Abstract
Fibrosis is a final stage of many lung diseases, with no effective treatment. Plasminogen activator inhibitor–1 (PAI-1), a primary inhibitor of tissue-type and urokinase-type plasminogen activators (tPA and uPA, respectively), plays a critical role in the development of fibrosis. In this study, we explored the therapeutic potential of an orally effective small molecule PAI-1 inhibitor, TM5275, in a model of lung fibrosis induced by transforming growth factor–β1 (TGF-β1), the most potent and ubiquitous profibrogenic cytokine, and in human lung fibroblasts (CCL-210 cells). The results show that an intranasal instillation of AdTGF-β1223/225, an adenovirus expressing constitutively active TGF-β1, increased the expression of PAI-1 and induced fibrosis in murine lung tissue. On the other hand, treating mice with 40 mg/kg of TM5275 for 10 days, starting 4 days after the instillation of AdTGF-β1223/225, restored the activities of uPA and tPA and almost completely blocked TGF-β1–induced lung fibrosis, as shown by collagen staining, Western blotting, and the measurement of hydroxyproline. No loss of body weight was evident under these treatment conditions with TM5275. Furthermore, we show that TM5275 induced apoptosis in both myofibroblasts (TGF-β1–treated) and naive (TGF-β1–untreated) human lung fibroblasts, and this apoptosis was associated with the activation of caspase-3/7, the induction of p53, and the inhibition of α–smooth muscle actin, fibronectin, and PAI-1 expression. Such an inhibition of fibrotic responses by TM5275 occurred even in cells pretreated with TGF-β1 for 6 hours. Together, the results suggest that TM5275 is a relatively safe and potent antifibrotic agent, with therapeutic potential in fibrotic lung disease.
Keywords: PAI-1 inhibitor, lung fibrosis therapy, (myo)fibroblast apoptosis, TGF-β1, animal model
Clinical Relevance
The findings of this study may lead to the development of new therapeutic drugs for lung fibrotic diseases. The results also shed new light on the mechanism whereby plasminogen activator inhibitor–1 promotes fibrosis.
Pulmonary fibrosis is a characteristic feature and final stage of many lung diseases, including idiopathic pulmonary fibrosis, cystic fibrosis, acute respiratory distress syndrome, severe acute respiratory syndrome, sarcoidosis, silicosis, and asbestosis. The most enigmatic and fatal form of pulmonary fibrosis is idiopathic pulmonary fibrosis (IPF). Despite decades of intensive study, no effective treatment for these devastating lung diseases has been developed because of a poor understanding of their complex pathological process. In the past, lung fibrosis was speculated to result from an unremitting inflammatory response to an exogenous insult, leading to the activation and proliferation of fibroblasts, and eventually culminating in progressive fibrosis. Therefore, anti-inflammatory agents, alone or in combination with cytotoxic drugs, have been used in the clinic as a standard therapeutic regimen for the treatment of lung fibrotic diseases. However, little evidence indicates that these agents alter the natural history of the disease or improve the survival of patients (1–4). Although other therapeutic strategies were used in clinical trials, including interferon-γ and anti–transforming growth factor–β or anti–connective tissue growth factor antibody, the efficacies of these treatments remain unclear (5–8).
Plasminogen activator inhibitor 1 (PAI-1) is a primary inhibitor of urokinase-type and tissue-type plasminogen activators (uPA and tPA, respectively), and plays a critical role in wound healing and tissue remodeling. The expression of PAI-1 is increased in many fibrotic diseases, including IPF (9–14), and in experimental models of fibrosis (15, 16). Knockout of the PAI-1 gene or the administration of PAI-1 small interfering RNA attenuates, whereas the overexpression of PAI-1 protein enhances, fibrotic responses induced by different stimuli (13, 17–19). All these lines of evidence suggest that PAI-1 plays an essential role in the development of lung fibrosis. Nonetheless, the mechanism whereby PAI-1 promotes fibrosis is still poorly understood, and most importantly, no PAI-1 inhibitor has been developed for the treatment of lung fibrotic diseases.
In this study, we explored the therapeutic potential of an orally effective small molecule PAI-1 inhibitor, TM5275, for lung fibrosis, using human lung fibroblasts and a well-established lung fibrosis model induced by transforming growth factor–β1 (TGF-β1), the most potent and ubiquitous profibrogenic cytokine. The results show that an oral administration of TM5275, 4 days after mice were challenged with TGF-β1, almost completely blocked TGF-β1–induced lung fibrosis, with no significant effect on body weight. We further show that TM5275 induced (myo)fibroblast apoptosis and suppressed TGF-β1–induced fibrotic responses in human lung fibroblasts. Together, the results suggest that TM5275, a small molecule PAI-1 inhibitor, is a promising therapeutic agent for lung fibrotic diseases.
Materials and Methods
Animal Treatment
Male C57BL/6 mice (6–8 weeks old) were challenged with 109 plaque-forming units of AdTGF-β1223/225, an adenovirus expressing constitutively active TGF-β1, AdDL70-3, a virus vector, or saline by intranasal instillation. Four days later, the mice were treated with 40 mg/kg of TM5275 (dissolved in 2% DMSO) or solvent by gavage daily for 10 days. Mice were killed 7 or 14 days after the instillation of AdTGF-β1223/225. Bronchoalveolar lavage was performed, the left lungs were fixed with 4% paraformaldehyde, and the rest of the lung tissue was frozen immediately in liquid nitrogen. All animals were maintained on a 12-hour light/dark cycle at 22°C in the specific pathogen free facility, and all procedures involving animals were approved by the Institutional Animal Care and Use Committees at the University of Alabama at Birmingham.
Cell Culture and Treatment
CCL-210 cells, which are normal human lung fibroblasts from the American Type Culture Collection (Manassas, VA), were cultured in Eagle's minimum essential medium formulated by the American Type Culture Collection, as we described previously (20). At 70–80% confluence, cells were treated with 1 ng/ml of TGF-β1 (R&D, Minneapolis, MN) for various periods of time, and then with 75 μM TM5275 for 24 hours.
ELISA
The amounts of total and active TGF-β proteins in the bronchoalveolar lavage fluid (BALF) were measured using a ELISA kit (catalogue number 84-7344-88) from eBioscience (San Diego, CA), according to the protocol provided by the manufacturer. The amounts of PAI-1 antigen in the BALF were determined using a ELISA kit from Molecular Innovations (Novi, MI), as we described previously (21).
Northern Blot Hybridization
Total RNA was isolated from lung tissue, using TRIzol reagent. The mRNA of procollagen α2 (I), procollagen α1 (III), and PAI-1 was assessed by Northern blot hybridization, as we described previously (22).
Lung Histology and Collagen Staining
The deposition of collagen in the lung was revealed by Masson trichrome staining, and quantified by morphometric techniques, as we described previously (23).
Immunohistochemical Staining
α–smooth muscle actin (α-SMA) in murine lung tissue was stained with monoclonal anti-mouse α-SMA antibody (catalogue number CM001B; Biocare Medical, Concord, CA) and semiquantified, as we previously described (23).
Measurement of Hydroxyproline
The hydroxyproline content in lung tissue was determined as described elsewhere (24), and calculated according to the standard curves derived from 4-hydroxy-L-proline.
Reverse Zymography and Zymography
The activities of PAI-1 and tPA/uPA were determined by reverse zymography and zymography, respectively, as we described previously (21). The intensities of bands were semiquantified using Image J software (from the National Institutes of Health website).
Western Blot Analyses
Western blot analyses of protein abundance were conducted as we described previously (25), using the antibodies collagen 1α1 (catalogue number sc8784; Santa Cruz Biotechnology, Santa Cruz, CA), PAI-1 (ASMPAI-GF; Molecular Innovations), α-SMA (catalogue number CM001B; Biocare Medical), fibronectin (catalogue number 610077; BD Biosciences, Franklin Lakes, NJ), and β-actin (protein loading control). Protein bands were semiquantified using Image J software.
Apoptosis Analysis
Apoptosis was analyzed by flow cytometry techniques, using an Alexa Fluor 488 Annexin V Kit (Invitrogen, Carlsbad, CA), following the protocol provided by the manufacturer.
Activity of Caspase-3/7
The activity of caspase-3/7 was determined with an Apo-ONE Homogenous Caspase-3/7 Assay (catalogue number G7790; Promega, Madison, WI), according to the protocol provided by the manufacturer. The fluorescence was measured at excitation/emission wavelengths of 485/528 nm, and the results were normalized according to protein concentrations.
Statistical Analysis
Data are presented as means ± SEM, and were evaluated by one-way ANOVA. Statistical significance was determined post hoc by Tukey's test.
Results
Intranasal Instillation of AdTGF-β1223/225 Increased the Expression of PAI-1 and Induced Lung Fibrosis in Mice
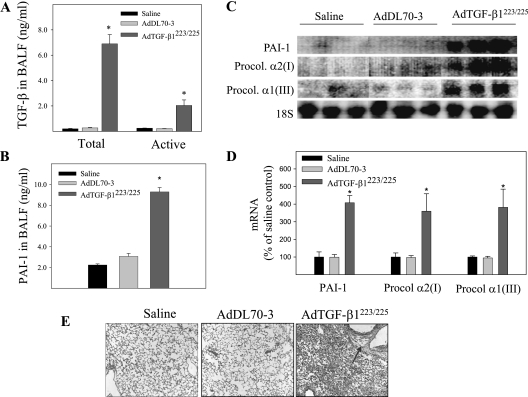
TGF-β1 is the most potent and ubiquitous profibrogenic cytokine, and is increased in almost all fibrotic diseases. To explore the therapeutic potential of the PAI-1 inhibitor TM5275 in lung fibrotic diseases, we first examined whether an intranasal instillation (a noninvasive lung drug-delivery technique) of AdTGF-β1223/225, an adenovirus expressing constitutively active TGF-β1, would effectively induce the expression of PAI-1 and lung fibrosis in mice. The results show that the concentrations of protein in both active and latent forms of TGF-β1 in the BALF were dramatically increased 7 days after an intranasal instillation of AdTGF-β1223/225 (Figure 1A). Associated with the increase in expression of TGF-β1, the instillation of AdTGF-β1223/225 significantly increased concentrations of PAI-1 protein and mRNA in BALF and lung tissue (Figures 1B–1D), as well as the expression of procollagen mRNA and deposition of collagen in the lung (Figures 1C–1E). Virus vector alone (AdDL70-3), on the other hand, exerted no significant effect on the expression of TGF-β or PAI-1, and neither did it increase the deposition of collagen in the lung, suggesting that fibrotic responses are induced by TGF-β1 and not adenovirus. Importantly, such fibrotic responses induced by an intranasal instillation of AdTGF-β1223/225 persisted for at least 21 days (data not shown). These results suggest that an intranasal instillation of AdTGF-β1223/225 is an effective and reliable means of inducing lung fibrosis. Because the expression of TGF-β is increased in almost all fibrotic diseases, TGF-β–induced lung fibrosis serves as a good animal model for testing the therapeutic potential of an antifibrotic drug, and was used in this study.
Figure 1.
Effects of intranasal instillation of an adenovirus expressing constitutively active transforming growth factor–β1 (AdTGF-β1223/225) on the expression of plasminogen activator inhibitor 1 (PAI-1) and the accumulation of collagen in murine lung tissue. AdTGF-β1223/225, the adenovirus vector (AdDL70-3), or saline was administered to murine lungs by intranasal instillation. Seven days after instillation, the mice were killed. The amounts of total and active transforming growth factor–β1 (TGF-β1) (A) as well as total PAI-1 protein (B) in the bronchoalveolar lavage fluid (BALF) were determined by ELISA. (C) Representative Northern blotting image of procollagen (Procol) and PAI-1 mRNAs in murine lung tissue. 18S was used as an RNA loading control. (D) Quantitative data of the radioactivity of Northern blots from Instant Image (Packard Instrument Co., Meriden, CT). (E) Trichrome staining of collagens in murine lung tissue (arrow indicates collagen). *Significantly different from saline-treated mice (P < 0.05, n = 5–8).
Effects of PAI-1 Inhibitor on Activities of PAI-1 and tPA/uPA in Lungs of Mice Treated with or without TGF-β1
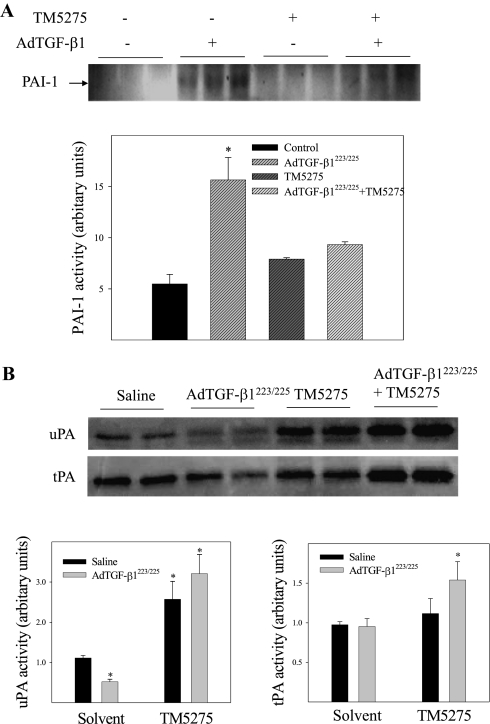
TM5275, a novel, orally effective PAI-1 inhibitor, was shown to exert potent antithrombotic effects in both rats and monkey models, without causing overt toxicity or effects on bleeding time (26). To test its antifibrotic potential, we first examined the effects of an oral administration of TM5275 on the activities of PAI-1, tPA, and uPA in murine lung tissue. The results show that an administration of AdTGF-β1223/225 increased the activity of PAI-1 (Figure 2A) and suppressed the activity of uPA, although it exerted no significant effect on the activity of tPA (Figure 2B). Treatment with TM5275, on the other hand, inhibited the TGF-β1–induced activity of PAI-1 (Figure 2A), and completely reversed the inhibitory effect of TGF-β1 on uPA activity (Figure 2B). We also observed that the activities of tPA and uPA in mice treated with TGF-β1 plus TM5275 were significantly higher than those treated with TM5275 alone. These results suggest that the expression of tPA and uPA proteins may be increased in TGF-β1–treated mice, even though their activities were inhibited because of a simultaneous increase in the expression and activity of PAI-1. No obvious body weight loss was evident upon treatment with TM5275 (data not shown), suggesting that TM5275 did not cause obvious toxicity under the conditions used in this study.
Figure 2.
Effects of small molecule PAI-1 inhibitor TM5275 on the activities of PAI-1, tissue-type plasminogen activator (tPA), and urokinase-type plasminogen activator (uPA) in the lungs of mice intranasally instilled with AdTGF-β1223/225 or saline. (A) The activity of PAI-1 was determined by reverse zymography. (B) The activities of tPA and uPA were determined by zymography, and photo-negative images of the gels are presented here. Dark bands attributable to an inhibition of casein degradation by PAI-1 in reverse zymography and protein lytic bands in zymography were semiquantified with Image J software. Semiquantified data are presented at the bottoms of A and B. *Significantly different from saline + solvent–treated control mice (P < 0.05, n = 4–6).
Effects of PAI-1 Inhibitor on TGF-β1–Induced Lung Fibrosis in Mice
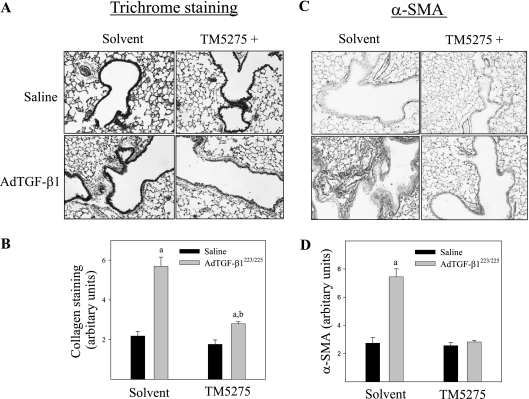
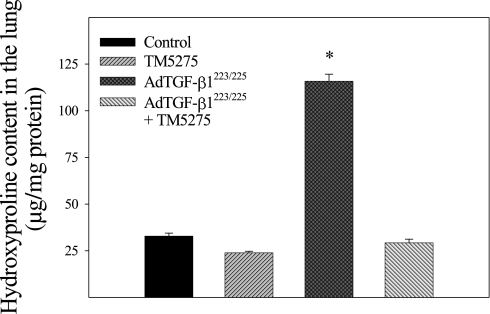
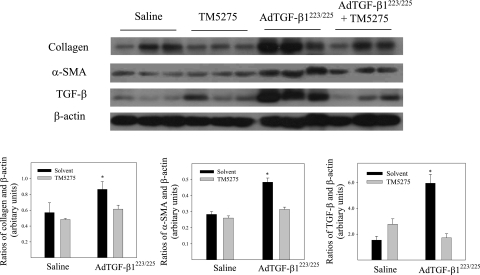
The effects of TM5275 on TGF-β1–induced lung fibrosis were explored further by measuring the deposition of collagen and hydroxyproline content in murine lung tissue. The results show that an administration of TM5275 significantly reduced the TGF-β1–induced accumulation of collagen in the lung, as shown by trichrome staining, Western blotting, and hydroxyproline measurement (Figures 3–5). Immunohistochemical staining and Western blotting further showed that TM5275 suppressed TGF-β1–induced α–smooth muscle actin (α-SMA), a marker of myofibroblast differentiation in murine lungs (Figures 3 and 4), suggesting that TM5275 may prevent the TGF-β1–induced differentiation of myofibroblasts, or induce the apoptosis of myofibroblasts. Most interestingly, Western blotting analysis showed that TM5275 almost completely blocked the AdTGF-β1223/225–induced expression of TGF-β1. These data clearly demonstrate the potent antifibrotic activity of TM5275 in our model of TGF-β1–induced lung fibrosis.
Figure 3.
Effects of TM5275 on TGF-β1–induced accumulation of collagen and α–smooth muscle actin (α-SMA) in murine lung tissue. (A) Representative images of trichrome staining of collagen in murine lung. (B) Summary of semiquantified collagen staining data. (C) Representative immunohistochemical staining images of α-SMA in murine lung tissue. (D) Summary of semiquantification data of α-SMA immunostaining. aSignificantly different from saline + solvent–treated control group. bSignificantly different from group with AdTGF-β1223/225 alone (P < 0.05, n = 4–5).
Figure 5.
Effect of TM5275 on accumulation of hydroxyproline in lungs of TGF-β1–treated mice. The amount of hydroxyproline was determined by a color reaction assay, as described in Materials and Methods. *Significantly different from saline + solvent–treated control group (P < 0.05, n = 3–6).
Figure 4.
Western blot analyses of effects of TM5275 on the expression of collagen, α-SMA, and TGF-β proteins in lungs of TGF-β1–treated mice. Top, representative Western blotting images of collagen, α-SMA, and TGF-β. β-actin was used to show equal protein loading. Bottom, summaries of semiquantified data of collagen, α-SMA, and TGF-β band densities relative to β-actin, as determined by Image J software. *Significantly different from saline + solvent–treated control group (P < 0.05, n = 4–5).
Effects of PAI-1 Inhibitor on TGF-β1–Induced Fibrotic Responses in Human Lung Fibroblasts
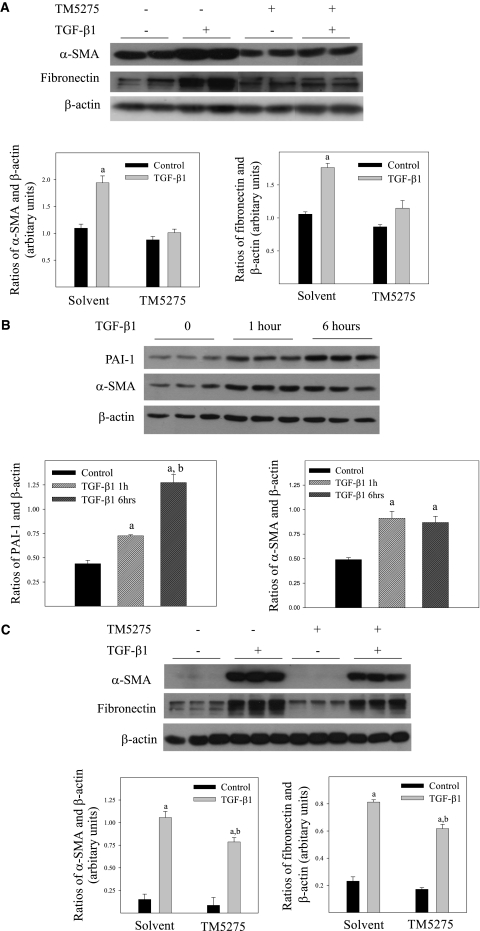
To explore further the therapeutic potential of TM5275 in the treatment of lung fibrotic diseases and the mechanism whereby TM5275 blocks AdTGF-β1223/225–induced lung fibrosis, we assessed the effects of TM5275 on TGF-β1–induced fibrotic responses in human lung fibroblasts (CCL-210 cells). TGF-β1, not AdTGF-β1223/225, was used in all in vitro studies, because the results of in vivo studies indicate that TGF-β1, and not adenovirus, caused fibrosis in murine lung tissue (Figure 1). The results show that treatment with TGF-β1 increased the activity of PAI-1 and inhibited the activity of uPA, whereas treating cells with TM5275 blocked the TGF-β1–induced activity of PAI-1 (13.7128 ± 0.3012, 19.9487 ± 1.1632, 13.5068 ± 0.3808, and 12.8755 ± 2.0121 arbitrary units for cells treated with solvent, TGF-β1, TM5275, and TGF-β1 plus TM5275, respectively) and stimulated the activity of uPA (1.0925 ± 0.0424, 0.3678 ± 0.0256, 1.2443 ± 0.1651, and 1.7916 ± 0.3619 arbitrary units for cells treated with solvent, TGF-β1, TM5275, and TGF-β1 plus TM5275, respectively). Neither TGF-β1 nor TM5275 alone exerted a significant effect on the activity of tPA. Most importantly, Western blot analyses showed that TM5275, when added to culture medium simultaneously with TGF-β1, almost completely blocked the TGF-β1–induced expression of α-SMA, fibronectin, and PAI-1 (Figure 6A).
Figure 6.
Effects of TM5275 on TGF-β1–induced expression of α-SMA, fibronectin, and PAI-1 in human lung fibroblasts. (A) Human lung fibroblasts (CCL-210 cells) were treated with 1 ng/ml of TGF-β1 in the presence or absence of 75 μM of TM5275 for 24 hours. (B) CCL-210 cells were treated with 1 ng/nl of TGF-β1 for 1 or 6 hours. (C) CCL-210 cells were pretreated with 1 ng/ml of TGF-β1 for 6 hours, and then with 75 μM of TM5275 for 24 hours. Top, representative Western blotting images. Bottom, summaries of the semiquantified data of band densities relative to β-actin band densities. aSignificantly different from solvent alone group. bSignificantly different from TGF-β1 alone–treated group (P < 0.05, n = 6).
To explore further whether TM5275 can suppress the progression of fibrosis after it is initiated, we pretreated CCL-210 cells with TGF-β1 for 1 or 6 hours, and then with 75 μM of TM5275 for 24 hours. The results show that the expression of PAI-1 and α-SMA was increased as early as 1 hour after treatment with TGF-β1 (Figure 6B). TM5275, however, significantly reduced the TGF-β1–induced expression of α-SMA and fibronectin, even after cells were pretreated with TGF-β1 for 6 hours (Figure 6B). These data further suggest that TM5275 can resolve preexisting fibrosis or block the progression of fibrosis.
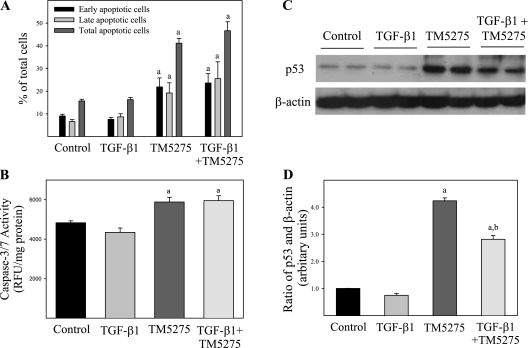
Induction of Apoptosis by PAI-1 Inhibitor in Human Lung Fibroblasts Treated with or without TGF-β1
Although the traditional view, supported by our previous studies, suggests that PAI-1 promotes fibrosis by suppressing the degradation of extracellular matrix (ECM) (21, 27), emerging evidence suggests that PAI-1 may exert its profibrogenic effects by preventing apoptosis in (myo)fibroblasts (14, 28). Therefore, we examined whether TM5275 induced apoptosis in human lung fibroblasts. Our results indicate that TGF-β1 alone exerted no significant effect on the apoptosis of CCL-210 cells, whereas TM5275 significantly increased apoptotic cell numbers in both naive (TGF-β1–untreated) and activated (TGF-β1–treated) fibroblasts (Figure 7A). Interestingly, CCL-210 cells that were treated with TGF-β1 and therefore underwent myofibroblast differentiation seemed more sensitive to TM5275-induced apoptosis than were naive fibroblasts (Figure 7A). The apoptosis induced by TM5275 also activated caspase-3 (Figure 7B) and increased the expression of p53, a tumor repressor and initiator of apoptosis (Figures 7C and 7D). Together, these data suggest that TM5275 blocked TGF-β1–induced fibrosis, probably by inducing apoptosis in (myo)fibroblasts.
Figure 7.
Induction of apoptosis by TM5275 in human lung fibroblasts treated with or without TGF-β1. CCL-210 cells were treated with 1 ng/ml of TGF-β1 in the presence or absence of 75 μM of TM5275 for 24 hours. (A) Apoptotic cell death was analyzed by flow cytometry techniques, using an Alexa Fluor 488 Annexin V/Dead Cell Apoptosis Kit from Invitrogen. Early and late stages of apoptotic cells were calculated separately or in combination. The results are expressed as percentages of total cell number. (B) The activity of caspase-3/7 was determined with an Apo-ONE Homogenous Caspase-3/7 Assay Kit from Promega. (C) Concentrations of p53 protein were determined by Western blot analysis, and the results were semiquantified with Image J software (D). aSignificantly different from corresponding untreated control group. bSignificantly different from corresponding group treated with TM5275 alone (P < 0.05, n = 7–8). RFU, relative fluorescence units.
Discussion
Fibrosis is a final stage of many diseases involved in almost all organ systems, including the lung, and no effective treatment is available for these devastating diseases. PAI-1 plays a key role in the development of fibrosis, and therefore is an ideal therapeutic target in the treatment of fibrotic diseases. Nonetheless, after the performance of intensive studies, no PAI-1 inhibitors have been developed for such purposes. Here we report for the first time, to the best of our knowledge, that TM5275, an orally effective novel small molecule PAI-1 inhibitor that demonstrated potent antithrombotic activity but low toxicity in several animal species/models (26), almost completely blocked TGF-β1–induced lung fibrosis in an animal model. In vitro studies further show that TM5275 induced the apoptosis of (myo)fibroblasts and significantly reduced fibrotic responses, even after cells were pretreated with TGF-β1 for 6 hours. These data suggest that TM5275 is a promising antifibrotic agent that can block the progression of fibrosis.
The mechanism whereby TM5275 blocked TGF-β1–induced lung fibrosis is unclear at present. TM5275 was administrated 4 days after mice were challenged with AdTGF-β1223/225. Although we did not monitor fibrotic changes until 7 days after the instillation of AdTGF-β1223/225, Sime and colleagues (29), who generated the AdTGF-β1223/225 adenovirus, reported that an extensive deposition of ECM proteins occurred, including collagen, elastin, and fibronectin, starting on Day 3 after the instillation of AdTGF-β1223/225. The induction of α-SMA, a marker of fibroblast activation, was also evident from Day 3 onward (29), suggesting that fibrosis began to develop as early as 3 days after the instillation of AdTGF-β1223/225 in this model. Importantly, a comparable amount of total (latent + active) TGF-β1 was detected in BALF after the instillation of AdTGF-β1223/225 in our study, as it was in the study of Sime and colleagues (29), suggesting that fibrotic tissue had likely been deposited in lung tissue by Day 4 after the instillation of AdTGF-β1223/225, when treatment with TM5275 began. Our new in vitro data further show that TGF-β1 induced fibrotic responses, including the expression of PAI-1 and α-SMA, as early as 1 hour after treatment. TM5275, on the other hand, significantly reduced such fibrotic responses, even after cells were pretreated with TGF-β1 for 6 hours (Figures 6B and 6C). Based on these data, we conclude that TM5275 blocks TGF-β1–induced lung fibrosis, at least in part, by resolving established fibrosis or blocking the progression of fibrosis.
TGF-β plays a critical role in the development of fibrosis. A positive feedback loop between PAI-1 and TGF-β1 was described elsewhere (30, 31). Matsuo and colleagues reported that upon unilateral ureteral obstruction, PAI-1 transgenic mice showed increased numbers of interstitial myofibroblasts and higher concentrations of TGF-β1 mRNA (30). Seo and colleagues further showed that knockout of the PAI-1 gene suppressed the expression of high glucose-induced TGF-β1 mRNA, whereas recombinant PAI-1 restored the inducibility of TGF-β1 by high glucose in PAI-1 knockout mesangial cells (31). Furthermore, they showed that recombinant PAI-1 protein stimulated TGF-β promoter activity, and that the induction of fibronectin and collagen I by recombinant PAI-1 was abrogated by the TGF-β1 receptor inhibitor or anti–TGF-β antibody (31). Together, the data strongly suggest that PAI-1 positively regulates TGF-β1 gene expression. In the present study, the intranasal instillation of AdTGF-β1223/225, but not control virus (AdDL70-3), induced endogenous TGF-β1 as well as the expression of PAI-1 in murine lungs, suggesting that the induction was caused by TGF-β1 and not adenovirus. Although the precise mechanism whereby TGF-β1 (AdTGF-β1223/225) induced its own gene expression is unclear, our data suggest that PAI-1 may be involved, because TM5275 completely blocked such an induction. These results further support the notion of a positive feedback loop between PAI-1 and TGF-β1. The data also suggest that TM5275 blocks TGF-β1–induced lung fibrosis in mice, at least in part, by breaking up this positive feedback loop.
Myofibroblasts are the major producers of ECM, and therefore contribute importantly to the development of fibrosis. Upon the resolution of normal wound healing, myofibroblasts undergo apoptosis. The dysregulation of apoptosis, therefore, leads to impaired wound healing (fibrosis) because of the prolonged activity of myofibroblasts. The mechanisms regulating the apoptosis of myofibroblasts are largely unknown at present. Interestingly, plasmin was reported to induce apoptosis, whereas PAI-1 protects (myo)fibroblasts from the apoptosis induced by different stimuli (14, 28, 32–35). The density of myofibroblasts is greater in PAI-1–overexpressing mice (30) and lower in PAI-1 knockout mice (36) upon fibrotic stimulation, which further suggests that PAI-1 may promote fibrosis by protecting myofibroblast from apoptosis. In this study, TGF-β1 increased the expression of α-SMA, a marker of myofibroblasts, in murine lungs and in human lung fibroblasts, whereas TM5275 suppressed the TGF-β1–induced expression of α-SMA both in vivo and in vitro. These data suggest that another potential mechanism, whereby TM5275 blocks TGF-β1–induced lung fibrosis, induces the apoptosis of myofibroblasts. This notion is further supported by our flow cytometry data, which show that TM5275 induced apoptosis in both naive fibroblasts (TGF-β1–untreated) and myofibroblasts (TGF-β1–treated fibroblasts). Moreover, the flow cytometry data indicate that myofibroblasts were more sensitive to TM5275-induced apoptosis than were naive fibroblasts. Because myofibroblasts are the major producers of ECM and produce much more ECM than naive fibroblasts, and because myofibroblasts can be derived from different types of cells in addition to resident fibroblasts, a drug targeting myofibroblasts should demonstrate better therapeutic potential than a drug targeting naive fibroblasts. These data suggest that TM5275 is a promising antifibrotic agent. Nonetheless, whether TM5275 has the same selectivity in vivo is unknown, as is the mechanism underlying such selectivity, and further investigation is warranted. The results from these studies will aid in the development of more effective antifibrotic drugs.
Different hypotheses were proposed to elucidate the mechanisms whereby PAI-1 protects cells from apoptosis. One potential mechanism involves the inhibition of caspase-3 activity by directly binding to caspse-3 protein (32, 37, 38). In this study, TM5275, with or without TGF-β1, slightly but significantly increased the activity of caspase-3/7. Although the underlying mechanism is unknown, the results suggest that TM5275 induced the apoptosis of (myo)fibroblasts in part by the activation of caspase-3/7 pathways. Horowitz and colleagues showed that in addition to binding to and inhibiting the activity of caspase-3, plasminogen and plasmin induced the apoptosis of fibroblasts, which was associated with pericellular fibronectin proteolysis (28). They also showed that PAI-1 protected fibroblasts from the apoptosis induced by plasminogen but not by plasmin, suggesting that PAI-1 protects fibroblasts from apoptosis by inhibiting the activation of plasminogen (28). Although we did not measure the activity of plasmin, our data show that TM5275 treatment restored or increased the activity of uPA and tPA in both TGF-β1–challenged mice and cultured human lung fibroblasts, suggesting that the induction of (myo)fibroblast apoptosis by TM5275 may result from increased plasmin activity.
p53 is a master controller of apoptosis. Here we showed for the first time, to the best of our knowledge, that the expression of p53 was increased in human lung fibroblasts by TM5275, with or without TGF-β1 treatment. TGF-β1 alone exerted no significant effect on p53 expression, but slightly reduced the TM5275-stimulated expression of p53. The data suggest that the activation of p53 is involved in TM5275-induced apoptosis in human lung fibroblasts, although the mechanism underlying the activation of p53 pathway by TM5275 remains unclear. Our results are consistent with data reported by other investigators (39, 40), indicating that the activation of p53 was associated with the apoptosis of fibroblasts induced by gallic acid or silica. p53 was also shown to prevent apoptosis or lung injury. Davis and colleagues showed that bleomycin induced more apoptosis in macrophages and lung injury in p53 null mice than in p53 heterozygous or wild-type mice (41). Ghosh and colleagues also reported that knockdown of the p53 gene in epithelial cells (surfactant protein C–expressing cells), using dominant-negative techniques, enhanced the sensitivity of mice to bleomycin-induced lung fibrosis (42). These results suggest that p53 protects macrophages and lung epithelial cells from apoptosis and thereby lung injury and fibrosis. Such contradictory effects of p53 in apoptosis and lung injury/fibrosis can be explained by the diverse functions of p53 and the role of different types of cells in the development of fibrosis. Because (myo)fibroblasts are the major producers of ECM, the induction of (myo)fibroblast apoptosis is expected to attenuate fibrosis progression. Nonetheless, whether TM5275 induces p53 and apoptosis in lung (myo)fibroblasts in vivo, and whether the induction of p53 and the apoptosis of (myo)fibroblasts underlie the protective effects of TM5275, remain to be explored further.
Finally, we point out that PAI-1 performs multiple functions and is involved in the pathogenesis of various diseases, including thrombosis, arthrosclerosis, cancer, and fibrosis. Therefore, the development of a specific PAI-1 inhibitor has been a major focus of many studies. Several strategies, involving PAI-1 inhibitory antibody (43, 44), mutant PAI-1 proteins (27, 45, 46), and PAI-1 small interfering RNA (13), were proposed to inhibit the activity of PAI-1 in the treatment of these diseases. However, the application of these large molecules in clinical settings will be limited because of potential delivery problems. Small molecule PAI-1 inhibitors offer great therapeutic potential, because they can be delivered easily to the body, and most are orally effective (47–50). Several of these small molecule PAI-1 inhibitors proved effective in the treatment of thrombosis (the major area of interest in PAI-1 inhibitor research) (47–50), but whether they demonstratee therapeutic potential for lung fibrotic diseases remains unclear. In this study, we show for the first time, to the best of our knowledge, that TM5275 at a dose of 40 mg/kg/day for 10 days, a dose much lower than that (2,000 mg/kg/day for 2 weeks) used in the previous toxicity study and shown to cause no obvious toxicity (26), almost completely blocked TGF-β1–induced lung fibrosis. These data suggest that TM5275 is a relatively safe and potent antifibrotic agent, with promising therapeutic potential in lung fibrotic diseases.
Acknowledgments
The authors thank Dr. Jack Gauldie (Department of Pathology and Molecular Medicine, McMaster University, Hamilton, Ontario, Canada) for providing the AdTGF-β1223/225 and AdDL70-3 virus, Dr. Joanne Murphy-Ullrich for insightful suggestions on the project, and Dr. Mark MacEven and Miss Kimberly Gaston Pravia for their technical assistance in the analysis of apoptosis.
Footnotes
This work was supported by grants ES011831 and 5R01HL088141 from the National Institute of Environmental Health Sciences of the National Institutes of Health and by the American Lung Association (R.-M.L.), and by National Heart, Lung, and Blood Institute grant HL 082818 (J.H.).
Originally Published in Press as DOI: 10.1165/rcmb.2011-0139OC on August 18, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Sime PJ, O'Reilly KM. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin Immunol 2001;99:308–319 [DOI] [PubMed] [Google Scholar]
- 2.Selman M, Thannickal VJ, Pardo A, Zisman DA, Martinez FJ, Lynch JP III. Idiopathic pulmonary fibrosis: pathogenesis and therapeutic approaches. Drugs 2004;64:405–430 [DOI] [PubMed] [Google Scholar]
- 3.Hunninghake GW. Antioxidant therapy for idiopathic pulmonary fibrosis. N Engl J Med 2005;353:2285–2287 [DOI] [PubMed] [Google Scholar]
- 4.King TE Jr. Clinical advances in the diagnosis and therapy of the interstitial lung diseases. Am J Respir Crit Care Med 2005;172:268–279 [DOI] [PubMed] [Google Scholar]
- 5.Ziesche R, Hofbauer E, Wittmann K, Petkov V, Block LH. A preliminary study of long-term treatment with interferon gamma–1b and low-dose prednisolone in patients with idiopathic pulmonary fibrosis. N Engl J Med 1999;341:1264–1269 [DOI] [PubMed] [Google Scholar]
- 6.Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, King TE Jr. A placebo-controlled trial of interferon gamma–1B in patients with idiopathic pulmonary fibrosis. N Engl J Med 2004;350:125–133 [DOI] [PubMed] [Google Scholar]
- 7.Prasse A, Muller KM, Kurz C, Hamm H, Virchow JC Jr. Does interferon-gamma improve pulmonary function in idiopathic pulmonary fibrosis? Eur Respir J 2003;22:906–911 [DOI] [PubMed] [Google Scholar]
- 8.Selman M. A dark side of interferon-gamma in the treatment of idiopathic pulmonary fibrosis? Am J Respir Crit Care Med 2003;167:945–946 [DOI] [PubMed] [Google Scholar]
- 9.Lardot C, Heusterpreute M, Mertens P, Philippe M, Lison D. Expression of plasminogen activator inhibitors Type-1 and Type-2 in the mouse lung after administration of crystalline silica. Eur Respir J 1998;11:912–921 [DOI] [PubMed] [Google Scholar]
- 10.El Solh AA, Bhora M, Pineda L, Aquilina A, Abbetessa L, Berbary E. Alveolar plasminogen activator inhibitor–1 predicts ARDS in aspiration pneumonitis. Intensive Care Med 2006;32:110–115 [DOI] [PubMed] [Google Scholar]
- 11.Idell S, Koenig KB, Fair DS, Martin TR, McLarty J, Maunder RJ. Serial abnormalities of fibrin turnover in evolving adult respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 1991;261:L240–L248 [DOI] [PubMed] [Google Scholar]
- 12.Kotani I, Sato A, Hayakawa H, Urano T, Takada Y, Takada A. Increased procoagulant and antifibrinolytic activities in the lungs with idiopathic pulmonary fibrosis. Thromb Res 1995;77:493–504 [DOI] [PubMed] [Google Scholar]
- 13.Senoo T, Hattori N, Tanimoto T, Furonaka M, Ishikawa N, Fujitaka K, Haruta Y, Murai H, Yokoyama A, Kohno N. Suppression of plasminogen activator inhibitor–1 by RNA interference attenuates pulmonary fibrosis. Thorax 2010;65:334–340 [DOI] [PubMed] [Google Scholar]
- 14.Chang W, Wei K, Jacobs SS, Upadhyay D, Weill D, Rosen GD. SPARC suppresses apoptosis of idiopathic pulmonary fibrosis fibroblasts through constitutive activation of beta-catenin. J Biol Chem 2010;285:8196–8206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olman MA, Mackman N, Gladson CL, Moser KM, Loskutoff DJ. Changes in procoagulant and fibrinolytic gene expression during bleomycin-induced lung injury in the mouse. J Clin Invest 1995;96:1621–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Ma J. Changes of coagulation and fibrinolysis system in bronchoalveolar lavage fluid in lung fibrosis. Beijing Da Xue Xue Bao 2005;37:516–519 [PubMed] [Google Scholar]
- 17.Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, Simon RH. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor–1 gene. J Clin Invest 1996;97:232–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, Simon RH, Drew AF. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest 2000;106:1341–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuang-Tsai S, Sisson TH, Hattori N, Tsai CG, Subbotina NM, Hanson KE, Simon RH. Reduction in fibrotic tissue formation in mice genetically deficient in plasminogen activator inhibitor–1. Am J Pathol 2003;163:445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu RM, Choi J, Wu JH, Gaston Pravia KA, Lewis KM, Brand JD, Mochel NS, Krzywanski DM, Lambeth JD, Hagood JS, et al. Oxidative modification of nuclear mitogen-activated protein kinase phosphatase 1 is involved in transforming growth factor beta1–induced expression of plasminogen activator inhibitor 1 in fibroblasts. J Biol Chem 2010;285:16239–16247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vayalil PK, Olman M, Murphy-Ullrich JE, Postlethwait EM, Liu RM. Glutathione restores collagen degradation in TGF-beta–treated fibroblasts by blocking plasminogen activator inhibitor–1 expression and activating plasminogen. Am J Physiol Lung Cell Mol Physiol 2005;289:L937–L945 [DOI] [PubMed] [Google Scholar]
- 22.Liu R-M, Liu Y, Forman HJ, Olman M, Tarpey MM. Glutathione regulates transforming growth factor–{beta}–stimulated collagen production in fibroblasts. Am J Physiol Lung Cell Mol Physiol 2004;286:L121–L128 [DOI] [PubMed] [Google Scholar]
- 23.Katre A, Ballinger C, Akhter H, Fanucchi M, Kim D-K, Postlethwait E, Liu R-M. Increased transforming growth factor beta 1 expression mediates ozone-induced airway fibrosis in mice. Inhal Toxicol 2011;23:486–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woessner JF Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 1961;93:440–447 [DOI] [PubMed] [Google Scholar]
- 25.Vayalil PK, Iles KE, Choi J, Yi A-K, Postlethwait EM, Liu R-M. Glutathione suppresses TGF-beta–induced PAI-1 expression by inhibiting p38 and JNK MAPK and the binding of AP-1, SP-1, and SMAD to the PAI-1 promoter. Am J Physiol Lung Cell Mol Physiol 2007;293:L1281–L1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izuhara Y, Yamaoka N, Kodama H, Dan T, Takizawa S, Hirayama N, Meguro K, van Ypersele de Strihou C, Miyata T. A novel inhibitor of plasminogen activator inhibitor–1 provides antithrombotic benefits devoid of bleeding effect in nonhuman primates. J Cereb Blood Flow Metab 2010;30:904–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Y, Border WA, Lawrence DA, Noble NA. Noninhibitory PAI-1 enhances plasmin-mediated matrix degradation both in vitro and in experimental nephritis. Kidney Int 2006;70:515–522 [DOI] [PubMed] [Google Scholar]
- 28.Horowitz JC, Rogers DS, Simon RH, Sisson TH, Thannickal VJ. Plasminogen activation induced pericellular fibronectin proteolysis promotes fibroblast apoptosis. Am J Respir Cell Mol Biol 2008;38:78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor–beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 1997;100:768–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuo S, Lopez-Guisa JM, Cai X, Okamura DM, Alpers CE, Bumgarner RE, Peters MA, Zhang G, Eddy AA. Multifunctionality of PAI-1 in fibrogenesis: evidence from obstructive nephropathy in PAI-1–overexpressing mice. Kidney Int 2005;67:2221–2238 [DOI] [PubMed] [Google Scholar]
- 31.Seo JY, Park J, Yu MR, Kim YS, Ha H, Lee HB. Positive feedback loop between plasminogen activator inhibitor–1 and transforming growth factor–beta1 during renal fibrosis in diabetes. Am J Nephrol 2009;30:481–490 [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Kelm RJ Jr, Budd RC, Sobel BE, Schneider DJ. Inhibition of apoptosis and caspase-3 in vascular smooth muscle cells by plasminogen activator inhibitor Type–1. J Cell Biochem 2004;92:178–188 [DOI] [PubMed] [Google Scholar]
- 33.Rossignol P, Ho-Tin-Noe B, Vranckx R, Bouton MC, Meilhac O, Lijnen HR, Guillin MC, Michel JB, Angles-Cano E. Protease nexin–1 inhibits plasminogen activation–induced apoptosis of adherent cells. J Biol Chem 2004;279:10346–10356 [DOI] [PubMed] [Google Scholar]
- 34.Lademann UA, Romer MU. Regulation of programmed cell death by plasminogen activator inhibitor Type 1 (PAI-1). Thromb Haemost 2008;100:1041–1046 [PubMed] [Google Scholar]
- 35.Higgins SP, Samarakoon R, Higgins CE, Freytag J, Wilkins-Port CE, Higgins PJ. TGF-beta1–induced expression of the anti-apoptotic PAI-1 protein requires EGFR signaling. Cell Commun Insights 2009;2:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oda T, Jung YO, Kim HS, Cai X, Lopez-Guisa JM, Ikeda Y, Eddy AA. PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int 2001;60:587–596 [DOI] [PubMed] [Google Scholar]
- 37.Schneider DJ, Chen Y, Sobel BE. The effect of plasminogen activator inhibitor Type 1 on apoptosis. Thromb Haemost 2008;100:1037–1040 [PubMed] [Google Scholar]
- 38.Balsara RD, Ploplis VA. Plasminogen activator inhibitor–1: the double-edged sword in apoptosis. Thromb Haemost 2008;100:1029–1036 [PMC free article] [PubMed] [Google Scholar]
- 39.Chuang CY, Liu HC, Wu LC, Chen CY, Chang JT, Hsu SL. Gallic acid induces apoptosis of lung fibroblasts via a reactive oxygen species–dependent ataxia telangiectasia mutated-p53 activation pathway. J Agric Food Chem 2010;58:2943–2951 [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Bowman L, Lu Y, Rojanasakul Y, Mercer RR, Castranova V, Ding M. Essential role of p53 in silica-induced apoptosis. Am J Physiol Lung Cell Mol Physiol 2005;288:L488–L496 [DOI] [PubMed] [Google Scholar]
- 41.Davis DW, Weidner DA, Holian A, McConkey DJ. Nitric oxide–dependent activation of p53 suppresses bleomycin-induced apoptosis in the lung. J Exp Med 2000;192:857–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghosh S, Mendoza T, Ortiz LA, Hoyle GW, Fermin CD, Brody AR, Friedman M, Morris GF. Bleomycin sensitivity of mice expressing dominant-negative p53 in the lung epithelium. Am J Respir Crit Care Med 2002;166:890–897 [DOI] [PubMed] [Google Scholar]
- 43.Rupin A, Martin F, Vallez MO, Bonhomme E, Verbeuren TJ. Inactivation of plasminogen activator inhibitor–1 accelerates thrombolysis of a platelet-rich thrombus in rat mesenteric arterioles. Thromb Haemost 2001;86:1528–1531 [PubMed] [Google Scholar]
- 44.Levi M, Biemond BJ, van Zonneveld AJ, ten Cate JW, Pannekoek H. Inhibition of plasminogen activator inhibitor–1 activity results in promotion of endogenous thrombolysis and inhibition of thrombus extension in models of experimental thrombosis. Circulation 1992;85:305–312 [DOI] [PubMed] [Google Scholar]
- 45.Stefansson S, Yepes M, Gorlatova N, Day DE, Moore EG, Zabaleta A, McMahon GA, Lawrence DA. Mutants of plasminogen activator inhibitor–1 designed to inhibit neutrophil elastase and cathepsin G are more effective in vivo than their endogenous inhibitors. J Biol Chem 2004;279:29981–29987 [DOI] [PubMed] [Google Scholar]
- 46.Huang Y, Haraguchi M, Lawrence DA, Border WA, Yu L, Noble NA. A mutant, noninhibitory plasminogen activator inhibitor Type 1 decreases matrix accumulation in experimental glomerulonephritis. J Clin Invest 2003;112:379–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friederich PW, Levi M, Biemond BJ, Charlton P, Templeton D, van Zonneveld AJ, Bevan P, Pannekoek H, ten Cate JW. Novel low-molecular-weight inhibitor of PAI-1 (XR5118) promotes endogenous fibrinolysis and reduces postthrombolysis thrombus growth in rabbits. Circulation 1997;96:916–921 [PubMed] [Google Scholar]
- 48.Crandall DL, Elokdah H, Di L, Hennan JK, Gorlatova NV, Lawrence DA. Characterization and comparative evaluation of a structurally unique PAI-1 inhibitor exhibiting oral in-vivo efficacy. J Thromb Haemost 2004;2:1422–1428 [DOI] [PubMed] [Google Scholar]
- 49.Hennan JK, Elokdah H, Leal M, Ji A, Friedrichs GS, Morgan GA, Swillo RE, Antrilli TM, Hreha A, Crandall DL. Evaluation of PAI-039 [{1-benzyl-5-[4-(trifluoromethoxy)phenyl]-1H-indol-3-YL}(oxo)acetic acid], a novel plasminogen activator inhibitor–1 inhibitor, in a canine model of coronary artery thrombosis. J Pharmacol Exp Ther 2005;314:710–716 [DOI] [PubMed] [Google Scholar]
- 50.Baxi S, Crandall DL, Meier TR, Wrobleski S, Hawley A, Farris D, Elokdah H, Sigler R, Schaub RG, Wakefield T, et al. Dose-dependent thrombus resolution due to oral plasminogen activator inhibitor (PAI)–1 inhibition with tiplaxtinin in a rat stenosis model of venous thrombosis. Thromb Haemost 2008;99:749–758 [DOI] [PubMed] [Google Scholar]