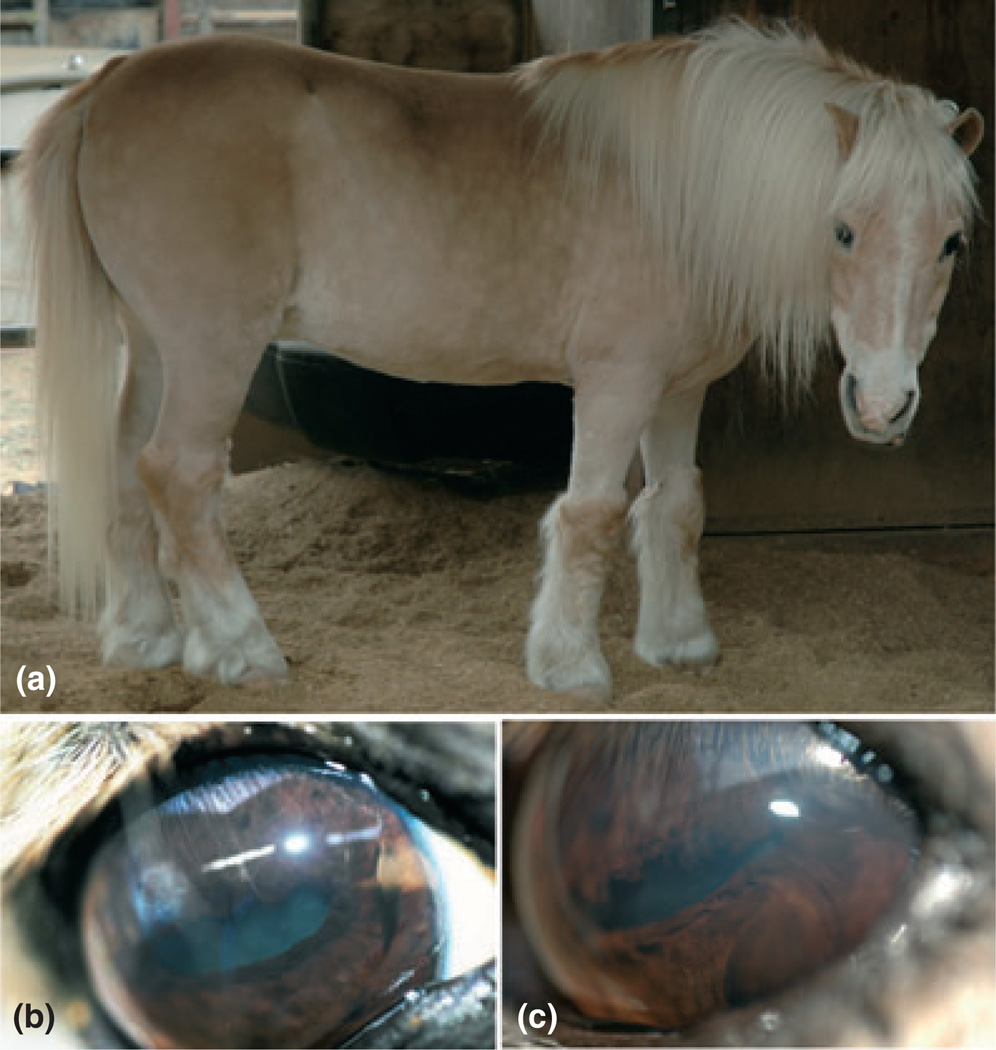
An approximately 23-year-old Shetland pony gelding was presented for cataract evaluation because the owner noticed that the pony was visually impaired under dim light conditions. There was no information about pedigree or prior medical history. The animal had a silver dapple coat color with a white mane and tail (Fig. 1a). The ophthalmic examination revealed bilateral positive menace responses and dazzle reflexes. Both pupils were miotic with decreased pupillary light reflexes. Overall, the findings were identical for both eyes (OU), except for a ventral strabismus that was only present on the left side (Fig. 1b). Testing of the optokinetic reflexes ruled out muscle paresis as the cause of ventral strabismus. Intraocular pressures were normal at 19 mmHg in the right eye (OD) and 20 mmHg in the left eye (OS). The corneas of this pony protruded excessively, and slit-lamp biomicroscopy revealed abnormally deep anterior chambers, confirming the presence of bilateral cornea globosa (Fig. 1c,d,e). Pharmacologic mydriasis was not achieved with application of either tropicamide or atropine sulfate 1% and the pupils remained miotic (Fig. 1f). The persistent bilateral miosis was not caused by anterior uveitis or prior drug applications. A diagnosis of iris hypoplasia was presumed based upon decreased pupillary light reflexes, and the lack of pupil dilation following topical application of mydriatics. The granula iridica encircled both miotic pupils through which nuclear cataracts were clearly distinguishable (Fig. 1f). Only the most peripheral parts of the ocular fundi could be appreciated by indirect ophthalmoscopy and they appeared unremarkable. The combination of bilateral cornea globosa, iris hypoplasia, and nuclear cataracts in a silver dapple pony was most consistent with MCOA (Table 2).1,2,5,6 The pathogenesis of the ventral strabismus of the left globe was not determined.
Figure 1.
Case 1. (a) A 23-year-old pony gelding with a black and silver coat color with dappling, and a white mane and tail. (b) The left globe with a ventral strabismus. Both the right (c) and left corneas (d) were protruding with resultant deep anterior chambers (cornea globosa). (e) A normal control eye of an unrelated horse is shown for comparison of the corneal curvature. (f) The pony exhibited bilateral iris hypoplasia, encircling granula iridica along the pupillary ruff, and nuclear cataracts.
Table 2.
Summary of clinical phenotypes and genotypes
| Case | Cornea globosa | Iris hypoplasia | Uveal cysts | Cataract | Phenotype | Genotype PMEL17ex11 marker |
|---|---|---|---|---|---|---|
| 1 | + | + | ND | + | MCOA | T/T homozygous |
| 2 | − | − | + | − | Cyst | T/C heterozygous |
| 3 | − | ND | ND | + | Possible MCOA | T/T homozygous |
| 4 | + | + | − | + | MCOA | T/T homozygous |
| 5 | + | + | ND | + | MCOA | T/T homozygous |
+, present; −, absent; ND, no data.
MCOA, severe multiple congenital ocular anomalies phenotype; Cyst, mild Cyst phenotype.
Nucleotides: T, thymine; C, cytosine.
Case 2
An approximately 4-year-old pony mare was examined following acquisition from an auction. Prior medical history and breed information was unknown. The owners did not observe any ocular abnormalities or signs of visual deficits. The pony had a chocolate-colored coat, with faint to absent dappling, and a flaxen-colored mane and tail (Fig. 2a). Examination of the anterior and posterior segments was unremarkable except for cysts arising from the lateral ciliary body OU (Fig. 2b). The pupils dilated normally following the topical application of tropicamide 1% ophthalmic solution. The combination of anterior uveal cysts and diluted hair color was most consistent with the Cyst phenotype of MCOA, as previously described in Rocky Mountain horses (Table 2).1,2,5,6
Figure 2.
Case 2. (a) A young pony mare with a chocolate-colored coat, very faint dappling, and a flaxen-colored mane and tail. (b) Ocular abnormalities consisted of temporal multiloculated uveal cysts (arrows) appreciated after pharmacologic mydriasis.
Case 3
An approximately 35-year-old Shetland pony gelding was presented with blindness and right eye cloudiness with intermittent epiphora. A detailed medical history and pedigree were not available. The pony had been previously diagnosed with Cushing’s disease (hyperadrenocorticism) but was not receiving any medication for the condition. Prior episodes of laminitis had resolved. The pony was silver dapple in color (Fig. 3a,b). Menace responses, pupillary light, and dazzle reflexes were not present OU. The right eye was buphthalmic with diffuse corneal edema and superficial corneal neovascularization (Fig. 3c,d). Intraocular structures could not be examined in detail OD due to the severity of the corneal edema. However, the right pupil was dilated with diffuse goniosynechiae, and the right lens was cataractous (Fig. 3d). There was a ventral strabismus on the left side (Fig. 3c,e). Muscle paresis was not the cause for the ventral strabismus based upon normal optokinetic reflexes. The left pupil was dilated and nonresponsive, and there was a diffuse immature cataract (Fig. 3e). The ocular fundi could not be examined due to the cataracts OU and the severe corneal edema OD. Both eyes did not stain with fluorescein. Intraocular pressures as measured by applanation tonometry were slightly elevated at 32 mmHg OD and 29 mmHg OS. There were no obvious clinical signs of chronic uveitis OU.
Figure 3.
Case 3. (a) A blind silver dapple pony with white mane and tail. (b) Detailed view of dappling. (c) The right buphthalmic eye with diffuse corneal edema, and the left eye with a ventral strabismus. (d) A close-up of the right eye revealing diffuse corneal edema and neovascularization, as well as the goniosynechiae (arrows). (e) The mydriatic left pupil with a cataractous lens.
Both eyes were treated with topical dorzolamide/timolol combination ophthalmic solution 2–3 times daily to address the elevated intraocular pressures. After 14 days the pony was re-examined. Intraocular pressures had reduced to 18 mmHg OU, and the right cornea was less edematous as appreciated by slit-lamp biomicroscopy. Otherwise, the results of the ophthalmic examination were the same as 2 weeks earlier. At the time of manuscript submission, the pony’s eyes were maintained on the topical medication for over 1 year. The clinical signs seen in this animal are commonly acquired anomalies in horses with chronic, recurrent anterior uveitis.7 Because of the pony’s coat color, MCOA was considered as a differential diagnosis (Table 2). The lack of previous ophthalmic examination data prevented the unequivocal determination of the pathogenesis. The presumed secondary chronic ocular hypertension may have caused damage to the retina and optic nerve resulting in blindness. The reason for the ventral strabismus of the left globe was also not determined.
Case 4
A 7-year-old silver dapple pony stallion initially presented with a painful right eye due to a superficial corneal stromal abscess. The pony had a white mane and tail (Fig. 4a). The abscess did not respond to medical therapy. Subsequently, a superficial keratectomy was performed to remove the abscess, and a conjunctival pedicle graft was placed on the exposed corneal stroma (Fig. 4b). Aerobic and fungal culture did not produce any viable organisms. Topical and systemic medical therapy was continued, and the cornea healed without complication. The treatment included the use of topical atropine sulfate 1% solution for iridocycloplegia, but the pupil did not dilate. Over the following 1.5 years, the pony’s right eye was intermittently treated topically with ophthalmic flurbiprofen 0.03% and atropine sulfate 1% solutions as well as systemically with oral flunixin meglumine because of recurrent episodes of mild ocular discomfort. The animal remained sighted OU.
Figure 4.
Case 4. (a) A 7-year-old pony stallion with silver dapple coat color including a white mane and tail. The animal was affected by the more severe MCOA phenotype, which included (b) iris hypoplasia, flattened and encircling granula iridica, cataracts, and (c) corneal globosa. A conjunctival pedicle graft is visible on the right cornea 3 years after surgery (b).
Simultaneously to the diagnosis and treatment of the right corneal stromal abscess, abnormally protruded corneas with deep anterior chambers (cornea globosa) and immature cortical cataracts were observed OU; the right lens was subluxated (Fig. 4b,c). Both irides appeared hypoplastic, with miotic pupils that were minimally responsive to light and unresponsive to topical atropine sulfate; the granula iridica were flattened and encircled the pupils (Fig. 4b). Schirmer tear tests (22 mm/min OU) and intraocular pressures (multiple measurements over 2 years averaged 25–29 mmHg OU) were normal after the right cornea had healed. The regions of the ocular fundi that could be visualized through the cataractous lenses appeared unremarkable. Ocular B-scan ultrasonography did not reveal any additional anomalies. The clinical findings in this silver dapple pony were most consistent with MCOA (Table 2).1,2,5,6
Case 5
An approximately 25-year-old Shetland pony gelding presented for blepharospasm and epiphora OS. He was a palomino with dappling (Fig. 5a). The owner also commented that the pony’s vision was abnormal, especially into the distance. The animal was receiving medication for the previously diagnosed Cushing’s disease (hyperadrenocorticism). Positive fluorescein staining revealed the presence of a superficial corneal ulcer OS associated with mild corneal edema and several bullae. The right cornea had central stromal fibrosis from a previous injury. The pony had normal menace responses OU, but both pupils were miotic, nonresponsive to light, and could not be dilated with topical tropicamide 1%, or with the combination of tropicamide and phenylephrine HCl 10% ophthalmic solutions. There was no evidence of posterior synechiae. Furthermore, the granula iridica appeared flattened and were circumferentially oriented at the pupillary ruff (Fig. 5b,c). Despite the small pupil size mild anterior cortical and nuclear cataracts were visible in both eyes (Fig. 5b,c). Only a limited view of the posterior segments was possible, but there were no obvious abnormalities. Applanation tonometry indicated normal intraocular pressures of 21 mmHg OU.
Figure 5.
Case 5. (a) Even though this 25-year-old palomino Shetland pony gelding was homozygous for the PMEL17 (Silver) mutation, it did not show the silver coat color. Eumelanin expression is blocked in palomino horses, which masks the phenotypic effect of the PMEL17 mutation. (b,c) Both eyes showed multiple anterior segment anomalies, including iris hypoplasia, flattened and circumferentially oriented granula iridica, and cataracts.
Treatment of the ulcerative keratitis and corneal edema with mild bullae OS consisted of neomycin-polymixin B-bacitracin, sodium chloride 5%, and atropine sulfate 1% ophthalmic ointments. At a 5-day recheck the corneal ulcer and bullae had healed, and the blepharospasm and epiphora had resolved. Intraocular pressures were 21 mmHg OD, and 22 mmHg OS. Despite three applications of atropine sulfate ophthalmic ointment miosis of the left pupil persisted. To rule out treatment inconsistency atropine sulfate 1% ophthalmic solution was applied to each eye. The pupils failed to dilate, which was consistent with iris hypoplasia. The combination of iris hypoplasia, flattened granula iridica, and cataracts was most consistent with MCOA (Table 2).1,2,5,6
Genotyping
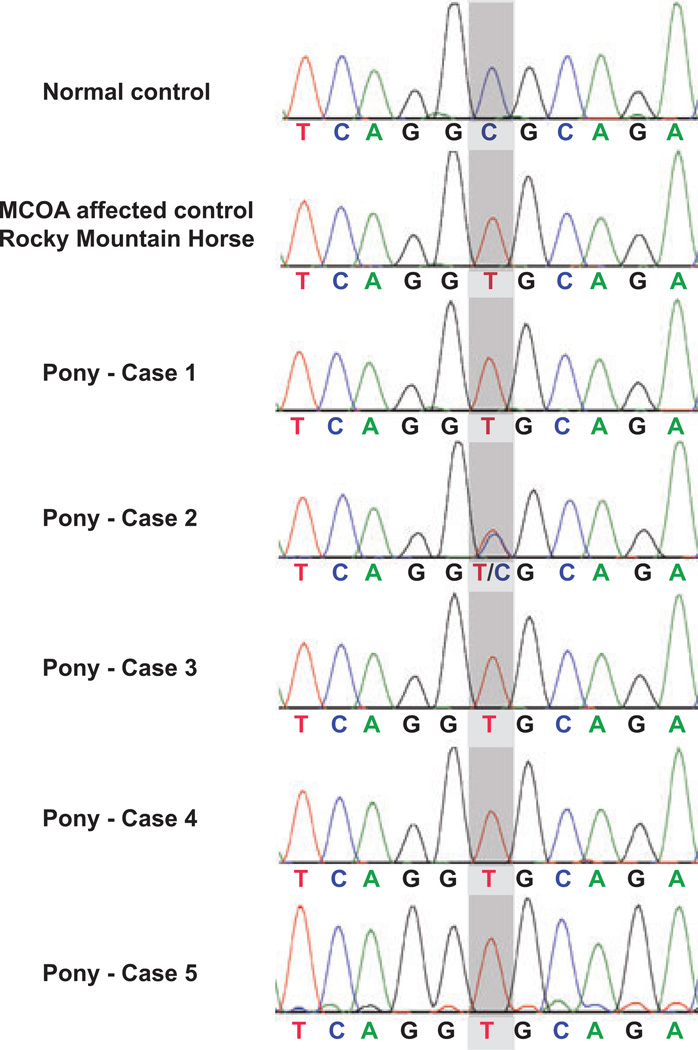
The results of the PMEL17ex11 genotyping are shown in the chromatograms of Fig. 6. The sequences were evaluated for the presence or absence of the C (wildtype) to T (Silver) missense mutation of the 5th nucleotide within exon 11 of the PMEL17 (Silver) gene. This Silver mutation is responsible for the silver dapple coat color and at the same time a marker for MCOA.4 Cases 1, 3, 4, and 5 were homozygous for the mutant MCOA allele as indicated by a single T-peak in their chromatograms (Fig. 6) (Table 2). Case 2 was heterozygous with overlapping peaks for C and T (Fig. 6) (Table 2). Both homozygous and heterozygous animals have a silver or chocolate coat color because the Silver mutation is inherited as an autosomal dominant trait.4 Exceptions to this mutation’s effect on coloration are found in red horses, such as chestnuts and palominos (for example case 5), that do not express eumelanin.4,8 Heterozygous horses manifest with the milder Cyst phenotype of MCOA whereas homozygous animal present with multiple anterior segment anomalies.5
Figure 6.
PMEL17ex11 genotyping. The chromatograms show the variations in the DNA sequence at the critical site (gray shading) of exon 11 within the PMEL17 (Silver) gene. A normal, nonsilver control horse is homozygous for the wildtype allele with cytosine (C) at the critical location. For comparison, a color-dilute, silver Rocky Mountain horse with multiple congenital ocular anomalies is homozygous for the mutant allele with thymine (T) at the critical site. The chromatograms show that cases 1, 3, 4, and 5 were also homozygous for the mutant allele with T. Only case 2 was heterozygous with both a C and a T at the same location.
DISCUSSION
Equine MCOA syndrome is characterized by the presence of temporal anterior uveal cysts with or without a combination of other anterior segment anomalies.1,2,6 It has been previously described in both Rocky Mountain and Kentucky Mountain Saddle horses.1,2,5 The condition was initially named Anterior Segment Dysgenesis (ASD) but it has been more appropriately termed as MCOA.1,2 Equine MCOA has been appreciated in other breeds including Morgan Horses, Belgian Draft horses, ponies and miniatures.1 However, to the best of our knowledge, our finding is the first formal documentation of the condition in ponies. We have presented five presumably unrelated ponies with clinical signs resembling the MCOA of affected Rocky Mountain horses.
The most common symptom of MCOA is the presence of temporal cysts of the posterior iris, ciliary body, and peripheral retina.1,2,6 The retinal cysts are considered extensions of uveal cysts and may manifest as retinal dysplasia or retinal detachment.1 If these cysts are the only ocular anomalies in an animal, the clinical findings are classified as the less severe Cyst phenotype of equine MCOA.1,2,6 Horses with the Cyst phenotype appear normal and are unlikely to be identified, except inadvertently, by their owners or veterinarians. In case 2, cysts were only discovered during a routine health check. Horses are classified as having the more severe MCOA phenotype if the temporal ciliary cysts are combined with other congenital ocular anomalies, including cornea globosa, iridocorneal angle abnormalities, iris hypoplasia, cataracts, lens subluxation, or macropalpebral fissure and craniofacial abnormalities.1,2,6 In four of our ponies, the ophthalmic findings were consistent with the MCOA phenotype. Although undocumented, we suspect that in some of these animals temporal uveal cysts were present but not visible under field conditions. Ocular ultrasonography might reveal these cysts.
While cataracts and lens luxations in adult horses are most commonly caused by chronic uveitis,9 we suspect that these abnormalities were part of the MCOA phenotype in cases 1, 4, 5 and possibly even 3.2,10 Subjectively, we did not perceive any enlargement of the palpebral fissures (macroblepharon) in any of our animals.2,10
One pony’s glaucoma (case 3) could have been a consequence of MCOA-associated goniosynechiae, especially since the animal was homozygous at the MCOA locus. While equine MCOA is generally considered a nonprogressive condition,1,2,5 chronic glaucoma may have caused visual loss and corneal disease in this case. In the same pony, iris hypoplasia could have been masked by glaucoma-induced mydriasis.11 In general, equine glaucoma is most commonly a complication of chronic anterior uveitis in adult horses,11 a possibility that we could not rule out in case 3 even with the suspected presence of MCOA. We believe that the corneal stromal abscess and ulcers seen in cases 4 and 5 were unlikely to be related to MCOA.
We could not associate the ventral strabismus in the left eyes of two ponies with MCOA. The strabismus could have been congenital or acquired. Acquired abnormalities in the visual axes of horses are typically caused by severe head trauma with skull fractures or central nervous system infections.12 There were no other clinical signs supporting a prior trauma or extraocular muscle palsy. It is possible, but rather unlikely, that in these ponies a misrouting of optic nerve fibers was responsible for the strabismus. This phenomenon has been previously described in color-dilute Siamese cats.13
Similar to the Rocky Mountain and Kentucky Mountain Saddle horses,2,10 we found an association between MCOA and the diluted silver or chocolate coat color, with white or flaxen-colored manes and tails. The silver coat color is characterized by dilution of the black pigment eumelanin.4 The gene mutation responsible for the silver coat color has recently been identified as a missense mutation in exon 11 of the Silver or PMEL17 gene on equine chromosome 6 (ECA6q23).4,14 PMEL17 encodes the pre-melanosomal protein 17 (PMEL17), a transmembrane protein of melanosomes that plays an important role in the formation of the characteristic fibrillar structure of these organelles.15,16 The exchange of a single nucleotide from C to T within exon 11 leads to a missense change of the second amino acid of PMEL17’s cytoplasmic region from an arginine to a cysteine (Arg618Cys).4 This mutation in PMEL17 leads to a dilution or inhibition of the black pigment eumelanin, but does not affect the synthesis of the red pheomelanin.16–18 Black and bay horses become silver dappled, but chestnut and palomino horses (such as case 5) are unaffected because they do not express eumelanin.4 Similar hypopigmented phenotypes have been described in PMEL17-mutant chickens and mice.4,17,18 In dogs, a mutation in PMEL17 results in the merle coat color, characterized by patches of diluted pigment intermingled with normal melanin.19,20
The silver coat color of horses is inherited as an autosomal dominant trait with color dilution in both homozygous and heterozygous animals.4 Homozygous animals may be more color diluted than heterozygotes,4 consistent with a darker coat color and underappreciated dappling, as seen in our heterozygous case 2. The other ponies in this study were homozygous for the Silver mutation and therefore more color dilute with clearly visible dappling. The exception was case 5 with the palomino coat color that masked the effect of the Silver mutation.
It has recently been shown that the missense mutation in PMEL17 (Silver) showed complete linkage with the MCOA locus in Rocky Mountain horses and therefore serves as a genetic marker (PMELex11) for MCOA.5 The MCOA locus has been narrowed to a 4.9 megabase (Mb) interval on the equine chromosome 6 (ECA6q) that includes the PMEL17 gene.5 The close proximity of the PMEL17 gene to the MCOA locus explains why coat color appears to be linked to the expression of ocular anomalies.5 The mutated PMEL17 (Silver) gene is presumably linked with the unknown mutated MCOA gene, and the wildtype PMEL17 gene is similarly linked with the wildtype form of the MCOA gene. Both the Silver mutation and the MCOA mutation are dominantly inherited traits, but there is incomplete penetrance for the MCOA mutation.2 This means that a heterozygous animal will always be a silver dapple but is less severely affected by MCOA with only uveal cysts (Cyst phenotype).2,5,6 A homozygously affected animal will be a silver dapple and have multiple anterior segment anomalies (MCOA phenotype). Even though we did not have any pedigree information for our ponies, the genotyping allowed us to confirm the same inheritance pattern as in Rocky Mountain horses. Multiple bilateral abnormalities (including cornea globosa, iris hypoplasia, and nuclear cataracts) were identified in cases 1, 3, 4, and 5, consistent with the MCOA phenotype. These animals were homozygous for the mutant MCOA allele (Table 2). In case 3 the diagnosis was not unequivocal since all the ocular anomalies could also have been acquired. It is remotely possible that case 3 was not affected by MCOA despite being homozygous for the in PMEL17 (Silver) mutation; a scenario that could be explained by recombination events between the PMEL17 (Silver) and the MCOA genes. Case 2 presented only with temporal ciliary body cysts, which was suggestive of the Cyst phenotype and associated with a heterozygous MCOA genotype (Table 2).
Despite the explanations in the previous paragraph, the PMEL17 (Silver) mutation has not been excluded as the causative gene for MCOA.5 Our case series supports the notion that the (still unknown) MCOA gene and the PMEL17 (Silver) gene are the same, or at least overlap on ECA6q23. Mutations in PMEL17 have also been associated with an abnormal ocular phenotype of zebrafish known as the fading vision mutation (fdv).21 Canine merle coat coloration has also been associated with ocular defects including coloboma, and microphthalmia.22,23 However, there have been indications in horses about a dissociation of the PMEL17 and MCOA mutations by recombination: for example, MCOA-like abnormalities have not been seen in some PMEL17 mutant horses, including some silver-colored Icelandic ponies1,2,5,6,10 and possibly our case 3, and a few nonsilver dapple horses have expressed the MCOA phenotype.1,2,5,6,10
The prevalence of MCOA in purebred and crossbred Rocky Mountain and Kentucky Mountain horses has been estimated to be ~50% of their populations.1,2 This relatively high incidence of MCOA may be a founder effect based on the popularity of silver-colored horses.1,2,4–6 The breeding of individuals with desired characteristics (silver dapple) propagated an undesirable linked trait (MCOA). We suspect that any breed with a high frequency of silver-colored animals will be associated with an increased incidence of MCOA due to this linkage. The percentage of ponies with MCOA still needs to be determined.
CONCLUSIONS
We present here the first formal report of equine MCOA syndrome in ponies. We show that both the clinical phenotypes and the genetics of the condition in ponies are in agreement with the previously published findings for Rocky Mountain and Kentucky Mountain horses. MCOA is inherited as an autosomal dominant trait with incomplete penetrance. Homozygous ponies showed multiple congenital anterior segment anomalies, whereas a heterozygous pony only presented with temporal uveal cysts. Because of close linkage the PMEL17 or Silver mutation currently serves as one possible genetic marker for MCOA.
ACKNOWLEDGMENTS
The authors thank the owners of the ponies for providing permission to include their animals in this study and Dr. Jay Wayne for critical review of the manuscript. This study was partially funded by the National Institutes of Health, National Eye Institute (K12 EY015398 to Dr. Komáromy).
Footnotes
Presented in part at the Annual Conference of the American College of Veterinary Ophthalmologists, Chicago, IL, USA, November 2009.
REFERENCES
- 1.Ramsey DT, Ewart SL, Render JA, et al. Congenital ocular abnormalities of Rocky Mountain horses. Veterinary Ophthalmology. 1999;2:47–59. doi: 10.1046/j.1463-5224.1999.00050.x. [DOI] [PubMed] [Google Scholar]
- 2.Grahn BH, Pinard C, Archer S, et al. Congenital ocular anomalies in purebred and crossbred Rocky and Kentucky Mountain horses in Canada. The Canadian Veterinary Journal. 2008;49:675–681. [PMC free article] [PubMed] [Google Scholar]
- 3.Parker R. Equine Science. 2nd edn. Clifton Park, NY: Delmar Learning; 2003. [Google Scholar]
- 4.Brunberg E, Andersson L, Cothran G, et al. A missense mutation in PMEL17 is associated with the silver coat color in the horse. BMC Genetics. 2006;7:46. doi: 10.1186/1471-2156-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson LS, Juras R, Ramsey DT, et al. Equine multiple congenital ocular anomalies maps to a 4.9 megabase interval on horse chromosome 6. BMC Genetics. 2008;9:88. doi: 10.1186/1471-2156-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ewart SL, Ramsey DT, Xu J, et al. The horse homolog of congenital aniridia conforms to codominant inheritance. The Journal of Heredity. 2000;91:93–98. doi: 10.1093/jhered/91.2.93. [DOI] [PubMed] [Google Scholar]
- 7.Gilger BC, Deeg C. Equine recurrent uveitis. In: Gilger BC, editor. Equine Ophthalmology. 2nd edn. Maryland Heights, MO: Elsevier, Inc.; 2011. pp. 317–349. [Google Scholar]
- 8.Mariat D, Taourit S, Guerin G. A mutation in the MATP gene causes the cream coat colour in the horse. Genetics, Selection, Evolution: GSE. 2003;35:119–133. doi: 10.1186/1297-9686-35-1-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colitz CMH, McMullen RJ., Jr . Diseases and surgery of the lens. In: Gilger BC, editor. Equine Ophthalmology. 2nd edn. Maryland Heights, MO: Elsevier, Inc.; 2011. pp. 282–316. [Google Scholar]
- 10.Ramsey DT, Hauptman JG, Petersen-Jones SM. Corneal thickness, intraocular pressure, and optical corneal diameter in Rocky Mountain horses with cornea globosa or clinically normal corneas. American Journal of Veterinary Research. 1999;60:1317–1321. [PubMed] [Google Scholar]
- 11.Utter ME, Brooks DE. Glaucoma. In: Gilger BC, editor. Equine Ophthalmology. 2nd edn. Maryland Heights, MO: Elsevier, Inc.; 2011. pp. 350–366. [Google Scholar]
- 12.Gilger BC. Diseases and surgery of the globe and orbit. In: Gilger BC, editor. Equine Ophthalmology. 2nd edn. Maryland Heights, MO: Elsevier, Inc.; 2011. pp. 93–132. [Google Scholar]
- 13.Shatz CJ, LeVay S. Siamese cat: altered connections of visual cortex. Science. 1979;204:328–330. doi: 10.1126/science.432647. [DOI] [PubMed] [Google Scholar]
- 14.Reissmann M, Bierwolf J, Brockmann GA. Two SNPs in the SILV gene are associated with silver coat colour in ponies. Animal Genetics. 2007;38:1–6. doi: 10.1111/j.1365-2052.2006.01553.x. [DOI] [PubMed] [Google Scholar]
- 15.Hearing VJ. Biogenesis of pigment granules: a sensitive way to regulate melanocyte function. Journal of Dermatological Science. 2005;37:3–14. doi: 10.1016/j.jdermsci.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Theos AC, Truschel ST, Raposo G, et al. The silver locus product Pmel17/gp100/Silv/ME20: controversial in name and in function. Pigment Cell Research. 2005;18:322–336. doi: 10.1111/j.1600-0749.2005.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez-Esparza M, Jimenez-Cervantes C, Bennett DC, et al. The mouse silver locus encodes a single transcript truncated by the silver mutation. Mammalian Genome. 1999;10:1168–1171. doi: 10.1007/s003359901184. [DOI] [PubMed] [Google Scholar]
- 18.Kerje S, Sharma P, Gunnarsson U, et al. The dominant white, dun and smoky color variants in chicken are associated with insertion/deletion polymorphisms in the PMEL17 gene. Genetics. 2004;168:1507–1518. doi: 10.1534/genetics.104.027995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedan B, Corre S, Hitte C, et al. Coat colour in dogs: identification of the merle locus in the Australian shepherd breed. BMC Veterinary Research. 2006;2:9. doi: 10.1186/1746-6148-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark LA, Wahl JM, Rees CA, et al. Retrotransposon insertion in SILV is responsible for merle patterning of the domestic dog. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1376–1381. doi: 10.1073/pnas.0506940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schonthaler HB, Lampert JM, von Lintig J, et al. A mutation in the silver gene leads to defects in melanosome biogenesis and alterations in the visual system in the zebrafish mutant fading vision. Developmental Biology. 2005;284:421–436. doi: 10.1016/j.ydbio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Gelatt KN, McGill LD. Clinical characteristics of microphthalmia with colobomas of the Australian shepherd dog. Journal of the American Veterinary Medical Association. 1973;162:393–396. [PubMed] [Google Scholar]
- 23.Gelatt KN, Powell NG, Huston K. Inheritance of microphthalmia with coloboma in the Australian shepherd dog. American Journal of Veterinary Research. 1981;42:1686–1690. [PubMed] [Google Scholar]