Abstract
Gut hormones play a key role in the regulation of food intake, energy expenditure, glucose homeostasis, lipid metabolism, and a wide range of metabolic functions in response to food ingestion. These hormones are altered in metabolic diseases, such as obesity and type 2 diabetes, and are thus proposed to be possible targets for the prevention or treatment of these diseases. It is clear that food composition, macronutrients, and other non-nutrient components as well as the physical properties of food not only modulate the secretion of gut peptides but also modulate transcription and enteroendocrine cell differentiation, which ultimately modifies gut hormone response. The specific mechanisms or sensing machinery that respond to the different components of the diet have been studied for many years; however, over the last few years, new molecular genetic techniques have led to important advances, thereby allowing a deeper understanding of these mechanisms. This review addresses the current knowledge regarding enteroendocrine cells and how diet interacts with this machinery to stimulate and regulate the secretion of gut peptides. The potential for diet interventions as a promising strategy for modulating gut hormone responses to food ingestion and, ultimately, preventing or treating metabolic diseases is being emphasized considering that these diseases are currently a public health burden.
Introduction
In recent years, there has been an increasing awareness of the role of the gut (intestine) in metabolism. Gut cells are probably the first point of contact with ingested food; therefore, it is clear that they must prepare the organism for the nutrients that are entering the system. Of the cells that comprise the gut, there are many different subtypes of enteroendocrine cells. These cells have become increasingly more important over the past few years, because the production of their hormones plays a key role not only in the regulation of food intake but also in energy expenditure, glucose homeostasis, and a range of metabolic functions in response to food ingestion (1, 2). For instance, some of these hormones, such as GLP-1,5 GIP, or PYY, seem to be altered in metabolic disease states such as obesity and type 2 diabetes (3, 4). Whether this alteration is causal or is a consequence remains to be determined. In addition, dietary factors have been shown to strongly influence the regulation of these peptides. Oligofructosaccharides, e.g., have been shown to mediate obesity-associated inflammation states in rodents via the modulation of gut hormones levels, such as GLP-2 (5). In the context of the recent epidemic of obesity and diet-related diseases, it is important to understand the nature of the different enteroendocrine cells and how diet interacts with them for the secretion and regulation of gut peptides.
The specific mechanisms underlying the sensing machinery that responds to different nutrients and non-nutrient components of the diet have been studied for many years; however, due to the dispersed nature of enteroendocrine cells as well as the inability to distinguish the different cell types morphologically for single cell analysis (6), the characterization of this machinery has been restricted to cell line models, which are not always accurate models of native enteroendocrine cells. However, over the past few years, new molecular genetic techniques, such as fluorescent protein expression in mice under the control of a promoter for a peptide hormone precursor, have enabled the in vivo identification, primary cell isolation, and culturing of each type of enteroendocrine cell. These techniques have also allowed the detection of proteins that could be involved in the sensing mechanisms and a deeper exploration of the morphology of enteroendocrine cells. This characterization has led to important advances over the past years. Therefore, the purpose of this review is to focus on the current knowledge concerning enteroendocrine cells and how diet interacts with their machinery to stimulate and regulate the secretion of gut peptides.
Enteroendocrine cells
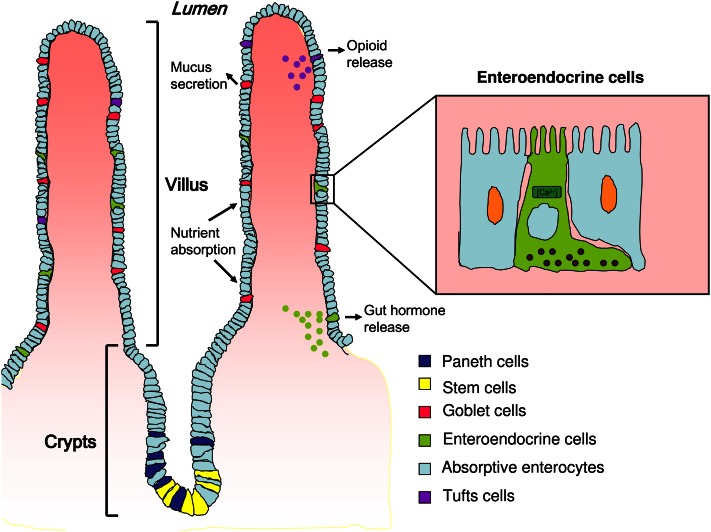
The intestinal surface has a unique architecture consisting of villus and crypt structures. The villus is a finger-like projection protruding into the lumen to increase the surface area of the small intestine by almost 30-fold (Fig. 1). Gut stem cells reside in crypts and are differentiated by notch signaling into absorptive enterocytes, paneth cells, goblet cells, tuft cells, and enteroendocrine cells (7, 8). The latter are fully differentiated cells that, along with goblet and paneth cells, constitute the secretory cell types in the small intestine, whereas absorptive enterocytes comprise 90% of the epithelium (9).
FIGURE 1.
Small intestine morphology and distribution of epithelial cell types. Paneth and stem cells are localized in the crypts. Upon differentiation, enterocytes and goblet, enteroendocrine, and tuft cells migrate toward the villus. Enteroendocrine cells are scattered throughout the intestinal tract and are embedded primarily in enterocytes. Enteroendocrine cells release their contents via the basolateral surface upon interaction with food components.
With the exception of paneth cells, epithelial cell turnover occurs quite rapidly, with a lifespan of 3–5 d. This turnover occurs via exfoliation at the tips of the villi, i.e. the cells presumably undergo apoptosis and are extruded into the lumen in distinction to nondiffuse endocrine organs, perhaps suggesting more plasticity (10, 11). Unlike many other endocrine cells that differentiate early in life and turn over slowly, enteroendocrine cells self-renew and differentiate from a large reservoir of stem cells throughout their lifespans (7).
Enteroendocrine cells are scattered throughout the intestinal tract and are embedded in a majority of nonendocrine cells, including absorptive enterocytes, goblet cells, and paneth cells. Although they represent ~1% of the epithelial cell population, they are considered the largest endocrine relative to the total numbers of cells (11, 12). There are at least 15 subtypes of enteroendocrine cells that secrete a wide range of peptide hormones, which, in a complex manner, control physiological and homeostatic functions in the digestive tract, particularly postprandial secretion and motility (13, 14). A summary of some of the different enteroendocrine cell subtypes, secreted peptides, and their functions is provided in Table 1. Initially, these cells were classified primarily by their secreted peptide; however, it is now clear that some of these cells are able to secrete more than one hormone.
TABLE 1.
Summary of the different subset of enteroendocrine cells, localization, hormone secretion, and function
| Cell | Localization | Peptide | Function |
| G cell | Pyloric antral part | Gastrin | Regulation of acid secretion |
| X or A like cells | Stomach | Ghrelin | Food intake stimulation |
| K cell | Proximal intestine | GIP1 | Enhancement of insulin secretion and gastric acid secretion and reduction of LPL activity in adipose tissue |
| I cell | Proximal intestine | CCK | Gallbladder contraction, stimulation of pancreatic enzyme secretion, inhibition of food intake |
| S cell | Proximal intestine | Secretin | Stimulation of bicarbonate secretion and inhibition of gastric acid secretion, colonic contraction, and motility |
| M cell | Proximal intestine | Motilin | Gut motility |
| N cell | Distal intestine | Neurotensin | Gastric acid secretion, biliary secretion, and intestinal mucosal growth |
| L cell | Distal intestine and colon | PYY, GLP-1, GLP-2, glicentin | Inhibition of gastric acid secretion and gastric emptying and enhancement of insulin secretion |
CCK, cholecystokinin; GLP, glucagon-like peptide; GIP, glucose-dependent insulinotropic peptide; LPL, lipoprotein lipase; PYY, peptide tyrosine tyrosine.
As summarized in Table 1, the primary functions of hormones secreted by the enteroendocrine cells are to coordinate the response of the gastrointestinal tract to food ingestion. Therefore, it has become clear that a key function of enteroendocrine cells is to act as sensors of luminal contents, thereby functioning as transepithelial signal transducers with their physicochemical signals, resulting in exocytosis of biological mediators into the circulation (11).
In terms of physiology, most enteroendocrine cells have been described as open cells with the ability to interact with luminal content. More recently, different transporters, such as SGLT1, taste receptors, and other GPR have been identified in some enteroendocrine cells as part of the machinery that interacts with chemical food components to trigger gut peptide release (15–18). In the context of the functions of gut peptides in satiety and metabolism as well as their alterations in obesity and metabolic-associated diseases, it is critical to understand how food components interact with enteroendocrine cells to regulate the secretion of these peptides. An understanding of this process could help with determining dietary strategies that modulate the secretion of these peptides. Such dietary modifications could be used as a preventive measure against or as a part of a treatment for metabolic diseases.
Due to the nature of the peptides that enteroendocrine cells secrete, the most studied cells are K, L, and I cells. Thus, this review will cover the characteristics and diet-cell interactions of these 3 cell types.
K cells and GIP
K cell overview.
After the conceptualization of the gut as an endocrine organ, Brown et al. (19) in 1971 purified a peptide capable of inhibiting gastric emptying and called it GIP. Later in 1973, Dupre et al. (20) determined that this peptide was also responsible for the enhancement of glucose-induced insulin secretion, the so-called incretin effect. GIP secretion was shown to be stimulated by ingestion of glucose and fat; however, the insulin response due to fat ingestion was observed only if glucose was also administered (20). The latter effect seems to be a safety mechanism for the inappropriate stimulation of insulin release in a high-fat, low-carbohydrate meal (10).
Localization.
K cells were originally identified in the small intestine via their ultra-structural features. However, it was not until 1975 that Buffa et al. (21) showed that these K cells in the small intestine reacted to GIP antisera. The characteristic appearance of these cells was later described as intracellular secretory granules with a small electron-dense core surrounded by a concentric electron-lucent halo (10).
Similar to other enteroendocrine cells, K cells are scattered along the intestinal tract; however, the greatest density of K cells is found in the duodenal mucosa and has been estimated at 13/1000 epithelial cells (22). More recently, a subset of enteroendocrine cells coexpressing GIP and GLP-1 in the mid-intestine has been found and is typically referred to as K/L or L/K cells for convenience (22–25).
K cells are an open type of enteroendocrine cell with a connection to the gut lumen. The cell contains inner secretory granules where the gut peptide GIP is stored. Upon electrophysiological stimulation, these granules fuse with the basolateral membrane to release their contents.
GIP biological actions.
Although the prime physiological role of GIP is to increase insulin secretion induced by intestinally absorbed nutrients, the GIP receptor has been found in different tissues, suggesting that it has variable extra-pancreatic functions. Indeed, it has been shown that GIP modulates calcium deposition in bone and bone formation (26, 27) and has an important role in lipid metabolism. GIP has been reported to stimulate fat deposition in adipocytes by increasing the activity of lipoprotein lipase (28–30). Moreover, high fat-fed and ob/ob mice that are knocked-out for the GIP receptor, although mildly glucose intolerant, were protected against obesity (31). In the same line, GIP receptor antagonism reversed obesity and metabolic-associated abnormalities in high fat-fed rats (32). These and other data suggest that GIP as is a metabolically thrifty gene and postulate it as possible link between overnutrition and obesity (31, 33, 34).
Diet effects and mechanisms.
Fasting plasma concentrations of GIP are relatively low, with values of ~10 pmol/L. However, upon food ingestion, GIP concentration rises several-fold, usually within 10–20 min (10). The specific components responsible for the secretion of GIP are associated with the nature of the food composition. For example, GIP secretion is strongly stimulated by fat and carbohydrates, whereas proteins are less potent inducers of the hormone (35, 36).
Dietary fat.
Dietary fat is the most potent stimulus for GIP secretion (10). TG hydrolysis appears to be necessary for the induction of GIP secretion, because the ingestion of orlistat, an inhibitor of lipoprotein lipase, with a mixed meal significantly attenuated the GIP response (37). Moreover, the finding that olive oil induced a greater secretion of GIP in healthy participants compared to butter suggested that the fatty acid composition of dietary fat may have an effect on GIP secretion (38). Linoleic acid induced a significant GIP response in primary K cell culture. These cells express GPR40, -120, and -119, which are receptors known to be involved in fatty acid sensing, thereby raising the possibility that the fatty acid-sensing mechanisms of these cells could be mediated by these receptors (10, 25). For instance, Edfalk et al. (16) showed that the GIP response induced by fat was impaired in GPR40 mutant mice.
Carbohydrates.
The mechanism behind GIP secretion by carbohydrates is associated with intestinal SGLT1. The fact that phlorizdin, a SGLT1 inhibitor, impairs glucose-stimulated GIP release supports this idea. However, because stimulation with α-methylglucopyranoside, a nonmetabolizable substrate of SGLT1, gives a significantly lower GIP response compared to glucose, a further glucose metabolism is thought to be needed to fully induce GIP secretion (25).
Jang et al. (39) found that mice knocked-out for α-gustducin (a component of the sweet taste receptor signaling cascade) had deficiencies in GIP secretion as well as decreased insulin response and impaired glucose tolerance. Based on the latter fact, a mechanism related to the presence of sweet taste receptors in the gut has been thought to be involved in carbohydrate-induced GIP secretion. Although the same research group found that sucralose, an artificial sweetener, induced GIP secretion in a murine cell line (40), other authors did not demonstrate the induction of GIP secretion in vitro and in vivo by such artificial sweeteners as acesulfame K, saccharin, or sucralose (41). Therefore, it seems that the evidence for the role of sweet taste receptors in GIP induction is inconclusive and more studies are needed to determine the exact role of sweet taste receptors in the carbohydrate induction of GIP secretion (42).
Protein.
The effect of protein on GIP secretion has been poorly studied. Although some studies report no effect of whole protein on GIP secretion (35), the administration of protein hydrolyzate to rats more recently induced a significant response on GIP secretion, and the administration of omeprazole, an inhibitor of gastric acid secretion, severely diminished the response, suggesting that the effect could be partially mediated by the acid-stimulatory properties of protein (43). The administration of a protein beverage in healthy subjects stimulated modest GIP secretion alone or in combination with glucose (44). Later, Carr et al. (45) showed strong GIP secretion after the administration of a protein-rich meal in healthy participants. Another study even observed that GIP stimulation in healthy individuals was different with differing types of proteins, with whey being the most potent inducer (46). A similar effect was observed in patients with type 2 diabetes in whom the addition of whey protein to the breakfast or lunch meal induced a greater response in GIP secretion compared with other sources of protein (47). The latter suggests that the amino acid pattern can modify the postprandial GIP response. For instance, prior studies have reported the induction of GIP secretion by certain amino acids (10, 48), whereas Parker et al. (25) showed a potent induction of GIP secretion by glutamine in K primary cells.
Other diet components.
Other non-nutrient components of the diet, such as coffee, are known to effect GIP secretion. In a study by Johnston et al. (49), the secretion of GIP was significantly decreased when coffee plus glucose was ingested in contrast to glucose alone, and this effect was even greater with decaffeinated coffee. These researchers suggested that the phenolic compounds present in coffee inhibit glucose transport, thereby attenuating GIP secretion (49). In contrast, Beaudoin et al. (50) showed that the consumption of coffee (regular or decaffeinated) before an oral glucose tolerance test and after an oral fat tolerance test increased the GIP response. The difference in findings could be due to the distinct methodologies of the studies. Therefore, more studies are needed to characterize the role of coffee in GIP secretion. However, it seems likely that coffee, whether caffeinated or decaffeinated, interacts with enteroendocrine cells to modify the GIP response.
I cells and CCK
Overview.
CCK, as mentioned by Liddle et al. (51), was first described by Ivy and Oldberg in 1928 as a stimulant of gall bladder contraction. However, it is now known that CCK also stimulates pancreatic enzyme secretion and inhibits gastric emptying and food intake. Collectively, these actions fulfill the primary role of CCK in the gastrointestinal tract, which is to regulate protein and fat digestion in the upper small intestine (51–53).
Localization.
I cells, which are localized primarily in the upper and intermediate gastrointestinal tract (duodenum and upper jejunum) as well as to the enteric nerves of the colonic wall, are responsible for CCK production and secretion. Importantly, CCK is also released from the central nervous system; however, due to the nature of this review, we will focus primarily on the CCK produced by I cells (54).
I cells are open cells oriented toward the intestinal lumen. They are roughly triangular or flask-shaped with the apical surfaces oriented toward the lumen, while the membrane-bound cytoplasmic granules containing CCK are concentrated around the basal surface (51, 54, 55). This allows the cell to be stimulated by intestinal nutrients and release their content into the blood and/or surrounding tissue. I cells have been found to be more abundant in intestinal crypts than in the villi, and the presence of pseudopod-like basal processes has recently been described in ~50–65% of the I cell population. Although the functionality of these structures is unknown at present, the authors suggest that these structures could receive information from neighboring cells, which are primarily enterocytes (55).
Similar to other enteroendocrine cells, the I cell turnover time is only a few days and the cells appear to differentiate from an enteroendocrine lineage, which also includes S cells (12).
Biological actions of CCK.
Selective processing of proCCK, which is a 115-amino acid precursor, leads to multiple bioactive CCK forms of different lengths. The major circulating forms in humans are CCK-58, -33, -22, and -8, all ligands of the CCK1R (52, 54).
As previously mentioned, the primary action of CCK is in gallbladder contraction, which is mediated through CCK-A receptors on the surface of gallbladder smooth muscle (54).
Another role of CCK is to stimulate pancreatic enzyme secretion. Prolonged administration of CCK has been shown to stimulate the synthesis of pancreatic enzymes and cellular proliferation (52). CCK also plays an important role in inhibiting gastric emptying; in fact, blocking CCK-A receptor inhibits the effect of fat and protein on gastric emptying, emphasizing that the ability of fats and proteins to inhibit gastric empting is CCK dependent (54, 56, 57).
CCK has been shown to inhibit food intake in multiple test situations and species, including humans. The food intake inhibition effect is observed relatively shortly after food ingestion; however, its duration is also brief. This effect is mediated via activation of vagal afferent mechanosensitive and chemosensitive fibers in the stomach and duodenum (52, 54).
More recently, a novel role of CCK was discovered in rodents. Cheung et al. (58) showed that CCK-8 was capable of lowering glucose production via triggering the gut-brain-liver neuronal axis. This effect was absent in CCK receptor-deficient rats (OLETF rats) and rats fed a high-fat diet, suggesting that intestinal CCK resistance leads to the dysregulation of glucose production and homeostasis in diet-induced obesity.
Diet effects and mechanisms.
Fasting plasma CCK concentrations are ∼1 pmol/L in most species and rise to 5–10 pmol/L following meal ingestion. Dietary fat and proteins are the most potent stimulators of CCK release, whereas carbohydrates are weak stimulants (48, 51, 54).
Dietary fat.
Dietary fat has been shown to stimulate CCK release in animals and humans. In both species, TG must be hydrolyzed to fatty acids to stimulate CCK secretion. For instance, pancreatic lipase insufficiency is associated with a poor CCK response to TG but a normal response to oleic acid (48, 54, 59). In addition, intestinal infusion of the lipase inhibitor, orlistat, together with TG virtually abolishes the postprandial release of CCK (60, 61). The length of the fatty acid carbon chain also determines CCK release. Most research studies have shown that fatty acids with an acyl chain length < 10 carbon atoms do not induce CCK secretion in humans, whereas long-chain fatty acids with at least 12 carbons are the most potent stimulants of CCK (48, 62). The sensing mechanism by which I cells detect long-chain fatty acids and trigger CCK secretion has long been associated with GPR. In the intestine cell line STC-1, Tanaka et al. (63) showed that long-chain fatty acid-induced CCK secretion was mediated via GPR120 (63). However, recently, using fluorescence-activated cell sorting isolation of CCK-producing cells (I cells), Liou et al. (18) demonstrated that the expression of GPR40 was 100-fold greater in cells producing CCK compared to cells that did not express it. They also showed that CCK secretion in vitro in response to linolenic was absent in GPR40−/− cells, whereas GPR40 null mice had a severely impaired CCK response induced by long-chain fatty acids. Because the response in mice was not completely blunted, Liou et al. (18) discussed that it was likely that an indirect fatty acid-sensing mechanism may be associated with chylomicron component ApoA-IV. In fact, ApoA-IV secreted by enterocytes in response to absorption of long-chain fatty acids previously has been associated with a satiety factor and an activator of CCK-responsive vagal afferents; however, its exact role remains to be elucidated (64–67).
Carbohydrates.
Carbohydrates have been shown to mildly induce CCK release; however, the sensing mechanisms have not yet been elucidated. Although taste receptors have been associated with gut hormone-releasing mechanisms from the enteroendocrine cell, Gerspach et al. (68) recently showed that inhibition of the sweet taste receptor T1R2/T1R3 by lactisole with a glucose load in healthy humans did not modify the CCK response, suggesting that glucose-induced CCK secretion is not mediated by these sweet taste receptors and that other glucose-sensing receptors must be involved.
Proteins.
Proteins and, more specifically, digested proteins (protein hydrolyzates) are more effective stimulants of CCK secretion than are carbohydrates (69, 70). Amino acids have been shown to have a direct effect on I cells, being the most effective aromatic amino acids. The specific sensing mechanism of these amino acids was recently demonstrated to occur via a GPR, the CaSR. Wang et al. (70) suggested that CaSR modulates signaling pathways that, upon l-phenylalanine and tryptophan stimulation in the presence of calcium, open calcium channels, causing an increase in intracellular Ca2+, and, in parallel, inhibit basal K+ channel activity, causing I cell depolarization, which is consistent with changes necessary for hormone secretion. This effect is abolished in native I cells with deletion of CaSR without affecting basal CKK secretion, suggesting that CaSR is required for CCK secretion in response to these aromatic amino acids (70). Additionally, Nakajima et al. (71) showed that in the STC-1 enteroendocrine cell line, the CCK secretion and Ca2+ mobilization induced by an arginine-rich β51–63 peptide from soybean β-conglycin was blocked when incubated with a CaSR antagonist, suggesting that this effect is also mediated by the CaSR.
Luminal protein hydrolysate (peptone) increases not only the secretion of CCK but also its transcription (15). The sensing mechanism of peptone is related to GPR93, because in the overexpression of GPR93 in STC-1 cells (an enteroendocrine cell line), a significant increase in CCK transcription and secretion was observed upon peptone stimulation. In parallel, an increase in cAMP level was observed, suggesting that the peptone-induced secretion of CCK in STC-1 cells could be mediated via cAMP; however, whether this mechanism is functional in the enteroendocrine I cell remains to be elucidated (15, 18).
Other dietary components.
Dietary fiber has been shown to affect postprandial CCK release. In several studies, mainly viscous soluble fibers produced a greater effect on postprandial CCK secretion compared to low fiber or control meal (72, 73).
Other dietary components, such as steroid glycosides from H. gordonii, which is a plant grown in Africa that has been shown to decrease food intake, as well as other bitter tastants, have been shown to induce CCK release in enteroendocrine cells lines. The mechanism was recently suggested to be associated with bitter taste-sensing mechanisms coupled to hormone release (74); however, their relevance in vivo remains to be determined.
L cells: GLP-1, PYY, and GLP-2
L cells overview.
GLP-1 was first described in 1983 by Bell et al. (75) as a fragment of the proglucagon molecule. In a later study in 1985, Schmidt et al. (76) described the potent insulinotropic actions of GLP-1 and it was therefore characterized as the second incretin hormone (77).
Although L cells were initially classified due to the secretion of GLP-1, they also secrete GLP-2, PYY, and oxyntumodulin. PYY was initially isolated in the 1980s from porcine intestine. Although PYY is released from L cells as a 36-amino acid peptide, it is readily truncated by dipeptidylpeptidase IV, resulting in 2 circulating isoforms, PYY1–36 and PYY3–36 (78, 79).
Localization.
The L cell is an open-type intestinal cell that makes direct contact with the lumen of the gut and senses the passage of nutrients along the gastrointestinal tract (6). In keeping with K cells, L cells are scattered along the gastrointestinal tract; however, the highest density has been found in the distal ileum and colon (80) and, as with other enteroendocrine cells, their secretory granules are harbored at their base.
Recently, the morphology of L cells from the ileum and colon has been studied more thoroughly by Bohorquez et al. (80). They found that L cells in the ileum have a classic L shape form with long extending basal processes, whereas those present in the colon have a spindle- or sigmoidal-like contour that weaves between epithelial cells while also maintaining contact with both the lumen and lamina propria. These researchers suggest that this particular shape may allow nutrient absorption and may result in gut hormones being secreted in an endocrine and paracrine manner.
Biological actions: GLP-1, PYY, and GLP-2.
GLP-1 contributes to glucose homeostasis not only by potentiating insulin release but also via its pancreatic and extra-pancreatic actions, which contribute to the pleiotropic effects of this hormone. GLP-1 has been shown to stimulate GIP secretion, induce β-cell proliferation, increase insulin gene transcription and mRNA stability, and suppress glucagon secretion via direct interaction with the GLP-1 receptor in pancreatic α-cells and indirectly via stimulation of somatostatin and insulin secretion. Its extra-pancreatic actions include the inhibition of gastric acid secretion, gastric emptying and motility, and modification of the nutrient entry rate into the circulation; thus, it plays a key role in the regulation of blood glucose levels (77). GLP-1 has also been characterized as a key regulator of appetite and food intake by directly stimulating anorectic pathways in the hypothalamus and brainstem and by acting through the vagus nerve (1, 81).
The biological action of PYY involves a wide range of digestive functions, which include regulation of insulin secretion, inhibition of gastric acid secretion, gastric emptying, mouth-to-cecum transit time, and gallbladder contraction in the cephalic phase in humans, with the latter functions being carried out by the PYY1–36 isoform. On the other hand, PYY3–36 has been shown to potently decrease appetite and reduce weight gain at physiological levels (80, 82). These anorectic effects are mediated via interaction with the vagus nerve and the hypothalamus (48). Of the 2 PYY isoforms, PYY3–36, appears to be the dominant isoform in humans, constituting ~65% of the circulating PYY during the fasting state (83).
GLP-2 is a 33-amino acid peptide whose main biological role has been associated with intestinotrophic actions, such as crypt cell proliferation and reduction in enterocyte apoptosis as well as enhancement of hexose transport, reduction of epithelial permeability, improvement of gut barrier function, and reduction of gastric motility and acid secretion (84, 85).
Diet effects and mechanisms.
GLP-1.
GLP-1 levels in plasma in the fasting state are typically in the 5–10 pmol/L range and they rise within 10–15 min after meal ingestion, reaching peak levels of 15–50 pmol/L around min 40 (6, 81, 86).
Although all macronutrients (proteins, carbohydrates, and lipids) have been shown to stimulate GLP-1 secretion (87), the magnitude of the response varies between nutrients and the general consensus is that carbohydrates and lipids are more potent stimulators of GLP-1 release than are proteins (88–91).
Carbohydrates.
Carbohydrate, and more specifically glucose, is a potent stimulator of GLP-1 in vivo and in vitro, although the underlying mechanisms for its stimulation are still a matter of debate. The proposed mechanism includes the closure of the KATP channel, SGLT1 activity, activation of sweet taste receptors, and the proximal-distal loop pathway. The latter was initially proposed to explain the early phases of GLP-1 secretion, given that L cells are primarily located in the distal part of the small intestine and colon (6). However, it is now clear that direct exposure of enteroendocrine L cells to carbohydrates is also needed, because glucose-induced GLP-1 secretion was severely diminished when the infusion was limited to the proximal 60 cm of the intestine, thereby leaving the ileum and colon (the richest source of L cells) without stimulation (92).
Nielsen et al. (93) observed the presence of KIR6.2, a major subunit of the KATP channel, in human L cells via immunostaining. Later, Reimann et al. (94) demonstrated that L cells have functional KATP channels. However, because sulfonylureas, which stimulate insulin secretion of β-cells by inducing KATP channel closure, are unable to stimulate GLP-1 secretion in humans (95), the implication of these channels in glucose-stimulated GLP-1 secretion has been questioned.
Additionally, the principle glucose transporter on the luminal surface, SGLT1, has been implicated in glucose stimulation of GLP-1 secretion via its ability to trigger membrane depolarization through electrogenic action (96). In fact, Phloridzin, which blocks SGLT1, has been shown to inhibit the release of GLP-1 completely in response to glucose in animal models, demonstrating that contact with the intestinal mucosa is not sufficient to stimulate GLP-1 secretion (97).
As discussed earlier, the presence of the taste receptor machinery in enteroendocrine cell lines, animal models, and humans (39, 98, 99) as well as the impairment in secreting GLP-1 in response to an oral glucose load in α gustducin null mice (39, 100) has suggested a role for sweet taste receptors in the secretion of GLP-1. Although the ingestion of sweeteners alone did not stimulate GLP-1 secretion in humans, the observation that sweeteners in combination with carbohydrates stimulate glucose absorption (101) has raised the question of whether this combination may have a synergistic effect on incretin release (102). Brown et al. (103) observed a synergistic effect of 75 g of glucose and diet soda (sucralose + acesulfame K) in the secretion of GLP-1. In addition, Gerspach et al. (68) recently showed that glucose-induced GLP-1 secretion was significantly diminished when lactisole, a sweet taste receptor inhibitor, was also administered. The observed effect was different when glucose was administered i.g. or intraduodenally, which suggests a complex regulation mechanism (68). It seems that sweet taste receptors do play a role, at least in glucose-induced GLP-1 secretion; however, sweet taste receptors do not seem to be the only mechanism responsible for GLP-1 releaseand more studies are needed to reveal the exact role of these receptors in GLP-1 secretion.
Dietary fat.
Fat, as previously mentioned, is a potent inducer of GLP-1 release. However, the response to fat seems to be delayed compared to the response to carbohydrates. The length of fatty acids as well as the degree of unsaturation appears to influence the magnitude of the response (104, 105). Thomsen et al. (38) observed that GLP-1 secretion in healthy humans was greater in response to olive oil (rich in MUFA) than with butter (rich in SFA). The same effect, although to a lesser degree, was observed years later by the same author in patients with type 2 diabetes (106). In addition, Feltrin et al. (107) showed that when lauric acid (12 carbon atoms fatty acid) was administered intraduodenally, it induced a significant GLP-1 response, whereas decanoic acid (C10 fatty acid) did not. Another interesting finding by Yoder et al. (108) was that the lipid-induced response of GLP-1 secretion in rats appears to be dose dependent when administered intraduodenally.
In vitro and in vivo studies have tried to elucidate the mechanism for fat-induced GLP-1 secretion. The hydrolysis of TG into FFA appears to be necessary for its stimulation, because several authors have reported that in the presence of orlistat, a lipase inhibitor, the GLP-1 response in humans is significantly attenuated (61, 105, 109). Beglinger (105) also found that administration of a CCK1R antagonist with long-chain fatty acids, such as oleic acid (C18:1), severely diminished the secretion of GLP-1, suggesting that long-chain fatty acids first stimulate CCK secretion, which subsequently acts on CCK1R, initiating the response on GLP-1.
The presence of certain GPR, such as GPR119, -120, -40, and -43, which are known as signaling mediators of fatty acids, has been identified in the intestine and enteroendocrine cells and cell lines (16, 104, 110, 111), suggesting that these receptors play a role in the induction of fat-induced gut hormone secretion. Hirasawa et al. (104) observed that GPR120 functions as a receptor for unsaturated long-chain fatty acids, promoting the secretion of GLP-1 in vivo and in vitro with a parallel, transient increase in intracellular calcium (63). Edfalk et al. (16) showed coexpression of GPR40 with GLP-1 in GLP-1–producing cells and observed a decreased response to fat-induced GLP-1 in GPR40 null mice. Chu et al. (110) found that activating GPR119, a receptor of the naturally occurring lipid oleoylethanolamide, with an agonist significantly increased GLP-1 and improved glucose tolerance (112).
Proteins.
Proteins are weak stimulators of GLP-1 release (89, 90); however, it seems that protein hydrolysis is necessary. Different proteins have been shown to induce differential responses to GLP-1; for instance, whey protein exerts a significant major increase in postprandial GLP-1 levels compared to casein (113). This effect has been associated with the amino acid profile of the proteins. Reimann et al. (114) observed the effects of different amino acids in GLP-1 secretion in GLUTag cells, an enteroendocrine cell line, and found that glutamine was the most potent, whereas phenylalanine or arginine had no effect. The effect of glutamine was confirmed in healthy, obese, and diabetic participants (115). Another study even found that the consumption of glutamine before a mixed meal increased postprandial GLP-1 secretion, thereby improving glucose levels in type 2 diabetic individuals (116). More recently, Tolhurst et al. (117) confirmed this effect in isolated mouse L cells. They found that the mechanism was associated with an increase in cAMP and cytosolic Ca2+. Although it is not clear whether the stimulation is from the apical or basolateral surface, they suggest the possible existence of a glutamine-responsive membrane receptor coupled to Gs signaling pathways. Le Neve et al. (118) recently showed in the NCI-H716 cell line, a human enteroendocrine cell line, that GLP-1 secretion was induced by selective tetrapeptides and was accompanied by an increase in intracellular calcium. They ruled out any specific amino acid induction, because neither the shorter peptides nor the corresponding amino acids were able to elicit a response. In contrast to the effects seen in other cell lines, they observed no changes in cAMP. They discussed that although the nature of the sensor and the intracellular mechanisms underlying stimulus-secretion coupling remain unknown, it probably involves store-operated calcium channels, because the addition of 2-Aminoethoxydiphenyl borate, a store-operated calcium channel inhibitor, abolished both the [Ca2+]i response and the GLP-1 release from NCI-H716 cells (118).
Results found by other authors in STC-1 cells, an enteroendocrine cell line model (119), suggest that Na-coupled neutral amino acid transporter 2 could also be a possible candidate for sensing amino acids, resulting in membrane depolarization, and thereby allowing an increase in intracellular calcium in enteroendocrine cells. Despite the considerable advances made in our understanding of protein and amino acid sensing in enteroendocrine cells, it is clear that more research is needed to fully characterize the mechanisms underlying GLP-1 secretion induced by proteins.
Other diet components.
Other dietary components have been shown to influence GLP-1 levels. Basal GLP-1 levels have been observed to be significantly increased in obese rodents with chronic administration of the prebiotic fiber oligofructose compared to the nonfermentable fiber cellulose (120). In the same line, chronic administration of nondigestible carbohydrates in humans, which are fermented by intestinal microflora, have been shown to increase postprandial GLP-1 levels. Cani et al. (120) showed that the latter effect was positively associated with breath hydrogen excretion, thus suggesting that the increase in GLP-1 levels could be related to production of SCFA produced by bacterial fermentation. Indeed, the SCFA receptor GPR43 has been positively identified in L cells (121). Moreover, Kaji et al. (122) recently showed that feeding rats with a fructooligosaccharide-containing diet increased the densities of the GPR43-positive enteroendocrine cells in the proximal colon by over 2-fold compared to controls. Furthermore, the mechanisms of SCFA induction in GLP-1 levels have been associated with intestinal differentiation of stem cells into L cells as well as the promotion of GLP-1 synthesis in the lower part of the gut (123). Other dietary fibers, such as resistant starch, increased basal and postprandial levels of GLP-1 in rodents (124). The intake of wheat fiber for >9 mo in hyperinsulinemic subjects was sufficient to increase postprandial GLP-1 levels (4).
Other dietary components, such as capsaicin, the pungent principle in hot red peppers or chiles, have been shown to mildly increase the secretion of GLP-1. The authors suggested that these could be associated with increased gastric emptying, resulting in earlier stimulation of L cells (125). However, because this is the first study to observe this effect, it is clear that more studies are needed to elucidate the effect of capsaicin on gut hormone release.
PYY
PYY concentrations rise within 15–30 min after meal ingestion and appear to be initiated by an indirect neuronal reflex, because its release occurred before the meal could trigger PYY secretion from colon cells (126). PYY secretion is stimulated by different macronutrients and is partially related to the calories ingested. Although it has been generally observed that fat is the most potent stimulator of PYY release in humans (48, 127–129), other authors have reported that protein-rich diets cause the greatest increase in PYY levels in healthy and obese humans as well as in rodents. The latter is associated with a greater satiety effect (83). In most cases, carbohydrates appear to be the less potent secretagogues of PYY. Lomenick et al. (129) have also shown that the response to different macronutrients varies between normal weight and obese individuals. In obese subjects, fat- and carbohydrate-induced PYY secretion was significantly reduced compared to lean subjects, whereas the response induced by protein increased. Batterham et al. (83), on the other hand, found that PYY secretion decreased in obese subjects upon stimulation with all macronutrients. Although the variations observed in these 2 studies are inconsistent, it is clear that obese subjects have a modified response to PYY secretion induced by macronutrients. The reason for this effect, although unknown, could be associated with modifications to other physiological signals or alterations in the sensing mechanisms of enteroendocrine cells in the obese state.
Fat.
Fat has been shown to be a potent inductor of PYY secretion, and the hydrolysis of TG appears to be essential for this process, because the administration of orlistat almost completely abolished fat-induced PPY secretion. More recently, Hata et al. (130) found that inhibition of intestinal microsomal TG transfer protein, which is the protein responsible for packing TG into chylomicrons, produced an increase in PYY secretion. In parallel, they found an increased concentration of FFA in the intestinal lumen, suggesting that this increase may be associated with the observed increase in PYY. However, the time course for both events was inconsistent; therefore, further investigation is needed (130). In the same study, the researchers also found elevated concentrations of TG in the intestinal tissue, an expected finding given that microsomal TG transfer protein catalyzes TG packing into chylomicrons. However, this result raises the question of whether the lipid content in the enterocyte may play a role in enteroendocrine cell stimulation.
The length of fatty acids also plays an important role, because only long-chain fatty acids (as oleic acid; C18:1) induced a response, whereas medium-chain fatty acids (caprilic acid; C8:0) did not induce any response. In addition, the administration of a CCK-1R antagonist in healthy subjects almost attenuated the long-chain fatty acid-induced PYY secretion, suggesting that the induction of PYY secretion by long-chain fatty acids is partly through CCK stimulation (60). As per the present data, it would be interesting to study fatty acid induction in PYY secretion in isolated L cells to determine whether CCK alone is responsible for the long-chain fatty acid-induced response in PYY.
Carbohydrates.
Recently, Gerspach et al. (68) showed that i.g. glucose-induced PYY secretion was significantly decreased in the presence of lactisole, a sweet taste receptor antagonist in healthy men; however, the latter effect was not seen with the administration of a mixed meal. Thus, they suggest that glucose-induced PYY secretion could be partially mediated by this sweet taste receptor; however, the secretion induced by the other components of the mixed meal (protein and fat) may involve other sensors present in enteroendocrine cells in addition to outweighing the effect induced by glucose.
Other dietary components.
Dietary fiber has been shown to modulate PYY secretion and this effect has been associated with the rheological properties of fiber. The reduction in viscosity in oat bran beverages significantly increased PYY secretion (131). Although another study also reported an increase in PYY secretion due to the addition of psyllium fiber (132), this effect was not notably clear, because the macronutrient composition between the test meals and the control meal differed with respect to carbohydrate and, more importantly, fat content. Given that fat is a potent inductor of PYY secretion, this effect may not be entirely due to the addition of fiber.
Chronic ingestion of prebiotics, such as fructooligosaccharides and resistant starch, has also been shown to increase levels of PYY in humans and rodents (120, 124, 133), This property has been associated with the fermentation of nondigestible polysaccharides by the gut microbiota, which produce SCFA that are thought to stimulate enteroendocrine cells (4).
GLP-2
Although the dietary sensing mechanisms of L cells in relation to GLP-1 secretion have been well studied, their specific effects on the secretion of GLP-2 have not been evaluated. Only Cani et al. (5) have shown that upon chronic administration of oligofructose, a prebiotic fiber to obese mice, basal levels of GLP-2 and GLP-1 increased (120). One can speculate that because they are secreted from the same enteroendocrine cell type, their secretion pathways are similar; however, further research is needed.
Conclusions
This review summarizes the current knowledge concerning dietary effects and interactions with enteroendocrine cells. In the last few years, new molecular techniques have resulted in important advances in our knowledge of the food-sensing mechanisms of enteroendocrine cells, allowing a deeper understanding of these processes. It is clear that food composition, macronutrients, and other non-nutrient components as well as the physical properties of food not only affect the secretion of gut peptides but also their transcription and the differentiation of enteroendocrine cells, which ultimately modifies gut hormone responses.
The role of gut hormones in the maintenance of metabolic homeostasis and their observed alterations in metabolic diseases, such as obesity and type 2 diabetes, has resulted in their being considered as possible targets for the prevention and treatment of these diseases (1). Data have shown that obese and type 2 diabetic subjects have an increased response to GIP secretion after the ingestion of a meal (10, 77). In fact, GIP has been established as a possible link between overnutrition and obesity. In a mouse model of obesity induced by a high-fat diet and in ob/ob mice, antagonism of the GIP receptor or GIP knockout was able to prevent or revert some of the metabolic disturbances (32), thereby raising the possibility of targeting GIP secretion or its activity to prevent the metabolic complications associated with obesity (30, 31). In addition, GLP-1 analogs are currently a line of treatment for type 2 diabetes due to their effects on incretin and in satiety mechanisms (134, 135). Finally, in bariatric surgeries, such as Roux-en-Y gastric by-pass or sleeve gastrectomy, the observed improvement in patients is accompanied by modifications in the gut hormone profile, suggesting a link between the observed weight reduction and the metabolic improvement of gut hormones (78, 79).
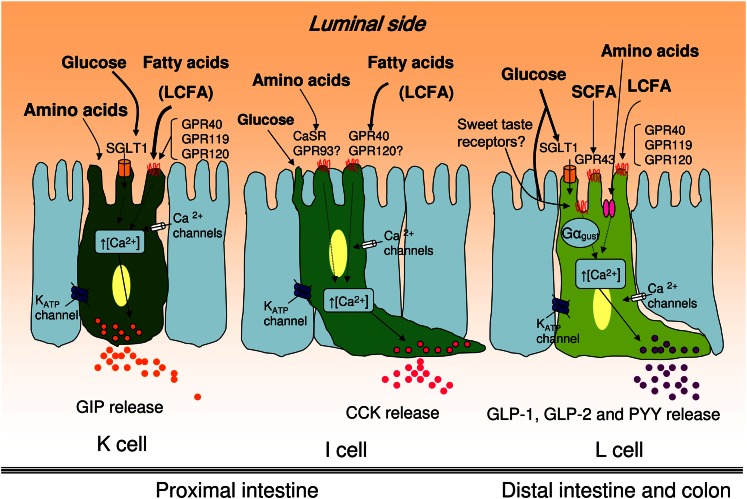
As discussed throughout this review, gut hormones, such as GIP, CCK, GLP-1, and PYY, play a key role in glucose homeostasis, lipid metabolism, energy expenditure, and food intake. The different macronutrients and non-nutrient components of the diet have a strong influence over the secretion of these hormones. Dietary fat is a potent inductor of GIP and CCK secretion and carbohydrates, specifically glucose, appear to be the major secretagogue of GLP-1, whereas PYY seems to be induced by fat and proteins. Moreover, SCFA produced by gut microbiota because of dietary fiber ingestion have also been shown to modify fasting levels of gut hormones secreted by L cells, highlighting the complex regulation of gastrointestinal peptides (Fig. 2).
FIGURE 2.
Food component stimulation of enteroendocrine cells and sensing machinery. K and I cells are primarily localized in the proximal intestine, whereas L cells are predominantly in the distal intestine and colon. A schematic representation of the enteroendocrine cell machinery for the different cell subtypes. See text for details. CaSR, calcium-sensing receptor; GPR, G protein-coupled receptor; LCFA, long-chain fatty acids; SGLT1, sodium-dependent glucose transporter 1.
An interesting finding is that GPR play a major role in the sensing mechanisms not only for fatty acids but also for carbohydrates and possibly for sweeteners in the form of taste receptors as well as for certain amino acids.
Although further research is still needed to completely elucidate the sensing mechanisms in these cells and to be able to modulate gut hormones specifically through diet, recent advances have revealed that dietary manipulations are a promising strategy for preventing and treating metabolic diseases, such as obesity and type 2 diabetes, which currently present a considerable public health burden. In addition, novel findings may help to elucidate whether the gut hormone modifications observed in metabolic diseases are a cause or a consequence of these disorders.
Acknowledgments
We are grateful to Dr. Adolfo Garcia-Sainz and Dr. Andrea Diaz-Villaseñor for graciously revising the manuscript. All authors have read and approved the final manuscript.
Footnotes
Supported by a CONACYT grant.
Author disclosures: S. Moran-Ramos, A. Tovar, and N. Torres, no conflicts of interest.
Abbreviations used: CaSR, calcium-sensing receptor; CCK, cholecystokinin; CCK1R, CCK 1 receptor; GIP, glucose-dependent insulinotropic peptide; GLP, glucagon-like peptide; GPR, G protein-coupled receptor; KATP, ATP-sensitive potassium; PYY, peptide tyrosine tyrosine; SGLT1, sodium-dependent glucose transporter.
Literature Cited
- 1.Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854–9 [DOI] [PubMed] [Google Scholar]
- 2.Murphy KG, Dhillo WS, Bloom SR. Gut peptides in the regulation of food intake and energy homeostasis. Endocr Rev. 2006;27:719–27 [DOI] [PubMed] [Google Scholar]
- 3.García-Martínez JM, Chocarro-Calvo A, Moya CM, Garcia-Jimenez C. WNT/beta-catenin increases the production of incretins by entero-endocrine cells. Diabetologia. 2009;52:1913–24 [DOI] [PubMed] [Google Scholar]
- 4.Freeland KR, Wilson C, Wolever TM. Adaptation of colonic fermentation and glucagon-like peptide-1 secretion with increased wheat fibre intake for 1 year in hyperinsulinaemic human subjects. Br J Nutr. 2010;103:82–90 [DOI] [PubMed] [Google Scholar]
- 5.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tolhurst G, Reimann F, Gribble FM. Nutritional regulation of glucagon-like peptide-1 secretion. J Physiol. 2009;587:27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schonhoff SE, Giel-Moloney M, Leiter AB. Minireview: development and differentiation of gut endocrine cells. Endocrinology. 2004;145:2639–44 [DOI] [PubMed] [Google Scholar]
- 8.Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux JF, Pignodel C, et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. 2011;192:767–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–59 [DOI] [PubMed] [Google Scholar]
- 10.Cho YM, Kieffer TJ. K-cells and glucose-dependent insulinotropic polypeptide in health and disease. Vitam Horm. 2010;84:111–50 [DOI] [PubMed] [Google Scholar]
- 11.Moran GW, Leslie FC, Levison SE, McLaughlin JT. Enteroendocrine cells: neglected players in gastrointestinal disorders? Therap Adv Gastroenterol. 2008;1:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002;21:6338–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Höcker M, Wiedenmann B. Molecular mechanisms of enteroendocrine differentiation. Ann N Y Acad Sci. 1998;859:160–74 [DOI] [PubMed] [Google Scholar]
- 14.Mellitzer G, Beucher A, Lobstein V, Michel P, Robine S, Kedinger M, Gradwohl G. Loss of enteroendocrine cells in mice alters lipid absorption and glucose homeostasis and impairs postnatal survival. J Clin Invest. 2010;120:1708–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi S, Lee M, Shiu AL, Yo SJ, Hallden G, Aponte GW. GPR93 activation by protein hydrolysate induces CCK transcription and secretion in STC-1 cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1366–75 [DOI] [PubMed] [Google Scholar]
- 16.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelstoft MS, Egerod KL, Holst B, Schwartz TW. A gut feeling for obesity: 7TM sensors on enteroendocrine cells. Cell Metab. 2008;8:447–9 [DOI] [PubMed] [Google Scholar]
- 18.Liou AP, Chavez DI, Espero E, Hao S, Wank SA, Raybould HE. Protein hydrolysate-induced cholecystokinin secretion from enteroendocrine cells is indirectly mediated by the intestinal oligopeptide transporter PepT1. Am J Physiol Gastrointest Liver Physiol. 2011;300:G895–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown JC. A gastric inhibitory polypeptide. I. The amino acid composition and the tryptic peptides. Can J Biochem. 1971;49:255–61 [DOI] [PubMed] [Google Scholar]
- 20.Dupre J, Ross SA, Watson D, Brown JC. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. 1973;37:826–8 [DOI] [PubMed] [Google Scholar]
- 21.Buffa R, Polak JM, Pearse AG, Solcia E, Grimelius L, Capella C. Identification of the intestinal cell storing gastric inhibitory peptide. Histochemistry. 1975;43:249–55 [DOI] [PubMed] [Google Scholar]
- 22.Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, Egan JM. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab. 2006;290:E550–9 [DOI] [PubMed] [Google Scholar]
- 23.Mortensen K, Christensen LL, Holst JJ, Orskov C. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul Pept. 2003;114:189–96 [DOI] [PubMed] [Google Scholar]
- 24.Fujita Y, Chui JW, King DS, Zhang T, Seufert J, Pownall S, Cheung AT, Kieffer TJ. Pax6 and Pdx1 are required for production of glucose-dependent insulinotropic polypeptide in proglucagon-expressing L cells. Am J Physiol Endocrinol Metab. 2008;295:E648–57 [DOI] [PubMed] [Google Scholar]
- 25.Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia. 2009;52:289–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsukiyama K, Yamada Y, Yamada C, Harada N, Kawasaki Y, Ogura M, Bessho K, Li M, Amizuka N, Sato M, et al. Gastric inhibitory polypeptide as an endogenous factor promoting new bone formation after food ingestion. Mol Endocrinol. 2006;20:1644–51 [DOI] [PubMed] [Google Scholar]
- 27.Ding KH, Shi XM, Zhong Q, Kang B, Xie D, Bollag WB, Bollag RJ, Hill W, Washington W, Mi QS, et al. Impact of glucose-dependent insulinotropic peptide on age-induced bone loss. J Bone Miner Res. 2008;23:536–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Getty-Kaushik L, Song DH, Boylan MO, Corkey BE, Wolfe MM. Glucose-dependent insulinotropic polypeptide modulates adipocyte lipolysis and reesterification. Obesity (Silver Spring). 2006;14:1124–31 [DOI] [PubMed] [Google Scholar]
- 29.Kim SJ, Nian C, McIntosh CH. Resistin is a key mediator of glucose-dependent insulinotropic polypeptide (GIP) stimulation of lipoprotein lipase (LPL) activity in adipocytes. J Biol Chem. 2007;282:34139–47 [DOI] [PubMed] [Google Scholar]
- 30.Irwin N, Flatt PR. Evidence for beneficial effects of compromised gastric inhibitory polypeptide action in obesity-related diabetes and possible therapeutic implications. Diabetologia. 2009;52:1724–31 [DOI] [PubMed] [Google Scholar]
- 31.Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K, Toyokuni S, et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med. 2002;8:738–42 [DOI] [PubMed] [Google Scholar]
- 32.McClean PL, Irwin N, Cassidy RS, Holst JJ, Gault VA, Flatt PR. GIP receptor antagonism reverses obesity, insulin resistance, and associated metabolic disturbances induced in mice by prolonged consumption of high-fat diet. Am J Physiol Endocrinol Metab. 2007;293:E1746–55 [DOI] [PubMed] [Google Scholar]
- 33.Song DH, Getty-Kaushik L, Tseng E, Simon J, Corkey BE, Wolfe MM. Glucose-dependent insulinotropic polypeptide enhances adipocyte development and glucose uptake in part through Akt activation. Gastroenterology. 2007;133:1796–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fulurija A, Lutz TA, Sladko K, Osto M, Wielinga PY, Bachmann MF, Saudan P. Vaccination against GIP for the treatment of obesity. PLoS ONE. 2008;3:e3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1 (7–36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol. 1993;138:159–66 [DOI] [PubMed] [Google Scholar]
- 36.Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Enç FY, Ones T, Akin HL, Dede F, Turoglu HT, Ulfer G, Bekiroglu N, Haklar G, Rehfeld JF, Holst JJ, et al. Orlistat accelerates gastric emptying and attenuates GIP release in healthy subjects. Am J Physiol Gastrointest Liver Physiol. 2009;296:G482–9 [DOI] [PubMed] [Google Scholar]
- 38.Thomsen C, Rasmussen O, Lousen T, Holst JJ, Fenselau S, Schrezenmeir J, Hermansen K. Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am J Clin Nutr. 1999;69:1135–43 [DOI] [PubMed] [Google Scholar]
- 39.Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA. 2007;104:15069–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA. 2007;104:15075–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujita Y, Wideman RD, Speck M, Asadi A, King DS, Webber TD, Haneda M, Kieffer TJ. Incretin release from gut is acutely enhanced by sugar but not by sweeteners in vivo. Am J Physiol Endocrinol Metab. 2009;296:E473–9 [DOI] [PubMed] [Google Scholar]
- 42.Renwick AG, Molinary SV. Sweet-taste receptors, low-energy sweeteners, glucose absorption and insulin release. Br J Nutr. 2010;104:1415–20 [DOI] [PubMed] [Google Scholar]
- 43.Wolfe MM, Zhao KB, Glazier KD, Jarboe LA, Tseng CC. Regulation of glucose-dependent insulinotropic polypeptide release by protein in the rat. Am J Physiol Gastrointest Liver Physiol. 2000;279:G561–6 [DOI] [PubMed] [Google Scholar]
- 44.Karamanlis A, Chaikomin R, Doran S, Bellon M, Bartholomeusz FD, Wishart JM, Jones KL, Horowitz M, Rayner CK. Effects of protein on glycemic and incretin responses and gastric emptying after oral glucose in healthy subjects. Am J Clin Nutr. 2007;86:1364–8 [DOI] [PubMed] [Google Scholar]
- 45.Carr RD, Larsen MO, Winzell MS, Jelic K, Lindgren O, Deacon CF, Ahren B. Incretin and islet hormonal responses to fat and protein ingestion in healthy men. Am J Physiol Endocrinol Metab. 2008;295:E779–84 [DOI] [PubMed] [Google Scholar]
- 46.Nilsson M, Stenberg M, Frid AH, Holst JJ, Bjorck IM. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr. 2004;80:1246–53 [DOI] [PubMed] [Google Scholar]
- 47.Frid AH, Nilsson M, Holst JJ, Bjorck IM. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am J Clin Nutr. 2005;82:69–75 [DOI] [PubMed] [Google Scholar]
- 48.Karhunen LJ, Juvonen KR, Huotari A, Purhonen AK, Herzig KH. Effect of protein, fat, carbohydrate and fibre on gastrointestinal peptide release in humans. Regul Pept. 2008;149:70–8 [DOI] [PubMed] [Google Scholar]
- 49.Johnston KL, Clifford MN, Morgan LM. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. Am J Clin Nutr. 2003;78:728–33 [DOI] [PubMed] [Google Scholar]
- 50.Beaudoin MS, Robinson LE, Graham TE. An oral lipid challenge and acute intake of caffeinated coffee additively decrease glucose tolerance in healthy men. J Nutr. 2011;141:574–81 [DOI] [PubMed] [Google Scholar]
- 51.Liddle RA. Cholecystokinin cells. Annu Rev Physiol. 1997;59:221–42 [DOI] [PubMed] [Google Scholar]
- 52.Johnson LR, editor Physiology of the gastrointestinal tract. 4th ed Burlington, MA: Elsevier Academic Press; 2006 [Google Scholar]
- 53.Dockray GJ. Cholecystokinin and gut-brain signalling. Regul Pept. 2009;155:6–10 [DOI] [PubMed] [Google Scholar]
- 54.Moran TH, Kinzig KP. Gastrointestinal satiety signals II. Cholecystokinin. Am J Physiol Gastrointest Liver Physiol. 2004;286:G183–8 [DOI] [PubMed] [Google Scholar]
- 55.Chandra R, Samsa LA, Vigna SR, Liddle RA. Pseudopod-like basal cell processes in intestinal cholecystokinin cells. Cell Tissue Res. 2010;341:289–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Green T, Dimaline R, Peikin S, Dockray GJ. Action of the cholecystokinin antagonist L364,718 on gastric emptying in the rat. Am J Physiol. 1988;255:G685–9 [DOI] [PubMed] [Google Scholar]
- 57.Hölzer HH, Turkelson CM, Solomon TE, Raybould HE. Intestinal lipid inhibits gastric emptying via CCK and a vagal capsaicin-sensitive afferent pathway in rats. Am J Physiol. 1994;267:G625–9 [DOI] [PubMed] [Google Scholar]
- 58.Cheung GW, Kokorovic A, Lam CK, Chari M, Lam TK. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab. 2009;10:99–109 [DOI] [PubMed] [Google Scholar]
- 59.McLaughlin J, Grazia Luca M, Jones MN, D'Amato M, Dockray GJ, Thompson DG. Fatty acid chain length determines cholecystokinin secretion and effect on human gastric motility. Gastroenterology. 1999;116:46–53 [DOI] [PubMed] [Google Scholar]
- 60.Degen L, Matzinger D, Drewe J, Nissle S, Maecke H, Lengsfeld H, Hadvary P, Beglinger C. Role of free fatty acids in regulating gastric emptying and gallbladder contraction. Digestion. 2006;74:131–9 [DOI] [PubMed] [Google Scholar]
- 61.Ellrichmann M, Kapelle M, Ritter PR, Holst JJ, Herzig KH, Schmidt WE, Schmitz F, Meier JJ. Orlistat inhibition of intestinal lipase acutely increases appetite and attenuates postprandial glucagon-like peptide-1-(7–36)-amide-1, cholecystokinin, and peptide YY concentrations. J Clin Endocrinol Metab. 2008;93:3995–8 [DOI] [PubMed] [Google Scholar]
- 62.McLaughlin J. Long-chain fatty acid sensing in the gastrointestinal tract. Biochem Soc Trans. 2007;35:1199–202 [DOI] [PubMed] [Google Scholar]
- 63.Tanaka T, Katsuma S, Adachi T, Koshimizu TA, Hirasawa A, Tsujimoto G. Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:523–7 [DOI] [PubMed] [Google Scholar]
- 64.Fujimoto K, Cardelli JA, Tso P. Increased apolipoprotein A-IV in rat mesenteric lymph after lipid meal acts as a physiological signal for satiation. Am J Physiol. 1992;262:G1002–6 [DOI] [PubMed] [Google Scholar]
- 65.Kalogeris TJ, Monroe F, Demichele SJ, Tso P. Intestinal synthesis and lymphatic secretion of apolipoprotein A-IV vary with chain length of intestinally infused fatty acids in rats. J Nutr. 1996;126:2720–9 [DOI] [PubMed] [Google Scholar]
- 66.Glatzle J, Darcel N, Rechs AJ, Kalogeris TJ, Tso P, Raybould HE. Apolipoprotein A-IV stimulates duodenal vagal afferent activity to inhibit gastric motility via a CCK1 pathway. Am J Physiol Regul Integr Comp Physiol. 2004;287:R354–9 [DOI] [PubMed] [Google Scholar]
- 67.Lo CM, Zhang DM, Pearson K, Ma L, Sun W, Sakai RR, Davidson WS, Liu M, Raybould HE, Woods SC, et al. Interaction of apolipoprotein AIV with cholecystokinin on the control of food intake. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1490–4 [DOI] [PubMed] [Google Scholar]
- 68.Gerspach AC, Steinert RE, Schonenberger L, Graber-Maier A, Beglinger C. The role of the gut sweet taste receptor in regulating GLP-1, PYY and CCK release in humans. Am J Physiol Endocrinol Metab. Epub 2011 May 1 [DOI] [PubMed] [Google Scholar]
- 69.Bowen J, Noakes M, Trenerry C, Clifton PM. Energy intake, ghrelin, and cholecystokinin after different carbohydrate and protein preloads in overweight men. J Clin Endocrinol Metab. 2006;91:1477–83 [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Chandra R, Samsa LA, Gooch B, Fee BE, Cook JM, Vigna SR, Grant AO, Liddle RA. Amino acids stimulate cholecystokinin release through the Ca2+-sensing receptor. Am J Physiol Gastrointest Liver Physiol. 2011;300:G528–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakajima S, Hira T, Eto Y, Asano K, Hara H. Soybean beta 51–63 peptide stimulates cholecystokinin secretion via a calcium-sensing receptor in enteroendocrine STC-1 cells. Regul Pept. 2010;159:148–55 [DOI] [PubMed] [Google Scholar]
- 72.Bourdon I, Yokoyama W, Davis P, Hudson C, Backus R, Richter D, Knuckles B, Schneeman BO. Postprandial lipid, glucose, insulin, and cholecystokinin responses in men fed barley pasta enriched with beta-glucan. Am J Clin Nutr. 1999;69:55–63 [DOI] [PubMed] [Google Scholar]
- 73.Bourdon I, Olson B, Backus R, Richter BD, Davis PA, Schneeman BO. Beans, as a source of dietary fiber, increase cholecystokinin and apolipoprotein b48 response to test meals in men. J Nutr. 2001;131:1485–90 [DOI] [PubMed] [Google Scholar]
- 74.Le Nevé B, Foltz M, Daniel H, Gouka R. The steroid glycoside H.g.-12 from Hoodia gordonii activates the human bitter receptor TAS2R14 and induces CCK release from HuTu-80 cells. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1368–75 [DOI] [PubMed] [Google Scholar]
- 75.Bell GI, Sanchez-Pescador R, Laybourn PJ, Najarian RC. Exon duplication and divergence in the human preproglucagon gene. Nature. 1983;304:368–71 [DOI] [PubMed] [Google Scholar]
- 76.Schmidt WE, Siegel EG, Creutzfeldt W. Glucagon-like peptide-1 but not glucagon-like peptide-2 stimulates insulin release from isolated rat pancreatic islets. Diabetologia. 1985;28:704–7 [DOI] [PubMed] [Google Scholar]
- 77.Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60:470–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116–30 [DOI] [PubMed] [Google Scholar]
- 79.Field BC, Chaudhri OB, Bloom SR. Bowels control brain: gut hormones and obesity. Nat Rev Endocrinol. 2010;6:444–53 [DOI] [PubMed] [Google Scholar]
- 80.Bohórquez DV, Chandra R, Samsa LA, Vigna SR, Liddle RA. Characterization of basal pseudopod-like processes in ileal and colonic PYY cells. J Mol Histol. 2011;42:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–65 [DOI] [PubMed] [Google Scholar]
- 82.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med. 2003;349:941–8 [DOI] [PubMed] [Google Scholar]
- 83.Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, Withers DJ. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4:223–33 [DOI] [PubMed] [Google Scholar]
- 84.Benjamin MA, McKay DM, Yang PC, Cameron H, Perdue MH. Glucagon-like peptide-2 enhances intestinal epithelial barrier function of both transcellular and paracellular pathways in the mouse. Gut. 2000;47:112–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burrin DG, Petersen Y, Stoll B, Sangild P. Glucagon-like peptide 2: a nutrient-responsive gut growth factor. J Nutr. 2001;131:709–12 [DOI] [PubMed] [Google Scholar]
- 86.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39 [DOI] [PubMed] [Google Scholar]
- 87.Reimann F. Molecular mechanisms underlying nutrient detection by incretin-secreting cells. Int Dairy J. 2010;20:236–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu WJ, Yang Q, Sun W, Woods SC, D'Alessio D, Tso P. The regulation of the lymphatic secretion of glucagon-like peptide-1 (GLP-1) by intestinal absorption of fat and carbohydrate. Am J Physiol Gastrointest Liver Physiol. 2007;293:G963–71 [DOI] [PubMed] [Google Scholar]
- 89.Wu T, Rayner CK, Jones K, Horowitz M. Dietary effects on incretin hormone secretion. Vitam Horm. 2010;84:81–110 [DOI] [PubMed] [Google Scholar]
- 90.Parker HE, Reimann F, Gribble FM. Molecular mechanisms underlying nutrient-stimulated incretin secretion. Expert Rev Mol Med. 2010;12:e1. [DOI] [PubMed] [Google Scholar]
- 91.Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia. 2011;54:10–8 [DOI] [PubMed] [Google Scholar]
- 92.Little TJ, Doran S, Meyer JH, Smout AJ, O'Donovan DG, Wu KL, Jones KL, Wishart J, Rayner CK, Horowitz M, et al. The release of GLP-1 and ghrelin, but not GIP and CCK, by glucose is dependent upon the length of small intestine exposed. Am J Physiol Endocrinol Metab. 2006;291:E647–55 [DOI] [PubMed] [Google Scholar]
- 93.Nielsen LB, Ploug KB, Swift P, Orskov C, Jansen-Olesen I, Chiarelli F, Holst JJ, Hougaard P, Porksen S, Holl R, et al. Co-localisation of the Kir6.2/SUR1 channel complex with glucagon-like peptide-1 and glucose-dependent insulinotrophic polypeptide expression in human ileal cells and implications for glycaemic control in new onset type 1 diabetes. Eur J Endocrinol. 2007;156:663–71 [DOI] [PubMed] [Google Scholar]
- 94.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8:532–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.El-Ouaghlidi A, Rehring E, Holst JJ, Schweizer A, Foley J, Holmes D, Nauck MA. The dipeptidyl peptidase 4 inhibitor vildagliptin does not accentuate glibenclamide-induced hypoglycemia but reduces glucose-induced glucagon-like peptide 1 and gastric inhibitory polypeptide secretion. J Clin Endocrinol Metab. 2007;92:4165–71 [DOI] [PubMed] [Google Scholar]
- 96.Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes. 2003;52:1147–54 [DOI] [PubMed] [Google Scholar]
- 97.Moriya R, Shirakura T, Ito J, Mashiko S, Seo T. Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am J Physiol Endocrinol Metab. 2009;297:E1358–65 [DOI] [PubMed] [Google Scholar]
- 98.Rozengurt E, Sternini C. Taste receptor signaling in the mammalian gut. Curr Opin Pharmacol. 2007;7:557–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Young RL, Sutherland K, Pezos N, Brierley SM, Horowitz M, Rayner CK, Blackshaw LA. Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut. 2009;58:337–46 [DOI] [PubMed] [Google Scholar]
- 100.Kokrashvili Z, Mosinger B, Margolskee RF. Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr. 2009;90:S822–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582:379–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ma J, Bellon M, Wishart JM, Young R, Blackshaw LA, Jones KL, Horowitz M, Rayner CK. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol. 2009;296:G735–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brown RJ, Walter M, Rother KI. Ingestion of diet soda before a glucose load augments glucagon-like peptide-1 secretion. Diabetes Care. 2009;32:2184–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–4 [DOI] [PubMed] [Google Scholar]
- 105.Beglinger S, Drewe J, Schirra J, Goke B, D'Amato M, Beglinger C. Role of fat hydrolysis in regulating glucagon-like peptide-1 secretion. J Clin Endocrinol Metab. 2010;95:879–86 [DOI] [PubMed] [Google Scholar]
- 106.Thomsen C, Storm H, Holst JJ, Hermansen K. Differential effects of saturated and monounsaturated fats on postprandial lipemia and glucagon-like peptide 1 responses in patients with type 2 diabetes. Am J Clin Nutr. 2003;77:605–11 [DOI] [PubMed] [Google Scholar]
- 107.Feltrin KL, Little TJ, Meyer JH, Horowitz M, Smout AJ, Wishart J, Pilichiewicz AN, Rades T, Chapman IM, Feinle-Bisset C. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am J Physiol Regul Integr Comp Physiol. 2004;287:R524–33 [DOI] [PubMed] [Google Scholar]
- 108.Yoder SM, Yang Q, Kindel TL, Tso P. Stimulation of incretin secretion by dietary lipid: is it dose dependent? Am J Physiol Gastrointest Liver Physiol. 2009;297:G299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.O'Donovan D, Horowitz M, Russo A, Feinle-Bisset C, Murolo N, Gentilcore D, Wishart JM, Morris HA, Jones KL. Effects of lipase inhibition on gastric emptying of, and on the glycaemic, insulin and cardiovascular responses to, a high-fat/carbohydrate meal in type 2 diabetes. Diabetologia. 2004;47:2208–14 [DOI] [PubMed] [Google Scholar]
- 110.Chu ZL, Carroll C, Alfonso J, Gutierrez V, He H, Lucman A, Pedraza M, Mondala H, Gao H, Bagnol D, et al. A role for intestinal endocrine cell-expressed g protein-coupled receptor 119 in glycemic control by enhancing glucagon-like Peptide-1 and glucose-dependent insulinotropic Peptide release. Endocrinology. 2008;149:2038–47 [DOI] [PubMed] [Google Scholar]
- 111.Tanaka T, Yano T, Adachi T, Koshimizu TA, Hirasawa A, Tsujimoto G. Cloning and characterization of the rat free fatty acid receptor GPR120: in vivo effect of the natural ligand on GLP-1 secretion and proliferation of pancreatic beta cells. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:515–22 [DOI] [PubMed] [Google Scholar]
- 112.Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes. 2009;58:1058–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hall WL, Millward DJ, Long SJ, Morgan LM. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br J Nutr. 2003;89:239–48 [DOI] [PubMed] [Google Scholar]
- 114.Reimann F, Williams L, da Silva Xavier G, Rutter GA, Gribble FM. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia. 2004;47:1592–601 [DOI] [PubMed] [Google Scholar]
- 115.Greenfield JR, Farooqi IS, Keogh JM, Henning E, Habib AM, Blackwood A, Reimann F, Holst JJ, Gribble FM. Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am J Clin Nutr. 2009;89:106–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Samocha-Bonet D, Wong O, Synnott EL, Piyaratna N, Douglas A, Gribble FM, Holst JJ, Chisholm DJ, Greenfield JR. Glutamine reduces postprandial glycemia and augments the glucagon-like peptide-1 response in type 2 diabetes patients. J Nutr. 2011;141:1233–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tolhurst G, Zheng Y, Parker HE, Habib AM, Reimann F, Gribble FM. Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology. 2011;152:405–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Le Nevé B, Daniel H. Selected tetrapeptides lead to a GLP-1 release from the human enteroendocrine cell line NCI-H716. Regul Pept. 2011;167:14–20 [DOI] [PubMed] [Google Scholar]
- 119.Young SH, Rey O, Sternini C, Rozengurt E. Amino acid sensing by enteroendocrine STC-1 cells: role of the Na+-coupled neutral amino acid transporter 2. Am J Physiol Cell Physiol. 2010;298:C1401–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D, De Backer F, Neyrinck AM, Delzenne NM. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr. 2009;90:1236–43 [DOI] [PubMed] [Google Scholar]
- 121.Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, Furness JB, Kuwahara A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324:353–60 [DOI] [PubMed] [Google Scholar]
- 122.Kaji I, Karaki S, Tanaka R, Kuwahara A. Density distribution of free fatty acid receptor 2 (FFA2)-expressing and GLP-1-producing enteroendocrine L cells in human and rat lower intestine, and increased cell numbers after ingestion of fructo-oligosaccharide. J Mol Histol. 2011;42:27–38 [DOI] [PubMed] [Google Scholar]
- 123.Delzenne NM, Cani PD, Neyrinck AM. Modulation of glucagon-like peptide 1 and energy metabolism by inulin and oligofructose: experimental data. J Nutr. 2007;137:S2547–51 [DOI] [PubMed] [Google Scholar]
- 124.Zhou J, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, Shen L, Danna SC, Tripathy S, Hegsted M, Keenan MJ. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab. 2008;295:E1160–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Smeets AJ, Westerterp-Plantenga MS. The acute effects of a lunch containing capsaicin on energy and substrate utilisation, hormones, and satiety. Eur J Nutr. 2009;48:229–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kirchner H, Tong J, Tschop MH, Pfluger PT. Ghrelin and PYY in the regulation of energy balance and metabolism: lessons from mouse mutants. Am J Physiol Endocrinol Metab. 2010;298:E909–19 [DOI] [PubMed] [Google Scholar]
- 127.Essah PA, Levy JR, Sistrun SN, Kelly SM, Nestler JE. Effect of macronutrient composition on postprandial peptide YY levels. J Clin Endocrinol Metab. 2007;92:4052–5 [DOI] [PubMed] [Google Scholar]
- 128.Helou N, Obeid O, Azar ST, Hwalla N. Variation of postprandial PYY 3–36 response following ingestion of differing macronutrient meals in obese females. Ann Nutr Metab. 2008;52:188–95 [DOI] [PubMed] [Google Scholar]
- 129.Lomenick JP, Melguizo MS, Mitchell SL, Summar ML, Anderson JW. Effects of meals high in carbohydrate, protein, and fat on ghrelin and peptide YY secretion in prepubertal children. J Clin Endocrinol Metab. 2009;94:4463–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hata T, Mera Y, Ishii Y, Tadaki H, Tomimoto D, Kuroki Y, Kawai T, Ohta T, Kakutani M. JTT-130, a novel intestine-specific inhibitor of microsomal triglyceride transfer protein, suppresses food intake and gastric emptying with the elevation of plasma peptide YY and glucagon-like peptide-1 in a dietary fat-dependent manner. J Pharmacol Exp Ther. 2011;336:850–6 [DOI] [PubMed] [Google Scholar]
- 131.Juvonen KR, Purhonen AK, Salmenkallio-Marttila M, Lahteenmaki L, Laaksonen DE, Herzig KH, Uusitupa MI, Poutanen KS, Karhunen LJ. Viscosity of oat bran-enriched beverages influences gastrointestinal hormonal responses in healthy humans. J Nutr. 2009;139:461–6 [DOI] [PubMed] [Google Scholar]
- 132.Karhunen LJ, Juvonen KR, Flander SM, Liukkonen KH, Lahteenmaki L, Siloaho M, Laaksonen DE, Herzig KH, Uusitupa MI, Poutanen KS. A psyllium fiber-enriched meal strongly attenuates postprandial gastrointestinal peptide release in healthy young adults. J Nutr. 2010;140:737–44 [DOI] [PubMed] [Google Scholar]
- 133.Cani PD, Dewever C, Delzenne NM. Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br J Nutr. 2004;92:521–6 [DOI] [PubMed] [Google Scholar]
- 134.Giorgino F, Natalicchio A, Leonardini A, Laviola L. Exploiting the pleiotropic actions of GLP-1 for the management of type 2 diabetes mellitus and its complications. Diabetes Res Clin Pract. 2007;78:S59–67 [Google Scholar]
- 135.Boyle PJ, Freeman JS. Application of incretin mimetics and dipeptidyl peptidase IV inhibitors in managing type 2 diabetes mellitus. J Am Osteopath Assoc. 2007;107 Suppl:S10–6 [PubMed] [Google Scholar]