Abstract
Oxidant stress, resulting from an excess of reactive electrophiles produced in the lung by both resident (epithelial and endothelial) and infiltrated leukocytes, is thought to play an obligatory role in tissue injury and abnormal repair. Previously, using a conventional (whole-body) knockout model, we showed that antioxidative gene induction regulated by the transcription factor Nrf2 is critical for mitigating oxidant-induced (hyperoxic) stress, as well as for preventing and resolving tissue injury and inflammation in vivo. However, the contribution to pathogenic acute lung injury (ALI) of the cellular stress produced by resident versus infiltrated leukocytes remains largely undefined in vivo. To address this critical gap in our knowledge, we generated mice with a conditional deletion of Nrf2 specifically in Clara cells, subjected these mice to hyperoxic insult, and allowed them to recover. We report that a deficiency of Nrf2 in airway epithelia alone is sufficient to contribute to the development and progression of ALI. When exposed to hyperoxia, mice lacking Nrf2 in Clara cells showed exacerbated lung injury, accompanied by greater levels of cell death and epithelial sloughing than in their wild-type littermates. In addition, we found that an Nrf2 deficiency in Clara cells is associated with a persistent inflammatory response and epithelial sloughing in the lungs during recovery from sublethal hyperoxic insult. Our results demonstrate (for the first time, to the best of our knowledge) that Nrf2 signaling in Clara cells is critical for conferring protection from hyperoxic lung injury and for resolving inflammation during the repair process.
Keywords: oxidative stress, lung injury and repair, inflammation
Clinical Relevance
The contribution of cellular stress, generated by the airway epithelium in response to injurious insults that promote acute lung injury, is largely undefined. Our data demonstrate that stress response–modifier Nrf2–regulated signaling in Clara cells is critical for conferring protection from oxidant-induced acute lung injury and for resolving inflammation during the repair process.
Acute lung injury (ALI) and its most severe form, acute respiratory distress syndrome (ARDS), are common clinical syndromes caused by various injurious insults. These syndromes can lead to the development of lung pathogenesis and cause major public health problems throughout the world (1, 2). Various studies, using various experimental models of ALI/ARDS (including the hyperoxic ALI model), established that oxidants inflict damage on lung tissue by generating reactive oxygen and nitrogen species (ROS/RNS), resulting in proteinaceous edema and inflammation and a subsequent impairment of respiratory function, as well as morbidity and mortality. The production of excessive ROS during prolonged exposure to hyperoxia can trigger damage to lung tissue and cause the death of both endothelial and alveolar Type I and Type II epithelial cells in vitro and in vivo (3–5). The infiltrated leukocytes, which are generally recruited to the lung during injury, are known to generate ROS/RNS, which contribute to or perpetuate lung injury in various experimental models of ALI/ARDS (6). However, the exact contribution of the oxidative stress generated by various cell types to ALI/ARDS and to the subsequent development of lung pathogenesis is not well-defined.
The Nrf2 transcription factor regulates the expression levels of several antioxidant enzymes and proteins that are required to protect cells against oxidant injury (7, 8). Nrf2 is ubiquitously expressed in various cell types, and is predominantly localized to the cytoplasm. However, in response to cellular stress, it is translocated to the nucleus and binds to the antioxidant response element (ARE) in the regulatory regions of various antioxidative genes, and activates their transcription. The conventional disruption of Nrf2 leads to greater susceptibility to various injurious insults, including hyperoxia (9). When exposed to chronic levels of hyperoxic stress, Nrf2-deficient mice display greater levels of lung injury and inflammation than do Nrf2-sufficient mice (10). The lack of Nrf2 also leads to an impairment of the resolution of lung injury and inflammation after sublethal hyperoxic insults (11). The administration of the antioxidant glutathione to Nrf2-deficient mice immediately after sublethal exposure to hyperoxia can block DNA damage, restore the proliferation of endothelial and Type II alveolar cells in the lungs, and attenuate lung inflammation (11). Although studies using a whole-body Nrf2-knockout model demonstrated a protective role for Nrf2 during oxidant injury, the compartmentalized effects of lung cell type–specific Nrf2 signaling during oxidant injury and repair process remain largely undefined.
Airway epithelial cells are essential for maintaining the integrity of lung structure and function. They also serve as the first line of defense against a variety of insults, including respiratory toxicants. In response to toxicant and oxidant stress, airway epithelial cells secrete growth factors and inflammatory cytokines that regulate lung inflammation and tissue repair (12). Although oxygen-induced cellular injury to the lung endothelium and alveolar epithelium is known to contribute to ALI and inflammation (13, 14), the contribution of cellular stress generated by the airway epithelium in response to oxidant exposure, particularly hyperoxic insult, to tissue injury and the repair process is largely undefined. To determine this contribution, we generated mice bearing a “floxed” allele of Nrf2, and deleted Nrf2 specifically in Clara cells by using a Cre-loxP recombination system, and then exposed the mice to hyperoxic insult. Here, we report that the disruption of Nrf2 in Clara cells is sufficient to exacerbate hyperoxic lung injury and to impair the resolution of inflammation during recovery.
Some of the results of these studies were previously reported in the form of an abstract at the ATS International Conference in May 2010 (15).
Materials and Methods
Mice
Mice carrying the Nrf2 “floxed” allele (Nrf2f/f) were generated on a C57BL/6 background. A targeting vector of Nrf2 was constructed by inserting LoxP sites flanking the DNA-binding domain (exon 5) of the Nrf2 gene and a neomycin cassette flanked by flp recombinase target sites for the selection of positive clones. The linearized vector was transfected into C57BL/6J embryonic stem (ES) cells. After selection with neomycin, surviving clones were expanded for PCR analysis to identify ES clones with homologous recombination at the Nrf2 locus. Two recombinant clones were identified and used for injection into recipient female mice. Chimeras with the targeted allele were backcrossed with C57BL/6 mice to generate the Nrf2flox/flox (hereafter referred to as Nrf2f/f) and neomycin casette (Neo)+ mice. Mice expressing Cre recombinase under the control of the Clara-cell secretory protein (CCSP) promoter on a C57BL/6 background were described elsewhere (16). All experimental animal protocols were performed in accordance with guidelines of the Animal Care and Use Committee at Johns Hopkins University (Baltimore, MD).
PCR Genotyping
We used N1: TCTTAGGCACCATTTGGGAGAG (forward) and N2: TACAGCAGGCA TACCATTGTGG (reverse) primers for the detection of “floxed” alleles. For the detection of Nrf2 disruption, N1, N2, and N3 (TGAGAGCTTCCCAGACTCA CTT [forward]) primers were used, as outlined in Figure 1B. For the detection of CCSP–Cre, CCSP: ACTGCCCATTGCCCAAACAC (forward) and Cre: ACGAACCTGGTCGAAATC AGTGCG (reverse) primers were used.
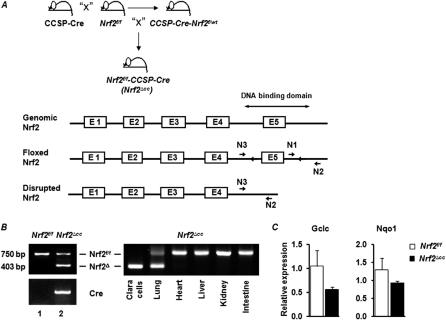
Figure 1.
Generation of mice lacking Nrf2 in Clara cells. (A) Schematic presentation shows the generation of Nrf2f/f-CCSP-Cre (Nrf2∆cc) mice. Mice with the Nrf2 “floxed” allele (Nrf2f/f) were bred with Clara-cell secretory protein (CCSP)–Cre mice, and the resulting Nrf2F/W/CCSP-Cre (F1) mice were backcrossed with Nrf2f/f mice to produce Nrf2f/f/CCSP-Cre (Nrf2∆cc) mice (top). Schematic presentation of the wild-type, floxed, and deleted locus of Nrf2. Location of loxP sites and PCR primer binding sites are indicated (bottom). (B) PCR amplification of genomic DNA isolated from lungs of Nrf2f/f and Nrf2∆cc mice (left). PCR amplification of genomic DNA isolated from Clara cells and different tissues of Nrf2∆cc mice (right), indicating Nrf2 disruption only in lungs, specifically in Clara cells. bp, base pairs. (C) Relative expression of glutamate-cysteine ligase, catalytic subunit (Gclc) and NAD(P)H dehydrogenase, quinone 1 (Nqo1) in tracheal bronchial epithelial cells isolated from Nrf2f/f and Nrf2∆cc mice.
Exposure to Hyperoxia and Assessment of Lung Injury and Inflammation
The Nrf2f/f and Nrf2f/f-CCSP-Cre (Nrf2∆cc) mice (8 weeks old) were exposed to hyperoxia or room air for 48 hours or 60 hours. A separate group of mice exposed to hyperoxia for 48 hours (n = 6) was allowed to recover in room air for 24 or 72 hours. Lung alveolar permeability and inflammation were assessed as previously described (11). To assess vascular permeability, mice were injected intraperitoneally with Evans blue dye (EBD, 75 mg/kg body weight; Sigma, St. Louis, MO). Lungs were perfused with warm PBS containing 20 U/ml of heparin, harvested en bloc, rinsed in PBS, and dabbed until dry. EBD from lungs and plasma was extracted in formamide. Concentrations of EBD from lung extracts and plasma extracts were measured at 620 nm. Lungs were dried at 65°C for 72 hours, and dry weights were measured. The ratio of lung EBD/plasma EBD/lung dry weight was calculated for each sample.
Analysis of Cell Death and mRNA Expression
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and real-time PCR assays were performed as previously described (11).
Isolation of Tracheal Bronchial Epithelial Cells
Tracheal bronchial epithelial cells (TBECs) were isolated as described previously (17).
Cytokine Analysis
Bronchoalveolar lavage (BAL) fluid samples from mice were analyzed according to the multiplex suspension array system, using Luminex beads (Bio-Rad Laboratories, Hercules, CA). BAL samples were thawed, diluted 1:1, and run according to the manufacturer's protocol, and were measured as picograms per milliliter of BAL fluid (n = 5).
Statistical Analysis
All data involving animal experimentation were collected in a double-blinded fashion. Data were expressed as mean ± SEM (n = 3–6 for each condition). ANOVA was used to compare means of multiple groups. For paired data, the Student t test was used. Significance was defined as P ≤ 0.05.
Results
Conditional Deletion of Nrf2 in Clara Cells
To determine the precise role of airway epithelia–specific Nrf2 signaling in providing protection against hyperoxia-induced ALI, we deleted Nrf2 specifically in Clara cells, using a Cre-loxP recombination approach. We first generated mice bearing a “floxed” allele of Nrf2 (Nrf2f/f) (Figure 1A), in which loxP sites were inserted flanking exon 5, the DNA-binding domain of Nrf2 (Figure 1A). Nrf2f/f mice were crossed with transgenic mice bearing cDNA encoding Cre recombinase under the control of the CCSP promoter. The resulting Nrf2f/wt–CCSP–Cre mice were then backcrossed to the parental Nrf2f/f mice to obtain Nrf2f/f–CCSP–Cre mice (hereafter referred to as Nrf2∆cc). Nrf2f/f and Nrf2∆cc mice were genotyped for the presence of the Cre gene and Nrf2 “floxed” alleles, as outlined in Figure 1B. As anticipated, we observed both deleted and undeleted Nrf2 “floxed” alleles in the lung tissue of Nrf2∆cc mice (Figure 1B, left, lane 2), because the CCSP promoter drives Cre expression predominantly in Clara cells but not in other cell types in the lung. The genomic DNA from Nrf2f/f mice yielded only a high molecular weight band with “floxed” sequences (750 base pairs [bp]; Figure 1B, left, lane 1). To confirm the specificity of the Nrf2 deletion further, we isolated genomic DNA from purified TBECs and other tissues such as heart, liver, kidney, and intestine from Nrf2∆cc mice and PCR-amplified it. As anticipated, the disrupted allele was amplified (403 bp) only in Clara cells and lung tissue, whereas the floxed allele (750 bp) was amplified in other tissues (Figure 1B, right). To determine the levels of Nrf2-dependent gene expression in the airway epithelium of Nrf2∆cc and Nrf2f/f mice, we analyzed glutamate-cysteine ligase, catalytic subunit (Gclc) and NAD(P)H dehydrogenase, quinone 1 (Nqo1) mRNA expression in TBECs by real-time RT-PCR analysis. As shown in Figure 1C, Gclc and Nqo1 mRNA expression levels were markedly lower in Nrf2∆cc mice than in Nrf2f/f mice. These results confirmed the deletion of Nrf2 in the Clara cells of Nrf2∆cc mice. We did not analyze the expression of Nrf2 because the probes used in Taqman assays hybridize to both the disrupted and native forms of Nrf2.
Nrf2∆cc Mice Exhibit Higher Levels of Hyperoxic Lung Injury Than Do Wild-Type Mice
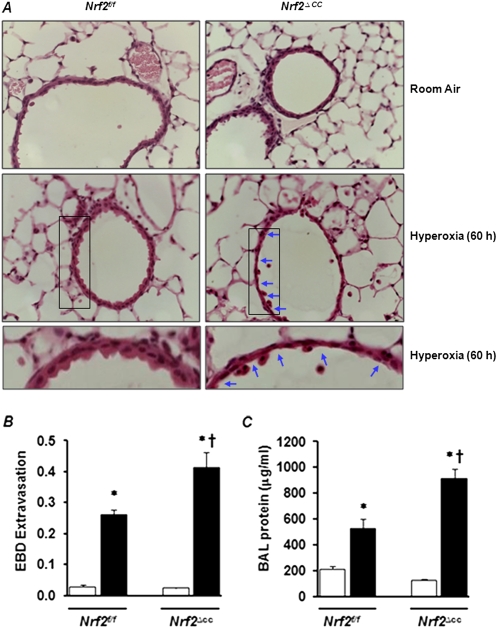
To examine the effects of Clara cell–specific Nrf2 deficiency on oxidant-induced ALI, we exposed Nrf2f/f and Nrf2∆cc mice to hyperoxia for 60 hours. This level of exposure is known to cause significant or severe forms of lung injury. In contrast, prolonged exposure (> 60 hours) causes lethality in C57BL6 mice. Lung injury was evaluated by (1) a histologic assessment of tissue sections, (2) quantifying vascular leakage with an EBD extravasation assay, and (3) analyzing alveolar permeability by measuring the protein concentration in BAL fluid. Lung inflammation was assessed by enumerating the number of inflammatory cells present in the BAL fluid obtained from the mice. As shown in Figure 2A, our histologic analysis revealed greater levels of hyperoxia-associated lung damage and epithelial sloughing in Nrf2∆cc mice than in their Nrf2f/f counterparts (Figure 2A, bottom, contains enlarged images of epithelial sloughing).
Figure 2.
Hyperoxia-induced lung injury in Nrf2f/f and Nrf2∆cc mice. Nrf2f/f and Nrf2∆cc mice were exposed to room air (RA) or hyperoxia for 60 hours and killed, and their left lungs were inflated and fixed in 1.5% paraformaldehyde. Lung tissue sections were prepared and stained with hematoxylin and eosin (H&E). (A) Representative images of H&E-stained lungs sections of three mice are shown. Bottom: Enlarged images of lung tissue sections of Nrf2f/f and Nrf2∆cc mice exposed to 60 hours of hyperoxia. Arrows indicate epithelial sloughing in Nrf2∆cc mice. (B) Evans blue dye (EBD) was injected intraperitoneally into the mice 2 hours before they were killed. Lungs were perfused, and lung EBD and serum EBD were extracted into formamide. Lungs were dried, and the ratios of lung EBD/serum EBD/dry ratios were calculated. The data shown represent means ± SEM (n = 5). (C) Bronchoalveolar lavage (BAL) fluid was collected from the right lung, and total protein was estimated in BAL fluids with a Bio-Rad protein assay. Data represent means ± SEM (n = 6). Significance was calculated using the Student t test. *P ≤ 0.05, room air versus hyperoxia or recovery. †P ≤ 0.05, Nrf2f/f mice versus Nrf2∆cc mice. Open bars, room-air controls; solid bars, hyperoxia.
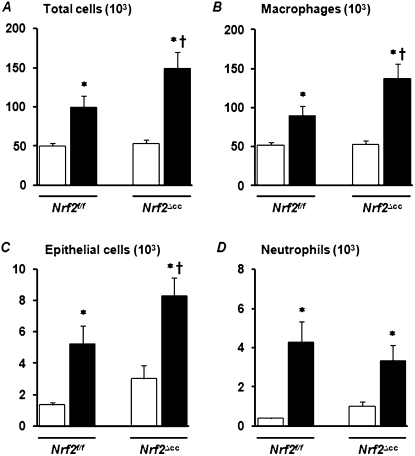
The assessment of lung vascular permeability by the method of EBD extravasation revealed higher levels of vascular leakage in the lungs of Nrf2∆cc mice than in those of their Nrf2f/f counterparts (Figure 2B). Consistent with these data, we found a significant increase in the accumulation of total protein in the BAL fluid of Nrf2∆cc mice compared with Nrf2f/f mice (Figure 2C). Likewise, the number of total cells (Figure 3A) in BAL fluid was significantly greater in Nrf2∆cc mice than in Nrf2f/f mice. This increase in the number of total cells is mainly attributable to the increase in the accumulation of macrophages (Figure 3B) and epithelial sloughing in the lungs (Figure 3C). The hyperoxia-induced infiltration of neutrophils occurred in both genotypes, with no significant difference in concentrations of neutrophils between genotypes (Figure 3D). Thus, these results suggest that Nrf2 disruption in Clara cells is sufficient to perpetuate lung injury in response to injurious hyperoxic exposure.
Figure 3.
Deletion of Nrf2 in Clara cells exacerbates hyperoxia-induced lung inflammation. Nrf2f/f mice and Nrf2∆cc mice were exposed to room air or hyperoxia for 60 hours. BAL fluid was collected from the right lung, centrifuged, and stained with a Diff-Quik stain kit. Differential cell counts were performed to analyze total cells (A), macrophages (B), epithelial cells (C), and neutrophils (D), and are expressed as means ± SEM. One-way ANOVA, followed by Newman-Keuls post hoc analysis, was performed for multiple group comparisons, whereas two-way ANOVA with a Bonferroni correction was used to compare interactions between genotype and hyperoxia. In both cases, P ≤ 0.05 was considered statistically significant. *Room air versus hyperoxia. †Nrf2f/f versus Nrf2∆cc counterparts. Open bars, room-air controls; solid bars, hyperoxia.
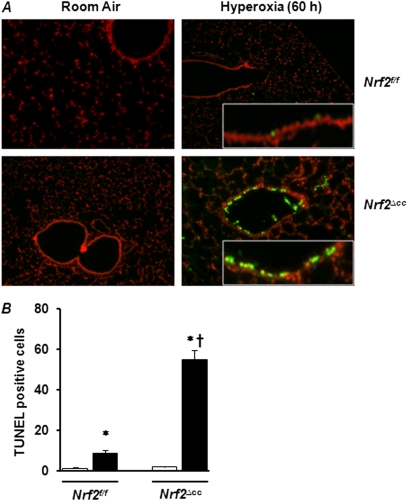
Hyperoxia Induces a Greater Level of Cell Death in Nrf2∆cc Than in Nf2f/f Mice
Because the DNA damage caused by hyperoxia can lead to cell death, we evaluated cell death by TUNEL staining in the lungs of Nrf2f/f and Nrf2∆cc mice after exposure to hyperoxia for 60 hours. As expected, we found approximately equal numbers of TUNEL-positive cells in the lung parenchyma of both genotypes after exposure to hyperoxia (Figure 4A). However, we observed a large number of TUNEL-positive cells in the airways of Nrf2∆cc mice (Figure 4A) because of the lack of Nrf2 in these cells. Only a very few TUNEL-positive cells (1–2 per field) were evident in the airways of Nrf2f/f mice exposed to hyperoxia. The quantification of total TUNEL-positive cells revealed a 6-fold increase in cell death in Nrf2∆cc mice compared with Nrf2f/f mice (Figure 4B; compare bar 2 and bar 4). No cell death was evident in the airways of room air–exposed mice of either genotype. Taken together, these results suggest that the Nrf2 deficiency in Clara cells makes them susceptible to hyperoxia-induced damage and leads to an impairment of Clara-cell function.
Figure 4.
Hyperoxia-induced cell death in airways of Nrf2f/f and Nrf2∆cc mice. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed, using an In Situ Cell Death Detection Kit on lung tissues of Nrf2f/f versus Nrf2∆cc mice exposed to room air and hyperoxia for 60 hours. (A) Representative images of TUNEL staining of lung tissue from mice in room air or exposed to 60 hours of hyperoxia are shown. (B) TUNEL-positive epithelial cells were enumerated (six fields/lung section), and the data represent the average of three mice ± SEM. Significance was calculated using the Student t test. *P ≤ 0.05, room air versus hyperoxia. †P ≤ 0.05, Nrf2f/f mice versus Nrf2∆cc counterparts. Open bars, room-air controls; solid bars, hyperoxia.
Nrf2 Signaling in Clara Cells Is Critical to Dampening the Inflammatory Response during Recovery from Lung Injury
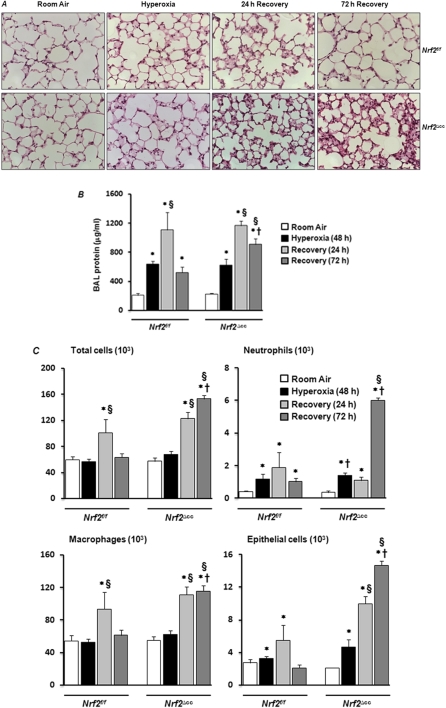
To determine the role of Clara cell–specific Nrf2 signaling in the resolution of the hyperoxia- induced inflammatory response, Nrf2f/f and Nrf2∆cc mice (n = 18) were exposed to sublethal (48-hour) hyperoxic insult. Some of these mice (n = 6) were immediately killed for analyses of lung injury, and some were allowed to recover in normoxia for 24 or 72 hours (n = 6 for each time point). Lung injury and inflammation were then assessed by histopathology and analysis of BAL fluid (Figure 5). Histopathologic examination revealed no significant difference in lung injuries between the Nrf2f/f and Nrf2∆cc mice immediately after 48-hour hyperoxia (Figure 5A). Consistent with the histopathology data, protein concentrations in BAL fluid did not differ significantly between the two genotypes immediately after hyperoxic exposure (Figure 5B). The analysis of BAL fluid revealed that the number of alveolar macrophages was unaltered in both genotypes after exposure to hyperoxia (Figure 5C). However, we found greater levels of neutrophil infiltration and epithelial sloughing in both genotypes after exposure to hyperoxia, compared with room air–exposed counterparts (Figure 5C).
Figure 5.
Effect of Nrf2-deficiency on lung injury and inflammation during recovery form hyperoxic injury. Nrf2f/f and Nrf2∆cc mice were exposed to room air or hyperoxia for 48 hours. Some mice (n = 6) were killed at the end of 48 hours of exposure, and some mice were allowed to recover for 24 hours (n = 6) or 72 hours (n = 6). (A) Left lungs were inflated and fixed. Lung sections were stained with H&E. Representative images of three mice are shown. (B) BAL fluid was collected from the right lung, and total protein was estimated. Data represent means ± SEM (n = 6). (C) Differential cell counts were performed to analyze total cells, neutrophils, macrophages, and epithelial cells. Data are expressed as means ± SEM. One-way ANOVA, followed by Newman-Keuls post hoc analysis, was performed for multiple group comparisons, whereas two-way ANOVA with a Bonferroni correction was used for comparing interactions between genotype and hyperoxia. *Room air versus 48-hour hyperoxia or recovery. §48 hours of hyperoxia versus 24 hours or 72 hours of recovery. †Nrf2f/f mice versus Nrf2∆cc counterparts.
On the other hand, when mice were allowed to recover for 24 hours after exposure to hyperoxia, we saw an increased level of cellularity in the lungs of both genotypes, compared with their respective room air–exposed or hyperoxia-exposed littermates (Figure 5A). Although the number of total cells was comparable after 24 hours of recovery in both genotypes, an increase in epithelial sloughing occurred in the Nrf2∆cc mice (Figure 5C). Alveolar permeability, as assessed by total protein concentration in the BAL, had increased significantly in both genotypes at 24 hours after hyperoxia (Figure 5B).
The cellular accumulation and proteinaceous edema in alveolar spaces in the lungs of Nrf2∆cc mice persisted at 72 hours after oxidant exposure (Figure 5A). In contrast, these injury-associated phenotypes were not evident in the lungs of Nrf2f/f mice. Consistent with our histologic data, the protein concentrations in BAL fluid remained high in the Nrf2∆cc mice, but not in the Nrf2f/f mice, at 72 hours after exposure (Figure 5B). Moreover, the cellular infiltration persisted in Nrf2∆cc mice at 72 hours after exposure, but had resolved in the Nrf2f/f mice (Figure 5C). These results demonstrate that the specific disruption of Nrf2 signaling in Clara cells leads to an impairment of the resolution of tissue repair, especially inflammation, after sublethal hyperoxic insult.
A Lack of Clara Cell–Specific Nrf2 Signaling Dysregulates the Expression of Inflammatory Cytokines Induced by Hyperoxia
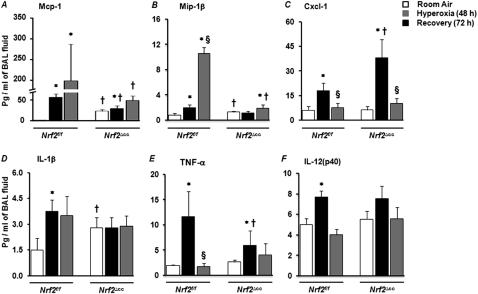
The inflammatory-cell influx induced by hyperoxia is orchestrated by several cytokines (18). We therefore used multiplex cytokine assays to measure the concentrations of various cytokines in the BAL fluid obtained from Nrf2f/f and Nrf2∆cc mice after exposure to room air or 48 hours of hyperoxia, and at 72 hours after injury. Among the cytokines measured, only the concentrations of macrophage chemotactic protein (Mcp1), macrophage inflammatory protein 1 beta (Mip1β), and chemokine (C-X-C motif) ligand 1 (Cxcl1) keratinocyte-derived chemokine were significantly different between the Nrf2f/f and Nrf2∆cc mice. Basal concentrations of Mcp1 were 10-fold higher in Nrf2∆cc mice (23 pg/ml) than in Nrf2f/f mice (2 pg/ml). Hyperoxia induced a 20-fold increase in Mcp1 concentrations in Nrf2f/f mice (56 pg/ml), and a modest alteration in Nrf2∆cc mice (30 pg/ml) (Figure 6A). When mice were allowed to recover, concentrations of Mcp1 robustly increased (to 200 pg/ml) in Nrf2f/f mice, whereas a 2-fold increase was observed in Nrf2∆cc mice (49 pg/ml) compared with room air–exposed control mice (Figure 6A). Similarly, concentrations of Mip1β increased 2-fold and 11-fold in the lungs of Nrf2f/f mice during exposure to hyperoxia and recovery, respectively. In contrast, concentrations of Mip1β in Nrf2∆cc mice were unaltered during hyperoxia, and only modestly increased (2-fold) during recovery (Figure 6B).
Figure 6.
Expression levels of inflammatory cytokines. Cytokines were measured in BAL fluids of Nrf2f/f mice versus Nrf2∆cc mice exposed to room air, 48 hours of hyperoxia, or 72 hours after injury. Analyses of cytokines were performed with a multiplex suspension array system, using Luminex beads. Mean values ± SEM are shown in picograms for macrophage chemotactic protein (Mcp1) (A), macrophage inflammatory protein 1 beta (Mip1β) (B), chemokine (C-X-C motif) ligand 1 (Cxcl1) keratinocyte-derived chemokine (KC) (C), IL-1β (D), TNF-α (E), and IL-12 (p20) (F) (n = 5). Significance was calculated using the Student t test. *P ≤ 0.05, room air versus hyperoxia or recovery. §P ≤ 0.05, hyperoxia versus recovery. †P ≤ 0.05, Nrf2f/f mice versus Nrf2∆cc counterparts.
Concentrations of Cxcl1, a neutrophil chemoattractant, in Nrf2f/f and Nrf2∆cc mice had significantly increased after 48 hours of hyperoxia, compared with those of room air–exposed mice (Figure 6C). In agreement with the enhanced infiltration of neutrophils, concentrations of Cxcl1 were approximately 2-fold higher in Nrf2∆cc mice than in Nrf2f/f mice. Concentrations of Cxcl1 had decreased to basal level in both genotypes at 72 hours of recovery. Concentrations of IL-1β were 2-fold higher in room air–exposed Nrf2∆cc mice than in Nrf2f/f mice. No induction of IL-1β was evident during hyperoxia or recovery in Nrf2∆cc mice. In contrast, concentrations of IL1β protein increased by 2.5-fold after exposure, and remained high (a 2-fold increase) during recovery in Nrf2f/f mice (Figure 6D). Hyperoxia significantly induced TNF-α in both wild-type and Nrf2 mutant mice. However, the increase in TNF-α was significantly higher in Nrf2f/f mice than in Nrf2∆cc mice (Figure 6E). Concentrations of TNF-α decreased to basal level in both genotypes during recovery. Concentrations of IL-12p40 did not differ between genotypes in room air–exposed or hyperoxia-exposed mice, either immediately after hyperoxia or after recovery (Figure 6F). These results indicate that the disruption of Nrf2 in Clara cells alters the expression levels of a variety of important cytokines.
Discussion
The oxidative stress produced by resident (lung epithelial and endothelial) cells and infiltrating leukocytes appears to play a major role in the development and perpetuation of oxidant-induced ALI, but the individual contributions of these cell types to pathogenic ALI remain largely undefined in vivo. In the present study, we provide experimental (genetic) evidence to support a pivotal role for airway epithelial (Clara) cell–specific Nrf2 signaling in conferring protection from oxidant-induced ALI. Dysfunction in the Nrf2-regulated antioxidant defense system in CCSP-expressing cells exacerbated lung injury (vascular and alveolar permeability) and increased the infiltration of inflammatory leukocytes into the lung in response to hyperoxic insult. These phenotypic changes were coincident with greater levels of apoptotic death, predominantly in Clara cells, and altered cytokine expression in the lung. Furthermore, a lack of Nrf2 signaling in Clara cells perpetuated lung injury and inflammation when mice were allowed to recover from sublethal hyperoxic insult, whereas these processes resolved in wild-type mice. Thus, our data illuminate a novel role for Clara cell–specific Nrf2 signaling, both during and after hyperoxic lung injury and repair.
Reactive electrophiles, generated during prolonged exposure to hyperoxia, induce apoptotic death in both endothelial cells and alveolar Type I and Type II epithelial cells (19, 20). However, the lung's epithelial lining fluid is known to possess a superior antioxidant system, and epithelial cells are relatively resistant to oxidant stress (21). Consistent with this notion, TUNEL staining revealed moderate levels of cell death in the airways of wild-type mice exposed to hyperoxia. However, we observed higher numbers of TUNEL-positive cells in the airways of Nrf2 mutant mice (Figure 4). The DNA damage induced by hyperoxia was reported to promote growth arrest in Type II alveolar epithelial cells in vivo and in vitro (22, 23). We previously showed that a deficiency of Nrf2 causes cellular stress and DNA damage, and impairs the proliferation of Type II alveolar cells as a result of a deregulation of expression of genes involved in cell-cycle progression (24). Moreover, we found an up-regulation of cytokine expression in Nrf2-deficient Type II epithelial cells (25). Thus, the lack of an Nrf2 transcription factor in Clara cells caused an increase in cellular stresses, as a result of their insufficient antioxidant defense system. This vulnerability led to cell death and a deregulated inflammatory response, which ultimately impaired the resolution of inflammation and processes of tissue repair in Nrf2∆cc mice.
Resident epithelial cells secrete various chemotactic inflammatory cytokines in response to pro-oxidant exposure. These cytokines play an important role in the augmentation and the resolution of inflammation (26, 27). The activation of circulating neutrophils and their subsequent transmigration into the alveolar air space after an injurious insult were linked to the development of ALI. The transmigration of neutrophils into the alveolar compartment of the lung is regulated by Cxcl1 through receptor-mediated signaling (i.e., binding to its receptor, CXCR2) (28). Consistent with this mechanism, our analysis of cytokines revealed higher concentrations of Cxcl1 in the BAL fluid obtained from Nrf2∆cc mice after hyperoxic injury, which was correlated with the increased concentrations of neutrophils seen in these mice when compared with their wild-type littermates (Nrf2f/f) (Figure 6). We also found that Mcp1, a chemokine required for the resolution of inflammation (29, 30), was induced several-fold in Nrf2f/f mice compared with Nrf2∆cc mice. Mcp1 is expressed in airway epithelial cells in response to oxidant stress, and regulates the production of mucus in lungs (31). Similarly, concentrations of Mip1β protein were not induced in Nrf2∆cc mice, but were elevated in Nrf2f/f mice during exposure to hyperoxia and recovery. Mip1β is mainly produced by hematopoietic cells and to a lesser extent by epithelial cells, and it regulates a wide range of target cell functions, including chemotaxis, degranulation, and phagocytosis (32). Nrf2 binds to the ARE, and activates transcription after challenge with a pro-oxidant or toxic stimulus. The analysis of transcription factor motifs revealed the presence of consensus AREs in both murine and human Mcp1 and Mip1β gene promoters. Although our results suggest that the expression of Mcp1 and Mip1β is regulated by Nrf2, whether these genes are direct or indirect targets of Nrf2 and their exact roles in resolving inflammation in our experimental conditions warrant further study.
Macrophages regulate both the progression and resolution of inflammation. They are also essential for the clearance of apoptotic granulocytes and cell debris and the resolution of lung injury and inflammation (33, 34). However, the accumulation of macrophages releases additional ROS and inflammatory cytokines, which contribute to the development of acute lung injury (35). We found no change in the number of macrophages in Nrf2f/f mice immediately after 48 hours of hyperoxia or during recovery, when compared with room air–exposed controls. Although the number of macrophages in Nrf2∆cc mice immediately after 48 hours of hyperoxia was comparable to that in Nrf2f/f mice, we observed a greater level of macrophage influx into the BAL fluid and interstitium of the lungs in Nrf2∆cc mice. Notably, our data revealed an increased level of cellular infiltration in Nrf2∆cc mice during recovery after injury, although concentrations of cytokines had decreased to basal level by this time. The increased level of cellular inflammation in Nrf2∆cc mice could be attributed either to the initial release of chemokines in the lungs of these mice in response to hyperoxia, or to a lack of the specific cytokines, such as Mcp1, required for the resolution of macrophage inflammation. Nonetheless, our results suggest that the Clara cell–specific Nrf2 transcriptional response may play a role in limiting the inflammatory response in the lung, most likely by modulating macrophage functions.
Clara cells are critical for airway repair after injury as well for the modulation of airway inflammation (36, 37). The CCSP secreted by Clara cells attenuates the inflammatory response induced by various stimuli by modulating the activity of leukocytes through the regulation of inflammatory cytokine expression. For example, CCSP increases neutrophil phagocytosis and decreases the oxidative activity of neutrophils (38), and the deletion of CCSP augments LPS-induced ALI and inflammation in mice (37). The activation of NF-κB in airway epithelia in response to endotoxin and nitric oxide toxicity was attributed to lung injury and inflammation (39–41). The overexpression of transcription factors such as signal-transducer and activator of transcription 3C and CCAAT/enhancer binding protein alpha in airway epithelia protects mice from hyperoxia-induced ALI (42, 43), whereas the deletion of signal-transducer and activator of transcription 3 specifically in Clara cells impairs epithelial repair after exposure to naphthalene in vivo (44). Although our data demonstrate that the Nrf2 transcriptional response in Clara cells is critical for mitigating hyperoxia-induced ALI, whether Nrf2 acts as a functional effector in regulating these proteins remains to be investigated under our experimental conditions. Our data suggest that Nrf2 signaling in Clara cells is critical in conferring protection from hyperoxia-induced ALI and in the resolution of inflammation during recovery from injury. Under physiological conditions, other transcriptional factors such as Nrf3 and the activator protein-1 family of proteins, such as c-Jun and Jun-D, may compensate for the functions of Nrf2, to maintain a basal level of expression in the antioxidant defense system (45, 46). However, the deletion of Nrf2 is unlikely to lead to an up-regulation of a compensatory pathway to mitigate cellular stress in Clara cells in response to oxidant stress, because the specific deletion of Nrf2 resulted in a greater level of cellular damage, specifically to airway epithelia, after exposure to hyperoxia.
In conclusion, our findings indicate that airway epithelial (Clara cell)–specific Nrf2 signaling is important for mitigating oxidant (hyperoxia)–induced ALI as well as for resolving lung inflammation after injury. Activation of the Nrf2 pathway in airway epithelia may offer a promising approach to attenuating the lung injury and inflammatory responses seen in ALI/ARDS.
Acknowledgments
The authors thank the Pathology Core of the Acute Lung Injury-Specialized Centers of Clinically Oriented Research (through National Institutes of Health grant P50-HL073994) for providing their services on a charge basis, Dr. Steven Kleeberger (National Institute of Environment Health Sciences, National Institutes of Health) for his continued interest in and support of this work, and Deborah McClellan for editorial assistance.
Footnotes
This work was supported by National Institutes of Health grants HL66109, ES11863, and P50-HL073994 (S.P.R.), and GM079239 and HL081205 (S.B.).
N.M.R. and H.P.R. performed the experiments and analyzed the data. N.M.R. and S.P.R. conceived the study, designed the experiments, analyzed and interpreted the data, and wrote the manuscript. S.B. contributed to the generation of Nrf2flox/flox mice. T.J.M. provided transgenic mice expressing Cre recombinase under the Clara cell–specific promoter.
The current address of Narsa M. Reddy and Haranatha R. Potteti is the Department of Pediatrics, MC856, College of Medicine, University of Illinois at Chicago, 840 S. Wood St., Chicago, IL 60612.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0144OC on June 9, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349 [DOI] [PubMed] [Google Scholar]
- 2.Matthay MA, Bhattacharya S, Gaver D, Ware LB, Lim LH, Syrkina O, Eyal F, Hubmayr R. Ventilator-induced lung injury: in vivo and in vitro mechanisms. Am J Physiol Lung Cell Mol Physiol 2002;283:L678–L682 [DOI] [PubMed] [Google Scholar]
- 3.Sanders SP, Zweier JL, Kuppusamy P, Harrison SJ, Bassett DJ, Gabrielson EW, Sylvester JT. Hyperoxic sheep pulmonary microvascular endothelial cells generate free radicals via mitochondrial electron transport. J Clin Invest 1993;91:46–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waxman AB, Einarsson O, Seres T, Knickelbein RG, Warshaw JB, Johnston R, Homer RJ, Elias JA. Targeted lung expression of interleukin-11 enhances murine tolerance of 100% oxygen and diminishes hyperoxia-induced DNA fragmentation. J Clin Invest 1998;101:1970–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantell LL, Horowitz S, Davis JM, Kazzaz JA. Hyperoxia-induced cell death in the lung: the correlation of apoptosis, necrosis, and inflammation. Ann N Y Acad Sci 1999;887:171–180 [DOI] [PubMed] [Google Scholar]
- 6.Reddy SP, Hassoun PM, Brower R. Redox imbalance and ventilator-induced lung injury. Antioxid Redox Signal 2007;9:2003–2012 [DOI] [PubMed] [Google Scholar]
- 7.Reddy SP. The antioxidant response element and oxidative stress modifiers in airway diseases. Curr Mol Med 2008;8:376–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol 2003;43:233–260 [DOI] [PubMed] [Google Scholar]
- 9.Cho HY, Jedlicka AE, Reddy SP, Zhang LY, Kensler TW, Kleeberger SR. Linkage analysis of susceptibility to hyperoxia: Nrf2 is a candidate gene. Am J Respir Cell Mol Biol 2002;26:42–51 [DOI] [PubMed] [Google Scholar]
- 10.Cho H-Y, Jedlicka AE, Reddy SPM, Kensler TW, Yamamoto M, Zhang L-Y, Kleeberger SR. Role of Nrf2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol 2002;26:175–182 [DOI] [PubMed] [Google Scholar]
- 11.Reddy NM, Kleeberger SR, Kensler TW, Yamamoto M, Hassoun PM, Reddy SP. Disruption of Nrf2 impairs the resolution of hyperoxia-induced acute lung injury and inflammation in mice. J Immunol 2009;182:7264–7271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schleimer RP, Kato A, Kern R, Kuperman D, Avila PC. Epithelium: at the interface of innate and adaptive immune responses. J Allergy Clin Immunol 2007;120:1279–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maniatis NA, Kotanidou A, Catravas JD, Orfanos SE. Endothelial pathomechanisms in acute lung injury. Vascul Pharmacol 2008;49:119–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagano A, Barazzone-Argiroffo C. Alveolar cell death in hyperoxia-induced lung injury. Ann N Y Acad Sci 2003;1010:405–416 [DOI] [PubMed] [Google Scholar]
- 15.Reddy NMHP, Mariani TJ, Biswal S, Reddy SP. Deficiency of Nrf2-signaling in airway epithelium impairs the resolution of acute lung injury and inflammation in mice. Am J Respir Crit Care Med 2010;181:A2324 [Google Scholar]
- 16.Simon DM, Arikan MC, Srisuma S, Bhattacharya S, Tsai LW, Ingenito EP, Gonzalez F, Shapiro SD, Mariani TJ. Epithelial cell PPAR[gamma] contributes to normal lung maturation. FASEB J 2006;20:1507–1509 [DOI] [PubMed] [Google Scholar]
- 17.Que J, Luo X, Schwartz RJ, Hogan BL. Multiple roles for Sox2 in the developing and adult mouse trachea. Development 2009;136:1899–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhandari V, Elias JA. Cytokines in tolerance to hyperoxia-induced injury in the developing and adult lung. Free Radic Biol Med 2006;41:4–18 [DOI] [PubMed] [Google Scholar]
- 19.Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem 1981;256:10986–10992 [PubMed] [Google Scholar]
- 20.Barazzone C, Horowitz S, Donati YR, Rodriguez I, Piguet PF. Oxygen toxicity in mouse lung: pathways to cell death. Am J Respir Cell Mol Biol 1998;19:573–581 [DOI] [PubMed] [Google Scholar]
- 21.van Klaveren RJ, Demedts M, Nemery B. Cellular glutathione turnover in vitro, with emphasis on Type II pneumocytes. Eur Respir J 1997;10:1392–1400 [DOI] [PubMed] [Google Scholar]
- 22.Roper JM, Mazzatti DJ, Watkins RH, Maniscalco WM, Keng PC, O'Reilly MA. In vivo exposure to hyperoxia induces DNA damage in a population of alveolar Type II epithelial cells. Am J Physiol Lung Cell Mol Physiol 2004;286:L1045–L1054 [DOI] [PubMed] [Google Scholar]
- 23.O'Reilly MA, Staversky RJ, Finkelstein JN, Keng PC. Activation of the G2 cell cycle checkpoint enhances survival of epithelial cells exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol 2003;284:L368–L375 [DOI] [PubMed] [Google Scholar]
- 24.Reddy NM, Kleeberger SR, Bream JH, Fallon PG, Kensler TW, Yamamoto M, Reddy SP. Genetic disruption of the Nrf2 compromises cell-cycle progression by impairing GSH-induced redox signaling. Oncogene 2008;27:5821–5832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy NM, Kleeberger SR, Yamamoto M, Kensler TW, Scollick C, Biswal S, Reddy SP. Genetic dissection of the Nrf2-dependent redox signaling regulated transcriptional programs of cell proliferation and cytoprotection. Physiol Genomics 2007;32:74–81 [DOI] [PubMed] [Google Scholar]
- 26.Ryan RM, Ahmed Q, Lakshminrusimha S. Inflammatory mediators in the immunobiology of bronchopulmonary dysplasia. Clin Rev Allergy Immunol 2008;34:174–190 [DOI] [PubMed] [Google Scholar]
- 27.Goodman RB, Pugin J, Lee JS, Matthay MA. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev 2003;14:523–535 [DOI] [PubMed] [Google Scholar]
- 28.Reutershan J, Ley K. Bench-to-bedside review: acute respiratory distress syndrome: how neutrophils migrate into the lung. Crit Care 2004;8:453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amano H, Morimoto K, Senba M, Wang H, Ishida Y, Kumatori A, Yoshimine H, Oishi K, Mukaida N, Nagatake T. Essential contribution of monocyte chemoattractant protein–1/C–C chemokine ligand–2 to resolution and repair processes in acute bacterial pneumonia. J Immunol 2004;172:398–409 [DOI] [PubMed] [Google Scholar]
- 30.Narasaraju T, Ng HH, Phoon MC, Chow VT. MCP-1 antibody treatment enhances damage and impedes repair of the alveolar epithelium in influenza pneumonitis. Am J Respir Cell Mol Biol 2010;42:732–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monzon ME, Forteza RM, Casalino-Matsuda SM. MCP-1/CCR2B–dependent loop upregulates MUC5AC and MUC5B in human airway epithelium. Am J Physiol Lung Cell Mol Physiol 2011;300:L204–L215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maurer M, von Stebut E. Macrophage inflammatory protein–1. Int J Biochem Cell Biol 2004;36:1882–1886 [DOI] [PubMed] [Google Scholar]
- 33.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 1998;101:890–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature 1997;390:350–351 [DOI] [PubMed] [Google Scholar]
- 35.Auten RL, Whorton MH, Nicholas Mason S. Blocking neutrophil influx reduces DNA damage in hyperoxia-exposed newborn rat lung. Am J Respir Cell Mol Biol 2002;26:391–397 [DOI] [PubMed] [Google Scholar]
- 36.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of SCGB1A1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 2009;4:525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snyder JC, Reynolds SD, Hollingsworth JW, Li Z, Kaminski N, Stripp BR. Clara cells attenuate the inflammatory response through regulation of macrophage behavior. Am J Respir Cell Mol Biol 2010;42:161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katavolos P, Ackerley CA, Clark ME, Bienzle D. Clara cell secretory protein increases phagocytic and decreases oxidative activity of neutrophils. Vet Immunol Immunopathol 2011;139:1–9 [DOI] [PubMed] [Google Scholar]
- 39.Ather JL, Alcorn JF, Brown AL, Guala AS, Suratt BT, Janssen-Heininger YM, Poynter ME. Distinct functions of airway epithelial nuclear factor–kappaB activity regulate nitrogen dioxide–induced acute lung injury. Am J Respir Cell Mol Biol 2010;43:443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadikot RT, Zeng H, Joo M, Everhart MB, Sherrill TP, Li B, Cheng DS, Yull FE, Christman JW, Blackwell TS. Targeted immunomodulation of the NF-kappaB pathway in airway epithelium impacts host defense against Pseudomonas aeruginosa. J Immunol 2006;176:4923–4930 [DOI] [PubMed] [Google Scholar]
- 41.Cheng DS, Han W, Chen SM, Sherrill TP, Chont M, Park GY, Sheller JR, Polosukhin VV, Christman JW, Yull FE, et al. Airway epithelium controls lung inflammation and injury through the NF-kappa B pathway. J Immunol 2007;178:6504–6513 [DOI] [PubMed] [Google Scholar]
- 42.Xu Y, Saegusa C, Schehr A, Grant S, Whitsett JA, Ikegami M. C/EBP{alpha} is required for pulmonary cytoprotection during hyperoxia. Am J Physiol Lung Cell Mol Physiol 2009;297:L286–L298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lian X, Qin Y, Hossain SA, Yang L, White A, Xu H, Shipley JM, Li T, Senior RM, Du H, et al. Overexpression of Stat3C in pulmonary epithelium protects against hyperoxic lung injury. J Immunol 2005;174:7250–7256 [DOI] [PubMed] [Google Scholar]
- 44.Kida H, Mucenski ML, Thitoff AR, Le Cras TD, Park KS, Ikegami M, Muller W, Whitsett JA. Gp130–Stat3 regulates epithelial cell migration and is required for repair of the bronchiolar epithelium. Am J Pathol 2008;172:1542–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braun S, Hanselmann C, Gassmann MG, auf dem Keller U, Born-Berclaz C, Chan K, Kan YW, Werner S. Nrf2 transcription factor: a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol Cell Biol 2002;22:5492–5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meixner A, Karreth F, Kenner L, Penninger JM, Wagner EF. Jun and JunD-dependent functions in cell proliferation and stress response. Cell Death Differ 2010;17:1409–1419 [DOI] [PubMed] [Google Scholar]