Abstract
Epidemiological studies beginning in the 1990s have reported that intake of quercetin, a polyphenolic flavonoid found in a wide variety of plant-based foods, such as apples, onions, berries, and red wine, is inversely related to cardiovascular disease. More recent work using hypertensive animals and humans (>140 mm Hg systolic and >90 mm Hg diastolic) indicates a decrease in blood pressure after quercetin supplementation. A number of proposed mechanisms may be responsible for the observed blood pressure decrease such as antioxidant effects, inhibition of angiotensin-converting enzyme activity, and improved endothelium-dependent and -independent function. The majority of these mechanisms have been identified using animal models treated with quercetin, and relatively few have been corroborated in human studies. The purpose of this review is to examine the evidence supporting the role of quercetin as a potential therapeutic agent and the mechanisms by which quercetin might exert its blood pressure–lowering effect.
Introduction
The American Heart Association estimates that 74.5 million Americans have hypertension (1), which is most commonly defined as systolic blood pressure >140 mm Hg and diastolic blood pressure >90 mm Hg. Equally alarming are recent estimates that ∼25% of the US population has prehypertension, which is defined as untreated blood pressure of 120–139 mm Hg systolic or 80–89 mm Hg diastolic (1). Although hypertension can frequently exist with other cardiovascular disease (CVD)5 risk factors such as metabolic syndrome, it is usually asymptomatic. Importantly, there is a positive and direct correlation between hypertension and the risk of other CVDs such as cardiac arrhythmia, coronary artery disease, cardiac hypertrophy, myocardial infarction, and heart failure (1). Taken together, it is estimated that, in 2010, the total direct and indirect costs of CVD was $503.2 billion (1).
Blood pressure is controlled by neural mechanisms carried out by the autonomic nervous system and humoral mechanisms involving substances such as nitric oxide (NO) and endothelin-1 (ET-1) that are released by different cell types. Decreased vasodilation, increased vasoconstriction, and greater vascular peripheral resistance characterize hypertension. Treatment of hypertension depends on the etiology of the disease and includes diet alterations, weight loss, exercise, and pharmacological interventions. Pharmacological therapies [e.g., angiotensin-converting enzyme (ACE) inhibition, diuretics, and calcium channel blockers] to treat hypertension are successful but may be associated with negative side effects such as persistent cough, dry throat, allergic reactions, dizziness, angioedema, and kidney failure (2). Lifestyle interventions (either alone or in combination) such as reduced sodium intake (3), the Dietary Interventions to Stop Hypertension diet (4), and increased physical activity (5) are also known to decrease blood pressure in hypertensive patients. Dietary antioxidant vitamins and supplements have also been reported to decrease blood pressure in both animals and humans. For example, both human and animal studies have used vitamins C (6, 7) and E (8, 9) and polyphenolic flavonoids (10–13) to decrease blood pressure and improve endothelial function. With regard to polyphenolic flavonoids, several epidemiological studies found significant correlations between flavonoid intake and CVD. Evidence from the Zutphen Elderly Study suggests a strong cardioprotective effect of several flavonoids, including quercetin (14). In this study, the risk of death from coronary heart disease was decreased by as much as 68% in men who consumed >29 mg of flavonols daily compared with men who consumed <10 mg of flavonols daily (14). Although the specific association between quercetin intake and blood pressure was not examined in this study, the authors did report an inverse relationship between foods high in quercetin and blood pressure. Similarly, other studies examining flavonoids found that increased flavonoid intake is inversely related to chronic disease, such as CVD (15–18). Given the public interest in alternative therapies for chronic disease coupled with the increasing number of studies reporting beneficial effects of natural products on prevention of disease, it is not surprising that vitamins and polyphenolic flavonoids have become increasingly popular in the treatment and prevention of hypertension among the public.
One particular flavonoid, quercetin, has been studied in cell-based assays, experimental animal models, and human clinical trials in recent years and is gaining popularity as a natural therapy for hypertension and vascular health. The purpose of this review is to examine the existing evidence that may support the use of quercetin as an antihypertensive agent and to review the possible mechanisms by which quercetin might decrease blood pressure.
Current status of knowledge
Quercetin: Food sources, chemistry, and health benefits
Phytochemicals are produced in plants and have innate biological activity to protect the plant against insect invasion, ultraviolet light damage, infection, and diseases. Plants also derive characteristics of color, flavor, and aroma from phytochemicals. Flavonoids constitute a class of phytochemical and are divided into various subclasses based on their molecular structure. The contributions of flavonoids to health are in part based on their molecular structure (19). Quercetin is a flavonol and is widely found in many plant-based foods such as apples, onions, citrus fruits, berries, red grapes, red wine, broccoli, bark roots, flowers, and tea (20) (Table 1). The average Western diet supplies 15–40 mg/d of quercetin, and dietary levels >33 mg/d have been associated with a decreased risk of CVD (14, 16, 17). Although the quercetin content of the diet may be estimated using databases available from the USDA (20), there is some variability in the reported flavonoid content of foods because of variations in soil, harvest, and storage conditions (21). At present, quercetin supplements are widely available through commercial sources in doses ranging from 250 to 1500 mg of quercetin. Typically, these commercial sources use 1 or more of the various isoforms of quercetin such as quercetin aglycone, rutin, and other glycoside versions. In general, quercetin supplements are marketed to the public as alternative therapy for treating allergies, asthma, bacterial infections, arthritis, gout, eye disorders, hypertension, and neurodegenerative disorders. Because few controlled, randomized trials have been performed, there is a lack of data to provide a solid scientific basis for many of these treatment claims. However, with regard to hypertension, there is a body of evidence from cell-based assays, experimental animal models, and human clinical trials that supports a possible therapeutic role for quercetin in the treatment of hypertension.
Table 1.
Amount of quercetin in selected foods1
| Food source | Quercetin content (mg/100 g) |
| Capers | 233.00 |
| Onions | 22.00 |
| Cocoa powder | 20.00 |
| Cranberries | 14.00 |
| Lingonberries | 7.40 |
| Asparagus, cooked | 7.61 |
| Blueberries | 5.05 |
| Apple, Red Delicious | 4.70 |
| Cherries | 2.64 |
| Broccoli, raw | 2.51 |
| Apple, Fuji | 2.02 |
| Green tea | 2.69 |
| Black tea | 1.99 |
| Red grapes | 1.38 |
Adapted from USDA database (20).
Bioavailability and safety of quercetin
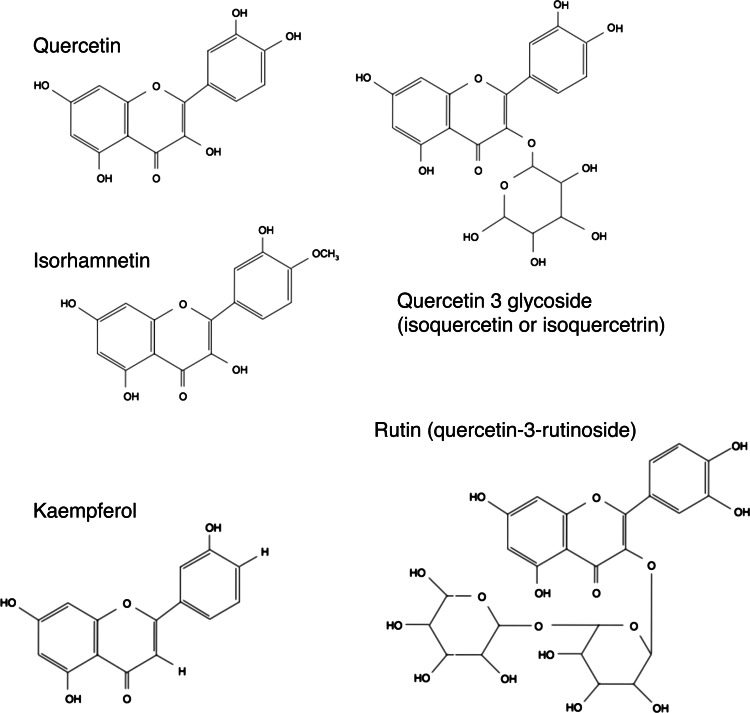
The chemical structure of pure quercetin is an unconjugated aglycone that does not have a carbohydrate moiety (Fig. 1). Quercetin in foods, such as onions, has a sugar group in its structure, which is known as a glycoside form (Fig. 2). Supplemental forms of quercetin are usually quercetin aglycone, although some products have small amounts of a glycoside called rutin as well. The bioavailability of quercetin is dependent on the form of quercetin that is ingested, with the glycoside forms of quercetin resulting in better absorption than quercetin aglycone (21). However, studies found that both forms are readily bioavailable (10, 21–23). For example, we found that a single 1095-mg dose of quercetin aglycone supplement (24) and a 4-wk regimen of 730 mg quercetin/d (10) can significantly increase plasma quercetin concentrations 3-fold over baseline.
Figure 1.
Chemical structure of quercetin and its various forms.
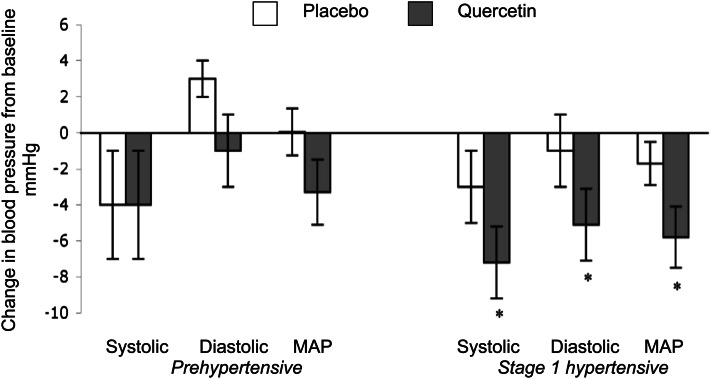
Figure 2.
Summary of blood pressure changes observed after a double-blind, placebo-controlled trial comparing 4 wk of 760 mg/d quercetin supplementation vs. placebo in male and female patients with hypertension. MAP, mean arterial pressure. Values are mean ± SE, n = 22 stage 1 hypertensive, n = 19 Prehypertensive. *Different from placebo; P < 0.05. Adapted with permission from (10).
Other factors such as intestinal flora and dietary components can affect the bioavailability of various isoforms of quercetin. For example, supplementing pectin and rutin together results in increased quercetin plasma concentrations in mice (25). Similar results were observed in rats fed a diet high in pectin that were orally administered a single 50-mg/kg dose of quercetin (26), although enhancement of absorption in this study was not attributed to alterations in intestinal flora. Consumption of dietary fat was shown to enhance absorption of both quercetin aglycone and quercetin-3-O-glycoside in pigs (27, 28). Few studies examined the ability of other polyphenolic compounds to influence quercetin absorption; however, it was reported that cosupplementation of epigallocatechin gallate and quercetin can increase epigallocatechin gallate absorption in rats (29).
All forms of quercetin (aglycone and quercetin with glycosides) are absorbed in the small intestine and colon. Studies using rodents and humans indicate that quercetin glycosides are hydrolyzed before absorption by a lactase phloridzin hydrolase enzyme before being absorbed as quercetin aglycone, whereas quercetin aglycone is absorbed intact (30–34). Once in the plasma, quercetin is bound to albumin (35) and transported to the liver (19). In the liver, quercetin (aglycone or with glycosides) is rapidly converted to one or more metabolites including isorhamnetin, kaempferol, and tamarixetin. In our laboratory, we found high plasma concentrations of isorhamnetin and, to a lesser extent, kaempferol in rats fed quercetin-supplemented (0.15%) diets for 11 wk (36). Others found isorhamnetin and tamarixetin in human plasma after quercetin aglycone supplementation (22). These stable metabolites of quercetin are also distributed throughout the body tissues via albumin (37).
Quercetin is believed to be antimutagenic in vivo, and long-term studies have not supported a carcinogenic role for quercetin (38). Few negative side effects have been noted with short-term (<3 mo), high intake of quercetin aglycone (10, 22, 39), but there have been signs of nephrotoxicity (increased serum creatinine) after large doses (∼3600 mg) were administered intravenously to patients being treated for cancer (reviewed in 38]). Quercetin is also an inhibitor of CYP3A4, an enzyme that breaks down many commonly prescribed drugs in the body; therefore, quercetin should not be taken with drugs that depend on this enzyme for metabolism. Because many flavonoids were found to inhibit platelet aggregation (via inhibition of thromboxane A2) (40, 41), it is also possible that pharmacological doses of quercetin could increase the risk of bleeding when taken with anticoagulant drugs.
Therapeutic role of quercetin to decrease blood pressure in animals and humans
Please note that all studies reviewed here used the aglycone form of quercetin (Fig. 1) unless otherwise noted. Quercetin has been shown to have vasodilator effects in vitro using isolated rat arteries (42–44). This vasodilatory effect is also observed using metabolites of quercetin such as isorhamnetin, tamarixetin, and kaempferol and is reported to be independent of the endothelium (42, 44). It should also be noted that the vasodilatory effect of quercetin appears to be more pronounced in resistance (e.g., mesenteric arteries) compared with conductance (e.g., aorta) arteries (42, 44). Given the data obtained from these in vitro studies, it is not surprising that a number of laboratories, including our own, have reported that quercetin lowers blood pressure in spontaneously hypertensive (11, 45) and Dahl salt-sensitive rats (46) as well as rats that consume a high-fat, high-sucrose diet (47), are deficient in NO (48), are infused with angiotensin I (49), or have experimentally induced pressure overload using aortic constriction (12). The aforementioned animal studies have provided important proof-of-principle that quercetin may be efficacious in decreasing blood pressure in humans with hypertension.
The antihypertensive effects of quercetin found in animals have also been reproduced in humans. The magnitude of blood pressure reduction is often greater in animal studies, and one could speculate that this may be related to factors such as the dose used (greater in animal studies than in human studies), and the severity of hypertension present in the animal models. Nevertheless, it is noteworthy that across models, quercetin has consistently been demonstrated to decrease blood pressure.
Supplementation of the diet with quercetin aglycone has been shown to decrease blood pressure in hypertensive individuals, but not those with prehypertension or normal blood pressure. For example, we conducted a randomized, double-blind, placebo-controlled, crossover trial to investigate the efficacy of quercetin supplements on stage 1 hypertensive (140–159 mm Hg systolic and 90–99 mm Hg diastolic, n = 22) and prehypertensive (20–139 mm Hg systolic and 80–89 mm Hg diastolic, n = 19) participants. Decreases (P < 0.01) in systolic (−7 ± 2 mm Hg), diastolic (−5 ± 2 mm Hg), and mean arterial blood pressure (−5 ± 2 mm Hg) were observed in subjects with hypertension after supplementation with 730 mg/d of quercetin for 28 d vs. placebo. In our study, we did not find any change in blood pressure in subjects with prehypertension (Fig. 2). Similar results have been observed in normotensive, healthy humans supplemented daily with 1000 mg of quercetin and 200 mg of rutin (quercetin-3-rutinoside) who demonstrate a marked increase in plasma quercetin levels after 4 wk, but no change in blood pressure (39). Likewise we have observed that quercetin does not decrease blood pressure in normotensive rodents, but only in hypertensive ones (12, 36).
In contrast to our work, 2 studies conducted by Egert et al. (50, 51) found that 150 mg/d of quercetin for 6 wk decreased blood pressure in overweight and obese prehypertensive individuals. Both studies by Egert et al. found statistically significant decreases in systolic pressure of ∼3 mm Hg; however, it was found that the effect was only present in subjects that were homozygous for the apolipoprotein E3 genotype (51). Subjects carrying a copy of apolipoprotein E4 did not have lower blood pressure after quercetin supplementation (51). There are several possible explanations for the discrepancies in the effect and magnitude of the decrease in blood pressure between our studies and those of Egert et al. For example, the differences may be related to the dose of quercetin used (150 vs. 730 mg/d), the duration of supplementation (42 vs. 28 d), the genotype of participants, and/or the sample size used (n = 93 vs. n = 19). However, all human studies to date are in broad agreement that quercetin supplementation can decrease blood pressure in hypertensive individuals.
Potential mechanisms for blood pressure reduction
Evidence exists to support several potential mechanisms whereby quercetin might decrease blood pressure and decrease the severity of hypertension in animals and humans. These mechanisms are a decrease in oxidative stress, interference with the renin-angiotensin-aldosterone system (RAAS), and /or improving vascular function in an endothelium-dependent or -independent manner.
Quercetin and oxidative stress.
Because oxidative stress has been linked to impaired vasodilation and kidney function in animal models of hypertension (52), many studies have examined the role of antioxidants to treat hypertension. Previous studies found that a decrease in blood pressure in hypertensive animals and humans treated with quercetin is due to a decrease in oxidative stress. For example, Duarte et al. (11) found that treatment of spontaneously hypertensive rats with quercetin improved endothelium-dependent vasorelaxation of aortic segments and decreased systolic blood pressure by 18%. Because indices of oxidative stress (e.g., urinary F2 isoprostane and plasma malondialdehyde) were lowered after quercetin treatment, the improvement in endothelial function was attributed to the antioxidant effect of quercetin. Other animal studies have also found that decreases in blood pressure after quercetin supplementation occur in conjunction with improved indices of oxidative stress such as plasma lipid peroxides and urinary isoprostanes (marker of lipid peroxidation) (45, 47, 48). In these studies, the authors concluded that the antioxidant effect of quercetin might have been the mechanism for blood pressure reduction. However, not all animal studies found that quercetin exerts a strong antioxidant effect. In our laboratory, we found that quercetin-supplemented chow can decrease blood pressure and hepatic malondialdehyde levels in pressure-overloaded (12), but not spontaneously hypertensive rats (36).
Similar to the equivocal findings in animals studies, human investigations using various quercetin doses have not consistently demonstrated an antioxidant effect of quercetin. Egert et al. (22) demonstrated that 150 mg of quercetin for 2 wk in healthy, normotensive individuals did not affect plasma oxidized low-density lipoprotein, ferric-reducing antioxidant potential, oxygen radical absorbance capacity, or inflammatory markers, In a later study conducted by the same group using 93 hypertensive individuals with symptoms of metabolic syndrome, 150 mg of quercetin for 6 wk was enough to decrease systolic blood pressure by 2.6 mm Hg (P < 0.05) and oxidized low-density lipoprotein, but not inflammatory markers or plasma antioxidant capacity (50). We found similar results that quercetin lowers systolic blood pressure by 7 mm Hg, but not indicators of oxidant load (plasma antioxidant capacity or urinary isoprostane concentration) in hypertensive humans with metabolic syndrome (10).
Although a great deal of evidence obtained from hypertensive animal models indicates that quercetin might be effective in decreasing oxidant load, available data from humans is equivocal. In general, higher doses of quercetin have been evaluated in animals compared with humans, and this may lead to significant differences in the intracellular concentrations of quercetin and subsequent antioxidant effects. In our previous studies, we noted decreases in liver malondialdehyde levels in rats fed quercetin-supplemented diets (150 mg/kg of quercetin) (12), but not in hypertensive humans in whom ∼8.1 mg quercetin/kg body weight failed to alter plasma antioxidant power or urinary isoprostanes (10). One key difference between these studies is the fact that plasma quercetin levels were 3.96 μg/mL in rats, but only 0.48 μg/mL in humans (corresponding to a 2-fold increase from baseline). This raises the possibility that the difference in plasma quercetin concentrations was responsible for the disparate effects on oxidative stress. Because inconsistent evidence exists for the ability of quercetin to act as an antioxidant in vivo, it remains possible that the blood pressure–lowering effect of quercetin may be due to other mechanisms.
Quercetin and RAAS.
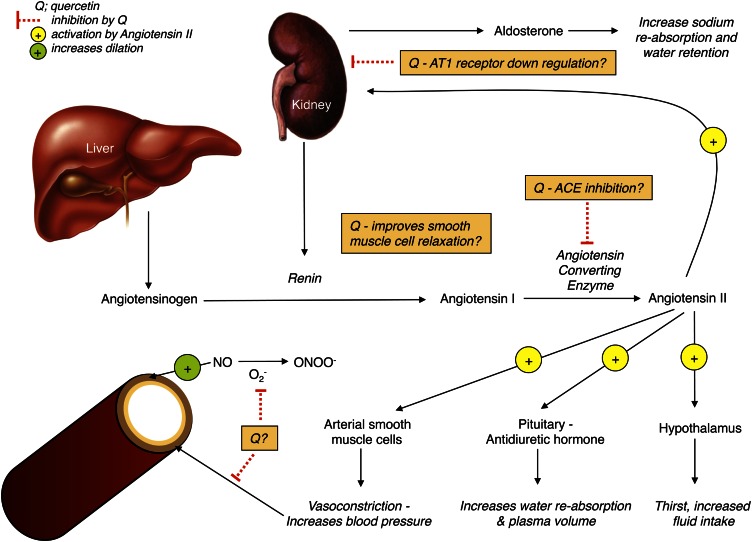
The RAAS (Fig. 3) plays a major role in the regulation of blood pressure. There is a great deal of evidence that long-term overactivation of this system leads to hypertension and other CVDs (reviewed in [53]). The use of ACE inhibitors or subtype-specific angiotensin receptor blockers can interfere with the RAAS and lead to a decrease in both blood pressure and cardiovascular events in high-risk populations (53, 54). Quercetin has been shown to inhibit ACE in vitro (55), presumably through its ability to chelate metal ions such as zinc (43). Interestingly, the mechanism of action by which ACE inhibitors such as captopril and imidapril inhibit ACE is by binding a zinc atom at the active site of the enzyme, which slows conversion of angiotensin I to angiotensin II (56). Häckl et al. (49) demonstrated that both oral and intravenous administration of quercetin in Wistar rats attenuates the increase in blood pressure evoked by intravenous infusion of angiotensin I. This study also reported a 31% decrease in ACE activity after quercetin treatment compared with baseline (49), suggesting that quercetin acted as an ACE inhibitor. Mackraj et al. (46) also compared the long-term antihypertensive effects of captopril with those of quercetin using Dahl salt-sensitive rats that were given daily injections for 4 wk of captopril (ACE inhibitor), quercetin, or vehicle. Although blood pressure increased in vehicle-treated Dahl rats during the 4-wk period, it was significantly decreased compared with baseline in both quercetin- and captopril-treated groups. The decrease in blood pressure occurred in parallel with down-regulation of the angiotensin-I receptor in the kidney, increased urine volume, and increased urinary sodium excretion (46), thus providing a potential mechanism for the long-term blood pressure–lowering effects of quercetin. Taken together, the aforementioned studies indicate that quercetin may engage in multiple points in the RAAS to decrease blood pressure (Fig. 3). Given the proven efficacy of pharmacological ACE inhibition in humans and the animal-based evidence suggesting quercetin may act as an ACE inhibitor, there is a need to conduct clinical trials to determine whether quercetin supplementation can decrease ACE activity in hypertensive humans.
Figure 3.
Overview of possible mechanisms based on available in vitro and in vivo evidence by which quercetin (Q) may interact with the renin-angiotensin-aldosterone system to decrease blood pressure, as reviewed in the text.
Quercetin and vascular function.
The innermost lining of the blood vessel wall is composed of a single-cell layer–thick structure called the endothelium. The endothelium plays a very important role in maintaining vascular homeostasis, vascular tone, and cardiovascular and microvascular health. Endothelial dysfunction is a critical event in the pathogenesis of CVD and an independent predictor of cardiovascular events and is a common feature in all forms of CVD, including hypertension (57). Endothelial cells synthesize and release a variety of substances that cause the vascular smooth muscle to dilate (e.g., NO) or constrict (e.g., ET-1) the blood vessel, which thereby regulates blood pressure and blood flow (58.59). A common feature of endothelial dysfunction is decreased bioavailability of vasodilator NO, which results in less endogenous opposition to circulating vasoconstrictors [e.g., ET-1 (4)]. NO and ET-1 appear to have a reciprocal regulation; as NO bioavailability decreases, there is enhanced synthesis and/or response to ET-1 (59). Endothelial dysfunction is reversible, and interventions that decrease the risk of CVD, such as exercise and pharmaceuticals, are associated with improved endothelial function; it has become a surrogate biomarker for determining the efficacy of CVD interventions (59).
Quercetin decreased blood pressure in rodents that was accompanied by improvements in endothelial function (11, 47, 48). For example, Duarte et al. (11) reported that spontaneously hypertensive rats given 10 mg quercetin/kg body weight (via gavage) have improved endothelium-dependent vasorelaxation in isolated aorta and decreased blood pressure. Similar results have been found when quercetin is administered to rats with hypertension that is produced by NO synthase inhibition (48). Using a dietary model of hypertension (high-fat, high-sucrose diet for 4 wk), Yamamoto and Oue (47) observed hypertension, low aortic NO synthase activity, and low urinary NO metabolites, all of which were reversed by quercetin consumption. Collectively, these studies provide evidence that one potential mechanism by which quercetin can decrease blood pressure is through improved endothelial function evoked by increased NO bioavailability and/or NO production.
Previous studies focused on determining whether quercetin improves vascular function due to NO-dependent mechanisms; however, findings of a recent study by Loke et al. (60) suggest that decreased levels of ET-1 may also contribute to quercetin’s antihypertensive effect. In this study, the acute effects of a single 200-mg dose of quercetin were examined in healthy, normotensive males. Although blood pressure was not measured, it was reported that 2 h after quercetin supplementation, plasma ET-1 was lower, and after 5 h, urinary metabolites of NO were higher with quercetin treatment compared with placebo treatment (60). Along these lines, in vitro studies also found that quercetin can decrease ET-1 expression and release in human umbilical vein endothelial cells in a dose-dependent manner (61, 62). Even though the study by Loke et al. was conducted in healthy males rather than individuals with hypertension, the data indicate that improving the balance between vasoconstrictors (ET-1) and vasodilators (NO) may be another possible mechanism by which quercetin could improve vascular function and decrease blood pressure.
There is also evidence that quercetin may decrease blood pressure through mechanisms independent of the endothelium by directly acting on the vascular smooth muscle. In this regard, both quercetin and isorhamnetin (a metabolite of quercetin) can evoke vasorelaxation in the aorta and smaller resistance arteries of rodents regardless of whether the endothelial layer of vessels is intact or denuded (42, 44). Likewise, it has been reported that flow-mediated vasodilation is improved in humans who consume quercetin-rich foods, but with no corresponding change in endothelium-dependent function (41). It remains unclear how quercetin evokes endothelium-independent relaxation, but it has been speculated that it results from inhibition of protein kinases involved in the Ca2+-sensitizing mechanisms responsible for smooth muscle contraction (44).
Conclusions
In vitro and in vivo research conducted using animal models has shown multiple potential mechanisms of action that could produce the blood pressure–lowering effect of quercetin seen in hypertensive humans. However, definitive evidence of a precise mechanism of action by which quercetin might decrease blood pressure in humans remains elusive. In conjunction with discovering mechanisms of action, more controlled, randomized human research studies are needed to confirm quercetin’s efficacy, magnitude of effect, and optimal dosing schedule. Furthermore, it needs to be determined whether quercetin is an effective treatment for all forms of hypertension regardless of pathological origin. However, despite the uncertainty of the mechanism of action of quercetin in humans, this flavonoid has promise for the treatment of hypertension and warrants larger scale clinical trials than have been done to date.
Footnotes
A.J.L. was supported by a grant from University of Utah PEAK Academy J.D.S. was supported by American Diabetes Association Research Grant 7-08-RA-164, and National Institutes of Health grant R15 HL 091493-01. T.J. was supported by a University of Utah College of Health grant, Melaluca Inc. Clinical Research Contract.
Author disclosures: A.J. Larson, J.D. Symons, and T. Jalili, no conflicts of interest.
Abbreviations used: ACE, angiotensin-converting enzyme; CVD, cardiovascular disease; ET-1, endothelin-1; NO, nitric oxide; RAAS, renin-angiotensin-aldosterone system.
Literature Cited
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, et al. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–215 Erratum in: Circulation. 2010;121:e260 [DOI] [PubMed] [Google Scholar]
- 2.Sica DA. Angiotensin-converting enzyme inhibitor use in the year 2005. J Clin Hypertens (Greenwich). 2005;7:8–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10 [DOI] [PubMed] [Google Scholar]
- 4.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–24 [DOI] [PubMed] [Google Scholar]
- 5.Padilla J, Wallace JP, Park S. Accumulation of physical activity reduces blood pressure in pre- and hypertension. Med Sci Sports Exerc. 2005;37:1264–75 [DOI] [PubMed] [Google Scholar]
- 6.Bell JP, Mosfer SI, Lang D, Donaldson F, Lewis MJ. Vitamin C and quinapril abrogate LVH and endothelial dysfunction in aortic-banded guinea pigs. Am J Physiol Heart Circ Physiol. 2001;281:H1704–10 [DOI] [PubMed] [Google Scholar]
- 7.Nishikawa Y, Tatsumi K, Matsuura T, Yamamoto A, Nadamoto T, Urabe K. Effects of vitamin C on high blood pressure induced by salt in spontaneously hypertensive rats. J Nutr Sci Vitaminol (Tokyo). 2003;49:301–9 [DOI] [PubMed] [Google Scholar]
- 8.Boshtam M, Rafiei M, Sadeghi K, Sarraf-Zadegan N. Vitamin E can reduce blood pressure in mild hypertensives. Int J Vitam Nutr Res. 2002;72:309–14 [DOI] [PubMed] [Google Scholar]
- 9.Koba K, Abe K, Ikeda I, Sugano M. Effects of alpha-tocopherol and tocotrienols on blood pressure and linoleic acid metabolism in the spontaneously hypertensive rat (SHR). Biosci Biotechnol Biochem. 1992;56:1420–3 [DOI] [PubMed] [Google Scholar]
- 10.Edwards RL, Lyon T, Litwin SE, Rabovsky A, Symons JD, Jalili T. Quercetin reduces blood pressure in hypertensive subjects. J Nutr. 2007;137:2405–11 [DOI] [PubMed] [Google Scholar]
- 11.Duarte J, Perez-Palencia R, Vargas F, Ocete MA, Perez-Vizcaino F, Zarzuelo A, Tamargo J. Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br J Pharmacol. 2001;133:117–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jalili T, Carlstrom J, Kim S, Freeman D, Jin H, Wu TC, Litwin SE, Symons JD. Quercetin-supplemented diets lower blood pressure and attenuate cardiac hypertrophy in rats with aortic constriction. J Cardiovasc Pharmacol. 2006;47:531–41 [DOI] [PubMed] [Google Scholar]
- 13.Machha A, Mustafa MR. Chronic treatment with flavonoids prevents endothelial dysfunction in spontaneously hypertensive rat aorta. J Cardiovasc Pharmacol. 2005;46:36–40 [DOI] [PubMed] [Google Scholar]
- 14.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–11 [DOI] [PubMed] [Google Scholar]
- 15.Yochum L, Kushi LH, Meyer K, Folsom AR. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am J Epidemiol. 1999;149:943–9 [DOI] [PubMed] [Google Scholar]
- 16.Geleijnse JM, Launer LJ, Van der Kuip DA, Hofman A, Witteman JC. Inverse association of tea and flavonoid intakes with incident myocardial infarction: the Rotterdam Study. Am J Clin Nutr. 2002;75:880–6 [DOI] [PubMed] [Google Scholar]
- 17.Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, Heliovaara M, Reunanen A, Hakulinen T, Aromaa A. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76:560–8 [DOI] [PubMed] [Google Scholar]
- 18.Knekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ. 1996;312:478–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–25 [DOI] [PubMed] [Google Scholar]
- 20.Nutrient Data Laboratory, Food Composition Laboratory USDA database for the flavonoid content of selected foods. Beltsville, MD: Beltsville Human Nutrition Research Center, Agriculture Research Service, USDA; January 2007 [Google Scholar]
- 21.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–42S [DOI] [PubMed] [Google Scholar]
- 22.Egert S, Wolffram S, Bosy-Westphal A, Boesch-Saadatmandi C, Wagner AE, Frank J, Rimbach G, Mueller MJ. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J Nutr. 2008;138:1615–21 [DOI] [PubMed] [Google Scholar]
- 23.Olthof MR, Hollman PC, Vree TB, Katan MB. Bioavailabilities of quercetin-3-glucoside and quercetin-4′-glucoside do not differ in humans. J Nutr. 2000;130:1200–3 [DOI] [PubMed] [Google Scholar]
- 24.Larson A, Bruno RS, Guo Y, Gale D, Tanner J, Jalili T, Symons JD. A single dose of quercetin lowers blood pressure in hypertensive, but not normotensive males. Experimental Biology 2010. Anaheim, CA: FASEB J; 2010. p. 230.6 [Google Scholar]
- 25.Tamura M, Nakagawa H, Tsushida T, Hirayama K, Itoh K. Effect of pectin enhancement on plasma quercetin and fecal flora in rutin-supplemented mice. J Food Sci. 2007;72:S648–51 [DOI] [PubMed] [Google Scholar]
- 26.Nishijima T, Iwai K, Saito Y, Takida Y, Matsue H. Chronic ingestion of apple pectin can enhance the absorption of quercetin. J Agric Food Chem. 2009;57:2583–7 [DOI] [PubMed] [Google Scholar]
- 27.Lesser S, Cermak R, Wolffram S. Bioavailability of quercetin in pigs is influenced by the dietary fat content. J Nutr. 2004;134:1508–11 [DOI] [PubMed] [Google Scholar]
- 28.Lesser S, Cermak R, Wolffram S. The fatty acid pattern of dietary fat influences the oral bioavailability of the flavonol quercetin in pigs. Br J Nutr. 2006;96:1047–52 [DOI] [PubMed] [Google Scholar]
- 29.Kale A, Gawande S, Kotwal S, Netke S, Roomi W, Ivanov V, Niedzwiecki A, Rath M. Studies on the effects of oral administration of nutrient mixture, quercetin and red onions on the bioavailability of epigallocatechin gallate from green tea extract. Phytother Res. 2010;24: Suppl 1:S48–55 [DOI] [PubMed] [Google Scholar]
- 30.Crespy V, Morand C, Besson C, Manach C, Demigne C, Remesy C. Comparison of the intestinal absorption of quercetin, phloretin and their glucosides in rats. J Nutr. 2001;131:2109–14 [DOI] [PubMed] [Google Scholar]
- 31.Day AJ, Canada FJ, Diaz JC, Kroon PA, McLauchlan R, Faulds CB, Plumb GW, Morgan MR, Williamson G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000;468:166–70 [DOI] [PubMed] [Google Scholar]
- 32.Day AJ, DuPont MS, Ridley S, Rhodes M, Rhodes MJ, Morgan MR, Williamson G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett. 1998;436:71–5 [DOI] [PubMed] [Google Scholar]
- 33.Ioku K, Pongpiriyadacha Y, Konishi Y, Takei Y, Nakatani N, Terao J. beta-Glucosidase activity in the rat small intestine toward quercetin monoglucosides. Biosci Biotechnol Biochem. 1998;62:1428–31 [DOI] [PubMed] [Google Scholar]
- 34.Sesink AL, Arts IC, Faassen-Peters M, Hollman PC. Intestinal uptake of quercetin-3-glucoside in rats involves hydrolysis by lactase phlorizin hydrolase. J Nutr. 2003;133:773–6 [DOI] [PubMed] [Google Scholar]
- 35.Manach C, Morand C, Texier O, Favier ML, Agullo G, Demigne C, Regerat F, Remesy C. Quercetin metabolites in plasma of rats fed diets containing rutin or quercetin. J Nutr. 1995;125:1911–22 [DOI] [PubMed] [Google Scholar]
- 36.Carlstrom J, Symons JD, Wu TC, Bruno RS, Litwin SE, Jalili T. A quercetin supplemented diet does not prevent cardiovascular complications in spontaneously hypertensive rats. J Nutr. 2007;137:628–33 [DOI] [PubMed] [Google Scholar]
- 37.Hollman PC. vd Gaag M, Mengelers MJ, van Trijp JM, de Vries JH, Katan MB. Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Radic Biol Med. 1996;21:703–7 [DOI] [PubMed] [Google Scholar]
- 38.Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45:2179–205 [DOI] [PubMed] [Google Scholar]
- 39.Conquer JA, Maiani G, Azzini E, Raguzzini A, Holub BJ. Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J Nutr. 1998;128:593–7 [DOI] [PubMed] [Google Scholar]
- 40.Perez-Vizcaino F, Duarte J, Andriantsitohaina R. Endothelial function and cardiovascular disease: effects of quercetin and wine polyphenols. Free Radic Res. 2006;40:1054–65 [DOI] [PubMed] [Google Scholar]
- 41.Vita JA. Polyphenols and cardiovascular disease: effects on endothelial and platelet function. Am J Clin Nutr. 2005;81:292S–7S [DOI] [PubMed] [Google Scholar]
- 42.Rendig SV, Symons JD, Longhurst JC, Amsterdam EA. Effects of red wine, alcohol, and quercetin on coronary resistance and conductance arteries. J Cardiovasc Pharmacol. 2001;38:219–27 [DOI] [PubMed] [Google Scholar]
- 43.Chen CK, Pace-Asciak CR. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen Pharmacol. 1996;27:363–6 [DOI] [PubMed] [Google Scholar]
- 44.Pérez-Vizcaino F, Ibarra M, Cogolludo AL, Duarte J, Zaragoza-Arnaez F, Moreno L, Lopez-Lopez G, Tamargo J. Endothelium-independent vasodilator effects of the flavonoid quercetin and its methylated metabolites in rat conductance and resistance arteries. J Pharmacol Exp Ther. 2002;302:66–72 [DOI] [PubMed] [Google Scholar]
- 45.Duarte J, Galisteo M, Ocete MA, Perez-Vizcaino F, Zarzuelo A, Tamargo J. Effects of chronic quercetin treatment on hepatic oxidative status of spontaneously hypertensive rats. Mol Cell Biochem. 2001;221:155–60 [DOI] [PubMed] [Google Scholar]
- 46.Mackraj I, Govender T, Ramesar S. The antihypertensive effects of quercetin in a salt-sensitive model of hypertension. J Cardiovasc Pharmacol. 2008;51:239–45 [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto Y, Oue E. Antihypertensive effect of quercetin in rats fed with a high-fat high-sucrose diet. Biosci Biotechnol Biochem. 2006;70:933–9 [DOI] [PubMed] [Google Scholar]
- 48.Duarte J, Jimenez R, O'Valle F, Galisteo M, Perez-Palencia R, Vargas F, Perez-Vizcaino F, Zarzuelo A, Tamargo J. Protective effects of the flavonoid quercetin in chronic nitric oxide deficient rats. J Hypertens. 2002;20:1843–54 [DOI] [PubMed] [Google Scholar]
- 49.Häckl LP, Cuttle G, Dovichi SS, Lima-Landman MT, Nicolau M. Inhibition of angiotensin-converting enzyme by quercetin alters the vascular response to bradykinin and angiotensin I. Pharmacology. 2002;65:182–6 [DOI] [PubMed] [Google Scholar]
- 50.Egert S, Bosy-Westphal A, Seiberl J, Kurbitz C, Settler U, Plachta-Danielzik S, Wagner AE, Frank J, Schrezenmeir J, Rimbach G, et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br J Nutr. 2009;102:1065–74 [DOI] [PubMed] [Google Scholar]
- 51.Egert S, Boesch-Saadatmandi C, Wolffram S, Rimbach G, Muller MJ. Serum lipid and blood pressure responses to quercetin vary in overweight patients by apolipoprotein E genotype. J Nutr. 2010;140:278–84 [DOI] [PubMed] [Google Scholar]
- 52.Vaziri ND, Rodriguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006;2:582–93 [DOI] [PubMed] [Google Scholar]
- 53.Cohn JN. Role of the renin-angiotensin system in cardiovascular disease. Cardiovasc Drugs Ther. Aug;24:341–4 [DOI] [PubMed] [Google Scholar]
- 54.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52 [DOI] [PubMed] [Google Scholar]
- 55.Loizzo MR, Said A, Tundis R, Rashed K, Statti GA, Hufner A, Menichini F. Inhibition of angiotensin converting enzyme (ACE) by flavonoids isolated from Ailanthus excelsa (Roxb) (Simaroubaceae). Phytother Res. 2007;21:32–6 [DOI] [PubMed] [Google Scholar]
- 56.Carretero OA. Novel mechanism of action of ACE and its inhibitors. Am J Physiol Heart Circ Physiol. 2005;289:H1796–7 [DOI] [PubMed] [Google Scholar]
- 57.Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–6 [DOI] [PubMed] [Google Scholar]
- 58.Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15:1983–92 [DOI] [PubMed] [Google Scholar]
- 59.Böhm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res. 2007;76:8–18 [DOI] [PubMed] [Google Scholar]
- 60.Loke WM, Hodgson JM, Proudfoot JM, McKinley AJ, Puddey IB, Croft KD. Pure dietary flavonoids quercetin and (-)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am J Clin Nutr. 2008;88:1018–25 [DOI] [PubMed] [Google Scholar]
- 61.Nicholson SK, Tucker GA, Brameld JM. Effects of dietary polyphenols on gene expression in human vascular endothelial cells. Proc Nutr Soc. 2008;67:42–7 [DOI] [PubMed] [Google Scholar]
- 62.Zhao XY, Gu ZL. Effects of quercetin on production and release of endothelin and cGMP from cultured endothelial cells. Zhongguo Yao Li Xue Bao. 1996;17:442–4 [PubMed] [Google Scholar]