Abstract
Stimulation by the ephrin-A1 ligand of the EphA2 receptor increases endothelial permeability. Lung injury increases the expression of EphA2, but the role of EphA2 in such injury is not well understood. To determine whether EphA2 contributes to changes in permeability and inflammation in the injured lung, we studied wild-type (WT) and EphA2 knockout (KO) mice, using isolated, perfused lung (IPL) preparations and a model of bleomycin-induced lung injury. We also studied the response of endothelial cells to ephrin-A1. In the IPL preparations, ephrin-A1 increased the filtration coefficient in WT mice, but not in EphA2 KO mice, demonstrating that EphA2 regulates vascular permeability. In early bleomycin injury in WT mice, the expression of both EphA2 and ephrin-A1 increased. EphA2 KO animals were protected from lung injury, showing less water and alveolar protein in the lungs than WT mice, consistent with reduced permeability. Bleomycin caused less accumulation of lung leukocytes in EphA2 KO animals than in WT animals, suggesting that EphA2 regulates inflammation. To determine whether EphA2 deficiency alters the production of chemokines, CXCL1 and CCL2 in the lungs were measured. After bleomycin injury, EphA2 KO animals produced less CXCL1 and CCL2 than WT animals. Because NF-κβ mediates the production of chemokines, the effect of the ephrin-A1 ligand on the activation of NF-κβ and the expression of chemokines was measured in endothelial cells. Ephrin-a1 significantly increased NF-κβ nuclear translocation and the expression of chemokine mRNA. This study demonstrates that the expression of EphA2 increases in the injured lung, and not only contributes to changes in permeability, but also plays a previously unrecognized role in promoting inflammatory responses.
Keywords: chemokine, ephrin-A1, inflammation, vascular leak
The ephrins are a large family of receptor tyrosine kinases and ligands with demonstrated importance in both neural and vascular development. Ephrin ligands and Eph receptors are both cell-surface molecules, and as such, are primarily described to mediate cell–cell interactions, leading to either repulsive or attractive cell contacts and the regulation of behaviors such as axonal pathfinding in the central nervous system. Although less is known about the role of ephrins in the vasculature, some members of the family have been implicated in postnatal angiogenesis. In particular, the EphA2 receptor and its cognate ligand ephrin-A1 were shown to contribute to migration and tube formation in lung endothelial cells (1–3).
Responses that promote cell migration and repulsive cell contacts are generally associated with a loss of cell–cell junctions, and might be expected to increase vascular permeability. We previously demonstrated that stimulation by the ephrin-A1 ligand leads both in vitro and in vivo to increases in lung endothelial permeability (4). These effects are associated with evidence of a breakdown of both adherens and tight junctions in endothelial cells. In addition, we recently presented data demonstrating that the expression of EphA2 and ephrin-A1 is increased in the setting of lung injury caused by viral infection combined with hypoxia, and that the antagonism of EphA2 signaling ameliorates the vascular leak seen in that model (5).
Acute lung injury is a clinical syndrome characterized not only by increased vascular and epithelial permeability, but also by a marked pulmonary inflammatory response. Data regarding the role of ephrins in inflammatory responses is limited and conflicting, although our previous findings led us to question whether EphA2 and ephrin-A1 also contribute to this aspect of lung injury. To answer these questions and better define the role of EphA2 in lung injury, we used a murine model of lung injury caused by the intratracheal instillation of bleomycin. This model of injury has similarities to human acute lung injury, in that it involves an early stage characterized by increased permeability and the formation of edema, with a later transition into a fibroproliferative condition (6, 7). Focusing on early-stage bleomycin injury, we found that the expression of EphA2 and its ligand ephrin-A1 are increased in the injured lung, and that the loss of EphA2 confers protection from both the permeability and the inflammatory changes associated with bleomycin injury.
Materials and Methods
Experimental Animals
Wild-type and EphA2-knockout mice were purchased from Jackson Laboratories (Bar Harbor, ME), and were bred in the vivarium of the University of Colorado at Denver. Bleomycin (4 U/kg) or sterile saline alone was administered into the lungs via the vocal cords, under direct vision. Tissue was collected 4 days after instillation. For bronchoalveolar lavage fluid (BALF) studies, lungs were lavaged via the trachea with iced saline in three 1-ml aliquots. The BALF was briefly centrifuged, and the cell pellet was used for cytospins and cell counts.
All animals were allowed free access to food and water, and were subjected to a similar day and night light cycle. The Institutional Animal Care and Use Committee of the University of Colorado at Denver approved all procedures and animal use.
Isolated, Perfused Murine Lung Model
The ex vivo, in situ isolation of murine lungs was performed as previously described (8). Immediately after lung isolation and perfusion, baseline pulmonary vascular resistance (PVR) was calculated. In the appropriate groups, ephrin-A1 was then added to the perfusate at a final concentration of 2 μg/ml. Measurements of PVR were repeated at 30 minutes and 60 minutes of perfusion and ventilation. At 60 minutes, experimental perfusion and ventilation ended, and the filtration coefficient (Kf) was calculated as previously described (8). Kf values are reported as ml/minute/mm Hg/100 g uninjured lung weight.
Western Blotting, Immunohistochemistry, and ELISAs
The antibodies used here included rabbit EphA2 and rabbit ephrin-A1 (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit ephrin-A1 (Zymed, Camarillo, CA), murine α-tubulin (Labvision), murine β-actin (Sigma Chemical Co., St. Louis, MO), and rabbit p65 NF-κβ (Labvision, Fremont, CA). Lung homogenates were studied by standard Western blotting techniques. For the Western blotting of BALF, 300 μl of fluid were concentrated in a spin column with a 10-kilodalton (kD) molecular weight cutoff membrane (Centricon 10; Millipore, Billerica, MA), and the entire concentrated sample was loaded onto a gel.
For histologic studies, lungs were inflation-fixed with 4% paraformaldehyde for 24 hours before paraffin-embedding and sectioning. For ELISA-based array measurements of chemokines (ELISArray; SABiosciences, Frederick, MD), aliquots of individual lung homogenates (n = 4 per group) were pooled to make the sample for each group. Measurements of keratinocyte chemoattractant (KC)/CXCL1 and monocyte chemoattractant protein 1 (MCP1)/CCL2 were performed on individual lung homogenates, using single-analyte ELISA kits (SABiosciences), according to the manufacturer's directions.
Cell Culture
Human umbilical-vein endothelial cells were purchased from Lonza (Walkersville, MD), and propagated according to the supplier's instructions. For immunofluorescence studies of p65, cells were fixed with 2% paraformaldehyde and permeabilized with 0.1% Triton-X before antibody incubation.
Real-Time PCR
For mRNA studies, the expression of mRNA was analyzed by real-time RT-PCR. Preliminary gradient optimization experiments (data not shown) for each primer set determined the ideal annealing temperature. Results were normalized to the expression of β-actin mRNA for each sample, and the relative expression was calculated as ddCt.
Statistical Analysis
Statistical analysis was performed using Prism 4.0 (GraphPad Software, Durham, NC). Multiple-group comparisons were performed using one-way ANOVA with Tukey post hoc testing. Two-group comparisons were performed using an unpaired Student t test. Differences were considered significant at P < 0.05. Results are expressed as means ± SEM, unless otherwise noted.
Results
Ligand Stimulation of EphA2 Increases Murine Lung Vascular Permeability
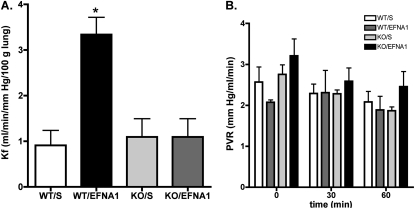
We previously reported that the injection of ephrin-A1 ligand increases albumin leak into the lungs in intact rats, and that EphA2 antagonism ameliorates vascular leak in hypoxic, virus-infected rats (4, 5). To verify that ephrin-A1 and EphA2 also regulate vascular permeability in the murine lung vasculature, we used an isolated, perfused murine lung preparation to measure the Kf. As shown in Figure 1A, in wild-type animals, perfusion of the lungs with perfusate containing 2 μg/ml ephrin-A1 led to a dramatic increase in Kf, compared with lungs perfused with buffer alone (P < 0.01), demonstrating that ephrin-A1 increases vascular permeability in the murine lung. To determine whether EphA2 was the receptor responsible for the changes in permeability induced by ephrin-A1 in the murine lung, we repeated these experiments in EphA2 knockout mice. As shown in Figure 1A, the Kf did not differ between buffer-perfused wild-type and EphA2-knockout mice, suggesting that baseline vascular permeability is not altered in EphA2-deficient animals. In contrast to the wild-type mice, the addition of ephrin-A1 to the perfusate exerted no effect on Kf in EphA2-knockout animals, demonstrating that EphA2 is responsible for the effects of ephrin-A1 on vascular permeability in the murine lung. Baseline PVR was slightly higher in the EphA2-knockout animals than in the wild-type mice, but ephrin-A1 exerted no significant effect on PVR in either genotype (Figure 1B). These results demonstrate that in the murine lung vasculature, ephrin-A1 increases endothelial permeability without changing the hemodynamic forces favoring the formation of edema.
Figure 1.
(A) Ephrin-A1 increases lung vascular permeability in wild-type but not in EphA2-deficient animals, as measured by the filtration coefficient (Kf) in isolated, perfused murine lungs. *P < 0.01 versus all other groups; n = 4–5 animals per group. (B) Pulmonary vascular resistance (PVR) does not significantly change with ephrin-A1 perfusion in isolated, perfused murine lungs. wt/s, wild-type, buffer-perfused; wt/EFNA1, wild-type, ephrin-A1–perfused; KO/s, EphA2-knockout, buffer-perfused; KO/EFNA1, EphA2-knockout, ephrin-A1–perfused.
Bleomycin Lung Injury Increases the Expression of Lung EphA2 and Ephrin-A1
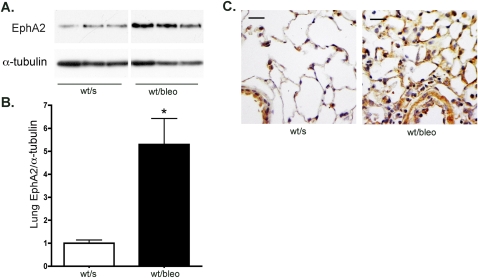
The early stages of lung injury caused by an intratracheal instillation of bleomycin are characterized by increased permeability and lung edema, as well as inflammation (6, 7). To determine whether the expression of EphA2 in the lung increases during early-stage, bleomycin-induced lung injury, wild-type mice underwent an intratracheal instillation of bleomycin or saline control. Lung tissue and lung lavage fluid were collected 4 days later. As measured by Western blotting, the expression of EphA2 protein in lung tissue was markedly increased in bleomycin-injured animals (Figure 2). Immunostaining paraformaldehyde-fixed lung tissue from saline-instilled animals demonstrated moderate EphA2 staining in the airway epithelium and alveolar macrophages, as well as weak staining in some alveolar septal corners. In contrast, bleomycin-injured mice demonstrated marked increases in EphA2 staining, most notably in edematous and inflamed alveolar septae, as well as in alveolar macrophages.
Figure 2.
The expression of EphA2 in the lung increases after bleomycin injury, as shown by representative Western blots (A) and densitometry (B). *P < 0.01; n = 4 per group. (C) Immunohistochemistry shows increased EphA2 protein (brown) in bleomycin-injured lung tissue. wt/s, wild-type, saline-instilled; wt/bleo, wild-type, bleomycin-instilled. Scale bar = 50 μm.
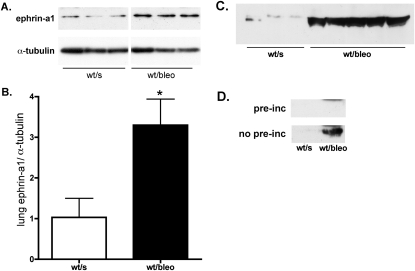
The principal ligand for EphA2 in the lung is ephrin-A1 (4). We measured the expression of ephrin-A1 in lung homogenates from control and bleomycin-injured animals, and as shown in Figure 3, concentrations of ephrin-A1 protein in lung tissue increased significantly with bleomycin injury. Ephrin-A1 has been described in both membrane-bound and soluble forms. To determine whether ephrin-A1 is released into the alveolar space during lung injury, we looked for ephrin-A1 protein in BALF from control and bleomycin-injured animals. A predominant 50-kD band was identified in the lavage fluid, using two different ephrin-A1–reactive antibodies (Figure 3C). Although this ephrin-A1 band was barely detectable in lavage fluid from control animals, markedly increased amounts of ephrin-A1 were evident in the lavage fluid of bleomycin animals. Because the molecular weight of ephrin-A1 is expected to be 25 kD, an additional experiment was performed to verify the identity of this 50-kD band. Preincubation of the detection antibody with ephrin-A1/Fc protein resulted in the loss of the 50-kD band (Figure 3D), suggesting that this band represents a higher molecular weight form of ephrin-A1, perhaps a dimer. These results suggest that in the bleomycin-injured lung, concentrations of ephrin-A1 protein are increased not only in the tissue, but also in the alveolar space.
Figure 3.
The expression of ephrin-A1 in the lung increases after bleomycin injury, as shown by representative Western blots of lung homogenates (A) and densitometry (B). *P < 0.01; n = 4 per group. (C) Ephrin-A1 immunoreactivity also increases in bronchoalveolar lavage fluid (BALF) from bleomycin-injured animals. This 50-kilodalton band disappears when the primary antibody is preincubated with ephrin-A1 (D), suggesting that it represents a soluble form of ephrin-A1 in BALF. pre-inc, preincubated; wt/s, wild-type, saline-instilled; wt/bleo, wild-type, bleomycin-instilled.
EphA2 Deficiency Reduces Bleomycin Lung Injury
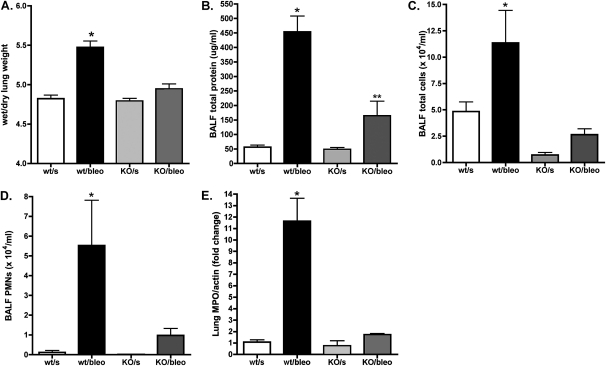
After finding that bleomycin injury is associated with the increased expression of EphA2 in the lung, we sought to determine whether EphA2 contributes to the edema and inflammation in those animals by comparing the responses to bleomycin in wild-type and EphA2-deficient mice. EphA2-deficient mice displayed no overt differences from wild-type animals in lung architecture, and in saline-instilled animals, wild-type mice and EphA2-deficient mice were similar in lung wet-to-dry weight ratio and concentrations of lung lavage fluid total protein (Figure 4).
Figure 4.
Bleomycin lung injury is reduced in EphA2-deficient animals, as demonstrated by (A) decreased lung wet-to-dry weight ratio (*P < 0.01, versus all other groups, n = 6 per group); (B) decreased BALF protein content (*P < 0.001, versus all other groups, **P < 0.05, versus wt/s and KO/s groups, n = 6 per group); (C) decreased BALF total cell count (*P < 0.01, versus all other groups, n = 6 per group); (D) decreased BALF neutrophil counts (*P < 0.05, versus all other groups, n = 6 per group); and (E) decreased lung myeloperoxidase (MPO) content (*P < 0.001, versus all other groups, n = 4 per group). wt/s, wild-type, saline-instilled; wt/bleo, wild-type, bleomycin-instilled; KO/s, EphA2-knockout, saline-instilled; KO/bleo, EphA2-knockout, bleomycin-instilled.
As shown in Figure 4, bleomycin injury in wild-type animals caused lung injury and the formation of edema, as demonstrated by a significant increase in lung water and concentrations of total protein in lung lavage fluid. As expected, bleomycin lung injury in wild-type animals was also characterized by a pronounced neutrophil influx into the lung, demonstrated both by lavage fluid cell counts and by lung myeloperoxidase content. In contrast, EphA2-deficient mice were largely protected from bleomycin lung injury. The lung wet-to-dry weight ratio did not increase in EphA2–deficient, bleomycin-injured animals, and those animals demonstrated a significantly smaller increase in concentrations of lung lavage fluid protein than did wild-type animals. The bleomycin-induced neutrophil influx was also markedly reduced in the EphA2-deficient animals, as evidenced by a lack of significant increase in lung myeloperoxidase content, in lung lavage fluid total cell count, and in lung lavage fluid neutrophil count. These findings suggest not only that EphA2 is involved in permeability responses to injury in the lung, but also that EphA2 modulates the recruitment of inflammatory cell to the injured lung.
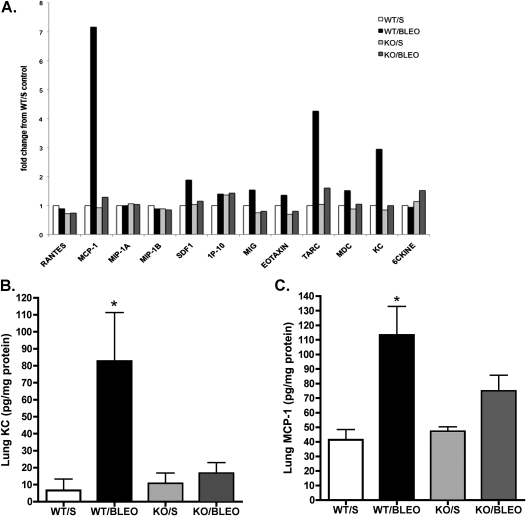
Alterations in the recruitment of inflammatory cells to the injured lung may be attributable to the altered production of chemokines. To investigate this possibility, we first used an ELISA-based array to screen for qualitative changes in the expression of lung chemokines. As shown in Figure 5, these results suggested that in wild-type mice but not in EphA2-deficient mice, bleomycin injury was associated with substantially increased concentrations of MCP-1/CCL2, KC/CXCL1, and thymus and activation-regulated chemokine (TARC)/CCL17 in the lungs. Smaller changes in a similar pattern were evident in concentrations of stromal cell–derived factor-1, MIG, eotaxin, and macrophage-derived chemokine (MDC)/CCL22, whereas concentrations of RANTES, MIP1a, MIP1b, IP10, and 6C-kine did not appear to change. To determine in a quantitative fashion whether the loss of EphA2 prevents the bleomycin-induced expression of chemokines, we used specific single-analyte ELISAs to measure lung concentrations of KC/CXCL1, a key neutrophil chemoattractant, and of MCP1/CCL2, a key monocyte chemoattractant. As shown in Figure 5, concentrations of KC/CXCL1 in the lungs increased substantially in bleomycin-injured, wild-type animals, but showed little change in EphA2-knockout animals. Similarly, concentrations of MCP1/CCL2 in lung tissue increased significantly with bleomycin injury in wild-type animals, but not in EphA2-knockout animals. These results suggest that EphA2 contributes to the inflammatory response in acute lung injury, at least in part, by modulating the production of important chemokines.
Figure 5.
The expression of chemokines during lung injury is reduced in EphA2-deficient animals. (A) ELISA-based array shows qualitative differences in the expression of chemokines in lung tissue. These results were validated with specific ELISA measurements (B, KC/CXCL1; C, MCP1/CCL2), again showing reduced of chemokines in response to bleomycin injury in EphA2-deficient animals compared with wild-type animals. *P < 0.05, versus all other groups; n = 6 per group. wt/s, wild-type, saline-instilled; wt/bleo, wild-type, bleomycin-instilled; KO/s, EphA2-knockout, saline-instilled; KO/bleo, EphA2-knockout, bleomycin-instilled.
Ephrin-A1 Increases Concentrations of Endothelial Nuclear NF-κβ
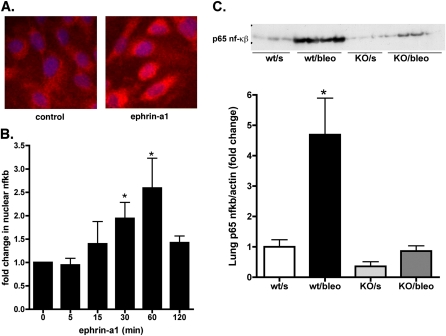
Endothelial cells are a potential source of chemokine production in the injured lung, and EphA2 is the principal EphA receptor expressed by endothelial cells. The production of many chemokines, including MCP-1/CCL2 and KC/CXCL1, is regulated by the transcription factor NF-κβ, which is viewed as a master transcriptional regulator of inflammation. To determine whether stimulation by ephrin-A1 directly causes the activation of NF-κβ, we used cultured human endothelial cells and assessed nuclear accumulations of the p65 subunit of NF-κβ by immunofluorescent staining after stimulation with ephrin-A1. As shown in Figure 6A, stimulation with ephrin-A1 caused a visible increase in concentrations of nuclear p65 protein within 30 minutes. We confirmed this finding in separate experiments with Western blots of nuclear extracts isolated from endothelial cells after stimulation with ephrin-A1. As shown in Figure 6B, stimulation with ephrin-A1 led to significant increases in nuclear concentrations of p65 after 30–60 minutes (P < 0.001). These results suggest that the stimulation of endothelial cells by the ephrin-A1 ligand contributes to inflammation, at least in part, by triggering the activation of NF-κβ.
Figure 6.
The stimulation by ephrin-A1 of endothelial cells causes the activation of NF-κβ. (A) Immunofluorescent staining for p65 NF-κβ after stimulation with ephrin-A1 (2 μg/ml) for 30 minutes, compared with unstimulated cells (red, p65 NF-κβ; blue, 4,6-diamidino-2-phenylindole nuclear counterstain). (B) Quantification of p65 NF-κβ protein concentrations in nuclear extracts of ephrin-A1–stimulated endothelial cells. *P < 0.05, versus unstimulated control sample, n = 5. (C) p65 NF-κβ protein in lung tissue increases with bleomycin injury in wild-type mice, but not in EphA2-knockout mice, as shown by Western blot (top) and densitometric analysis (bottom). *P < 0.01, versus all other groups; n = 4 per group. min, minutes; wt/s, wild-type, saline-instilled; wt/bleo, wild-type, bleomycin-instilled; KO/s, EphA2-knockout, saline-instilled; KO/bleo, EphA2-knockout, bleomycin-instilled.
Given these results, we wondered whether the loss of EphA2 altered the response of NF-κβ to injury in the intact animal. To answer this question, the expression of p65 protein in lung tissue was measured in saline and bleomycin-instilled wild-type and EphA2-deficient animals. As shown in Figure 6C, p65 was present in uninjured, EphA2-deficient animals in similar quantities as in uninjured, wild-type lung tissue and bleomycin injury led to a marked increase in lung p65 in wild-type animals, but very little change in EphA2-deficient animals.
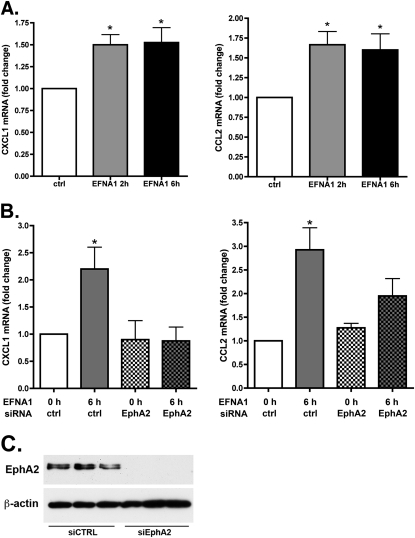
To determine whether stimulation by ephrin-A1 increases the production of chemokines by endothelial cells, we treated cultured human endothelial cells with ephrin-A1 and measured the transcription of mRNAs for the chemokines MCP1/CCL2, CXCL1, and CXCL8, and for the adhesion molecules ICAM1 and VCAM1, using real-time PCR. As shown in Figure 7, stimulation with ephrin-A1 led to modest but statistically significant increases in expression of endothelial-cell MCP1/CCL2 and KC/CXCL1 mRNA. Ephrin-A1 also caused a smaller but statistically significant increase in the expression of ICAM1 mRNA (1.45 ± 0.13–fold, P = 0.04), but did not significantly alter CXCL8 or VCAM1 transcription. To verify that EphA2 is required for the stimulation of chemokine production by ephrin-A1, endothelial cells were treated with noncoding control short interfering RNA (siRNA) or EphA2-specific siRNA, followed 48 hours later by stimulation with ephrin-A1 and measurement via PCR of chemokine mRNA expression. As shown in Figure 7, ephrin-A1 caused significant increases in the expression of CXCL1 and CCL2 mRNA in cells treated with control siRNA, but not in cells treated with EphA2 siRNA.
Figure 7.
Ephrin-A1 acts via EphA2 to increase endothelial cell chemokine expression. (A) Stimulation with ephrin-A1 increases the expression of CXCL1 and CCL2 mRNA. *P < 0.05, versus unstimulated control sample, n = 4. (B) Short interfering RNA-mediated knockdown of EphA2 blocks the effect of ephrin-A1 on the expression of endothelial CXCL1 and CCL2 mRNA. *P < 0.05 versus all other groups, n = 4. (C) Western blot of endothelial cell lystaes demonstrating knowckdown of EphA2 expression by EphA2-specific siRNA. siCTRL, noncoding control siRNA; siEphA2, EphA2-specific siRNA.
Discussion
These studies demonstrate that EphA2 contributes to the pathophysiology of experimental acute lung injury. The expression in lung tissue of both the EphA2 receptor and its principal ligand, ephrin-A1, is increased during the early stages of bleomycin-induced lung injury. More importantly, EphA2-deficient animals are protected from such injury. Mechanistically, acute lung injury is associated with changes in both vascular permeability and the recruitment of inflammatory cells and generation of chemokines. EphA2-deficient animals appear to be protected from both the permeability and the inflammatory changes associated with this experimental injury. These findings extend previous work that described a role for EphA2 in the regulation of pulmonary vascular permeability, and they constitute one of the first demonstrations of a role for EphA2 in the regulation of inflammatory responses to tissue injury.
The role of EphA2 in the postnatal lung remains poorly understood. We previously reported that the stimulation of EphA receptors in the pulmonary vasculature with the exogenous soluble ephrin-A1 ligand leads to increases in albumin extravasation into the lung (4). Although EphA2 is the most highly expressed EphA receptor in lung endothelial cells, the effect of ephrin-A1 on lung vascular permeability had not been shown definitively to require EphA2. Using the isolated, perfused lung approach in the present study, we confirmed that an infusion of ephrin-A1 ligand into the pulmonary vasculature increases vascular permeability, as measured by the filtration coefficient (Kf). In addition, the permeability response to ephrin-A1 was lost in EphA2-knockout mice, demonstrating that EphA2 transduces the permeability-increasing effect of ephrin-A1 in the pulmonary circulation. These results are consistent with our previously published work, and extend those findings to a second species. This effect of the ligand stimulation of EphA2 on vascular permeability suggests the hypothesis that changes in the expression of EphA2 could contribute to pathologic situations associated with increased vascular permeability, such as acute lung injury and sepsis.
Acute lung injury is a common and severe clinical problem in both adults and children. The acute lung injury caused by an instillation of bleomycin into the airways comprises a model that recapitulates several key features of human acute lung injury, most importantly an early edematous phase characterized by increased permeability and neutrophilic inflammatory cell influx (7, 9). As a result of these parallels with human lung injury, we studied the role of EphA2 in the early stages of bleomycin injury. Our results showed a clear and substantial increase in the expression of EphA2 in the distal lung of wild-type mice after bleomycin injury, localized to the alveolar septae and alveolar macrophages. These findings are consistent with previous results showing an increased expression of lung EphA2 after other insults, such as injections of LPS or exposure to hypoxia after a viral respiratory infection (5, 10). Of even greater interest, the response of EphA2-deficient animals to bleomycin injury suggests that EphA2 is not only increased in the injured lung, but that it also plays an important role in the development of such injury. Compared with wild-type mice, EphA2 -deficient mice accumulated less lung water and displayed less protein leak into the airspaces after bleomycin injury. These findings provide additional evidence that EphA2 contributes to the disruption of the alveolar–capillary barrier seen in acute lung injury, and are consistent with both our isolated, perfused lung experiments and our previous work describing the pro-permeability effects of the ligand stimulation of EphA2 in the lung endothelium. Although these data, as well as those in previous studies, clearly show that EphA2 is expressed in lung endothelial cells, it is noteworthy that EphA2 is also expressed in numerous other cell types that contribute to acute lung injury, including inflammatory cells and alveolar epithelial cells (11). Whether EphA2 also modulates lung epithelial permeability is not known, but such an effect is plausible, given that the activation of EphA2 was shown to disrupt adherens junctions in other epithelia (12–14).
The influx of inflammatory cells to the lung is a key element of acute lung injury, both in human patients and in our bleomycin model. We were intrigued to find that EphA2-deficient animals accumulated far fewer inflammatory cells and, in particular, neutrophils in their lungs in response to injury than did wild-type animals, suggesting that EphA2 contributes not just to changes in permeability but also to inflammatory responses. Chemokines are known as key mediators of leukocyte recruitment to the injured lung, and the altered elaboration of chemokines in EphA2-deficient animals provided a possible explanation for our findings. Indeed, concentrations in lung tissue of important neutrophil (KC/CXCL1) and monocyte (MCP1/CCL2) chemoattractants were markedly lower in EphA2-deficient mice after injury than in control animals. These results suggest a previously unrecognized contribution of ephrin signaling to the generation of chemokines and recruitment of leukocytes in the lung, in addition to effects on endothelial permeability. Whether EphA2 also contributes to the transmigration and retention of recruited leukocytes is not certain, although reduced permeability in EphA2-deficient vessels might also be expected to impede the transmigration of leukocytes. Mechanistically, the production of chemokines is generally regulated by the activation of proinflammatory transcription factors. The finding that the ligand stimulation by ephrin-A1 of endothelial cells triggers the activation of the prototypical proinflammatory transcription factor NF-κβ as well as the mRNA expression of CXCL1, CCL2, and ICAM1 is consistent with our observations on the expression of chemokines in intact animals. These results suggest that ligand stimulation of EphA2 has the capacity to regulate inflammatory responses transcriptionally. Whether other cell types respond similarly, and whether other transcription factors in addition to NF-κβ are activated by ephrin-A1, remain to be determined. Of further interest was a trend (that did not reach statistical significance) toward reduced macrophage numbers in the lavage fluid from uninjured EphA2-knockout animals compared with uninjured control animals. This observation, if borne out in future studies, could suggest that EphA2 also regulates leukocyte trafficking in the normal lung as well as in the setting of lung injury.
The mechanism by which EphA2 is activated in the injured lung remains uncertain. As already discussed, one likely possibility involves ligand stimulation. Ephrin-A1 is the principal ligand for EphA2, and it is known to be expressed by endothelial cells, epithelial cells, and some leukocytes (15–17). The expression of ephrin-A1 is also known to be induced by some cytokines, consistent with a possible role in inflammation (2, 15). In support of this idea, we found increased concentrations of ephrin-A1 ligand protein in the injured lungs. Whereas ephrin-A1 principally exists as a membrane-anchored protein, soluble forms (both monomeric and multimeric) have also been described (18–20). Our results suggest that such forms of ephrin-A1 are released into the alveolar space in the setting of lung injury. These results are most consistent with the idea that the increased expression of both ephrin-A1 ligand and the EphA2 receptor in the injured lung leads to the increased ligand-mediated activation of EphA2 in that setting. Alternatively, both the ligand-independent effects of EphA2 overexpression and the transactivation of EphA2 by non–ephrin ligands have also been described (including thrombin, another mediator known to be released in large amounts in the injured lung) (21, 22). Whether EphA2 acts via these additional mechanisms in the setting of lung injury will require further study. Given the demonstrated role of ephrins in the nervous system as key regulators of cell–cell contacts and cell–cell signaling, ephrin ligands and receptors seem likely to play a similar role in the lung, and to regulate responses to tissue injury via their role in cell-to-cell communication.
In conclusion, we demonstrate that EphA2 regulates permeability and inflammation in the injured lung, and that EphA2 thus contributes to the pathophysiology of acute lung injury. The mechanisms by which EphA2 regulates lung inflammation and leak and the possible role of EphA2 as a therapeutic target in the injured lung deserve further investigation.
Footnotes
This work was supported by National Institutes of Health/National Heart, Lung, and Blood Institute grants R01 HL077743 (T.C.C.), PPG HL014985-37 (K.R.S.), and P30 HL101295 (E.P.S.).
Originally Published in Press as DOI: 10.1165/rcmb.2011-0044OC on July 28, 2011
Author Disclosure: E.P.S. has received payment for service as a speaker on clinical topics relevant to critical care medicine for the Rocky Mountain Hospital Medicine Symposium. K.R.S. has received payment as a scientific consultant for Pfizer. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Brantley-Sieders DM, Caughron J, Hicks D, Pozzi A, Ruiz JC, Chen J. EphA2 receptor tyrosine kinase regulates endothelial cell migration and vascular assembly through phosphoinositide 3-kinase–mediated Rac1 GTPase activation. J Cell Sci 2004;117:2037–2049 [DOI] [PubMed] [Google Scholar]
- 2.Cheng N, Brantley DM, Liu H, Lin Q, Enriquez M, Gale N, Yancopoulos G, Cerretti DP, Daniel TO, Chen J. Blockade of EphA receptor tyrosine kinase activation inhibits vascular endothelial cell growth factor–induced angiogenesis. Mol Cancer Res 2002;1:2–11 [PubMed] [Google Scholar]
- 3.Hunter SG, Zhuang G, Brantley-Sieders D, Swat W, Cowan CW, Chen J. Essential role of Vav family guanine nucleotide exchange factors in EphA receptor–mediated angiogenesis. Mol Cell Biol 2006;26:4830–4842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larson J, Schomberg S, Schroeder W, Carpenter TC. Endothelial EphA receptor stimulation increases lung vascular permeability. Am J Physiol Lung Cell Mol Physiol 2008;295:L431–L439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cercone MA, Schroeder W, Schomberg S, Carpenter TC. EphA2 receptor mediates increased vascular permeability in lung injury due to viral infection and hypoxia. Am J Physiol Lung Cell Mol Physiol 2009;297:L856–L863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orr FW, Adamson IY, Young L. Promotion of pulmonary metastasis in mice by bleomycin-induced endothelial injury. Cancer Res 1986;46:891–897 [PubMed] [Google Scholar]
- 7.Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, et al. TGF-beta is a critical mediator of acute lung injury. J Clin Invest 2001;107:1537–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt EP, Damarla M, Rentsendorj O, Servinsky LE, Zhu B, Moldobaeva A, Gonzalez A, Hassoun PM, Pearse DB. Soluble guanylyl cyclase contributes to ventilator-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol 2008;295:L1056–L1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mutsaers SE, Foster ML, Chambers RC, Laurent GJ, McAnulty RJ. Increased endothelin-1 and its localization during the development of bleomycin-induced pulmonary fibrosis in rats. Am J Respir Cell Mol Biol 1998;18:611–619 [DOI] [PubMed] [Google Scholar]
- 10.Ivanov AI, Steiner AA, Scheck AC, Romanovsky AA. Expression of Eph receptors and their ligands, ephrins, during lipopolysaccharide fever in rats. Physiol Genomics 2005;21:152–160 [DOI] [PubMed] [Google Scholar]
- 11.Reddy NM, Kleeberger SR, Yamamoto M, Kensler TW, Scollick C, Biswal S, Reddy SP. Genetic dissection of the NRF2-dependent redox signaling–regulated transcriptional programs of cell proliferation and cytoprotection. Physiol Genomics 2007;32:74–81 [DOI] [PubMed] [Google Scholar]
- 12.Fang WB, Ireton RC, Zhuang G, Takahashi T, Reynolds A, Chen J. Overexpression of EPHA2 receptor destabilizes adherens junctions via a RhoA-dependent mechanism. J Cell Sci 2008;121:358–368 [DOI] [PubMed] [Google Scholar]
- 13.Tanaka M, Kamata R, Sakai R. EphA2 phosphorylates the cytoplasmic tail of claudin-4 and mediates paracellular permeability. J Biol Chem 2005;280:42375–42382 [DOI] [PubMed] [Google Scholar]
- 14.Miura K, Nam JM, Kojima C, Mochizuki N, Sabe H. EphA2 engages GIT1 to suppress ARF6 activity modulating epithelial cell–cell contacts. Mol Biol Cell 2009;20:1949–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandey A, Shao H, Marks RM, Polverini PJ, Dixit VM. Role of B61, the ligand for the ECK receptor tyrosine kinase, in TNF-alpha–induced angiogenesis. Science 1995;268:567–569 [DOI] [PubMed] [Google Scholar]
- 16.dos Santos CC, Han B, Andrade CF, Bai X, Uhlig S, Hubmayr R, Tsang M, Lodyga M, Keshavjee S, Slutsky AS, et al. DNA microarray analysis of gene expression in alveolar epithelial cells in response to TNF-alpha, LPS, and cyclic stretch. Physiol Genomics 2004;19:331–342 [DOI] [PubMed] [Google Scholar]
- 17.Sharfe N, Nikolic M, Cimpeon L, Van De Kratts A, Freywald A, Roifman CM. EphA and ephrin-A proteins regulate integrin-mediated T lymphocyte interactions. Mol Immunol 2008;45:1208–1220 [DOI] [PubMed] [Google Scholar]
- 18.Alford SC, Bazowski J, Lorimer H, Elowe S, Howard PL. Tissue transglutaminase clusters soluble A-type ephrins into functionally active high molecular weight oligomers. Exp Cell Res 2007;313:4170–4179 [DOI] [PubMed] [Google Scholar]
- 19.Alford S, Watson-Hurthig A, Scott N, Carette A, Lorimer H, Bazowski J, Howard PL. Soluble ephrin A1 is necessary for the growth of HeLa and SK-BR3 cells. Cancer Cell Int 2010;10:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wykosky J, Palma E, Gibo DM, Ringler S, Turner CP, Debinski W. Soluble monomeric ephrin-A1 is released from tumor cells and is a functional ligand for the EphA2 receptor. Oncogene 2008;27:7260–7273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan B, Sukhatme VP. Receptor tyrosine kinase EphA2 mediates thrombin-induced upregulation of ICAM-1 in endothelial cells in vitro. Thromb Res 2009;123:745–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao H, Li DQ, Mukherjee A, Guo H, Petty A, Cutter J, Basilion JP, Sedor J, Wu J, Danielpour D, et al. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell 2009;16:9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]