Abstract
S-Nitrosoglutathione (GSNO) reductase regulates cell signaling pathways relevant to asthma and protects cells from nitrosative stress. Recent evidence suggests that this enzyme may prevent human hepatocellular carcinoma arising in the setting of chronic hepatitis. We hypothesized that GSNO reductase may also protect the lung against potentially carcinogenic reactions associated with nitrosative stress. We report that wild-type Ras is S-nitrosylated and activated by nitrosative stress and that it is denitrosylated by GSNO reductase. In human lung cancer, the activity and expression of GSNO reductase are decreased. Further, the distribution of the enzyme (including its colocalization with wild-type Ras) is abnormal. We conclude that decreased activity of GSNO reductase could leave the human lung vulnerable to the oncogenic effects of nitrosative stress, as is the case in the liver. This potential should be considered when developing therapies that inhibit pulmonary GSNO reductase to treat asthma and other conditions.
Keywords: lung cancer, S-nitrosoglutathione reductase, Ras
Protein S-nitrosylation, the post-translational modification of a cysteine by the attachment of an NO group, is a regulated pathway that is responsible for a variety of signaling effects (1, 2). S-nitrosylation, caused both by exposure to exogenous nitrogen oxides and by the activity of nitric oxide synthase (NOS), is involved in the regulation of gene expression, cell division, and a spectrum of other processes in cell biology. For example, the S-nitrosylation of wild-type Ras by endothelial NOS (eNOS) is required for cell proliferation and tumor growth in a common model of tumorigenesis (3). Specifically, oncogenic K-Ras–GTP activates proteins to initiate human tumor growth. Of these proteins, only the phosphatidylinositol 3 kinase (PI3 kinase)/Akt pathway is indispensable for tumor maintenance (3). The essential Akt substrate for this process is eNOS. The activation of NOS, in turn, S-nitrosylates and activates wild-type (wt) H-Ras and N-Ras proteins at cysteine 118. Either the knockdown of eNOS or the mutation of wt Ras cysteine 118 (the site of S-nitrosylation) prevents the activation of Ras and the formation of tumors (3).
Although the activation of NOS leads to wt Ras S-nitrosylation, the mechanism by which Ras can be denitrosylated is not known. As with phosphorylation/dephosphorylation coupling, the addition and removal of NO from cysteines are normally regulated in cell biology, and several enzymes serve as denitrosylases (1, 2, 4, 5). In the human airway, S-nitrosoglutathione (GSNO) reductase is an important enzyme responsible for denitrosylation (1, 4, 6, 7). This highly conserved enzyme is traditionally regarded as an aldehyde dehydrogenase, although it is somewhat more efficient as GSNO reductase (5, 8, 9). Its relative redox activities depend on substrate concentration and the local nicotinamide adenine dinucleotide/nicotinamide adenine dinucleotide reduced (NAD+/NADH) ratio.
Our evidence suggests that decreased activity of GSNO reductase may be associated with human lung cancer. Recently, mice deficient in GSNO reductase were found to exhibit an increased risk for the development of hepatocellular carcinoma (10). This risk is directly related to endogenous nitrosative stress. If these mice are crossed with inducible NOS−/− mice, they no longer develop the cancer (10). The activity of GSNO reductase is also decreased in human hepatocellular carcinoma, possibly because of chromosome deletions and a loss of heterozygosity (LOH) at or near 4q23, similar to the deletions and LOH in human lung cancers (11–14). Here, we show that human lung cancers also exhibit abnormal expression of GSNO reductase and decreased activity of GSNO reductase. Further, we show that exposure to exogenous nitrosative stress can increase wt Ras S-nitrosylation and activity and that GSNO reductase serves as a Ras denitrosylase. Taken together, these data suggest that (as in the case of hepatocellular carcinoma) the decreased activity of GSNO reductase has the potential to contribute to cancer risk in the human lung.
Materials and Methods
Cells and Tissues
Human lung adenocarcinoma cell lines (Calu-3, H1650, and A549) and squamous-cell carcinoma cell lines (H157, Calu-1, and H226) were acquired from the American Type Culture Collection (Manassas, VA). Human bronchial epithelial cells (CFB41o−) were obtained from Dr. D. Gruenert (15). A human lung cancer tissue array (73 triplicate samples) also contained normal tissue (16). Normal human airway was acquired from the Severe Asthma Research Program (17). Lung cancer tissue blocks and unaffected margins were obtained from operative lobectomies. The Human Investigation Committee of the University of Virginia approved both protocols.
Biochemical Assays
H-Ras was immunoprecipitated from overexpressing cells, treated with S-nitroso-N-acetyl cysteine (24), and washed three times with PBS. It was assayed for denitrosylation in 2 mM glutathione (GSH) and 300 μM NADH (TRIS; pH 7.4), with or without GSNO reductase isolated from CFBE41− cells (4, 5). S-nitrosothiols were assayed as described elsewhere (18). The activity of Ras was assayed by ELISA (catalogue number 17-497; Millipore, Jaffrey, NH) by Raf-1RBD binding, using Fluostar Omega (BMG, Offenburg, Germany) in the presence of GSH (2 mM) and NADH (300 μM), with or without either preincubation with S-nitrosocysteine (CSNO, 10 μM) or the GSNO reductase inhibitor, 5-chloro-3{2-[4-ethoxyphenyl)(ethyl)amino]-2-oxoethyl}-1H-indole-2-carboxylic acid (C2, 100 μM; gift of P. Sanghani [26]).
Real-Time PCR
The IQ SVBR Green Supermix (catalogue number 170–8882; Bio-Rad, Hercules, CA) was used according to the supplier's instructions (15) with the GSNO reductase primers 5′-CGATGCCTATACCCTG- 3′ and 3′-CACTGTGGGTGTAAAGTG-5′.
Mice
GSNO reductase–deficient mice were gifts from Dr. S. Stamler and Dr. L. Liu (4, 5, 10, 21, 24). Lungs were used with or without an intratracheal pre-instillation of the S-nitrosylating agent, ethyl nitrite (EtONO, 1 mM, 5 μl; 5 minutes) (31).
Transfection and Immunoprecipitation
In cells transfected with a hemagglutinin-tagged (HA) wt H-Ras construct (from Dr. C.M. Counter) (3) using the Effectin Transfection reagent (Qiagen, Valencia, CA), HA antibody–immunoprecipitated (1:250; Sigma Chemical Co., St. Louis, MO) fractions (3) underwent immunoblotting with an anti-Ras monoclonal antibody (1:1,000; ABCAM, Cambridge, MA), or were incubated with 100 μM S-nitroso-N-acetylcysteine (24) for 30 minutes, washed three times in PBS, and assayed for the activity of GSNO reductase.
Biotin Substitution of S-Nitrosothiol Bonds
Biotin-substituted samples, prepared as described elsewhere (15), underwent SDS gel electrophoresis and were blotted with anti-biotin antibodies (1:500) and secondary AB-HRP conjugate (1:500), or with anti-Ras (1:250; Santa Cruz Biotechnology, Santa Cruz, CA), anti-GSNO reductase (1:1,000; Protein Tech Group, Chicago, IL), anti-eNOS, anti-nNOS, anti-iNOS, or anti-actin (1:1,000 each; BD Transductional Laboratories, San Jose, CA).
Exposure to Nitric Oxide
Cells were exposed to NO (19) in a gas-tight chamber (37°C) and assayed for [NO] by chemiluminescence (18).
Immunohistochemistry
Deparaffinized tissues were incubated in anti-GSNO reductase rabbit antibody (1:50; catalogue number 11051-1-AP; Protein Tech Group), and then with ABC reagents (Vector Laboratories, Burlingame, CA). They were analyzed for the expression of GSNO reductase by two observers (N.V.M. and B.G.) who were blinded to tissue type and diagnosis.
Immunofluorescence
Cells were fixed in 4% paraformaldehyde for 20 minutes, permeabilized in 0.05% Triton-X for 5 minutes, rinsed, and incubated overnight with primary antibodies to GSNO reductase (1:50; Protein Tech Group) or to Ras (1:100; ABCAM). After rinsing, they were incubated with secondary antibodies (Alexa Fluor 568, 1:100 dilution; Alexa Fluor 488, 1:500 dilution; Invitrogen, Carlsbad, CA) and visualized with confocal microscopy.
Statistical Analysis
The nonparametric rank-sum test, t test, and Fisher exact test for proportions were used as appropriate. The relationship of GSNO reductase score to risk factors was studied with multiple logistic regressions. Analyses were performed using SAS 9.2 (SAS Institute, Inc., Cary, NC).
Results
The Activity and Expression of GSNO Reductase Are Decreased in Human Squamous-Cell Lung Carcinoma
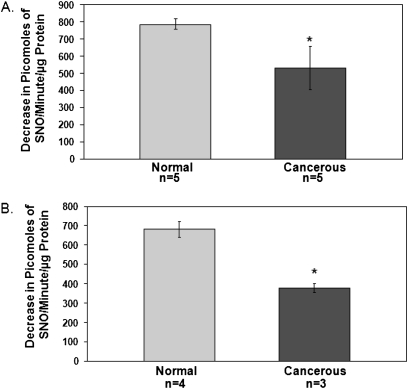
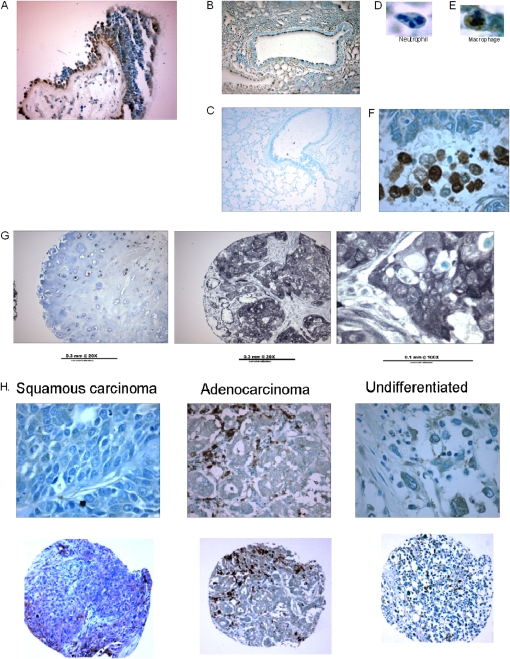
The activity of GSNO reductase was decreased in squamous-cell carcinoma lung specimens relative to adjacent normal lung tissue (Figure 1A; n = 5 each; P < 0.05), and in the squamous-cell lung carcinoma cell line H226 (n = 3) relative to nonmalignant airway epithelial cells in culture (CFBE41o−; n = 4; P < 0.05; Figure 1B). Furthermore, the frequency of positive GSNO reductase immunostaining was decreased in all non–small cell lung carcinoma surgical specimens in our array (20 out of 65), relative both to 100% positive expression in human spleen (n = 4), liver (n = 3), and kidney (n = 3) and to 100% positive expression in normal, nonsmoking human endobronchial biopsies from the Severe Asthma Research Program (SARP) (n = 6; P = 0.0016; Figures 2A, 2G, and 2H and Table 1). The antibody stained wt murine lungs (Figure 2B), but not those from GSNO reductase−/− mice (Figure 2C). Note that among the NSCLC tumors studied, GSNO reductase expression did not vary significantly with histological diagnosis (Table 1); nor did expression vary with age, sex, or smoking history (Table 2). However, by multiple linear regression analysis, GSNO reductase expression was less in tumors in which the patient was clinical Stage III and above (n = 10) than in Stage IA (n = 25; odds ratio, 0.088; P = 0.04).
Figure 1.
Decreased activity of S-nitrosoglutathione (GSNO) reductase in non–small cell lung carcinoma. (A) Squamous-cell lung-cancer tissue homogenates were compared with homogenates of adjacent normal lung parenchyma for the activity of GSNO reductase ex vivo. (B) Cultured squamous-cell lung carcinoma cells (H226) were compared with nonmalignant human airway cell cultures (CFBE41o−) for the activity of GSNO reductase. *P < 0.05.
Figure 2.
Decreased GSNO reductase immunoreactivity in lung-cancer specimens. (A) Airway tissue from healthy, nonsmoking adults expresses epithelial GSNO reductase (brown stain; ×20). (B and C) Wild-type (wt) mice (B), but not GSNO reductase−/− mice (C), express pulmonary GSNO reductase in their lungs. (D) A representative neutrophil is GSNO reductase–negative. (E) A representative macrophage is GSNO reductase–positive. (F) Inflammatory cells that immunostain positively serve as a positive internal control in lung-cancer specimens (in this case, squamous-cell carcinoma). (G) Examples of squamous-cell carcinoma specimens with reduced GSNO reductase immunoreactivity. (H) This decreased GSNO reductase expression is true for a range of cancers, including squamous-cell carcinoma, adenocarcinoma and large-cell (undifferentiated) carcinoma (top, ×40; bottom, ×10).
TABLE 1.
GSNO REDUCTASE EXPRESSION IN LUNG CANCER
| Normal Lung (Asthma Study) | Lung Cancer (Surgical Specimens) | P Value | |
| Positive staining | |||
| 6 | 20 | ||
| Tumor histology | |||
| SCC 9 | |||
| AD* 11 | |||
| Large cell 0 | |||
| Negative staining | |||
| 0 | 45 | ||
| Tumor histology | |||
| SCC 21 | |||
| AD* 20 | |||
| Large cell 4 | |||
| Total | |||
| 6 | 65 | ||
| Percentage of positive specimens | 100 | 31 | P = 0.00 |
Definition of abbreviations: AD, adenocarcinoma; SCC, squamous cell carcinmoma.
Includes bronchoalveolar adenocarcinoma.
One-tailed Fisher exact test.
TABLE 2.
PATIENT CHARACTERISTICS
| Clinical Parameters | Number (%) of Patients | |
| Age | ||
| ≤65 yr | 34 | (52.3) |
| >65 yr | 31 | (47.7) |
| Sex | ||
| Men | 36 | (55.4) |
| Women | 29 | (44.6) |
| Smoking history | ||
| Yes | 57 | (87.7) |
| No | 5 | (7.7) |
| Unknown | 3 | (4.6) |
| Received neoadjuvant chemoradiation | ||
| Yes | 6 | (9.2) |
| No | 59 | (90.8) |
| Histology | ||
| Adenocarcinoma | 29 | (44.6) |
| Squamous | 30 | (46.2) |
| Bronchoalveolar carcinoma | 2 | (3) |
| Large cell | 4 | (6.2) |
| IASLC Stage | ||
| IA | 24 | (36.9) |
| IB | 15 | (23.1) |
| IIA | 13 | (20) |
| IIB | 3 | (4.6) |
| III | 7 | (10.8) |
| IV | 3 | (4.6) |
| Lymph node metastasis | ||
| Yes | 15 | (23.1) |
| No | 48 | (73.8) |
| Inadequate sampling | 2 | (3.1) |
Definition of abbreviation: IASLC, International Association for the Study of Lung Cancer.
Vascular endothelial cells and macrophages immunostained positively for GSNO reductase and provided positive internal controls within each tumor (Figures 2E and 2F). Normal human bronchial epithelial cells also immunostained apically for GSNO reductase in the SARP biopsies (Figure 2A), as did the murine epithelium (Figure 2B). We did not have normal alveolar cells to evaluate. The expression of GSNO reductase mRNA did not differ between H226 cancer cells (n = 2; 30.9 and 31.2) and nonmalignant CFBE41o− cells (n = 2; 27.6 and 29.9; P = NS), implying that the reduction in GSNO protein expression may be attributable to post-translational events. Mutant GSNO reductase primers (negative controls) did not amplify by PCR.
GSNO Reductase Cellular Distribution Is Abnormal in Lung Cancer Cells
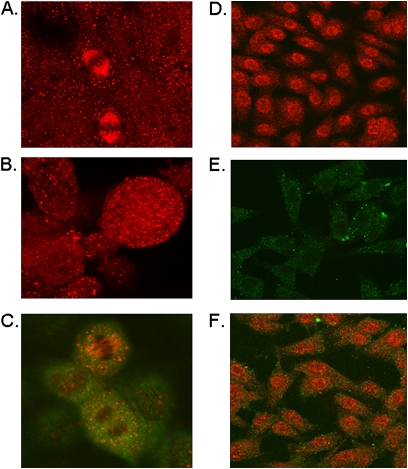
By confocal microscopy, GSNO reductase exhibited a diffuse, punctate staining pattern, and colocalized with cytosolic structures in cultured, nonmalignant airway epithelial cells. It also localized to mitotic spindles during anaphase and telaphase of mitosis (Figures 3A and 3C), an effect abrogated by treatment with colchicine, which inhibits microtubule polymerization (Figure 3B). In contrast, in the cancer cell line H226, localization was primarily perinuclear, and no association with mitotic spindles was evident (Figures 3D–3F). Taken together, these data suggest that the expression level of GSNO reductase, its activity, and its dynamic pattern of localization during mitosis are altered in human lung cancer.
Figure 3.
Confocal imaging shows altered distribution of GSNO reductase in squamous-cell lung carcinoma cells. (A) GSNO reductase is localized in punctate cytoplasmic structures and in mitotic spindles in nonmalignant bronchoepithelial cells (CFBE41o−). (B) GSNO reductase colocalization with mitotic spindles is inhibited by colchicine. (C) GSNO reductase (red) is colocalized with wt Ras (green) in CFBE41o− cells, but not (F) in squamous-cell carcinoma cells (H226). (D) The localization of GSNO reductase is perinuclear in primary squamous-cell lung carcinoma cells. (E) Background fluorescence (negative control).
Ras S-Nitrosylation and Activation
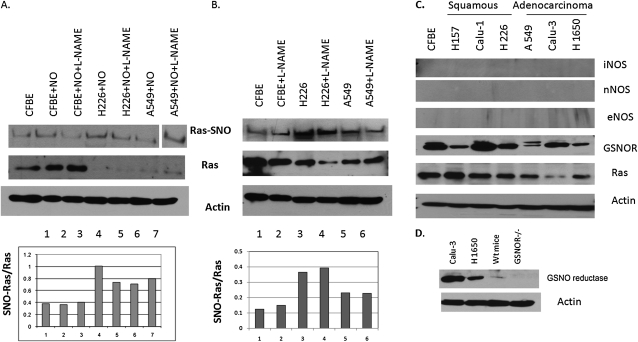
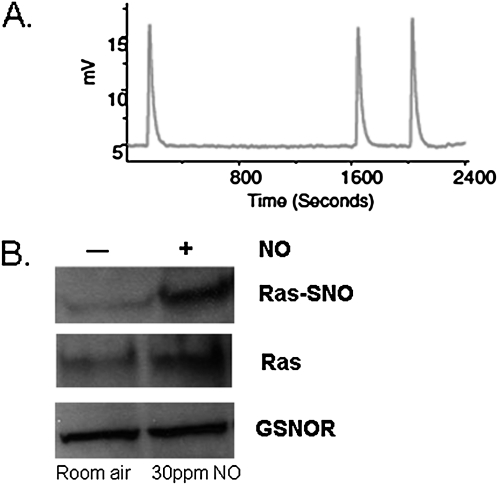
Ras S-nitrosylation was increased relative to Ras expression in most cancer cell lines, although the overall expression of wt Ras was lower in the cancer cell lines (Figure 4). The antibody immunostained murine GSNO reductase weakly in immunoblots of murine wt lung homogenates, but did not stain lung homogenates from GSNO reductase−/− mice (Figure 4D). Two days of exposure to NO did not further increase Ras S-nitrosylation relative to the expression of Ras in H226 or A549 cells in vitro, possibly reflecting a lower exposure to nitrosative stress (Figure 4A). NOS isoforms were not expressed in these cancer cell lines, and the NOS inhibitor, L-N-monomethyl arginine (100 μM), did not significantly inhibit Ras S-nitrosylation (Figures 4B and 4C). Exposure to NO for 5 days did not affect the expression of GSNO reductase. However, wt Ras S-nitrosylation was increased by 5 days of exposure to exogenous NO (∼ 30 ppm in the gas phase; Figure 5) in CFBE41o− cells, as it also was by the activation of eNOS in cells that contain eNOS and by exogenous GSNO (3). The S-nitrosylating agent, ethyl nitrite, increased Ras S-nitrosylation in murine lungs in vivo; and the S-nitrosylating agent CSNO increased the activity of Ras in vitro (Figure 6).
Figure 4.
Ras S-nitrosylation is increased in most lung-cancer cell lines, but is minimally affected by nitric oxide synthase inhibition or 2 days of exposure to NO. (A) The immunoprecipate (IP) of Ras was biotin-substituted from cell lines exposed with or without NO (30 ppm) for 2 days or to L-N-monomethyl arginine (L-NMMA) (100 μM). The biotin-substituted IP and—on a separate gel—total cell extract underwent immunoblotting for Ras and for actin (loading control). Densitometry revealed an increased SNO-Ras/Ras ratio in tumor cell lines (lanes 4–7) relative to nonmalignant cells (lanes 1–3), with a minimal effect of 3 days of NO or L-NMMA. Note that the molecular weight marker ran between lanes 6 and 7, but all bands are from the same gel. (B) The experiments in A were repeated without exposure to NO; again, SNO-Ras/Ras is increased in the cancer cells. (C) Lung-cancer cell lines in culture do not express NOS isoforms and express less GSNO reductase than nonmalignant CFBE41o− cells, with the exception of Calu-1 cells. (D) Our polyclonal antibody was highly reactive against human GSNO reductase, but much less so against the murine enzyme, and not at all against the GSNO reductase−/− mouse lung homogenates.
Figure 5.
Exposure to nitrosative stress for 5 days increases Ras S-nitrosylation, but does not alter the expression of S-nitrosoglutathione reductase (GSNOR). (A) In a sealed NO cell-exposure chamber, a steady concentration of NO at 30 ppm (400 nM in the gas phase) was measured using serial 0.2-ml gas samples from the chamber and was analyzed by chemiluminescence. A representative chemiluminescent signal is shown. (B) Nonmalignant epithelial cells (CFBE41o−) were incubated for 5 days in this chamber, and exhibited no change in the expression of GSNOR, but did exhibit increased Ras S-nitrosylation.
Figure 6.
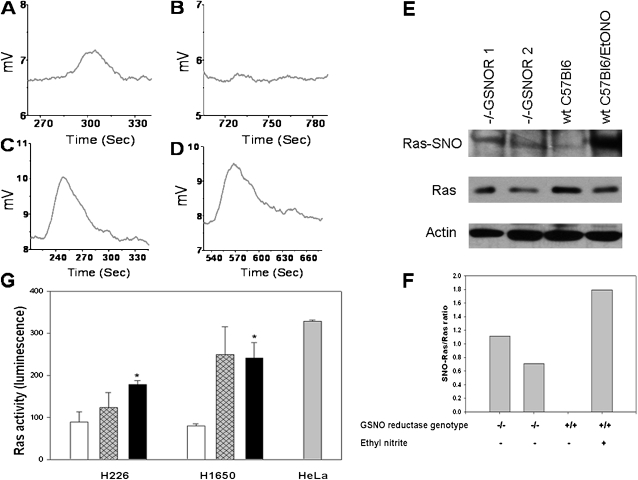
The Ras denitrosylase function of GSNO reductase. (A–D) Immunoprecipitated H-Ras was S-nitrosylated with 100 μM S-nitroso-N-acetyl cysteine, and washed three times in PBS. SNO–H-Ras was exposed to glutathione (GSH) and nicotinamide adenine dinucleotide reduced (NADH), with (A and B) or without (C and D) GSNO reductase. SNO–H-Ras was measured by reduction chemoluminescence at 0 minutes (A and C) and 10 minutes (B and D). B and D represent identical conditions, except that GSNO reductase is present in B. The GSNO formed by transnitrosation from SNO-Ras requires GSNO reductase for its breakdown. (E) GSNOR−/− mice (lanes 1 and 2) had higher concentrations of SNO-Ras relative to total Ras than did background wt (lane 3) when wild-type mouse lungs were pretreated with 1 mM ethyl nitrite (EtONO; 5 μl). However, concentrations of SNO-Ras increased (lane 4). (F) Concentrations in E were quantitated by densitometry (background subtracted). (G) The activity of Ras was increased by S-nitrosocysteine (CSNO, 10 μM; hatched and solid bars) relative to baseline (open bars) in the presence of GSH and NADH in two non–small cell lung carcinoma cell lines. This effect tended to be augmented by the GSNO reductase inhibitor, C3 (solid bars; 100 μM; 26), in H226 cells, but the effect was not significant, consistent with the decreased activity of baseline GSNO reductase. HeLa cells stimulated with epidermal growth factor (gray bar) served as positive internal controls for the ELISA assay. *n = 3 each; P < 0.03, relative to baseline, according to ANOVA.
S-Nitrosylated Ras Is a Denitrosylation Target for Denitrosylation and Inactivation by GSNO Reductase
In the presence of GSH, GSNO reductase from CFBE41o− cells denitrosylated SNO-Ras, whereas GSH alone was inactive (Figures 6A–6D). Wild-type Ras was immunoprecipitated from cystic fibrosis pancreatic adenocarcinoma cell 1 (CFPAC1) cells in which it was overexpressed, treated with 100 μM S-nitroso-N-acetyl cysteine, and washed three times in PBS. GSNO reductase was colocalized with H-Ras in nonmalignant airway epithelial cells in culture, but not in a primary squamous-cell carcinoma cell line (Figures 3C and 3F). The S-nitrosylation of Ras relative to the total expression of Ras was increased in the lungs of GSNO reductase−/− mice, and was also increased in wt mice by treatment with EtONO (Figures 6E and 6F). Consistent with a previous report (3), CSNO increased the activity of Ras (Figure 6G). In the H1650 cancer cell line, this increase tended to be augmented by the GSNO reductase inhibitor C3 (26), although this change did not reach significance (Figure 6G). The weak effect of C3 may reflect the decreased expression and activity of GSNO reductase in cancer cells at baseline (Figures 1, 2, and 4).
Discussion
Lung cancer remains the leading cause of cancer death for both men and women and is the most preventable form of cancer death (19). The expected 5-year survival rate for all patients with lung cancer is less than 15%, compared with 65% for colon cancer and 89% for breast cancer. According to the American Cancer Society, tobacco use is associated with the development of lung cancer, and it accounts for 87% of lung cancer deaths. Among smokers, squamous-cell lung carcinoma accounts for the plurality (42%) of non–small cell cancer (19, 20). Nitrogen oxides (particularly NO, NO2, and N2O3) in tobacco smoke can be anticipated to contribute to enhanced protein nitrosylation (S-nitrosylation), a key post-translation modification that frequently targets apoptotic and prosurvival proteins such as Ras, caspases, and IKKβ (1, 2). Cigarette smoke also contains oxidants (including formaldehyde) that are implicated in carcinogenesis (33, 34).
In this study, we found that the activity and expression of GSNO reductase (also known as ADHIII, or glutathione-dependent formaldehyde dehydrogenase) is decreased in lung cancer specimens compared with normal lung tissue. Furthermore, we found that GSNO reductase denitrosylates Ras both in vitro and in a murine model in vivo, and that Ras S-nitrosylation is increased in lung-cancer cell lines. These data suggest that GSNO reductase regulates the state of Ras S-nitrosylation and that the decreased activity of GSNO reductase may favor the increased activity of wt Ras that promotes tumor-cell survival in lung cancer. We focused on the denitrosylase activity of the enzyme, which, depending on substrate concentration, also functions in antioxidant defense. In particular, GSNO reductase may protect the airway from the carcinogenic effects of formaldehyde. Thus, although we focused on the role of GSNO reductase as a denitrosylase, its decreased activity may leave the airway more vulnerable to the carcinogenic effects of other components of cigarette smoke.
Recently, the decreased expression of GSNO reductase was observed in human and murine hepatocellular carcinomas (10). Specifically, GSNO reductase knockout mice are more susceptible to chemically induced hepatocellular carcinoma (10). As in viral hepatitis–associated hepatocellular carcinoma, chronic nitrosative stress promotes lung tumorigenesis. In the case of lung cancer, the unique source of the nitrosative stress is normally cigarette smoke (19, 20). Interestingly, LOH and structural chromosomal abnormalities in the GSNO reductase gene locus, 4q23, were reported in both lung cancers and hepatocellular carcinomas (11–14). We thus hypothesized that decreased protection from nitrosative stress by the activity of GSNO reductase may contribute to lung carcinogenesis, as seen in the development of experimentally induced hepatocellular carcinoma. We found that the activity of GSNO reductase was decreased both in human lung carcinoma specimens in situ and in cultured lung-cancer cell lines. In lung-cancer cell lines, GSNO reductase was found to exhibit abnormal cytosolic distribution and altered dynamic distribution during mitosis, compared with cultured, noncancerous bronchial epithelium. We speculate that the trafficking of GSNO reductase to mitotic spindles may play a role in the regulation of mitosis. However, little is known about the functional significance of the subcellular localization of the enzyme.
Malignant and benign cultured airway epithelial cells did not differ with regard to concentrations of GSNO reductase mRNA. Thus, the abnormal levels of protein expression and activity of GSNO reductase are likely attributable to post-transcriptional regulation or compartmental sequestration. The post-transcriptional regulation of GSNO reductase is poorly understood. Abnormal sequestration of GSNO reductase to the perinuclear region of the tumor cell may hinder its activity and perhaps make this protein difficult to identify by immunohistochemistry. Interestingly, more advanced tumors were less likely to demonstrate evidence of GSNO reductase by immunohistochemistry, reflecting either decreased expression or increased sequestration in advanced tumors.
Nitrosative and oxidative stress may be pro-oncogenic through several mechanisms. Here, we focused on nitrosative stress. Nitrogen oxides can directly modify DNA, leading in particular to cytidine to thymidine mutations (22). Nitrogen oxides can also modify c-Src tyrosine kinase (23), protein von Hippel Lindau (24), nuclear Cl− intracellular channel 4 (30), and alkyl guanine-DNA alkyl transferase (10), altering the activities of these proteins and potentially permitting malignant transformation. GSNO reductase may protect against these effects in airway epithelial cells exposed to nitrosative stress. We studied wt Ras because its activation by eNOS through S-nitrosylation was clearly demonstrated to be an effect downstream of the K-Ras–mediated activation of PI3 kinase/Akt (3), and of potential relevance to lung cancer (25). We found that GSNO reductase denitrosylates wt Ras in the presence of cellular concentrations of GSH and NADH; that wild-type Ras S-nitrosylation is increased in airway epithelial cells exposed to nitrosative stress; that nitrosative stress activates Ras in lung cancer cells, particularly in the absence of GSNO reductase activity; and that Ras S-nitrosylation, relative to Ras expression, is increased in several lung cancer cell lines. Thus, we propose that one mechanism by which the decreased activity of GSNO reductase may be permissive for lung carcinogenesis is through the failure to denitrosylate wild-type Ras.
Importantly, a deficiency of GSNO reductase may also protect against cancer by detoxifying formaldehyde (33) and protecting against oxidative stress (34). Clearly, exogenous/inhalational oxidative and nitrosative stress (rather than endogenous production) is normally required for the initiation of lung and airway cancer. The increased expression of NOS and maintenance of normal levels of nitrogen oxides may actually disfavor the progression of non–small cell lung carcinoma (35), consistent with the paradoxical effects of NOS and high concentrations of nitrogen oxides observed in other contexts (1).
Many issues remain to be resolved. (1) The differences in agent, dose, and time-course with regard to Ras S-nitrosylation in airway epithelial cells will need extensive study. These may reflect both cell permeability and the relative electropositivity of the NO moiety (i.e., EtONO, as EtO−–NO+ [31], appears to be more effective than NO itself). (2) The reasons for the loss of wt Ras in lung-cancer cell lines will need to be identified. We suspect that Ras S-nitrosylation, in addition to activating the enzyme, could target the protein for degradation or inhibit its translation, as is the case with other proteins (15, 24, 27). (3) The source of nitrogen oxides remains to be defined in these NOS-deficient cancer cell lines, although environmental nitrogen oxides promote S-nitrosylation, and certain cellular S-nitrosylated proteins are sequestered and quite stable after S-nitrosylation (1, 32). (4) Much work remains to be done on the human genetics and biology of GSNO reductase in the airway epithelium. (5) Finally, our data suggest that a long-term study of the oncogenic potential of exposure to cigarette smoke in GSNO reductase−/− mice would be worthwhile.
To our knowledge, these are the first data identifying a role for GSNO reductase as a denitrosylase with the potential to protect against lung cancer in the context of nitrosative stress. Inhibitors of GSNO reductase are in development (as are nitrogen oxide donors) for the treatment of asthma and cystic fibrosis (15, 26–29). Our data suggest that decreasing the activity of GSNO reductase may change airway biology to favor the development of lung cancer, particularly in the context of exposure to smoke.
Acknowledgments
The authors express their appreciation to Michael Forbes for assistance with immunohistochemistry, to Stephen J. Lewis for assistance with the murine experiments, and to Jonathan S. Stamler and Limin Liu for the GSNO reductase−/− mice.
Footnotes
This work was supported by National Institutes of Health/National Heart, Lung, and Blood Institute grants 3R01HL59337 and P01HL101871–01A1, and National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program grant 2R01HL69170.
Originally Published in Press as DOI: 10.1165/rcmb.2011-0147OC on August 4, 2011
Author Disclosure: N.V.M., S.Y., C.W., H.W., A.S.N., L.L., T.M., and D.R.J. do not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.G. owns intellectual property related to this manuscript that is licensed through his institution. Neither B.G. nor his institution profits from this license.
References
- 1.Gaston B, Doctor A, Singel D, Stamler JS. S-nitrosothiol signaling in respiratory biology. Am J Respir Crit Care Med 2006;173:1186–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med 2009;15:391–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim KH, Ancrile BB, Kashatus DF, Counter CM. Tumour maintenance is mediated by eNOS. Nature 2008;452:646–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang K, Johns R, Macdonald T, Kinter M, Gaston B. S-nitrosoglutathione breakdown prevents airway smooth muscle relaxation in the guinea-pig. Am J Physiol Lung Cell Mol Physiol 2000;279:L716–L21 [DOI] [PubMed] [Google Scholar]
- 5.Liu L, Hausladen A, Zeng M, Heitman J, Stamler JS. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 2001;410:490–494 [DOI] [PubMed] [Google Scholar]
- 6.Que LG, Liu L, Yan Y, Whitehead GS, Gavett SH, Schwartz DA, Stamler JS. Protection from experimental asthma by an endogenous bronchodilator. Science 2005;308:1618–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Que L, Zhonghui Y, Stamler JS, Lugogo NL, Kraft M. S-nitrosoglutathione reductase: an important regulator in human asthma. Am J Respir Crit Care Med 2009;180:226–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen DE, Belka GK, Du Bois GC. S-nitrosoglutathione is a substrate for rat alcohol dehydrogenase Class III isoenzyme. Biochem J 1998;331:659–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staab CA, Hellgren M, Höög J-O. Dual functions of alcohol dehydrogenase 3: implications with focus on formaldehyde dehydrogenase and S-nitrosoglutathione reductase activities. Cell Mol Life Sci 2008;65:3950–3960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei W, Li B, Hanes MA, Kakar S, Chen X, Liu L. S-nitrosylation from GSNOR deficiency impairs DNA repair and promotes hepatocarcinogenesis. Sci Transl Med 2010;2:19(ra)13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mimbacas AB, Cardoso JH. A recurrent chromosome 4 marker in primary squamous cell lung cancer. Cancer Detect Prev 2004;28:331–333 [DOI] [PubMed] [Google Scholar]
- 12.Forsyth NR, Morrison V, Craig NJ, Fitzsimmons SA, Barr NI, Ireland H, Gordon KE, Dowen S, Cuthbert AP, Newbold RF, et al. Functional evidence for a squamous cell carcinoma mortality gene(s) on human chromosome 4. Oncogene 2002;21:5135–5147 [DOI] [PubMed] [Google Scholar]
- 13.Shivapurkar N, Virmani AK, Wistuba LL, Milchgrub S, Mackay B, Minna JD, Gazdar AF. Deletions of chromosome 4 at multiple sites are frequent in malignant mesothelioma and small cell lung carcinoma. Clin Cancer Res 1999;5:17–23 [PubMed] [Google Scholar]
- 14.Cho ES, Chang J, Chung KY, Shin DH, Kim YS, Kim SK, Kim SK. Identification of tumor suppressor loci on the long arm of chromosome 4 in primary small cell lung cancers. Yonsei Med J 2002;43:145–151 [DOI] [PubMed] [Google Scholar]
- 15.Marozkina NV, Borowitz M, Yemen S, Liu L, Sun F, Islam R, Erdmann-Gilmore P, Townsend RR, Lichti CF, Mantri S, et al. S-nitrosoglutathione targets Hsp 70/Hsp 90 organizing protein as a corrector therapy for cystic fibrosis. Proc Natl Acad Sci USA 2010;107:11393–11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagji AS, Liu Y, Stelow EB, Stukenborg GJ, Jones DR. BRMS1 transcriptional repression correlates with CpG island methylation and advanced pathological stage in non–small cell lung cancer. J Pathol 2010;221:229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore WC, Bleecker ER, Curran-Everett DC, Teague WG, Li H, Li X, D'Agostino R, Jr, Castro M, Curran-Everett D, Fitzpatrick AM, et al. National Heart, Lung, and Blood Institute's Severe Asthma Research Program: Characterization of the severe asthma phenotype by the NHLBI Severe Asthma Research Program. J Allergy Clin Immunol 2007;119:405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang K, Ragsdale NV, Carey RM, MacDonald T, Gaston B. Reductive assays for S-nitrosothiols: implications for measurements in biological systems. Biochem Biophys Res Commun 1998;252:535–540 [DOI] [PubMed] [Google Scholar]
- 19.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics. CA 2009;59:225–249 [DOI] [PubMed] [Google Scholar]
- 20.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non–small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell 2004;116:617–628 [DOI] [PubMed] [Google Scholar]
- 22.Wink DA, Kasprzak KS, Maragos CM, Elespuru RK, Misra M, Dunams TM, Cebula TA, Koch WH, Andrews AW, Allen JS, et al. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science 1991;254:1001–1003 [DOI] [PubMed] [Google Scholar]
- 23.Rahman MA, Senga T, Ito S, Hyodo T, Hasegawa H, Hamaguchi M. S-nitrosylation at cysteine 498 of c-Src tyrosine kinase regulates nitric oxide–mediated cell invasion. J Biol Chem 2010;285:3806–3814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer L, Doctor A, Chhabra P, Sheram ML, Laubach V, Gaston B. S-nitrosothiols signal hypoxia-mimetic vascular pathology. J Clin Invest 2007;117:2592–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adjei AA. K-Ras as a target for lung cancer therapy. J Thorac Oncol 2008;3:S160–S163 [DOI] [PubMed] [Google Scholar]
- 26.Sanghani PC, Davis WI, Fears SL, Green SL, Zhai L, Tang Y, Martin E, Bryan NS, Sanghani SP. Kinetic and cellular characterization of novel inhibitors of S-nitrosoglutathione reductase. J Biol Chem 2009;284:24354–24362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaman K, Carraro L, Doherty J, Gainov IC, Turner R, Vaughan J, Hunt JF, Marquez J, Gaston B. A novel class of compounds that increase CFTR expression and maturation in epithelial cells. Mol Pharmacol 2006;70:1435–1442 [DOI] [PubMed] [Google Scholar]
- 28.Gaston B, Reilly J, Drazen JM, Fackler J, Ramdev P, Arnelle D, Mullins ME, Sugarbaker DJ, Chee C, Singel DJ, et al. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc Natl Acad Sci USA 1993;90:10957–10961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snyder A, McPherson ME, Hunt JF, Johnson M, Stamler JS, Gaston B. Acute effects of aerosolized S-nitrosoglutathione in cystic fibrosis. Am J Respir Crit Care Med 2002;165:922–926 [DOI] [PubMed] [Google Scholar]
- 30.Malik M, Shukla A, Amin P, Niedelman W, Lee J, Jividen K, Phang JM, Ding J, Suh KS, Curmi PM, et al. S-nitrosylation regulates nuclear translocation of chloride intracellular channel protein CLIC4. J Biol Chem 2010;285:23818–23828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moya MP, Gow AJ, McMahon TJ, Niedelman W, Lee J, Jividen K, Phang JM, Ding J, Suh KS, Curmi PM, et al. S-nitrosothiol repletion by an inhaled gas regulates pulmonary function. Proc Natl Acad Sci USA 2001;98:5792–5797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paige JS, Xu G, Stanecevic B, Jaffrey SR. Nitrosothiol reactivity profiling identifies S-nitrosylated proteins with unexpected stability. Chem Biol 2008;15:1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neilsen G, Wolkoff P. Cancer effects of formaldehyde: a proposal for an indoor air guideline value. Arch Toxicol 2010;84:423–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ilonen I, Rasanen J, Sihvo E, Knuuttila A, Salmenkivi K, Ahotupa M, Kinnula V, Salo J. Oxidative stress in non–small cell lung cancer: role of nicotinamide adenine dinucleotide phosphate oxidase and glutathione. Acta Oncol 2009;48:1054–1061 [DOI] [PubMed] [Google Scholar]
- 35.Puhakka A, Kinnula V, Näpänkangas U, Säily M, Koistinen P, Pääkkö P, Soini Y. High expression of nitric oxide synthases is a favorable prognostic sign in non–small cell lung carcinoma. APMIS 2003;111:1137–1146 [DOI] [PubMed] [Google Scholar]