Abstract
The role of thyroid hormone metabolism in clinical outcomes of the critically ill remains unclear. Using preclinical models of acute lung injury (ALI), we assessed the gene and protein expression of type 2 deiodinase (DIO2), a key driver for synthesis of biologically active triiodothyronine, and addressed potential association of DIO2 genetic variants with ALI in a multiethnic cohort. DIO2 gene and protein expression levels in murine lung were validated by microarrays and immunoblotting. Lung injury was assessed by levels of bronchoalveolar lavage protein and leukocytes. Single-nucleotide polymorphisms were genotyped and ALI susceptibility association assessed. Significant increases in both DIO2 gene and D2 protein expression were observed in lung tissues from murine ALI models (LPS- and ventilator-induced lung injury), with expression directly increasing with the extent of lung injury. Mice with reduced levels of DIO2 expression (by silencing RNA) demonstrated reduced thyroxine levels in plasma and increased lung injury (increased bronchoalveolar lavage protein and leukocytes), suggesting a protective role for DIO2 in ALI. The G (Ala) allele of the Thr92Ala coding single-nucleotide polymorphism (rs225014) was protective in severe sepsis and severe sepsis–associated ALI after adjustments for age, sex, and genetic ancestry in a logistic regression model in European Americans. Our studies indicate that DIO2 is a novel ALI candidate gene, the nonsynonymous Thr92Ala coding variant of which confers ALI protection. Increased DIO2 expression may dampen the ALI inflammatory response, thereby strengthening the premise that thyroid hormone metabolism is intimately linked to the integrated response to inflammatory injury in critically ill patients.
Keywords: acute respiratory distress syndrome, hypothyroidism, mechanical ventilation, sepsis
Acute lung injury (ALI) is one of the major causes of acute respiratory failure that usually develops in response to major insults, such as sepsis, trauma, pneumonia, and multiple transfusions (1). Although mechanical ventilation (MV) is indispensable for the survival of patients with ALI (2), clinical trials have shown that improperly delivered MV causes or worsens lung injury, a syndrome known as ventilator-induced lung injury (VILI). ALI and VILI are characterized by augmented capillary leakage, acute inflammation, and increases in inflammatory cytokine expression (3, 4). Microarray analyses have identified a number of ALI-associated candidate genes correlated with the outcome of both ALI and VILI (5–8). Case–control association studies further support that genetic variants in these genes contribute to susceptibility and survival of sepsis and ALI (8–12). Despite these increasing insights into ALI/VILI pathobiology, ALI continues to carry an unacceptably high mortality (>30%), and the basis for the increased ALI morbidity/mortality remains poorly understood.
Clinicians have had a long-standing interest in the enigmatic role of thyroid hormones (THs) in critically ill patients (13) who exhibit low concentrations of thyroid prohormone, thyroxine (T4), and active hormone, 3,3′,5-tri-iodothyronine (T3), in concert with elevated concentrations of inactive reverse TH, rT3 (14–16). These profound changes in thyroid function tests have been correlated directly with the severity of illness, implying the poorest prognosis for survival (17). In addition, hypothyroidism appears to aggravate the clinical setting of lung injury, and TH deprivation impedes alveolar fluid clearance, resulting in persistent hypoxia, reduced antioxidant profiles, and worsening lung function (18). In contrast, administration of TH to critically ill patients with ALI or sepsis improves lung mechanics (19).
The exact role of TH in ALI is likely related to the enzymatic activities of the three types of deiodinases, particularly the type 2 deiodinase (D2) encoded by the type 2 deiodinase gene (DIO2; HGNC:2884). D2 plays a vital role in the maintenance of circulating and tissue hormone levels via outer-ring deiodination of T4 to drive synthesis of the biologically active T3. More recently, D2 has been suggested to regulate locally TH signaling in a tissue- and time-specific fashion, independently of TH serum concentrations (20). Although TH metabolizing genes have not yet been explored in the context of sepsis or ALI, DIO2 variants are associated with multiple disorders, including bipolar disorder, diabetes, osteoarthritis, and mental retardation (21–24).
In this study, we identified a significant dysregulation of DIO2 expression in murine ALI models. Case–control association studies determined association of the DIO2 nonsynonymous Thr92Ala (rs225014) single-nucleotide polymorphism (SNP) with susceptibility to sepsis and ALI. As increases in DIO2 lung expression may serve to dampen the inflammatory response to ALI, these results strengthen the premise that TH metabolism is intimately linked with the integrated response to inflammatory injury in critically ill patients.
Materials and Methods
NCBI Genome-Wide Expression Profiles of Preclinical Murine ALI Models
Microarray datasets GSE2368 (6), GSE11662 (7), GSE9368 (5), and GSE14525 (25) generated in spontaneously breathing (SB), LPS-, or VILI-challenged mice were evaluated. Signal intensities were normalized using robust microarray analysis (26), analyzed by Spearman's ranked correlation test, and expressed as log2 values.
In Vivo DIO2 Silencing, Bronchoalveolar Lavage Protein, Leukocytes, and Western Blot Analysis in Murine Lungs
Silencing (si) DIO2 (siDIO2) or siCTRL (10 mg/kg) was delivered intratracheally to mice 72 hours before LPS or VILI challenge. Bronchoalveolar lavage (BAL), lungs, blood, and plasma were extracted. Protein and leukocytes in the BAL were assayed as previously described (27). D2 expression levels in lung lysates were assessed by Western blot as previously described (5–7). All animal procedures conformed to the University of Illinois at Chicago Animal Care Committee guidelines.
Prohormone T4 and Thyroid-Stimulating Hormone Levels in Plasma
T4 and thyroid-stimulating hormone (TSH) levels were measured using a DRI Thyroxine Assay kit (Microgenics Corporation, Fremont, CA) and an immunoradiometric assay (Gold Standard Diagnostics, Davis, CA), respectively.
Endothelial Cell Cultures and Cyclic Stretch Studies
Human microvascular endothelial cells (ECs) were exposed to 18% cyclic stretch (CS) or static conditions for 24 hours, and D2 content in cell lysates was analyzed by Western blotting, as described previously (13).
Study Populations and Demographics
A total of 327 European Americans (EAs) and 261 African Americans (AAs) were used in this study. Severe sepsis and ALI were defined using the American–European Consensus Criteria (28) and the Society of Critical Care Medicine Consensus statements (29).
SNP Selection and Genotyping
TagIT 3.03 software (30) was used to select tagging SNPs (tSNPs) from HapMap phase II data (31, 32). Genotyping was conducted using the iPLEX Gold platform (Sequenom Inc., San Diego, CA) and validated by the TaqMan allelic discrimination assay (Life Technologies Corporation, Carlsbad, CA).
Assessment of Population Stratification among Case–Control Samples
EAs were genotyped for 93 ancestry informative markers (33). AAs were genotyped for 96 ancestry informative markers selected with average allele frequency differences > 0.6 among European, West African, and Native American/Asian populations (34, 35). EIGENSOFT was used to perform principal component analysis (36).
Statistical Analysis
Animal results were analyzed using one-way ANOVA or Newman-Keuls test. Demographics were compared by two-tailed Mann-Whitney test, and all groups were compared across diagnosis groups by one-way ANOVA. Survival rates were compared by Chi-square tests. Departures from Hardy-Weinberg equilibrium (HWE) were calculated using the Exact test (37). Association of tSNPs with disease was assessed using the Cochran-Armitage trend test. Multiple logistic regression analysis was used to adjust for age, sex, and population stratification. SPSS (SPSS Inc., Chicago, IL) was used to estimate the odds ratio (OR) and 95% confidence intervals (CI). Testing UNtyped Alleles (TUNA) software version 1.1 (38) was used to estimate allele frequencies and to perform indirect association testing of untyped SNPs with minor allele frequency ≥ 5% and multilocus linkage disequilibrium values (MD) ≥ 0.7. PHASE 2.1 (39) was used to estimate the DIO2 haplotypes. False discovery rate was assessed by QVALUE (40). EPIDAT 3.0 (http://dxsp.sergas.es) was used to perform a joint analysis using the Mantel-Haenszel test. Haplotype associations were tested using Hapstats software (41). Detailed methods are described in the online supplement.
Results
Increased DIO2 Gene and D2 Protein Expression in Murine LPS/VILI Models and in CS-Activated Human Microvascular Endothelium
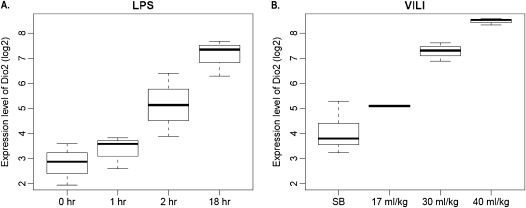
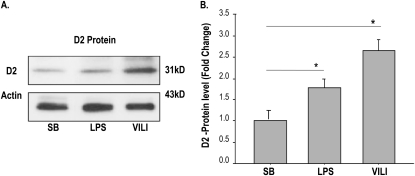
Lung expression of genes involved in TH synthesis or metabolism (DIO1, DIO2, and DIO3) were assessed in SB control and preclinical murine LPS/VILI models. Neither DIO1 nor DIO3 gene expression was significantly dysregulated (data not shown), whereas DIO2 expression was markedly up-regulated, with the magnitude correlated with lung injury. Gene expression increased with either increasing duration of LPS exposure (Figure 1A) or increasing tidal volumes (17 ml/kg << 30 ml/kg << 40 ml/kg) (Figure 1B), with maximal DIO2 expression in the LPS-challenged mouse model comparable to expression in the highest tidal volume used for MV (5- to 15-fold). D2 protein expression in lung homogenates was highly consistent with increased DIO2 gene lung expression after LPS or VILI (Figure 2). We next assessed D2 expression (Western blotting) in human microvascular ECs subjected to increased mechanical stress via 18% CS. D2 expression was significantly up-regulated in human ECs upon exposure to 18% stretch (24 h; n = 3; P < 0.05) relative to static conditions (see Figures E1A and E1B in the online supplement).
Figure 1.
Type 2 deiodinase gene (DIO2) expression in mouse lung tissues correlates with severity of LPS- and ventilation-induced lung injury (VILI). Expression profiling values (Affymetrix Microarray; Santa Clara, CA) were retrieved from the four datasets described in Materials and Methods and DIO2 expression levels assessed in two preclinical models of acute lung injury (ALI). (A) Box plot of duration-dependent DIO2 expression in LPS-induced lung injury lungs. (B) Box plot of tidal volume–dependent DIO2 expression in VILI lungs. Signal intensities were normalized using the Robust Multi-Array algorithm and expressed as log2 values.
Figure 2.
D2 protein expression in LPS- and ventilation-induced lung injury (VILI) models. (A) D2 protein and β-actin expression levels were detected by Western blotting from lung homogenates of spontaneous breathing (SB)–, LPS-, and VILI-challenged mice. (B) Graphical presentation of normalized D2 protein levels to β-actin and expressed in fold change showing significant increases of D2 in LPS- and VILI-challenged mice compared with SB mice. All values are presented as mean ± SD from five mice per experimental group. LPS denotes endotoxin-induced ALI; *P < 0.05.
siDIO2 Expression Augments LPS- and VILI-Mediated Lung Injury
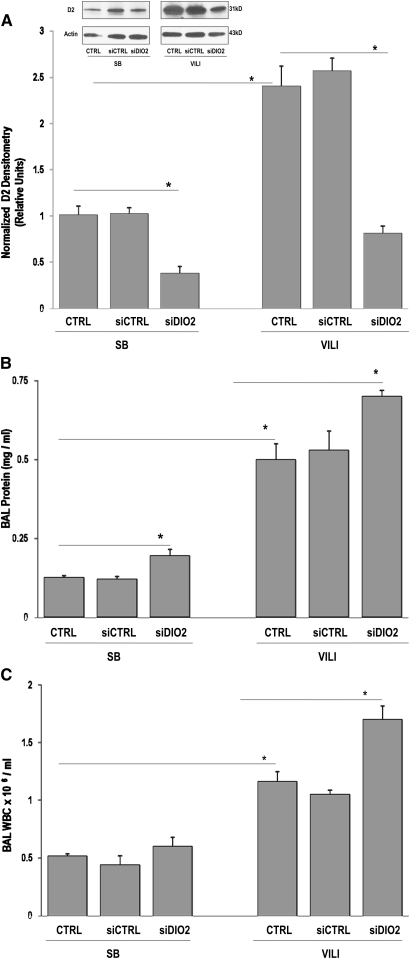
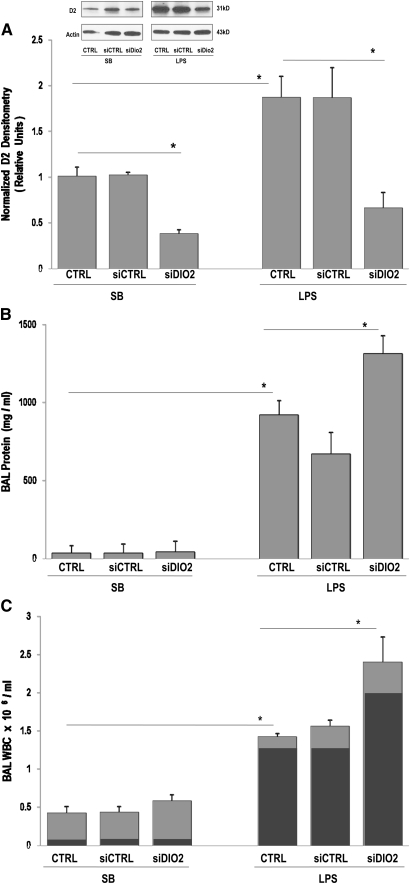
Exposure to a silencing RNA directed at DIO2 successfully reduced DIO2 gene and subsequent D2 protein expression (P < 0.0001 versus SB controls) (Figure 3A, inset). In addition, VILI-mediated increases in D2 protein expression were significantly reduced by siDIO2 pretreatment (P < 0.0001 versus VILI) (Figure 3A). DIO2-silenced VILI mice exhibited marked susceptibility to lung injury with increased BAL protein level (Figure 3B) and total leukocytes count compared with VILI CTRL or VILI siCTRL mice (Figure 3C), indicating augmentations of VILI by siDIO2. Similar to VILI, siDIO2 significantly attenuated LPS-induced increases in lung D2 protein levels (Figure 4A, inset) and exacerbated LPS-induced lung injury reflected by BAL protein (Figure 4B) and BAL leukocytes (Figure 4C), represented primarily by increased neutrophil influx (Figure 4C, inset).
Figure 3.
VILI is augmented by silencing (si) DIO2 (siDIO2). (A) In vivo silencing effect of custom-designed siDIO2 in lung homogenates was confirmed by Western blotting (inset), quantified by densitometry, and expressed in bar graphs as normalized D2 densitometry in relative units. Significant reduction of D2 expression was observed in siDIO2 pretreated SB mice. D2 level augmented by VILI was markedly reduced by siDIO2 compared with VILI vehicle control (CRTL) or scrambled siRNA (siCTRL) mice. (B) Bronchoalveolar lavage (BAL) protein levels in SB and VILI mice. Significant increase of BAL proteins was observed in siDIO2-pretreated SB or VILI mice compared with CRTL or siCTRL mice. (C) BAL leukocytes (white blood cells [WBCs]) were expressed as 106/ml in SB and VILI mice. A significant increase of BAL leukocytes was observed in siDIO2-pretreated SB or VILI mice compared with CRTL or siCTRL mice. A tidal volume of 30 ml/kg for 4 hours was used for the VILI model, which was evidenced by minimal inflammatory cells observed in SB mice treated with vehicle control (CTRL) or siCTRL, whereas inflammation and protein leak increased in VILI mice (CTRL or siCTRL). siDIO2 (10 mg/kg) or siCTRL was delivered intratracheally 72 hours before the experiments. Results are from at least five independent experiments; *P < 0.05.
Figure 4.
LPS-induced lung injury is augmented by siDIO2. (A) In vivo silencing effect of custom-designed siDIO2 in lung homogenates was confirmed by Western blotting (inset), quantified by densitometry, and expressed in bar graphs as normalized D2 densitometry in relative units. A significant reduction of D2 expression was observed in siDIO2-pretreated SB mice compared with vehicle control (CRTL) or scrambled siRNA (siCTRL) mice. D2 level augmented by LPS was markedly reduced by siDIO2 compared with LPS vehicle control (CRTL) or scrambled siRNA (siCTRL). (B) BAL protein levels in SB and LPS mice. Significant increase of BAL proteins was observed in siDIO2-pretreated SB or LPS mice compared with CRTL or siCTRL mice. (C) BAL leukocytes (WBC) were expressed as 106/ml in SB and LPS mice. A significant increase of BAL leukocytes (light gray bars) represented primarily by increased neutrophil influx (dark gray bars) was observed in siDIO2-pretreated SB or LPS mice compared with CRTL or siCTRL mice. LPS (1 mg/kg) was intratracheally administrated for 18 hours for the LPS model. Minimal inflammatory cells were observed in SB treated with vehicle control (CTRL) or siCTRL, whereas inflammation and protein leak were increased in LPS mice (CTRL or siCTRL). siDIO2 (10 mg/kg) or siCTRL was delivered intratracheally 72 hours before the experiments. Results are from at least five independent experiments; *P < 0.05.
T4 levels in plasma were reduced in either LPS- or VILI-challenged mice (CTRL, siCTRL) compared with SB controls, whereas T4 levels were significantly augmented in LPS- or VILI-challenged mice pretreated with siDIO2 compared with LPS- or VILI-challenged mice pretreated with vehicle (CTRL) or scrambled silencing RNA (siCTRL), respectively, confirming the potential protective role for D2 in ALI. T4 levels in each animal model under specific treatment are listed in Table 1. However, TSH levels measured were below detectable level (<0.03 ng/dl) within each experimental group (data not shown).
TABLE 1.
PROHORMONE THYROXINE BLOOD LEVELS IN SPONTANEOUS BREATHING, VENTILATION-INDUCED LUNG INJURY, AND LPS-INDUCED LUNG INJURY MURINE MODELS
| T4 Level (μg/dl) |
|||
| Treatment | SB Model | VILI Model | LPS Model |
| CTRL | 3.8 ± 05 | 1.5 ± 0.2* | 1.8 ± 0.2† |
| siCTRL | 4.9 ± 0.5 | 1.9 ± 0.2 | 1.7 ± 0.3 |
| siDIO2 | 3.5 ± 0.2 | 2.9 ± 0.3‡ | 2.6 ± 0.1§ |
Definition of abbreviations: CTRL, control; SB, spontaneous breathing; siCTRL, silencing control; siDIO2, silencing type 2 deiodinase; T4, thyroxine; VILI, ventilation-induced lung injury.
T4 level is presented as mean ± SE and analyzed by one-way ANOVA or nonparametric Newman-Keuls test.
P < 0.05 for VILI CTRL versus SB (CTRL, siCTRL, and siDIO2).
P < 0.05 for LPS CTRL versus SB (CTRL, siCTRL, and siDIO2).
P = 0.041 between VILI siDIO2, VILI CTRL, and VILI siCTRL.
P = 0.0039 between LPS siDIO2, LPS CTRL, and LPS siCRTL.
A DIO2 Coding SNP (Thr92Ala) Associates with Human Risk for ALI
We next performed a case–control association study to test whether DIO2 genetic variants were associated with susceptibility to sepsis-associated ALI. Demographic and disease severity characteristics are depicted in Table 2. All subjects with sepsis enrolled experienced either severe sepsis or septic shock, with the lung serving as the most common site of infection. Age, Acute Physiology and Chronic Health Evaluation II scores (mean ± SD), and comorbid factors were not significantly different between AAs and EAs. Hospital survival, measured up to 60 days, was reduced in patients with ALI versus those with sepsis for both ethnic groups.
TABLE 2.
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF THE STUDIED POPULATION
| SS |
SS with ALI/ARDS |
Controls |
||||
| Characteristics | AA | EA | AA | EA | AA | EA |
| Sample size | 78 | 139 | 41 | 78 | 187 | 188 |
| Sex, male/female | 32/43 | 78/64 | 19/23 | 43/36 | 9/124 | 56/76 |
| Age | 53.9 ± 19.3 | 62.0 ± 16.1 | 44.6 ± 15.4 | 58.6 ± 16.9 | 54.8 ± 17.4 | 55.6 ± 12.4 |
| APACHE II | 27.1 ± 7.0 | 28.4 ± 7.6 | 28.0 ± 7.7 | 29.7 ± 7.4 | NA | NA |
| Survival, % | 62.7 | 64.1 | 52.4 | 62.0 | NA | NA |
| Smoking history, % | 37.7 | 38.8 | 34.2 | 40.3 | Unavailable | Unavailable |
| Renal failure, % | 29.3 | 19.0 | 21.4 | 19.0 | 0.0 | 0.0 |
| Cancer, % | 10.7 | 21.3 | 11.9 | 21.8 | 0.0 | 0.0 |
| CLD, % | 13.5 | 12.0 | 4.9 | 13.9 | 0.0 | 0.0 |
| Diabetes, % | 20.0 | 19.7 | 16.7 | 19.0 | 0.0 | 0.0 |
| COPD, % | 8.0 | 20.3 | 2.4 | 14.3 | 0.0 | 0.0 |
| Anemia, % | 13.3 | 8.5 | 9.5 | 7.7 | 0.0 | 0.0 |
| AIDS, % | 13.3 | 1.4 | 19.0 | 2.5 | Unavailable | Unavailable |
| Site of Infection* | ||||||
| Lung, % | 57.3 | 63.4 | 71.4 | 73.4 | NA | NA |
| Abdomen/GI, % | 12.0 | 20.4 | 11.9 | 19.0 | NA | NA |
| UTI, % | 16.0 | 18.3 | 9.5 | 10.1 | NA | NA |
| Other, % | 28.0 | 26.8 | 21.4 | 21.5 | NA | NA |
Definition of abbreviations: AA, African American; AIDS, acquired immune deficiency virus; ALI, acute lung injury; APACHE II, Acute Physiology and Chronic Health Evaluation II; ARDS, acute respiratory distress syndrome; CLD, chronic liver disease; COPD, chronic obstructive pulmonary disease; EA, European American; GI, gastrointestinal; NA, not applicable; UTI, urinary tract infection; SS, severe sepsis.
More than one site of infection is listed for some patients; sum of percentages exceeds 100%.
Genotyping completion rate of all tSNPs was > 97% (see Table E1 in the online supplement). SNPs that showed a minor allele frequency < 1% in EAs (i.e., rs225019 and rs17110436) were excluded from analysis. Considering the number of tests performed, three SNPs (rs225017, rs225011, and rs12885300) significantly deviated from HWE in EA subjects with severe sepsis. However, these SNPs were retained for association analysis, as HWE deviations were observed only in ALI group, and association tests did not rely on the assumption of HWE. None of the tSNPs deviated from HWE in the control group or in AA samples. Genotyping was blinded to case–control status and ethnicity, and population stratification correction was performed in association tests.
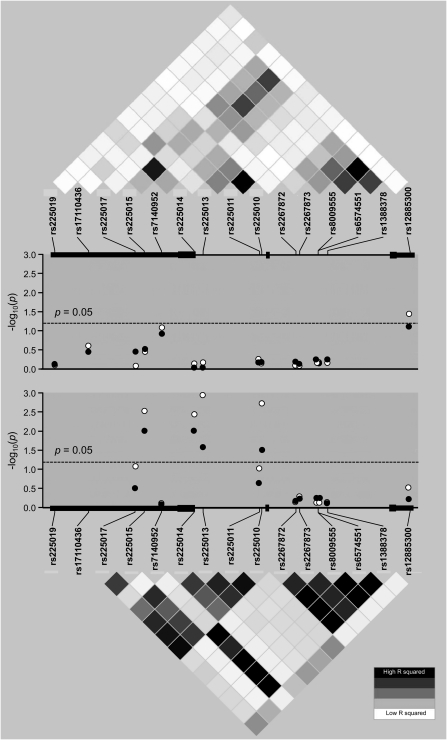
The strongest direct association in EAs was found for rs225014, predicting a coding SNP within exon 3 resulting in a nonsynonymous amino acid change (Thr92Ala). This SNP was associated with reduced susceptibility for severe sepsis (P = 0.009; q = 0.030) and severe sepsis–associated ALI (P = 0.004; q = 0.020) (Table E2, Figure 5), and remained significant after adjustments for age, sex, and population stratification using the first ancestral principal component scores in a logistic regression model (for G [Ala] allele: OR = 0.66; 95% CI = 0.48–0.92; P = 0.013 for severe sepsis; and OR = 0.53; 95% CI = 0.34–0.82; P = 0.003 for severe sepsis–associated ALI). Duplicate genotyping of rs225014 using a TaqMan assay showed a minimal discrepancy rate with the results obtained by iPLEX (0.15% [95% CI = 0.01–0.96%]), not affecting the association significance. Indirect association testing of untyped variants using TUNA showed three additional SNPs (rs225015 at 3′ flanking, rs225013, and rs225010 in intron 2) associated with severe sepsis and with severe sepsis–associated ALI (0.001 ≤ P ≤ 0.033; 0.020 ≤ q ≤ 0.083) (Table E2). However, all these three SNPs showed moderate-to-strong linkage disequilibrium with SNP rs225014 (0.73 ≤ D′ ≤1, 0.50 ≤ r2 ≤ 0.78), suggesting the existence of a single association. The results were largely similar if the three SNPs significantly deviating from HWE were excluded during TUNA computations (data not shown).
Figure 5.
Plots of association P values of tested DIO2 tagging and imputed single-nucleotide polymorphisms (SNPs) with severe sepsis and severe sepsis–associated ALI. Depicted are association P values of tested DIO2 tagging and imputed SNPs for African Americans (top panel) and European Americans (bottom panel). Dashed line represents a P value of 0.05. Closed circles depict association with severe sepsis, and open circles depict association with severe sepsis–associated ALI. For reference, a linkage disequilibrium (LD) plot of r2 values is shown for the SNPs of the gene. Each diamond of the LD plot represents a pairwise SNP comparison, with its r2 value schematically symbolized by a color gradient, ranging from black (r2 = 1, complete LD) to gray (1 ≤ r2 ≤ 0, moderate LD) and white (r2 = 0, absence of LD).
For AAs, only the rs12885300 SNP (a.k.a. [ORFa]-Gly3Asp) located on the 5′ flanking region of DIO2 was associated, albeit weakly, with severe sepsis–associated ALI (P = 0.039, q = 0.156), and failed to reach significance after covariate adjustments including population stratification (OR = 2.26; 95% CI = 0.92–5.51). Indirect association testing of untyped variants using TUNA did not show any other associated SNPs among AAs. Similarly, none of the DIO2 genetic variants associated significantly with sepsis survival (Table E3). A search for the presence of associations across combined EA and AA groups did not identify additional associated SNPs, and failed to increase the statistical significance of the four SNPs (rs225015, rs225014, rs225013, and rs225010) previously found to be associated with either severe sepsis or severe sepsis–associated ALI in EAs (Table E2).
Pleiotropic effects have been described for genetic variants and complex diseases for which any prior knowledge of being affected by overlapping pathways was lacking (42). Thus, we assumed that haplotypes of rs225014 and rs12885300 of DIO2 gene, which were previously associated with osteoarthritis, may exert similar effects in ALI. Subsequently, we tested if haplotypes of rs225014 and rs12885300 were associated with ALI (both associated in this study at least at nominal significance). Our data revealed three common haplotypes (frequency > 5%) in both EA and AA groups. The common haplotype, rs225014G-rs12885300C, showed a significant association with severe sepsis (P = 0.026) and severe sepsis–associated ALI (P = 0.005), but only among EAs. However, the association was weakened after adjusting for age, sex, and genetic ancestry (Table E4).
Discussion
The role of THs and metabolism in ALI outcomes, although likely important (43–45), is complex and incompletely understood. Our preclinical and clinical studies in this report are the first to directly implicate lung tissue expression and enzymatic activity of D2 in the response to ALI. Our microarray data show that DIO2 expression was increased during lung injury. It is also interesting to evaluate the expression of DIO2 in the context of other classes of genes in the family to determine whether DIO2 up-regulation is dependent on other genes. However, with the current microarray technology, we are unable to determine the dependence of dysregulation between genes. To ensure that DIO2 up-regulation is selective after LPS or VILI challenges, we assessed expression profiles for additional genes in the DIO family (i.e., DIO1 and DIO3), but failed to observe an expression pattern similar to DIO2. The DIO2 protein (D2) level was increased in LPS and VILI animal models. Similarly, an in vitro model exposing ECs to 18% CS, a strain relevant to pathophysiological conditions produced by MV and that causes progressive cell death (46), increases D2 expression in human lung endothelium. Reductions in DIO2 expression by siDIO2 augmented the magnitude of lung injury induced by LPS or ventilation reflected by increased BAL protein level and leukocytes. In most tissues, the majority of nuclear T3 is derived from intracellular T4 conversion to biologically active T3 (47) driven by D2. Reductions in T3 and TSH are observed in critically ill patients and in preclinical models of sepsis and ALI (48, 49), and correlate with the severity of illness and survival (17). In our in vivo experiments, we observed T4 levels to decline without a concomitant increase in TSH levels, likely reflecting the transitional phase of the disease. In addition, TSH exhibits a much shorter half-life (1 h), with short, pulsatile release or diurnal fluctuations in secretion compared with T4 half-life (1 wk), consistent with acute perturbation of the pituitary–thyroid axis, often resulting in transient non–steady-state conditions. Together, our findings support the hypothesis that lung tissue–specific expression of DIO2 may be an integral aspect of the compensatory response to reductions in local TH signaling (50, 51) due to severe inflammation and mechanical stress. Studies are underway to explore fully the potential mechanism of the protective effects of D2 in lung injury.
Consistent with this critical and protective role for D2 in the response to acute inflammatory lung injury, we identified DIO2 as an ALI candidate gene, with variants conferring significant risk for development of severe sepsis and severe sepsis–associated ALI in critically ill patients. We observed that European descent carriers of the G (Ala) allele at the nonsynonymous coding SNP (Thr92Ala, rs225014 A/G) were protected against development of both severe sepsis and severe sepsis–associated ALI, but not among subjects of African descent. The failure to replicate SNP associations with complex diseases between populations of European and African descent has been well recognized in the literature, even in well powered studies (52, 53). This may reflect population-specific gene–gene or gene–environment interactions, or complex pathways with exchangeable genetic variants. Furthermore, a post hoc calculation of statistical power for EA and AA samples indicated that the study attained power of 78% for EAs and 61% for AAs to detect effects of a minimum of 2.0 odd ratio with a two-sided P of 0.05 significance level for SNPs with minor allele frequencies of 0.25. Thus, the limited statistical power of the study in AA samples or the genetic heterogeneity among populations (32) could account for the lack of replication of rs225014 association in AAs. Although the substitution of Thr to Ala is nonconservative (aliphatic for polar), codon 92 does not reside in the active catalytic site of the enzyme, and is not phylogenetically conserved, as rodents exhibit proline in the homologous position. It is worth noting, however, that Thr92 is the first amino acid of the instability loop in D2, a key determinant of D2 turnover rate (54). Studies addressing the functional consequences of this coding SNP have yielded conflicting results, with in vitro studies failing to identify a functional effect of Thr92Ala (55). However, recent studies have demonstrated a strong allelic imbalance in particular cell types where the G allele was expressed at higher levels than the A allele (56), a finding that is congruent with the protective role of DIO2 in ALI. In addition, in vivo studies indicate that patients with athyroid 92Ala/92Ala require higher T4 dosing for optimal TSH suppression (57, 58). Furthermore, D2 reaction velocity is significantly lower in thyroid tissue and skeletal muscle samples from subjects with 92Ala/92Ala (21, 50). Most recently, the Thr92Ala SNP has been used to predict improved responses to combination T4/T3 replacement therapy (59).
Although the Thr92Ala SNP does not appear to influence serum TH levels in healthy subjects, a polymorphism in the 5′-untranslated region of the DIO2 gene (rs12885300 [a.k.a., ORFa-Gly3Asp]) is associated with altered circulating TH parameters (60, 61). Open reading frame a (ORFa) is the site among the three short ORFs primarily responsible for the inhibitory effect of this 5′-untranslated region SNP on D2 translation (62). Genotypes 3Gly–3Gly, 92Ala–92Ala, as well as the haplotype ORFa-3Gly–92Ala, decrease D2 enzymatic activity, alter absolute and relative T3 and T4 levels, and contribute to the pathogenesis of neurologic disease (23, 62), suggesting that other functional genetic variants of DIO2 other than Thr92Ala may be involved. Our results do not provide strong support for rs12885300 (ORFa-Gly3Asp) in ALI susceptibility given the nominal significance of association individually among AAs and when combined into haplotypes (92Ala-ORFa-3Gly) in EAs. Although the association is not significant after statistical adjustments for covariates, including genetic ancestry, this DIO2 haplotype associated with ALI protection is also associated with risk for osteoarthritis (24). In contrast, we identified several DIO2 SNPs to be strongly associated with severe sepsis and sepsis-associated ALI as well among EAs. These data suggest a more complex scenario underlying the association of DIO2 in ALI susceptibility, involving additional polymorphisms and/or epistatic interactions. Further studies are required to assess fully the impact of DIO2 genetic variants in sepsis and ALI.
In summary, our study sheds considerable light on the perplexing association of TH levels and metabolism with outcomes in critically ill patient with sepsis and ALI. Although further studies using D2 knockout mice or D2 overexpression models, as well as additional replication and functional studies of DIO2 genetic variants, would establish a direct role of D2 in ALI and sepsis, our genomic and genetic findings strongly indicate DIO2 as a novel susceptibility gene conferring risk to severe sepsis and to severe sepsis–associated ALI. Future investigations should focus on the mechanism by which TH might be causally related to the development of ALI, and examine gene–gene or gene–environment interactions. Strategies designed to increase DIO2 lung expression or TH levels as a platform for future targeted genotype-based therapy will benefit critically ill patients at risk for ALI and VILI.
Supplementary Material
Acknowledgments
The authors acknowledge the valuable input of Drs. Lawrence Frohman, Olga Barca-Mayo, Roy Weiss, and Samuel Refetoff.
Footnotes
Author Contributions: Every coauthor listed contributed significantly to this work.
This work was supported by National Institutes of Health grant HL 58094 (J.G.N.G.) and Instituto de Salud Carlos III and Gobierno de Canarias grant EMER07/001 (C.F.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0179OC on June 17, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med 1995;151:293–301 [DOI] [PubMed] [Google Scholar]
- 2.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 2007;369:1553–1564 [DOI] [PubMed] [Google Scholar]
- 3.Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 1999;282:54–61 [DOI] [PubMed] [Google Scholar]
- 4.Tremblay LN, Miatto D, Hamid Q, Govindarajan A, Slutsky AS. Injurious ventilation induces widespread pulmonary epithelial expression of tumor necrosis factor–alpha and interleukin-6 messenger RNA. Crit Care Med 2002;30:1693–1700 [DOI] [PubMed] [Google Scholar]
- 5.Hong SB, Huang Y, Moreno-Vinasco L, Sammani S, Moitra J, Barnard JW, Ma SF, Mirzapoiazova T, Evenoski C, Reeves RR, et al. Essential role of pre–B-cell colony enhancing factor in ventilator-induced lung injury. Am J Respir Crit Care Med 2008;178:605–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma SF, Grigoryev DN, Taylor AD, Nonas S, Sammani S, Ye SQ, Garcia JG. Bioinformatic identification of novel early stress response genes in rodent models of lung injury. Am J Physiol Lung Cell Mol Physiol 2005;289:L468–L477 [DOI] [PubMed] [Google Scholar]
- 7.Meyer NJ, Huang Y, Singleton PA, Sammani S, Moitra J, Evenoski CL, Husain AN, Mitra S, Moreno-Vinasco L, Jacobson JR, et al. GADD45a is a novel candidate gene in inflammatory lung injury via influences on Akt signaling. FASEB J 2009;23:1325–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye SQ, Simon BA, Maloney JP, Zambelli-Weiner A, Gao L, Grant A, Easley RB, McVerry BJ, Tuder RM, Standiford T, et al. Pre–B-cell colony–enhancing factor as a potential novel biomarker in acute lung injury. Am J Respir Crit Care Med 2005;171:361–370 [DOI] [PubMed] [Google Scholar]
- 9.Ma SF, Flores C, Wade MS, Dudek SM, Nicolae DL, Ober C, Garcia JG. A common cortactin gene variation confers differential susceptibility to severe asthma. Genet Epidemiol 2008;32:757–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flores C, Ma SF, Maresso K, Wade MS, Villar J, Garcia JG. IL6 gene–wide haplotype is associated with susceptibility to acute lung injury. Transl Res 2008;152:11–17 [DOI] [PubMed] [Google Scholar]
- 11.Gao L, Flores C, Ma SF, Miller EJ, Moitra J, Moreno L, Wadgaonkar R, Simon B, Brower R, Sevransky J, et al. Macrophage migration inhibitory factor in acute lung injury: expression, biomarker, and associations. Transl Res 2007;150:18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao L, Grant A, Halder I, Brower R, Sevransky J, Maloney JP, Moss M, Shanholtz C, Yates CR, Meduri GU, et al. Novel polymorphisms in the myosin light chain kinase gene confer risk for acute lung injury. Am J Respir Cell Mol Biol 2006;34:487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stathatos N, Levetan C, Burman KD, Wartofsky L. The controversy of the treatment of critically ill patients with thyroid hormone. Best Pract Res Clin Endocrinol Metab. 2001;15:465–478 [DOI] [PubMed] [Google Scholar]
- 14.Bermudez F, Surks MI, Oppenheimer JH. High incidence of decreased serum triiodothyronine concentration in patients with nonthyroidal disease. J Clin Endocrinol Metab 1975;41:27–40 [DOI] [PubMed] [Google Scholar]
- 15.Kaplan MM, Larsen PR, Crantz FR, Dzau VJ, Rossing TH, Haddow JE. Prevalence of abnormal thyroid function test results in patients with acute medical illnesses. Am J Med 1982;72:9–16 [DOI] [PubMed] [Google Scholar]
- 16.Peeters RP, Wouters PJ, Kaptein E, van Toor H, Visser TJ, Van den Berghe G. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J Clin Endocrinol Metab 2003;88:3202–3211 [DOI] [PubMed] [Google Scholar]
- 17.Kaptein EM, Weiner JM, Robinson WJ, Wheeler WS, Nicoloff JT. Relationship of altered thyroid hormone indices to survival in nonthyroidal illnesses. Clin Endocrinol (Oxf) 1982;16:565–574 [DOI] [PubMed] [Google Scholar]
- 18.Palmer KC, Mari F, Malian MS. Cadmium-induced acute lung injury: compromised repair response following thyroidectomy. Environ Res 1986;41:568–584 [DOI] [PubMed] [Google Scholar]
- 19.Inan M, Koyuncu A, Aydin C, Turan M, Gokgoz S, Sen M. Thyroid hormone supplementation in sepsis: an experimental study. Surg Today 2003;33:24–29 [DOI] [PubMed] [Google Scholar]
- 20.Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeold A, Bianco AC. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev 2008;29:898–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canani LH, Capp C, Dora JM, Meyer EL, Wagner MS, Harney JW, Larsen PR, Gross JL, Bianco AC, Maia AL. The type 2 deiodinase A/G (Thr92Ala) polymorphism is associated with decreased enzyme velocity and increased insulin resistance in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2005;90:3472–3478 [DOI] [PubMed] [Google Scholar]
- 22.Guo TW, Zhang FC, Yang MS, Gao XC, Bian L, Duan SW, Zheng ZJ, Gao JJ, Wang H, Li RL, et al. Positive association of the DIO2 (deiodinase type 2) gene with mental retardation in the iodine-deficient areas of China. J Med Genet 2004;41:585–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He B, Li J, Wang G, Ju W, Lu Y, Shi Y, He L, Zhong N. Association of genetic polymorphisms in the type II deiodinase gene with bipolar disorder in a subset of Chinese population. Prog Neuropsychopharmacol Biol Psychiatry 2009;33:986–990 [DOI] [PubMed] [Google Scholar]
- 24.Meulenbelt I, Min JL, Bos S, Riyazi N, Houwing-Duistermaat JJ, van der Wijk HJ, Kroon HM, Nakajima M, Ikegawa S, Uitterlinden AG, et al. Identification of DIO2 as a new susceptibility locus for symptomatic osteoarthritis. Hum Mol Genet 2008;17:1867–1875 [DOI] [PubMed] [Google Scholar]
- 25.Mirzapoiazova T, Moitra J, Moreno-Vinasco L, Sammani S, Turner JR, Chiang ET, Evenoski C, Wang T, Singleton PA, Huang Y, et al. Non–muscle myosin light chain kinase isoform is a viable molecular target in acute inflammatory lung injury. Am J Respir Cell Mol Biol 2010;44:40–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 2003;31:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin–induced inflammatory lung injury. Am J Respir Crit Care Med 2004;169:1245–1251 [DOI] [PubMed] [Google Scholar]
- 28.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American–European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818–824 [DOI] [PubMed] [Google Scholar]
- 29.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20:864–874 [PubMed] [Google Scholar]
- 30.Weale ME, Depondt C, Macdonald SJ, Smith A, Lai PS, Shorvon SD, Wood NW, Goldstein DB. Selection and evaluation of tagging SNPs in the neuronal-sodium-channel gene SCN1A: implications for linkage-disequilibrium gene mapping. Am J Hum Genet 2003;73:551–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA, Belmont JW, Boudreau A, Hardenbol P, Leal SM, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature 2007;449:851–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flores C, Ma SF, Maresso K, Ober C, Garcia JG. A variant of the myosin light chain kinase gene is associated with severe asthma in African Americans. Genet Epidemiol 2007;31:296–305 [DOI] [PubMed] [Google Scholar]
- 33.Price AL, Butler J, Patterson N, Capelli C, Pascali VL, Scarnicci F, Ruiz-Linares A, Groop L, Saetta AA, Korkolopoulou P, et al. Discerning the ancestry of European Americans in genetic association studies. PLoS Genet 2008;4:e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian C, Hinds DA, Shigeta R, Adler SG, Lee A, Pahl MV, Silva G, Belmont JW, Hanson RL, Knowler WC, et al. A genomewide single-nucleotide-polymorphism panel for Mexican American admixture mapping. Am J Hum Genet 2007;80:1014–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian C, Hinds DA, Shigeta R, Kittles R, Ballinger DG, Seldin MF. A genomewide single-nucleotide-polymorphism panel with high ancestry information for African American admixture mapping. Am J Hum Genet 2006;79:640–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 2006;38:904–909 [DOI] [PubMed] [Google Scholar]
- 37.Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet 2005;76:887–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wen X, Nicolae DL. Association studies for untyped markers with TUNA. Bioinformatics 2008;24:435–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 2001;68:978–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA 2003;100:9440–9445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng D, Lin DY, Avery CL, North KE, Bray MS. Efficient semiparametric estimation of haplotype–disease associations in case–cohort and nested case–control studies. Biostatistics 2006;7:486–502 [DOI] [PubMed] [Google Scholar]
- 42.Pino-Yanes M, Ma SF, Sun X, Tejera P, Corrales A, Blanco J, Perez-Mendez L, Espinosa E, Muriel A, Blanch L, Interleukin-1 receptor–associated kinase 3 gene associates with susceptibility to acute lung injury. Am J Respir Cell Mol Biol 2011;45:740–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monig H, Arendt T, Meyer M, Kloehn S, Bewig B. Activation of the hypothalamo–pituitary–adrenal axis in response to septic or non-septic diseases—implications for the euthyroid sick syndrome. Intensive Care Med 1999;25:1402–1406 [DOI] [PubMed] [Google Scholar]
- 44.Okutan O, Kartaloglu Z, Onde ME, Bozkanat E, Kunter E. Pulmonary function tests and thyroid hormone concentrations in patients with chronic obstructive pulmonary disease. Med Princ Pract 2004;13:126–128 [DOI] [PubMed] [Google Scholar]
- 45.Scoscia E, Baglioni S, Eslami A, Iervasi G, Monti S, Todisco T. Low triiodothyronine (T3) state: a predictor of outcome in respiratory failure? Results of a clinical pilot study. Eur J Endocrinol 2004;151:557–560 [DOI] [PubMed] [Google Scholar]
- 46.Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol 2003;285:L785–L797 [DOI] [PubMed] [Google Scholar]
- 47.Larsen PR, Silva JE, Kaplan MM. Relationships between circulating and intracellular thyroid hormones: physiological and clinical implications. Endocr Rev 1981;2:87–102 [DOI] [PubMed] [Google Scholar]
- 48.Sen S, Dulchavsky SA, Dutta S. Effects of triiodothyronine (T3) supplementation upon ozone-induced lung injury. Free Radic Res Commun 1993;18:299–308 [DOI] [PubMed] [Google Scholar]
- 49.Ture M, Memis D, Kurt I, Pamukcu Z. Predictive value of thyroid hormones on the first day in adult respiratory distress syndrome patients admitted to ICU: comparison with SOFA and APACHE II scores. Ann Saudi Med 2005;25:466–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mebis L, Langouche L, Visser TJ, Van den Berghe G. The type II iodothyronine deiodinase is up-regulated in skeletal muscle during prolonged critical illness. J Clin Endocrinol Metab 2007;92:3330–3333 [DOI] [PubMed] [Google Scholar]
- 51.Mebis L, van den Berghe G. The hypothalamus–pituitary–thyroid axis in critical illness. Neth J Med 2009;67:332–340 [PubMed] [Google Scholar]
- 52.Shriner D, Adeyemo A, Gerry NP, Herbert A, Chen G, Doumatey A, Huang H, Zhou J, Christman MF, Rotimi CN. Transferability and fine-mapping of genome-wide associated loci for adult height across human populations. PLoS ONE 2009;4:e8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathias RA, Grant AV, Rafaels N, Hand T, Gao L, Vergara C, Tsai YJ, Yang M, Campbell M, Foster C, et al. A genome-wide association study on African-ancestry populations for asthma. J Allergy Clin Immunol 2010;125:336–346e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dentice M, Bandyopadhyay A, Gereben B, Callebaut I, Christoffolete MA, Kim BW, Nissim S, Mornon JP, Zavacki AM, Zeold A, et al. The Hedgehog-inducible ubiquitin ligase subunit WSB-1 modulates thyroid hormone activation and PTHrP secretion in the developing growth plate. Nat Cell Biol 2005;7:698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chidakel A, Mentuccia D, Celi FS. Peripheral metabolism of thyroid hormone and glucose homeostasis. Thyroid 2005;15:899–903 [DOI] [PubMed] [Google Scholar]
- 56.Bos SD, Loughlin J, Nelissen RGHH, Slagboom PE, Meulenbelt I. Functional characterization of OA risk polymorphism rs225014 at DIO2 in human OA cartilage. Eur J Hum Genet 2011;19:381 [Google Scholar]
- 57.Heemstra KA, Hoftijzer HC, van der Deure WM, Peeters RP, Fliers E, Appelhof BC, Wiersinga WM, Corssmit EP, Visser TJ, Smit JW. Thr92Ala polymorphism in the type 2 deiodinase is not associated with T4 dose in athyroid patients or patients with Hashimoto thyroiditis. Clin Endocrinol (Oxf) 2009;71:279–283 [DOI] [PubMed] [Google Scholar]
- 58.Torlontano M, Durante C, Torrente I, Crocetti U, Augello G, Ronga G, Montesano T, Travascio L, Verrienti A, Bruno R, et al. Type 2 deiodinase polymorphism (threonine 92 alanine) predicts L-thyroxine dose to achieve target thyrotropin levels in thyroidectomized patients. J Clin Endocrinol Metab 2008;93:910–913 [DOI] [PubMed] [Google Scholar]
- 59.Panicker V, Saravanan P, Vaidya B, Evans J, Hattersley AT, Frayling TM, Dayan CM. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab 2009;94:1623–1629 [DOI] [PubMed] [Google Scholar]
- 60.Peeters RP, van den Beld AW, Attalki H, Toor H, de Rijke YB, Kuiper GG, Lamberts SW, Janssen JA, Uitterlinden AG, Visser TJ. A new polymorphism in the type II deiodinase gene is associated with circulating thyroid hormone parameters. Am J Physiol Endocrinol Metab 2005;289:E75–E81 [DOI] [PubMed] [Google Scholar]
- 61.Peeters RP, van Toor H, Klootwijk W, de Rijke YB, Kuiper GG, Uitterlinden AG, Visser TJ. Polymorphisms in thyroid hormone pathway genes are associated with plasma TSH and iodothyronine levels in healthy subjects. J Clin Endocrinol Metab 2003;88:2880–2888 [DOI] [PubMed] [Google Scholar]
- 62.Gereben B, Kollar A, Harney JW, Larsen PR. The mRNA structure has potent regulatory effects on type 2 iodothyronine deiodinase expression. Mol Endocrinol 2002;16:1667–1679 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.