Abstract
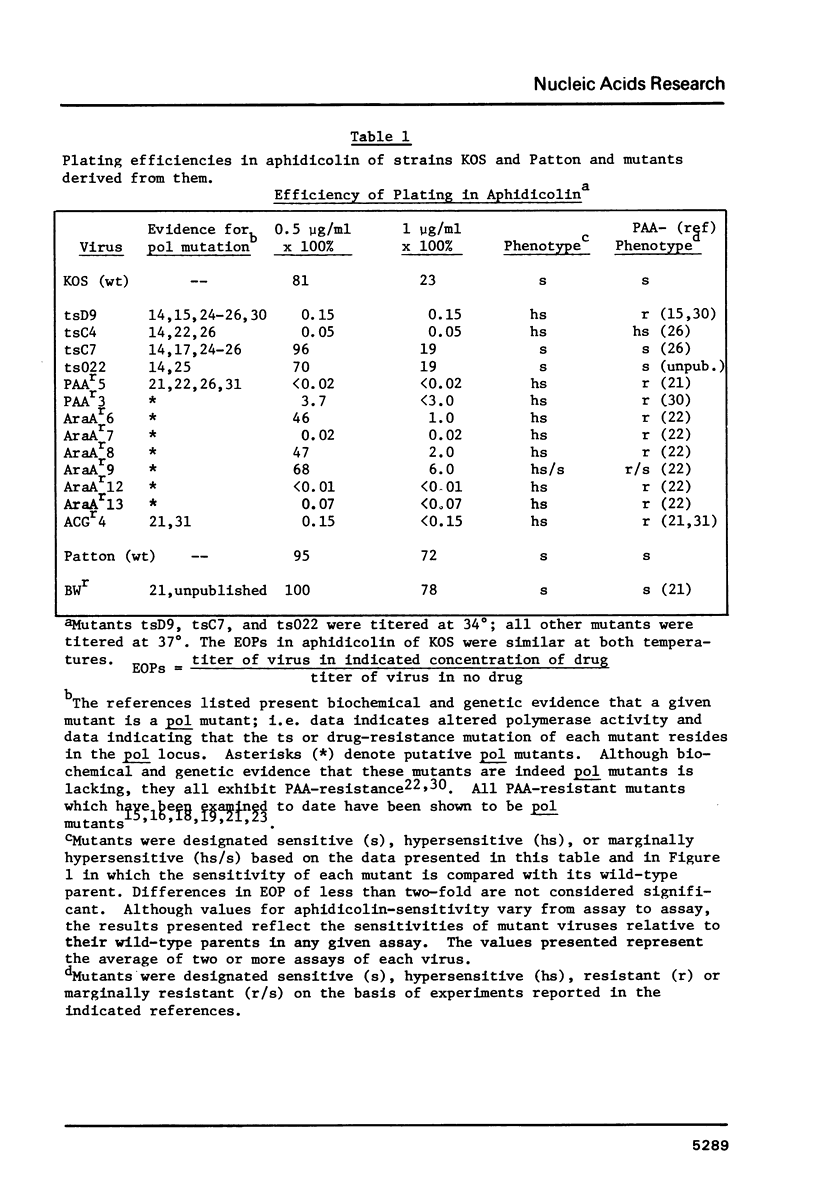
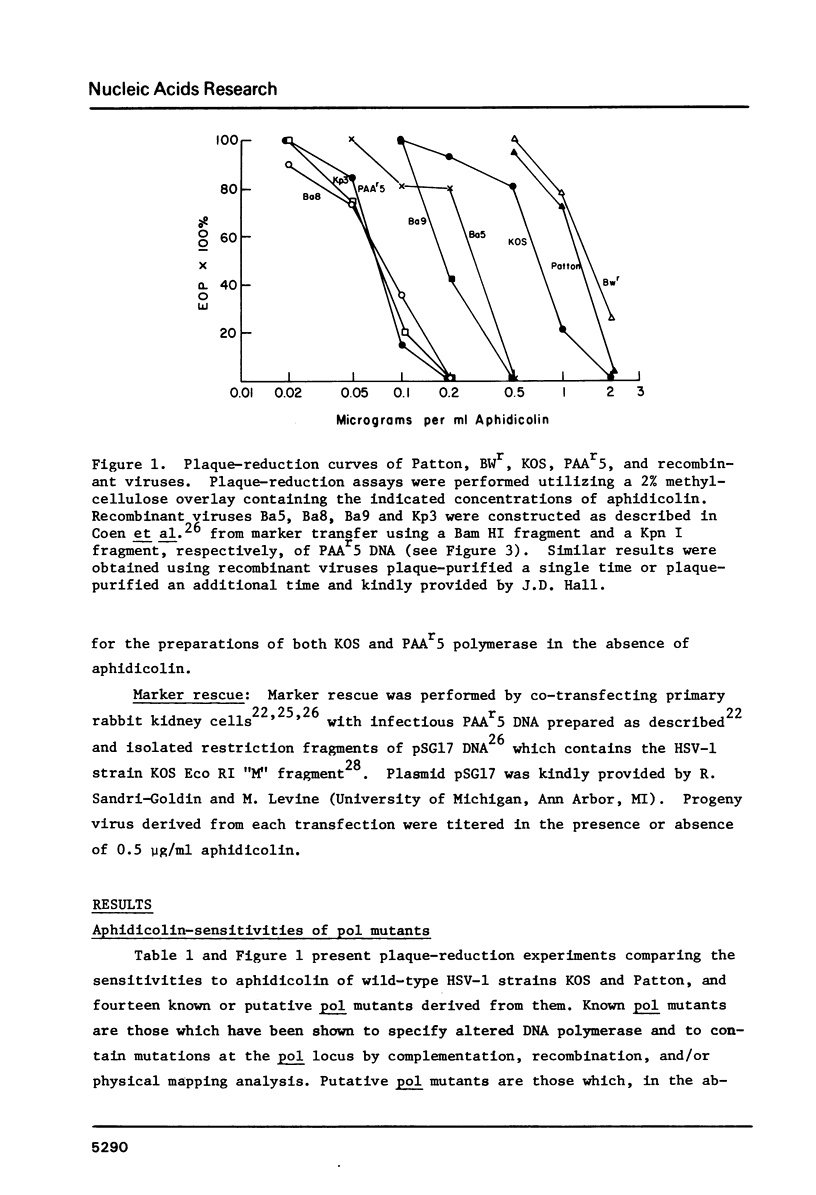
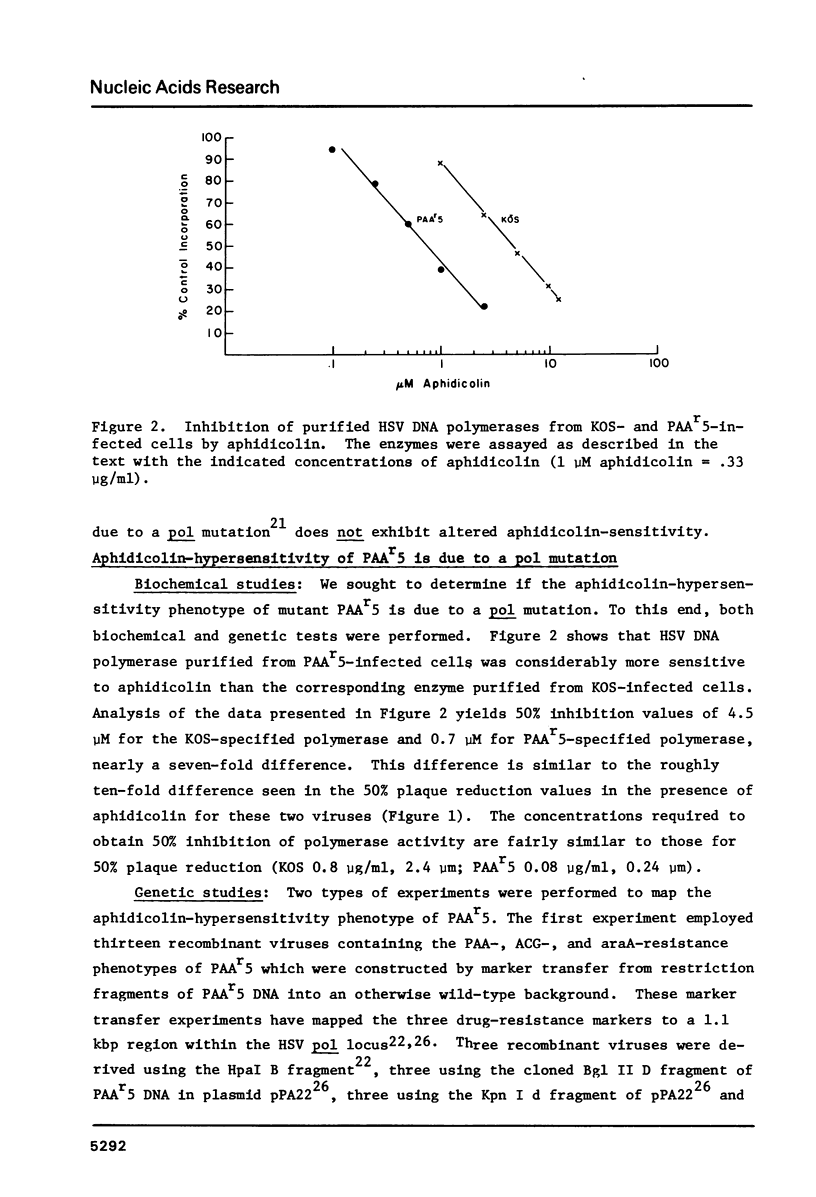
Fourteen mutants known or likely to contain mutations in the herpes simplex virus DNA polymerase gene were examined for their sensitivity to aphidicolin in plaque reduction assays. Eleven of these exhibited some degree of hypersensitivity to the drug; altered aphidicolin-sensitivity correlated with altered sensitivity to the pyrophosphate analog, phosphonoacetic acid. The DNA polymerase specified by one of these mutants, PAAr5, required roughly seven-fold less aphidicolin to inhibit its activity by 50% than did polymerase specified by its parental strain. Mutations responsible for the aphidicolin-hypersensitivity phenotype of PAAr5 were mapped to an 0.8 kbp region in the herpes simplex virus DNA polymerase locus. These data taken together indicate that 1) mutations in the herpes simplex virus DNA polymerase gene can confer altered sensitivity to aphidicolin, 2) that the HSV polymerase is sensitive to aphidicolin in vivo, and 3) that amino acid alterations which affect aphidicolin binding may affect the pyrophosphate exchange-release site as well, suggesting that aphidicolin binds in close proximity to this site.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aron G. M., Purifoy D. J., Schaffer P. A. DNA synthesis and DNA polymerase activity of herpes simplex virus type 1 temperature-sensitive mutants. J Virol. 1975 Sep;16(3):498–507. doi: 10.1128/jvi.16.3.498-507.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastow K. F., Derse D. D., Cheng Y. C. Susceptibility of phosphonoformic acid-resistant herpes simplex virus variants to arabinosylnucleosides and aphidicolin. Antimicrob Agents Chemother. 1983 Jun;23(6):914–917. doi: 10.1128/aac.23.6.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucknall R. A., Moores H., Simms R., Hesp B. Antiviral effects of aphidicolin, a new antibiotic produced by Cephalosporium aphidicola. Antimicrob Agents Chemother. 1973 Sep;4(3):294–298. doi: 10.1128/aac.4.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartrand P., Crumpacker C. S., Schaffer P. A., Wilkie N. M. Physical and genetic analysis of the herpes simplex virus DNA polymerase locus. Virology. 1980 Jun;103(2):311–326. doi: 10.1016/0042-6822(80)90190-7. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Furman P. A., Gelep P. T., Schaffer P. A. Mutations in the herpes simplex virus DNA polymerase gene can confer resistance to 9-beta-D-arabinofuranosyladenine. J Virol. 1982 Mar;41(3):909–918. doi: 10.1128/jvi.41.3.909-918.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Schaffer P. A. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2265–2269. doi: 10.1073/pnas.77.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derse D., Bastow K. F., Cheng Y. Characterization of the DNA polymerases induced by a group of herpes simplex virus type I variants selected for growth in the presence of phosphonoformic acid. J Biol Chem. 1982 Sep 10;257(17):10251–10260. [PubMed] [Google Scholar]
- Dicioccio R. A., Chadha K., Sahai Srivastava B. I. Inhibition of herpes simplex virus-induced DNA polymerase, cellular DNA polymerase alpha, and virus production by aphidicolin. Biochim Biophys Acta. 1980 Sep 19;609(2):224–231. doi: 10.1016/0005-2787(80)90233-6. [DOI] [PubMed] [Google Scholar]
- Dreesman G. R., Benyesh-Melnick M. Spectrum of human cytomegalovirus complement-fixing antigens. J Immunol. 1967 Dec;99(6):1106–1114. [PubMed] [Google Scholar]
- Furman P. A., Coen D. M., St Clair M. H., Schaffer P. A. Acyclovir-resistant mutants of herpes simplex virus type 1 express altered DNA polymerase or reduced acyclovir phosphorylating activities. J Virol. 1981 Dec;40(3):936–941. doi: 10.1128/jvi.40.3.936-941.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin A. L., Sandri-Goldin R. M., Levine M., Glorioso J. C. Cloning of herpes simplex virus type 1 sequences representing the whole genome. J Virol. 1981 Apr;38(1):50–58. doi: 10.1128/jvi.38.1.50-58.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay J., Moss H., Jamieson A. T., Timbury M. C. Herpesvirus proteins: DNA polymerase and pyrimidine deoxynucleoside kinase activities in temperature-sensitive mutants of herpes simplex virus type 2. J Gen Virol. 1976 Apr;31(1):65–73. doi: 10.1099/0022-1317-31-1-65. [DOI] [PubMed] [Google Scholar]
- Hay J., Subak-Sharpe J. H. Mutants of herpes simplex virus types 1 and 2 that are resistant to phosphonoacetic acid induce altered DNA polymerase activities in infected cells. J Gen Virol. 1976 Apr;31(1):145–148. doi: 10.1099/0022-1317-31-1-145. [DOI] [PubMed] [Google Scholar]
- Holmes A. M. Studies on the inhibition of highly purified calf thymus 8S and 7.3S DNA polymerase alpha by aphidicolin. Nucleic Acids Res. 1981 Jan 10;9(1):161–168. doi: 10.1093/nar/9.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Herpes simplex virus resistance and sensitivity to phosphonoacetic acid. J Virol. 1977 Feb;21(2):584–600. doi: 10.1128/jvi.21.2.584-600.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A. New views of the biochemistry of eucaryotic DNA replication revealed by aphidicolin, an unusual inhibitor of DNA polymerase alpha. Cell. 1981 Mar;23(3):647–648. doi: 10.1016/0092-8674(81)90426-8. [DOI] [PubMed] [Google Scholar]
- Ikegami S., Taguchi T., Ohashi M., Oguro M., Nagano H., Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978 Oct 5;275(5679):458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- Jofre J. T., Schaffer P. A., Parris D. S. Genetics of resistance to phosphonoacetic acid in strain KOS of herpes simplex virus type 1. J Virol. 1977 Sep;23(3):833–836. doi: 10.1128/jvi.23.3.833-836.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf K. W., Kaufman E. R., Crumpacker C. Physical mapping of drug resistance mutations defines an active center of the herpes simplex virus DNA polymerase enzyme. J Virol. 1981 Sep;39(3):746–757. doi: 10.1128/jvi.39.3.746-757.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krokan H., Schaffer P., DePamphilis M. L. Involvement of eucaryotic deoxyribonucleic acid polymerases alpha and gamma in the replication of cellular and viral deoxyribonucleic acid. Biochemistry. 1979 Oct 2;18(20):4431–4443. doi: 10.1021/bi00587a025. [DOI] [PubMed] [Google Scholar]
- Krokan H., Wist E., Krokan R. H. Aphidicolin inhibits DNA synthesis by DNA polymerase alpha and isolated nuclei by a similar mechanism. Nucleic Acids Res. 1981 Sep 25;9(18):4709–4719. doi: 10.1093/nar/9.18.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinbach S. S., Reno J. M., Lee L. F., Isbell A. F., Boezi J. A. Mechanism of phosphonoacetate inhibition of herpesvirus-induced DNA polymerase. Biochemistry. 1976 Jan 27;15(2):426–430. doi: 10.1021/bi00647a029. [DOI] [PubMed] [Google Scholar]
- Liu P. K., Chang C. C., Trosko J. E., Dube D. K., Martin G. M., Loeb L. A. Mammalian mutator mutant with an aphidicolin-resistant DNA polymerase alpha. Proc Natl Acad Sci U S A. 1983 Feb;80(3):797–801. doi: 10.1073/pnas.80.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longiaru M., Ikeda J. E., Jarkovsky Z., Horwitz S. B., Horwitz M. S. The effect of aphidicolin on adenovirus DNA synthesis. Nucleic Acids Res. 1979 Jul 25;6(10):3369–3386. doi: 10.1093/nar/6.10.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguro M., Suzuki-Hori C., Nagano H., Mano Y., Ikegami S. The mode of inhibitory action by aphidicolin on eukaryotic DNA polymerase alpha. Eur J Biochem. 1979 Jul;97(2):603–607. doi: 10.1111/j.1432-1033.1979.tb13149.x. [DOI] [PubMed] [Google Scholar]
- Ohashi M., Taguchi T., Ikegami S. Aphidicolin: a specific inhibitor of DNA polymerases in the cytosol of rat liver. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1084–1090. doi: 10.1016/0006-291x(78)90298-x. [DOI] [PubMed] [Google Scholar]
- Pedrali-Noy G., Spadari S. Mechanism of inhibition of herpes simplex virus and vaccinia virus DNA polymerases by aphidicolin, a highly specific inhibitor of DNA replication in eucaryotes. J Virol. 1980 Nov;36(2):457–464. doi: 10.1128/jvi.36.2.457-464.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purifoy D. J., Lewis R. B., Powell K. L. Identification of the herpes simplex virus DNA polymerase gene. Nature. 1977 Oct 13;269(5629):621–623. doi: 10.1038/269621a0. [DOI] [PubMed] [Google Scholar]
- Purifoy D. J., Powell K. L. Temperature-sensitive mutants in two distinct complementation groups of herpes simplex virus type 1 specify thermolabile DNA polymerase. J Gen Virol. 1981 May;54(Pt 1):219–222. doi: 10.1099/0022-1317-54-1-219. [DOI] [PubMed] [Google Scholar]
- Sala F., Parisi B., Burroni D., Amileni A. R., Pedrali-Noy G., Spadari S. Specific and reversible inhibition by aphidicolin in the alpha-like DNA polymerase of plant cells. FEBS Lett. 1980 Aug 11;117(1):93–98. doi: 10.1016/0014-5793(80)80920-3. [DOI] [PubMed] [Google Scholar]
- Schaffer P. A., Aron G. M., Biswal N., Benyesh-Melnick M. Temperature-sensitive mutants of herpes simplex virus type 1: isolation, complementation and partial characterization. Virology. 1973 Mar;52(1):57–71. doi: 10.1016/0042-6822(73)90398-x. [DOI] [PubMed] [Google Scholar]
- Sugino A., Nakayama K. DNA polymerase alpha mutants from a Drosophila melanogaster cell line. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7049–7053. doi: 10.1073/pnas.77.12.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]



