Abstract
γ-aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the mammalian central nervous system, and exerts its actions via both ionotropic (GABAA) and metabotropic (GABAB) receptors. Although the functional expression of GABAB receptors coupled to the Gi protein was reported for airway smooth muscle, the role of GABAB receptors in airway responsiveness remains unclear. We investigated whether Gi-coupled GABAB receptors cross-regulate phospholipase C (PLC), an enzyme classically regulated by Gq-coupled receptors in human airway smooth muscle cells. Both the GABAB-selective agonist baclofen and the endogenous ligand GABA significantly increased the synthesis of inositol phosphate, whereas GABAA receptor agonists, muscimol, and 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol exerted no effect. The baclofen-induced synthesis of inositol phosphate and transient increases in [Ca2+]i were blocked by CGP35348 and CGP55845 (selective GABAB antagonists), pertussis toxin (PTX, which inactivates the Gi protein), gallein (a Gβγ signaling inhibitor), U73122 (an inhibitor of PLC-β), and xestospongin C, an inositol 1,4,5-triphosphate receptor blocker. Baclofen also potentiated the bradykinin-induced synthesis of inositol phosphate and transient increases in [Ca2+]i, which were blocked by CGP35348 or PTX. Moreover, baclofen potentiated the substance P–induced contraction of airway smooth muscle in isolated guinea pig tracheal rings. In conclusion, the stimulation of GABAB receptors in human airway smooth muscle cells rapidly mobilizes intracellular Ca2+ stores by the synthesis of inositol phosphate via the activation of PLC-β, which is stimulated by Gβγ protein liberated from Gi proteins coupled to GABAB receptors. Furthermore, crosstalk between GABAB receptors and Gq-coupled receptors potentiates the synthesis of inositol phosphate, transient increases in [Ca2+]i, and smooth muscle contraction through Gi proteins.
Keywords: Gi protein, Gβγ, inositol phosphate, phospholipase C, airway smooth muscle
Clinical Relevance
The activation of γ-aminobutyric acid–B receptors in airway smooth muscle by therapeutic agents such as baclofen can potentiate intracellular calcium and the contraction of smooth muscle, and may potentiate bronchoconstriction in patents with asthma.
γ-aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the mammalian central nervous system. GABA acts at two distinct types of receptors, namely, ligand-gated ionotropic GABAA receptors and G-protein–linked metabotropic (GABAB) receptors. The GABAB receptor is composed of two subunits (GABABR1 and GABABR2), and typically functions as a Gi-protein–coupled receptor. In addition to their well-characterized expression and function in neurons, GABA and functional GABAB receptors were detected in peripheral tissues such as the adrenal medulla, islets of Langerhans, the placenta, and smooth muscle cells of the urinary bladder and uterus. In airways, a GABAB receptor agonist, baclofen, can worsen airway responses after the administration of methacholine to patients with asthma (1, 2). Although our recent findings suggest the functional expression of Gi-protein–coupled GABAB receptors in airway smooth muscle (3) and airway epithelial (4) cells, the functional signaling consequences of GABAB receptor activation on airway responsiveness remain unclear.
Airway contractile agents, such as acetylcholine, bradykinin, histamine, and tachykinins, initiate the contraction of airway smooth muscle by binding to Gq-coupled receptors. These agonists bind to their cognate heptahelical G-protein–coupled receptors (GPCRs) and activate the Gq protein, promoting its dissociation into Gqα and Gβγ and the exchange of guanosine diphosphate bound to Gαq for GTP. The resulting GTP-Gαq complex activates the β isoforms of phospholipase C (PLC) via the carboxy-terminal region of the enzyme (5). PLC-β catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) to generate inositol 1,4,5-triphosphate (IP3) and 1,2-diacylglycerol (DAG). IP3 binds to the IP3 receptor on the sarcoplasmic reticulum and releases Ca2+, leading to a rapid and transient increase in intracellular concentrations of Ca2+ ([Ca2+]i). Recently, the activation of the adenosine A1 receptor was reported to rapidly mobilize intracellular Ca2+ ([Ca2+]i) stores by a mechanism dependent on pertussis toxin (PTX)–sensitive Gi proteins and IP3 signaling in human bronchial smooth muscle cells (6). These findings indicate that not only Gq-coupled receptors but also Gi-coupled receptors can contribute to airway contraction through the synthesis of IP3 and release of [Ca2+]i from the sarcoplasmic reticulum. Because the GABAB receptor is Gi-coupled, these findings led us to hypothesize that the direct activation of the GABAB receptor may increase the synthesis of inositol phosphate, ([Ca2+]i and augment contraction in human airway smooth muscle.
In addition, many examples indicate that the synthesis of IP3 and [Ca2+]i signaling by one type of G-protein–coupled receptor can be influenced by the stimulation of a different type of GPCR (7–9). For example, in addition to directly activating PLC, the adenosine A1 receptor also “potentiates” the PLC responses mediated by a range of Gq-coupled receptors in several cell types (10, 11). Likewise, in smooth muscle cells, the dual activation of Gi-coupled adenosine A1 receptors and Gq-coupled bradykinin receptors synergistically stimulates the synthesis of IP3 and an increase in [Ca2+]i in the ductus deferens tumor cell line MF-2 smooth muscle cells (12). The crosstalk between Gi and Gq pathways in airway smooth muscle regulates bronchial contractility and relaxation (13). These findings led us to hypothesize that the activation of GABAB receptors could potentiate the Gq-coupled receptor–mediated synthesis of IP3, [Ca2+]i, and contraction signaling in airway smooth muscle cells.
Materials and Methods
Materials
SmGM-2 smooth muscle medium was obtained from Lonza (Walkersville, MD). Fluo-4AM and Pluronic F-127 were obtained from Molecular Probes (Eugene, OR). [3H]myo-inositol (20 Ci/mmol) was obtained from MP Biomedicals (Irvine, CA). CGP35348, CGP55845, gallein, and U73122 were obtained from Tocris Bioscience (Ellisville, MO). Pertussis toxin was obtained from Calbiochem (San Diego, CA). Xestospongin C was obtained from Cayman Chemical (Ann Arbor, MI). All other chemicals were obtained from Sigma (St. Louis, MO) unless otherwise stated.
Cell Culture
Primary cultures of human airway smooth muscle cells were obtained from lung transplant donors in accordance with procedures approved by the University of Pennsylvania Committee on Studies Involving Human Beings, as previously described (14). Cells were grown to confluence on 96-well (calcium study) or 24-well (inositol phosphate study) plates in culture medium (SmGM-2, supplemented with 5% FBS, 5 μg/ml insulin, 1 ng/ml human fibroblast growth factor, 500 pg/ml human epidermal growth factor, 30 μg/ml gentamicin, and 15 ng/ml amphotericin B; Lonza) at 37°C in an atmosphere of 5% CO2–95% air. Human airway smooth muscle cells cultured at these conditions retain the expression of both GABAA and GABAB receptors between passages 3 and 8 (3, 15).
Inositol Phosphate Assays
The synthesis of total 3H-inositol phosphates was measured in confluent human airway smooth muscle cells in 24-well tissue culture plates, as described elsewhere (16, 17). In brief, after overnight loading with 3H-myo-inositol (10 μCi/ml or 20 Ci/mmol) in inositol-free and serum-free Dulbecco's modified Eagle's medium (Chemicon, Temecula, CA), each well was washed three times (37°C, with 500 μl Hanks’ balanced salt solution and 10 mM LiCl). The incubation of cells with GABA receptor agonists (GABA, muscimol hydrobromide, 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol [THIP], or baclofen) in a final volume of 300 μl at 37°C for 30 minutes was performed in the absence or presence of 1 μM bradykinin. In some experiments, cells were pretreated with inhibitors, namely, 100 μM CGP35348 (a GABAB receptor antagonist, 30 minutes before the addition of baclofen), 10 nM CGP55845 (a potent GABAB receptor antagonist, 30 minutes before the addition of baclofen), 100 ng/ml pertussis toxin (PTX; 4 hours before the addition of baclofen), 100 μM gallein (a Gβγ signaling inhibitor (18, 19), 30 minutes before the addition of baclofen), or 5 μM U73122 (a PLC inhibitor, 30 minutes before the addition of baclofen). The reactions were terminated, and total [3H]inositol phosphates were recovered by chromatography (16). Experiments were repeated at least six times on human airway smooth muscle cells obtained from the same source.
Measurement of [Ca2+]i
Details regarding the measurement of [Ca2+]i are provided in the online supplement.
In Vitro Effects of GABAB Receptor Agonist on Guinea Pig Airway Smooth Muscle Contraction
Determination of the in vitro effects of the GABAB receptor agonist on guinea pig airway smooth muscle contraction was performed as previously described (17). Please see the online supplement for details.
Statistical Analysis
Statistical analysis was performed using repeated-measures of ANOVA, followed by a Bonferroni posttest comparison using GraphPad Instat version 3.0.6 software (GraphPad Software, Inc., San Diego, CA). Data are presented as means ± SEM. P < 0.05 was considered significant.
Results
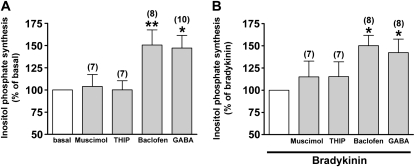
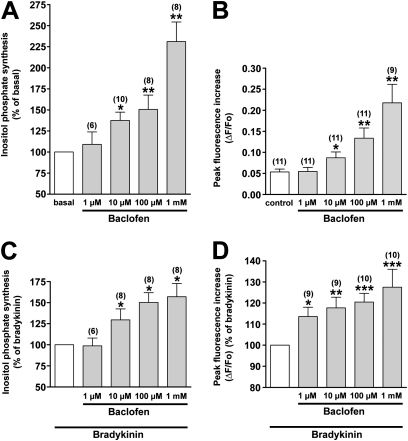
We first examined the effects of GABA receptor agonists (GABA, i.e., a nonselective GABA receptor agonist; muscimol hydrobromide and THIP, GABAA receptor agonists; and baclofen, a GABAB receptor agonist) on the synthesis of inositol phosphate in human airway smooth muscle cells. Both GABA (100 μM) and baclofen (100 μM) significantly increased the synthesis of inositol phosphate (P < 0.05, n = 10, and P < 0.01, n = 8, respectively), whereas GABAA receptor agonists (100 μM muscimol hydrobromide and 100 μM THIP) exerted no effect (Figure 1A). In addition, both GABA and baclofen-potentiated bradykinin (1 μM) induced the synthesis of inositol phosphate (P < 0.05, n = 8, and P < 0.05, n = 8, respectively), whereas GABAA receptor agonists did not affect bradykinin-induced synthesis of inositol phosphate (Figure 1B). Baclofen alone significantly stimulated both the synthesis of inositol phosphate (an increase of 231% ± 23.2%, compared with basal concentrations [P < 0.01, n = 8] at 1 mM baclofen) (Figure 2A) and transient increases in [Ca2+]i (ΔF/Fo = 0.218 ± 0.044 at 1 mM baclofen, P < 0.01, n = 9) (Figure 2B) at concentrations ranging from 10 μM to 1 mM in a concentration-dependent manner. Baclofen also elicited a concentration-dependent potentiation of the bradykinin (1 μM)–induced synthesis of inositol phosphate (an increase of 157% ± 15.8%, compared with bradykinin alone [P < 0.05, n = 8] at 1 mM baclofen) (Figure 2C) and a transient increase in [Ca2+]i (an increase of 128% ± 8.50%, compared with bradykinin alone [P < 0.001, n = 10] at 1 mM baclofen) (Figure 2D).
Figure 1.
(A) Effects of γ-aminobutyric acid (GABA) receptor agonists (i.e., GABAA receptor agonist; 100 μM muscimol hydrobromide or 100 μM 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol [THIP], GABAB receptor agonists; 100 μM baclofen, a nonselective GABA receptor agonist; and 100 μM GABA) on the synthesis of inositol phosphate in native cultured human airway smooth muscle cells. Data are shown as percentages of basal concentrations. *P < 0.05 and **P < 0.01, compared with basal concentrations. (B) Effects of GABA receptor agonists (GABAA receptor agonist; 100 μM muscimol hydrobromide or 100 μM THIP, GABAB receptor agonists; 100 μM baclofen, a nonselective GABA receptor agonist; and 100 μM GABA) on the synthesis of inositol phosphate stimulated by 1 μM bradykinin in native cultured human airway smooth muscle cells. Data are shown as percentage of bradykinin alone. *P < 0.05, compared with bradykinin alone (control). Data represent means ± SEM. Numbers of experiments are shown in parentheses.
Figure 2.
Dose-dependent effects of baclofen on (A) the synthesis of inositol phosphate (shown as percentages of basal concentrations) and (B) the peak (transient) increase in intracellular Ca2+ (shown as change in fluorescence [ΔF] from baseline fluorescence [Fo]) in human airway smooth muscle cells. *P < 0.05 and **P < 0.01, compared with basal concentrations. The dose-dependent effects of baclofen on (C) the bradykinin-stimulated synthesis of inositol phosphate and (D) the peak intracellular increase of Ca2+ in human airway smooth muscle cells are shown as a percentage of the response to 1 μM bradykinin. *P < 0.05, **P < 0.01, and ***P < 0.001, compared with bradykinin. Data represent means ± SEM. Numbers of experiments are shown in parentheses.
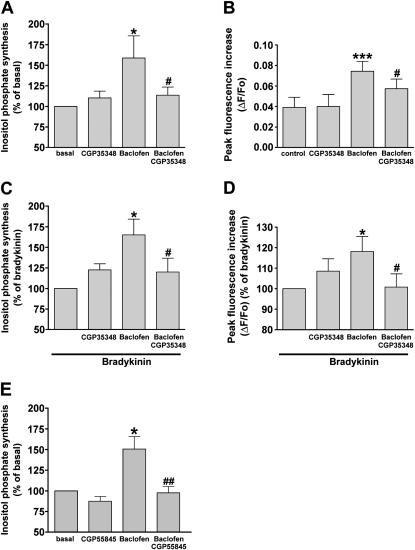
A GABAB receptor–selective antagonist, CGP35348 (100 μM), blocked the baclofen (100 μM)–stimulated synthesis of inositol phosphate (Figure 3A) and the transient increase in [Ca2+]i (P < 0.05, n = 8, and P < 0.05, n = 8, respectively) (Figure 3B). CGP35348 (100 μM) also blocked the baclofen (100 μM)–induced potentiation of the bradykinin-induced synthesis of inositol phosphate (Figure 3C) and the transient increase in [Ca2+]i (P < 0.05, n = 8 and P < 0.05, n = 7, respectively) (Figure 3D). In addition, another potent GABAB receptor–selective antagonist, CGP55845 (10 nM), blocked the baclofen (100 μM)–stimulated synthesis of inositol phosphate (P < 0.01, n = 7) (Figure 3E).
Figure 3.
Effects of selective GABAB receptor antagonist (100 μM CGP35348) on (A) the synthesis of inositol phosphate induced by 100 μM baclofen (shown as percentage of basal concentration) (n = 8) and (B) the peak increase in intracellular Ca2+ (shown as change in fluorescence [ΔF) from baseline fluorescence [Fo]) (n = 8) in human airway smooth muscle cells. *P < 0.05 and ***P < 0.001, compared with basal concentrations. #P < 0.05, compared with baclofen. Effects of a selective GABAB receptor antagonist (100 μM CGP35348) on the potentiation induced by 100 μM baclofen on the (C) synthesis of inositol phosphate (n = 8) and (D) peak increase in intracellular Ca2+ (n = 7) stimulated by 1 μM bradykinin in human airway smooth muscle cells, shown as a percentage of the response to 1 μM bradykinin. *P < 0.05, compared with bradykinin. #P < 0.05, compared with bradykinin/baclofen. (E) Effect of another potent selective GABAB receptor antagonist (10 nM CGP55845) on the synthesis of inositol phosphate induced by 100 μM baclofen (shown as percentage of basal concentration) (n = 7) in human airway smooth muscle cells. *P < 0.05, compared with basal concentration. ##P < 0.05, compared with baclofen. Data represent means ± SEM.
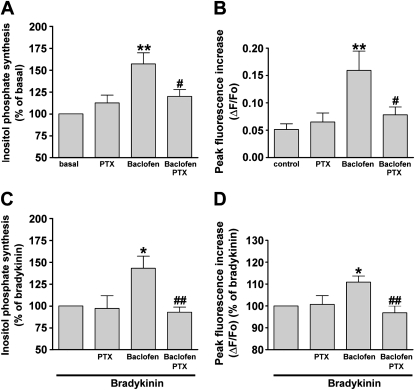
We examined whether the Gi protein takes part in the GABAB receptor–mediated activation of IP3 synthesis and the transient increase in [Ca2+]i. Pretreatment of human airway smooth muscle cells with pertussis toxin (PTX; 100 ng/ml; 4 hours), an inhibitor of Gi proteins, significantly blocked the synthesis of inositol phosphate (Figure 4A) and the transient increase in [Ca2+]i (Figure 4B) in response to 100 μM baclofen (P < 0.05, n = 12, and P < 0.05, n = 8, respectively). PTX also blocked the baclofen-induced potentiation of the bradykinin (1 μM)–induced synthesis of inositol phosphate (Figure 4C) and the transient in [Ca2+]i (P < 0.01, n = 7, and P < 0.01, n = 7, respectively) (Figure 4D). Pretreatment with PTX alone exerted no significant effect on the synthesis of inositol phosphate or the transient increase in [Ca2+]i in the presence or absence of bradykinin (Figure 4).
Figure 4.
Effects of 4-hour pretreatment with pertussis toxin (PTX; 100 ng/ml) on (A) the synthesis of inositol phosphate (shown as percentage of basal concentration) (n = 12) and (B) peak increase in intracellular Ca2+ (shown as change in fluorescence [ΔF] from baseline fluorescence [Fo]) (n = 8) induced by 100 μM baclofen in human airway smooth muscle cells. **P < 0.01, compared with basal concentration. #P < 0.05, compared with baclofen. (C and D) Effects of 4-hour pretreatment with 100 ng/ml PTX on the potentiation stimulated by 1 μM bradykinin in (C) the synthesis of inositol phosphate (n = 7) and (D) the peak increase in intracellular Ca2+ (n = 6) induced by 100 μM baclofen in human airway smooth muscle cells. *P < 0.05, compared with bradykinin. ##P < 0.01, compared with bradykinin/baclofen. The data are presented as a percentage of the response to 1 μM bradykinin. Data represent means ± SEM.
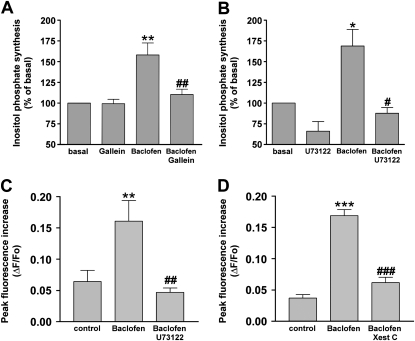
Gi-coupled receptors, including the GABAB receptor, have the capacity to initiate or modulate signaling through the actions of the liberated Giα or Giβγ subunits (20). Although PTX inhibits the activation of the Gi protein, it does not distinguish between signaling events mediated through Giα versus Giβγ subunits. Therefore, we examined whether Giβγ subunits participate in the GABAB receptor–mediated activation of inositol phosphate synthesis and the transient increase in [Ca2+]i. Pretreatment of human airway smooth muscle cells with gallein (100 μM; 30 minutes), a Gβγ inhibitor (18, 19), significantly inhibited the synthesis of inositol phosphate elicited by baclofen (100 μM). Pretreatment with gallein by itself did not exert any significant effect on the synthesis of inositol phosphate (Figure 5A).
Figure 5.
Effects of (A) Gβγ signaling inhibitor gallein (100 μM; 30-minute pretreatment; n = 5) and (B) the phospholipase C-β (PLC-β) inhibitor U73122 (5 μM; 30-minute pretreatment; n = 5) on the synthesis of inositol phosphate induced by 100 μM baclofen in human airway smooth muscle cells. *P < 0.05 and **P < 0.01, compared with basal concentration. #P < 0.05 and ##P < 0.01, compared with baclofen. Effects of (C) U73122 (5 μM; 30-minute pretreatment; n = 7) and (D) the inositol 1,4,5-triphosphate (IP3) receptor inhibitor xestospongin C (Xest C; 20 μM; 30-minute pretreatment; n = 6) on the peak intracellular Ca2+ increase induced by 100 μM baclofen (shown as change in fluorescence [ΔF] from baseline fluorescence [Fo]) in human airway smooth muscle cells. **P < 0.01 and ***P < 0.001, compared with basal concentration. ##P < 0.01 and ###P < 0.001, compared with baclofen. Data represent means ± SEM.
We next questioned whether Gβγ subunits liberated from Gi proteins after activation of the GABAB receptor cross-regulated the activity of PLC-β. PLC-β promotes the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into IP3 and DAG, and IP3 in turn liberates calcium from sarcoplasmic reticulum stores. Although Gqα and Gβγ dissociated from Gq-coupled receptors are the classic signaling molecules that activate PLC-β (5, 20), PLC-β was also shown to be activated by Gβγ proteins liberated from Gi proteins (21, 22). We therefore examined whether PLC-β modulates the baclofen-induced activation of inositol phosphate synthesis and the transient increase in [Ca2+]i. The presence of U73122 (5 μM), an inhibitor of PLC, significantly inhibited both the synthesis of inositol phosphate and the transient increase in [Ca2+]i in response to baclofen (100 μM) (P < 0.05, n = 5, and P < 0.01, n = 7, respectively) (Figures 5B and 5C). In addition, in the presence of xestospongin C (20 μM), a cell-permeable inhibitor of the IP3 receptor, the baclofen (100 μM)–induced transient increase in [Ca2+]I was significantly inhibited (P < 0.001, n = 6) (Figure 5D).
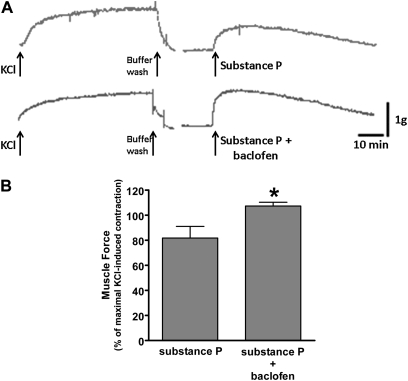
Finally, we examined whether baclofen potentiates the contraction of airway smooth muscle induced by substance P (1 μM). Baclofen (100 μM) significantly potentiated the substance P–induced contraction of guinea pig tracheal airway smooth muscle (Figure 6).
Figure 6.
The GABAB receptor agonist, baclofen, potentiates substance P–induced contractions in guinea pig tracheal rings. (A) Representative tension tracing in guinea pig tracheal ring illustrates the potentiation of substance P (1 μM)–induced contraction by the GABAB receptor agonist baclofen (100 μM). Upper tracing: control (substance P alone). Lower tracing: baclofen + substance P. (B) Baclofen (100 μM) significantly potentiated the contraction induced by substance P (1 μM). Data represent means ± SEM. *P < 0.05, compared with substance P (n = 7).
Discussion
To summarize the main findings of this study, GABAB receptor agonists stimulate the synthesis of inositol phosphate and the mobilization of [Ca2+]i in human airway smooth muscle cells. These responses are mediated by GABAB receptors that are coupled to PTX-sensitive Gi proteins, which crosstalk via Giβγ subunits to activate PLC-β. The activation of PLC-β, in turn, synthesizes inositol phosphates, which mobilize calcium from intracellular stores. In addition, the GABAB receptor agonist potentiates the bradykinin-stimulated synthesis of inositol phosphate and a transient increase in [Ca2+]i. Moreover, we demonstrated that the GABAB agonist baclofen potentiates a Gq-coupled contraction of guinea pig airway smooth muscle. Because [Ca2+]i is an important regulator of airway smooth muscle cell contraction, these findings suggest that the GABAB receptor may contribute to the regulation of bronchomotor tone in the airways.
In the present study, GABA, the natural endogenous ligand for GABAA and GABAB receptors, and baclofen, a selective GABAB receptor agonist, stimulated both the synthesis of inositol phosphate and the transient increase in [Ca2+]i, which were significantly blocked by two selective GABAB receptor antagonists (CGP35348 and CGP55845). In contrast, selective agonists for the GABAA receptor (muscimol and gaboxadol [THIP]) did not stimulate the synthesis of inositol phosphate and the transient increase in [Ca2+]i in human airway smooth muscle cells. These findings suggests that the GABAB receptor, but not the GABAA receptor, is the GABA receptor subtype that is coupled with the synthesis of inositol phosphate and the transient increase in [Ca2+]i in human airway smooth muscle cells. These findings are novel, because the synthesis of inositol phosphate and transient increases in calcium in direct response to the GABAB receptor agonist have not been previously demonstrated in airway smooth muscle. Because calcium is an important regulator of airway smooth muscle cell contraction, these findings suggest that the GABAB receptor may also exert direct effects that regulate bronchomotor tone in the airways.
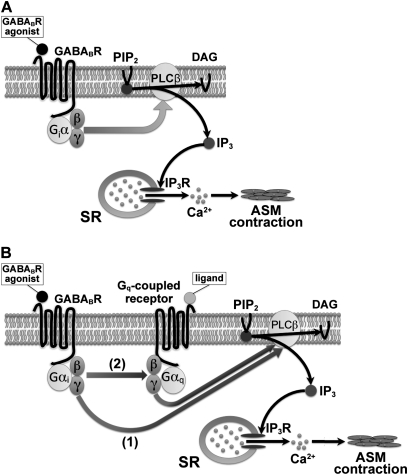
Classically, the activation of PLC-β, resulting in the synthesis of inositol phosphate and the release of sarcoplasmic reticulum calcium, was linked to Gq-coupled receptors. In the present study, the baclofen-induced synthesis of inositol phosphate and the transient increase in [Ca2+]i were attenuated by PTX (an inhibitor of Gi protein), gallein (a Gβγ signaling inhibitor), U73122 (a PLC inhibitor), and xestospongin C (an IP3 receptor inhibitor). Crosstalk among GABAB receptors to activate PLC-β through Gi-protein βγ subunits is possible, because several studies in a variety of cells and tissues demonstrated that the mobilization of [Ca2+]i is stimulated by Gi-coupled receptors such as the adenosine A1 receptor, involving the activation of PLC-β by Gβγ subunits (6, 21–26). In the airways, the activation of adenosine A1 receptors in allergic rabbit airway smooth muscle was shown to cause the production of IP3 via a PTX-sensitive Gi-protein–coupled PLC, and this signaling mechanism may be involved in airway contractile responses (27). Ethier and Madison also showed that adenosine A1 receptors induce the release of [Ca2+]i from the sarcoplasmic reticulum through the activation of PLC in human bronchial smooth muscle cells (6). Taken together, our findings suggest that baclofen stimulated the responses of [Ca2+]i via GABAB receptors, and dissociated Gβγ subunits from GABAB receptors activated PLC-β, which hydrolyzes PIP2 into DAG and IP3, followed by the mobilization of calcium from the sarcoplasmic reticulum (Figure 7A).
Figure 7.
Schematic drawings of GABAB receptor (GABABR)–mediated synthesis of IP3 and intracellular Ca2+ signaling under (A) basal or (B) Gq-coupled receptor activation in human airway smooth muscle. (A) The Gβγ subunit, dissociated from the GABAB receptor, activates PLC-β, which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) into 1,2-diacylglycerol (DAG) and IP3. IP3 binds to IP3 receptors (IP3R) located on the sarcoplasmic reticulum (SR). The activation of IP3 receptors results in an efflux of Ca2+ into the cytosol, which induces airway smooth muscle (ASM) contraction. (B) Activation of the GABAB receptor potentiates the Gq-coupled receptor–mediated synthesis of IP3 and intracellular Ca2+ signaling. Hypothetically, two mechanisms are involved: (1) the Gβγ subunit, dissociated from the GABAB receptor, directly activates PLC-β; and (2) the GABAB receptor provides the Gβγ subunit directly to the Gq-coupled receptor, which facilitates activation of the Gq signaling pathway.
In patients with asthma, the GABAB receptor agonist baclofen can worsen airway responses after the administration of methacholine, a Gq-coupled M3-muscarinic receptor agonist (2). This result indicates that activation of the Gi-coupled GABAB receptor could potentiate the airway contraction mediated via the activation of Gq-coupled receptors. Likewise, in the present study, the GABAB receptor agonist potentiated the guinea pig airway contraction induced by substance P, a Gq-coupled neurokinin receptor agonist. Moreover, the GABAB receptor agonist potentiated the synthesis of inositol phosphate and the [Ca2+]i transients induced by activation of the Gq-coupled bradykinin receptor in a PTX-sensitive manner. Previous findings suggest that the stimulation of Gi-coupled receptors can enhance the inositol phosphate signals triggered by an activation of Gq-coupled receptors, even if stimulation of the Gi-coupled receptors alone exerts no effect on inositol phosphates (28–30). In cerebellar Purkinje cells, activation of the GABAB receptor increases the calcium responses generated by the Gq-coupled metabotropic glutamate receptors, and Gβγ is responsible for this effect (31, 32). Quitterer and colleagues also proposed that crosstalk is mediated by Gβγ exchange between Gi-coupled and Gq-coupled receptors (33). In the present study, the Gβγ blocker significantly inhibited the baclofen-induced synthesis of inositol phosphate and the calcium transients, indicating that activation of the GABAB receptor dissociates the Gβγ subunit, which in turn enhances bradykinin-induced activities. However, the mechanism of such enhancement by Gβγ subunits continues to be debated (9). The direct stimulation of PLC-β by free Gβγ subunits was reported (34), and this stimulation can occur additively after that by Gqα (35). However, the question arises of whether the direct stimulation of PLC-β by liberated Gβγ constitutes the only mechanism for the enhancing effect caused by the activation of Gi-coupled receptors. Gβγ subunits derived from Gi-coupled adenosine A1 receptors were suggested to form a heterotrimeric complex with Gq-guanosine diphosphate that is activated by thyrotropin receptors and leads to potentiated PLC-β activity (22). Similarly, the adenosine A1 receptor and α2c-adrenoceptors provide Gβγ subunits directly to Gq-coupled bradykinin B2 or P2Y nucleotide receptors, resulting in an enhanced binding of GTP to Gq and enhanced signaling (33). These findings suggest that Gβγ subunits dissociated from Gi-coupled receptors could enhance the synthesis of inositol phosphate and [Ca2+]i signaling by accelerating G-protein reassociation and by facilitating activation of the Gq signaling pathway (Figure 7B).
Evidence is increasing that such crosstalk between the GABAB receptor and the metabotropic glutamate 1A receptor does not require their physical interaction, but relates instead to functional crosstalk between their signaling pathways (36, 37). These findings suggest that crosstalk between Gi-coupled and Gq-coupled receptors could exist, even though these receptors are not physically associated.
In conclusion, GABAB receptors are coupled to calcium signaling, a second messenger pathway important to the regulation of bronchomotor tone. Calcium responses are mediated by GABAB receptors coupled to PTX-sensitive G proteins and Gβγ subunits, and depend on the activation of PLC-β and the mobilization of calcium from the sarcoplasmic reticulum. Furthermore, activation of the GABAB receptor potentiates the synthesis of inositol phosphate, the mobilization of calcium, and the contraction in airway smooth muscle induced by activation of the Gq-coupled receptor. These findings indicate that activation of the GABAB receptor can directly regulate the mobilization of calcium in airway smooth muscle cells, and may induce bronchoconstriction.
Supplementary Material
Footnotes
This work was supported by Grant-in-Aid for Young Scientists A-20689036 from the Japanese Society for the Promotion of Science (K.M.), a research grant from the Uehara Memorial Foundation (K.M.), a research grant from the Takeda Science Foundation (K.M.), and National Institutes of Health grant GM065281 (C.W.E.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0088OC on June 30, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Dicpinigaitis PV, Nierman DM, Miller A. Baclofen-induced bronchoconstriction. Ann Pharmacother 1993;27:883–884 [DOI] [PubMed] [Google Scholar]
- 2.Dicpinigaitis PV. Effect of the GABA-agonist baclofen on bronchial responsiveness in asthmatics. Pulm Pharmacol Ther 1999;12:257–260 [DOI] [PubMed] [Google Scholar]
- 3.Osawa Y, Xu D, Sternberg D, Sonett JR, D'Armiento J, Panettieri RA, Emala CW. Functional expression of the GABAB receptor in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2006;291:L923–L931 [DOI] [PubMed] [Google Scholar]
- 4.Mizuta K, Osawa Y, Mizuta F, Xu D, Emala CW. Functional expression of GABAB receptors in airway epithelium. Am J Respir Cell Mol Biol 2008;39:296–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem 2001;70:281–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ethier MF, Madison JM. Adenosine A1 receptors mediate mobilization of calcium in human bronchial smooth muscle cells. Am J Respir Cell Mol Biol 2006;35:496–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drakulich DA, Walls AM, Toews ML, Hexum TD. Neuropeptide Y receptor–mediated sensitization of ATP-stimulated inositol phosphate formation. J Pharmacol Exp Ther 2003;307:559–565 [DOI] [PubMed] [Google Scholar]
- 8.Selbie LA, King NV, Dickenson JM, Hill SJ. Role of G-protein βγ subunits in the augmentation of P2Y2 (P2U)receptor–stimulated responses by neuropeptide Y Y1 Gi/o–coupled receptors. Biochem J 1997;328:153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werry TD, Wilkinson GF, Willars GB. Mechanisms of cross-talk between G-protein–coupled receptors resulting in enhanced release of intracellular Ca2+. Biochem J 2003;374:281–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biden TJ, Browne CL. Cross-talk between muscarinic- and adenosine-receptor signalling in the regulation of cytosolic free Ca2+ and insulin secretion. Biochem J 1993;293:721–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okajima F, Sato K, Sho K, Kondo Y. Stimulation of adenosine receptor enhances α1-adrenergic receptor–mediated activation of phospholipase C and Ca2+ mobilization in a pertussis toxin–sensitive manner in FRTL-5 thyroid cells. FEBS Lett 1989;248:145–149 [DOI] [PubMed] [Google Scholar]
- 12.Gerwins P, Fredholm BB. Stimulation of adenosine A1 receptors and bradykinin receptors, which act via different G proteins, synergistically raises inositol 1,4,5-trisphosphate and intracellular free calcium in DDT1 MF-2 smooth muscle cells. Proc Natl Acad Sci USA 1992;89:7330–7334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGraw DW, Elwing JM, Fogel KM, Wang WC, Glinka CB, Mihlbachler KA, Rothenberg ME, Liggett SB. Crosstalk between Gi and Gq/Gs pathways in airway smooth muscle regulates bronchial contractility and relaxation. J Clin Invest 2007;117:1391–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panettieri RA, Murray RK, DePalo LR, Yadvish PA, Kotlikoff MI. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol 1989;256:C329–C335 [DOI] [PubMed] [Google Scholar]
- 15.Mizuta K, Xu D, Pan Y, Comas G, Sonett JR, Zhang Y, Panettieri RA, Jr, Yang J, Emala CW, Sr, GABAA receptors are expressed and facilitate relaxation in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2008;294:L1206–L1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotta K, Emala CW, Hirshman CA. TNF-α upregulates Giα and Gqα protein expression and function in human airway smooth muscle cells. Am J Physiol 1999;276:L405–L411 [DOI] [PubMed] [Google Scholar]
- 17.Jooste E, Zhang Y, Emala CW. Rapacuronium preferentially antagonizes the function of M2 versus M3 muscarinic receptors in guinea pig airway smooth muscle. Anesthesiology 2005;102:117–124 [DOI] [PubMed] [Google Scholar]
- 18.Lehmann DM, Seneviratne AM, Smrcka AV. Small molecule disruption of G protein βγ subunit signaling inhibits neutrophil chemotaxis and inflammation. Mol Pharmacol 2008;73:410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato M, Jiao Q, Honda T, Kurotani R, Toyota E, Okumura S, Takeya T, Minamisawa S, Lanier SM, Ishikawa Y. Activator of G protein signaling 8 (AGS8) is required for hypoxia-induced apoptosis of cardiomyocytes: role of Gβγ and connexin 43 (CX43). J Biol Chem 2009;284:31431–31440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Billington CK, Penn RB. Signaling and regulation of G protein–coupled receptors in airway smooth muscle. Respir Res 2003;4:2. [PMC free article] [PubMed] [Google Scholar]
- 21.Dickenson JM, Hill SJ. Involvement of G-protein βγ subunits in coupling the adenosine A1 receptor to phospholipase C in transfected CHO cells. Eur J Pharmacol 1998;355:85–93 [DOI] [PubMed] [Google Scholar]
- 22.Tomura H, Itoh H, Sho K, Sato K, Nagao M, Ui M, Kondo Y, Okajima F. βγ subunits of pertussis toxin–sensitive G proteins mediate A1 adenosine receptor agonist-induced activation of phospholipase C in collaboration with thyrotropin: a novel stimulatory mechanism through the cross-talk of two types of receptors. J Biol Chem 1997;272:23130–23137 [DOI] [PubMed] [Google Scholar]
- 23.Biber K, Klotz KN, Berger M, Gebicke-Harter PJ, van Calker D. Adenosine A1 receptor–mediated activation of phospholipase C in cultured astrocytes depends on the level of receptor expression. J Neurosci 1997;17:4956–4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang H, Kuang Y, Wu Y, Smrcka A, Simon MI, Wu D. Pertussis toxin–sensitive activation of phospholipase C by the C5a and fMet-Leu-Phe receptors. J Biol Chem 1996;271:13430–13434 [DOI] [PubMed] [Google Scholar]
- 25.Katz A, Wu D, Simon MI. Subunits βγ of heterotrimeric G protein activate β2 isoform of phospholipase C. Nature 1992;360:686–689 [DOI] [PubMed] [Google Scholar]
- 26.Murthy KS, Coy DH, Makhlouf GM. Somatostatin receptor–mediated signaling in smooth muscle: activation of phospholipase C-β3 by Gβγ and inhibition of adenylyl cyclase by Gαi1 and Gαo. J Biol Chem 1996;271:23458–23463 [DOI] [PubMed] [Google Scholar]
- 27.Abebe W, Mustafa SJ. A1 adenosine receptor–mediated Ins(1,4,5)P3 generation in allergic rabbit airway smooth muscle. Am J Physiol 1998;275:L990–L997 [DOI] [PubMed] [Google Scholar]
- 28.Hollingsworth EB, De la Cruz RA, Daly JW. Accumulations of inositol phosphates and cyclic AMP in brain slices: synergistic interactions of histamine and 2-chloroadenosine. Eur J Pharmacol 1986;122:45–50 [DOI] [PubMed] [Google Scholar]
- 29.Muller S, Lohse MJ. The role of G-protein beta gamma subunits in signal transduction. Biochem Soc Trans 1995;23:141–148 [DOI] [PubMed] [Google Scholar]
- 30.Neer EJ. Heterotrimeric G proteins: organizers of transmembrane signals. Cell 1995;80:249–257 [DOI] [PubMed] [Google Scholar]
- 31.Hirono M, Yoshioka T, Konishi S. GABAB receptor activation enhances mGluR-mediated responses at cerebellar excitatory synapses. Nat Neurosci 2001;4:1207–1216 [DOI] [PubMed] [Google Scholar]
- 32.Tabata T, Araishi K, Hashimoto K, Hashimotodani Y, van der Putten H, Bettler B, Kano M. Ca2+ activity at GABAB receptors constitutively promotes metabotropic glutamate signaling in the absence of GABA. Proc Natl Acad Sci USA 2004;101:16952–16957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quitterer U, Lohse MJ. Crosstalk between Gαi- and Gαq-coupled receptors is mediated by Gβγ exchange. Proc Natl Acad Sci USA 1999;96:10626–10631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camps M, Carozzi A, Schnabel P, Scheer A, Parker PJ, Gierschik P. Isozyme-selective stimulation of phospholipase C-β2 by G protein βγ–subunits. Nature 1992;360:684–686 [DOI] [PubMed] [Google Scholar]
- 35.Smrcka AV, Sternweis PC. Regulation of purified subtypes of phosphatidylinositol-specific phospholipase C beta by G protein alpha and beta gamma subunits. J Biol Chem 1993;268:9667–9674 [PubMed] [Google Scholar]
- 36.Prezeau L, Rives ML, Comps-Agrar L, Maurel D, Kniazeff J, Pin JP. Functional crosstalk between GPCRs: with or without oligomerization. Curr Opin Pharmacol 2010;10:6–13 [DOI] [PubMed] [Google Scholar]
- 37.Rives ML, Vol C, Fukazawa Y, Tinel N, Trinquet E, Ayoub MA, Shigemoto R, Pin JP, Prezeau L. Crosstalk between GABAB and mGlu1a receptors reveals new insight into GPCR signal integration. EMBO J 2009;28:2195–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.