Abstract
In addition to immune cells, airway epithelial cells can contribute to and shape the immune response in the lung by secreting specific cytokines. IL-6 is a key factor in determining the effector fate of CD4+ T cells. Here we show that under basal conditions, the IL-6 gene is already highly expressed in lung epithelial cells, but not in immune cells resident in the lung. However, upon exposure of the lungs to fungal allergens, the direct contact of β-glucans present in the fungus cell wall with lung epithelial cells is sufficient to trigger the rapid synthesis and secretion of IL-6 protein. This posttranscriptional regulation of IL-6 in response to fungal extracts is mediated by the p38 mitogen-activated protein kinase pathway. The inhalation of β-glucans with a nonallergenic antigen is sufficient to provide an adjuvant effect that leads to mucous hyperplasia in the airways. Thus, β-glucans may constitute a common determinant of the fungal and plant-derived allergens responsible for some of the pathological features in allergic asthma.
Keywords: IL-6, p38 MAPK, lung epithelial cells, fungal allergens, β-glucans, asthma
Clinical Relevance
The inhalation of a number of allergens leads to airway inflammation and often atopic asthma because of the type of immune response that these allergens trigger in the lung microenvironment. The common component among these allergens responsible for this type of immune response remains unclear. Our study shows that the direct interaction of fungal β-glucans with lung epithelial cells triggers the production of IL-6. Because IL-6 is a key regulator of the CD4 T-cell response and β-glucans are present in the majority of allergens, our study suggests that β-glucans may be the common denominator by which inhaled allergens induce atopic asthma. Thus, blocking β-glucans could constitute an alternative therapeutic strategy to minimize the effects of inhaled allergens. In addition, our study shows that the pharmacological inhibition of p38 mitogen-activated protein kinase (MAPK) in vivo diminishes the amount of IL-6 triggered by inhaled allergens. Thus, p38 MAPK inhibitors may also be effective in treating atopic asthma.
Although IL-6 is classically considered a nonspecific inflammatory marker, together with TNF-α and IL-1β, a number of studies during the past decade revealed this cytokine to be an active modulator of the immune response. For example, IL-6 plays an important role as a regulator of the effector fate of CD4+ T cells (1). IL-6 can inhibit the production of IFN-γ by T helper 1 (Th1) cells and interfere with T-regulatory cell function, whereas it favors the production of IL-4 by Th2, and contributes to Th17 and T follicular helper cell differentiation. In addition to its pleiotropic character, IL-6 differs from many other cytokines because it is produced not only by a number of immune cells, but also by nonhematopoietic cells. It is generally believed that dendritic cells and macrophages are the major sources of early IL-6 production during an immune response to infection, immunization, or acute allergen exposure. In response to specific stimuli, however, epithelial cells, astrocytes, hepatocytes, endothelial cells, and other cell types can also produce IL-6. Thus, the presence of IL-6 in serum or a particular tissue does not necessarily indicate an ongoing inflammatory response, but rather represents the effect of an extrinsic stimulus on a tissue-specific cell type. IL-6 within the tissue microenvironment can then influence the type or magnitude of local immune response. Although a major effort has been underway during the last two decades to identify components in viruses, bacteria, and allergens that are recognized by receptors present in the innate immune cells (e.g., Toll-like receptor ligands), less is known about potential components that can bind to nonhematopoietic cells and trigger the production of IL-6 or other cytokines. In addition, the regulatory mechanisms for the production of IL-6 in these cells may also be distinct from those involved in macrophages or other innate immune cells.
Epithelial cells form a physical barrier that protects the host from mucosal infection. In addition to the skin, epithelial cells represent the major constituent of the lung, where they act as a first line of defense against inhaled particles or organisms. Lung epithelial cells may also contribute to allergic asthma, a chronic inflammatory disease of the airways characterized by inflammation, bronchoconstriction, and the hypersecretion of mucus in response to the inhalation of aeroallergens such as spore-forming fungi (e.g., Aspergillus fumigatus, Candida albicans, and Alternaria alternata), pollen (e.g., ragweed, grass, and tree), and house dust mite (2, 3). The cardinal features of allergic asthma seem to be the result of the development of a CD4+ Th2 type of immune response, and likely a Th17 response, according to more recent studies (4–6). It remains unclear why airway exposure to a broad range of allergens induces a similar immune response in the lung, suggesting a common component shared by these allergens that interacts with a specific cellular component in the lung.
IL-6 contributes to the production of IL-13 by CD4+ T cells and mucous hyperplasia within the allergic airway inflammatory response caused by direct, repeated exposure to inhaled inactive extracts of the allergen A. fumigatus (7). We and others showed that the in vivo inhalation of inactive extracts of A. fumigatus or other allergens rapidly triggers the secretion of high concentrations of IL-6 in lung airways (7–9). However, whether IL-6 is derived from lung resident/recruited inflammatory cells, or by lung epithelial cells, and which component within fungal extracts is responsible for triggering IL-6, remain unclear.
In this study, we show that the IL-6 gene is constitutively expressed in lung epithelial cells (LECs), but not in lung resident immune cells before exposure to allergens. Exposure to A. fumigatus extracts rapidly triggers the synthesis of IL-6 in lung epithelial cells through a translational–regulatory mechanism mediated by the p38 mitogen-activated protein kinase (MAPK) pathway. The β-glucans are the primary components in fungal extracts responsible for the induction of IL-6 synthesis in lung epithelial cells. The presence of β-glucans in most of known allergens and their effects on the production of IL-6 by lung epithelial cells could be the common feature responsible for the allergic airway inflammatory response caused by exposure to these allergens.
Materials And Methods
Mice and In Vivo Treatment
C57Bl/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). MAPK kinase 3−/− MAPK kinase 6+/− (MKK 3−/− MKK 6+/−) and Dectin-1 Knockout (KO) mice were previously described (10, 11). Mouse procedures were approved by the Institutional Animal Care and Use Committee at the University of Vermont. For in vivo experiments, mice received oropharyngeal A. fumigatus (A.f.) extracts (1 μg/mouse) and scleroglucan (10 μg) in PBS, as previously described (12). For the in vivo inhibition of p38 MAPK, mice received A.f. extracts (1 μg/mouse) and PBS or the p38 inhibitor SB203580 (200 μg/mouse). For the in vivo production of mucus, mice were oropharyngeally exposed to ovalbumin (2 μg) in the presence or absence of scleroglucan (10 μg) on Days 0, 7, and 14, as described elsewhere (7). Lungs were harvested for analysis 48 hours after the final challenge.
Cell Purification and Culture
Lungs were homogenized with collagenase (1 mg/ml) and DNase I (200 μg/ml). Tissue was physically disaggregated and filtered. Adherent cells were depleted (1 hour at 37°C), and nonadherent cells were incubated with anti-CD45–coated magnetic beads according to the manufacturer (Miltenyi Biotech, Cambridge, MA). The CD45− population (LECs) was collected after the first flow-through of the magnetic column. CD45+ cells were flushed off the column after removal of the magnet. Cell purity was assessed by staining with anti-CD45 antibody (Ab), and was evaluated by FACS (LSRII flow cytometer; BD Bioscience, San Jose, CA). The LEC population contained approximately 5% CD45+ cells. Most cells were cytokeratin-positive (> 85%), and 25–30% were Clara cell secretory protein (CCSP)–positive. LECs from unexposed mice were plated on collagen-coated Transwell (Costar, Lowell, MA) in airway media (DMEM, 5% FBS, and 0.12 U/ml insulin) for 4 days. Cells were washed and transferred to medium without insulin, and treated with different stimuli. The supernatant was collected 24 hours later. CD45+ cells were cultured in Bruff's medium. A.f. and C. albicans extracts were formalin-fixed or heat-inactivated to prevent infectivity. Other reagents included scleroglucan (CarboMer, San Diego, CA), p38 MAPK SB203580 inhibitor (Calbiochem, Rockland, MA), Mnk1 inhibitor CGP57380 (Sigma, St. Louis, MO), and L-threo-1-phenyl-2-decanoylamine-3-morpholino-1-propanol (PDMP) (Enzo Life Science, Farmingdale, NY). The β-glucanase treatment of fungal extracts was performed using A. niger β-glucanase (0.4 U/ml, 6 hours at 37°C, with heat-inactivation at 75°C for 20 minutes).
Immunofluorescence Staining and Confocal Microscopy
LECs and CD45+ cells from the lungs of unexposed mice were stained using an anti–cytokeratin-18 monoclonal antibody (mAb) (BD Bioscience). YOYO was used for nuclear staining (Invitrogen, Carlsbad, CA). Cells were examined by confocal microscopy, using a Zeiss LSM-510-META confocal microscope (Carl Zeiss, Thornwood, NY). Frozen lung sections were immunostained with anti–IL-6 and anti-CCSP Abs, and with 4′-6-diamidino-2-phenylindole for nuclear staining, and were analyzed by confocal microscopy.
ELISA
Cytokine detection in bronchoalveolar lavage fluid (BALF) and cell supernatants was performed by ELISA, using IL-6 Duoset and thymic stromal lymphopoietin (TSLP) Quantikine kits (R&D Systems, Minneapolis, MN) according to the manufacturer.
RNA Analysis
RNA was extracted, using the RNAeasy kit (Qiagen, Valencia, CA) as recommended (12). Quantitative RT-PCR was performed using IL-6, Dectin-1 (Clec7), and 18S Assay-on-Demand probe/primer sets (ABI Prism 7700; Applied Biosystems, Carlsbad, CA). The expression of IL-6 was normalized to 18S concentrations, using the comparative cycle threshold method.
Western Blot Analysis
Western blot analyses were performed as described elsewhere (13), using anti-p38, phospho-p38 (Cell Signaling, Danvers, MA), and actin (Santa Cruz Biotechnology, Santa Cruz, CA) Abs.
Statistical Analysis
Data represent means ± SEMs. The significance of differences between multiple groups was determined by ANOVA, followed by the Bonferroni post hoc test, and for two groups by the Student t test. P < 0.05 was considered statistically significant.
Results
Constitutive Expression of the IL-6 Gene in Primary Murine Lung Epithelial Cells
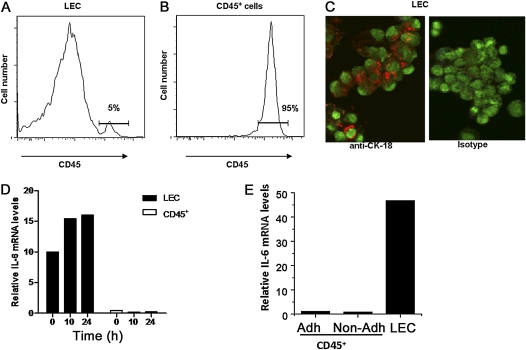
We previously showed that the in vivo oropharyngeal administration of inactive extracts from one of the most common environmental allergens, A.f., rapidly triggers the production of IL-6 and leads to high concentrations of IL-6 in BALF (7). As an inflammatory cytokine, IL-6 is considered to be produced primarily by cells of the innate immune system, but in vitro studies indicate that IL-6 can also be produced by human bronchial epithelial cell lines in response to different environmental stimuli (14–19). To investigate whether the IL-6 detected in BALF in response to the in vivo inhalation of allergens could be derived from immune cells or from lung epithelial cells, both cell types were isolated from mice before or after the oropharyngeal administration of A.f.. Lung epithelial cells were isolated from the lung cell suspension by the depletion of adherent cells (e.g., fibroblasts, endothelial cells, smooth muscle cells, and macrophages) by plastic adherence, followed by the depletion of CD45+ immune cells (e.g., dendritic cells, macrophages, granulocytes, B cells, T cells, and natural killer NK cells) through magnetic separation. The remaining population (defined as LECs) was enriched primarily in lung epithelial cells. The CD45+ cell population (leukocytes) was also collected. The FACS analysis of CD45 expression in the LEC-enriched population showed only a minimal (< 5%) contamination of CD45+ cells (Figure 1A). In contrast, most cells in the CD45+ isolated population expressed CD45 (Figure 1B). To show further the presence of epithelial cells in the LEC-enriched population, we performed intracellular staining for the epithelial cell marker cytokeratin-18 (CK-18), and cells were examined by confocal microscopy. The majority of cells in the LEC fraction expressed CK-18 (Figure 1C), further confirming that epithelial cells are the major component of this population. Total RNA was extracted from isolated LECs and CD45+ populations, and the expression of IL-6 was analyzed by real-time RT-PCR. Although concentrations of IL-6 mRNA were negligible in the CD45+ population, high concentrations of IL-6 mRNA were already present in LECs from unexposed mice (Figure 1D). After exposure to A.f., no significant difference in IL-6 mRNA concentrations in CD45+ cells was detected, with only a minimal increase in the LEC population relative to the already high concentrations present in unexposed LECs (Figure 1D).
Figure 1.
The IL-6 gene is constitutively expressed in primary lung epithelial cells. (A and B) Lung epithelial cells (LECs) and CD45+ cell populations were purified from total lung homogenate of wild-type mice. The expression of CD45 was examined by FACS analysis in the LECs (A) and CD45+ (B) populations. (C) Purified LECs were immunostained for CK-18 (red) and analyzed by confocal microscopy. Nuclear staining is shown in green. Original magnification, ×400. (D) Relative concentrations of IL-6 mRNA in LECs and CD45+ cells from the lungs of wild-type mice, 0, 10, or 24 hours after oropharyngeal exposure to A.f. extracts, as determined by real time RT-PCR. (E) The expression of IL-6 in CD45+ cells purified from unexposed lungs with (Adh) or without (Non-adh) plastic adherence and in LECs was determined by real time RT-PCR. Data are representative of two or three independent experiments.
Because alveolar macrophages are a potential source of IL-6 in the lung (20, 21) and were vastly depleted in the CD45+ population via the plastic adherence step before the purification of these cells, we measured concentrations of IL-6 mRNA in CD45+ cells isolated from lungs with or without plastic adherence. Real-time RT-PCR indicated very low concentrations of IL-6 mRNA in CD45+ cells, independent of cell purification, and with or without plastic adherence (Figure 1E), indicating that concentrations of IL-6 mRNA are marginal in macrophages before stimulation. Thus, the IL-6 gene is constitutively and highly expressed in LECs, but not in lung resident immune cells, before any type of stimulation.
Lung Epithelial Cells Are the Predominant Producers of IL-6 in the Lung in Response to the In Vivo Inhalation of Allergens
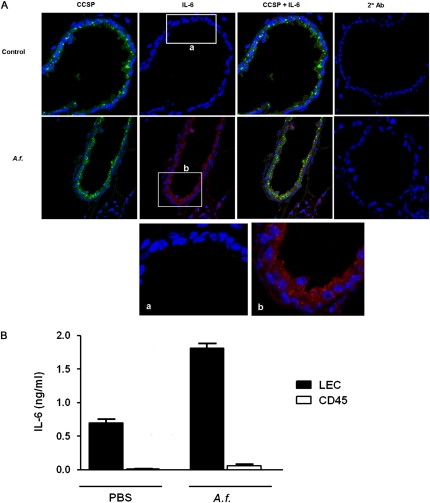
The constitutive expression of the IL-6 gene in LECs suggested that these cells are likely the major source of this cytokine after exposure to A.f. in vivo, because only the translation of preexisting mRNA would be required for the rapid production of IL-6. We therefore examined IL-6 protein concentrations in the lungs of unexposed mice and mice oropharyngeally exposed to A.f. extracts by IL-6 immunostaining and confocal microscopy. Immunostaining for CCSP was also performed, to identify airway epithelial cells. No IL-6 immunostaining was detected in the lungs of unexposed mice (Figure 2A). However, 8 hours after exposure to A.f., significant concentrations of IL-6 were detected in epithelial cells lining the small airways (Figure 2A). The colocalization of IL-6 with the CCSP marker indicated that IL-6 was produced predominantly by Clara cells. A few IL-6–positive cells were also detected in the lung parenchyma. To determine whether these cells were interstitial leukocytes or alveolar epithelial cells, we isolated LECs and the CD45+ population from in vivo A.f.–exposed mice and incubated them in medium alone (ex vivo) for 24 hours. The analysis of IL-6 concentrations in the supernatant by ELISA demonstrated the production of IL-6 in LECs but not in the CD45+ population (Figure 2B). Thus, the in vivo exposure to A.f. extracts triggers the production of IL-6 primarily in LECs.
Figure 2.
In vivo exposure to Aspergillus fumigatus (A.f.) extracts triggers the production of IL-6 in LECs. (A) Mice were oropharyngeally exposed to A.f. extracts (A.f.) (1 μg) or left unexposed (Control). After 8 hours, lung tissue was harvested, frozen lung sections were immunostained for Clara cell secretory protein (CCSP; green) and IL-6 (red) or secondary antibody alone (2° Ab, red), and were evaluated by confocal microscopy. 4′-6-diamidino-2-phenylindole was used for nuclear staining (blue). Original magnification, ×400. (a and b) Expansions of insets in A. Data are representative of two independent experiments. (B) LECs and CD45+ cell populations were purified from total lung homogenate of mice exposed to A.f., and were incubated in medium alone. Concentrations of IL-6 in culture supernatants were examined after 24 hours by ELISA.
Production of IL-6 in LECs Is Triggered by Direct Contact with A.f. Allergen
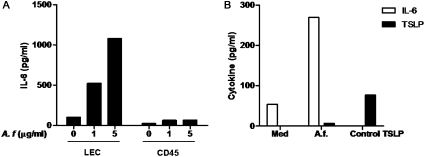
Because lung epithelial cells are one of the first barriers in the respiratory tract to come in contact with inhaled allergens, we tested whether the production of IL-6 by LECs was the result of a direct interaction of LECs with A.f extracts. Purified LECs from unexposed mice were cultured in an air–liquid interface and stimulated with different doses of A.f. extracts. The production of IL-6 was determined by ELISA. A.f. extracts triggered the production of IL-6 in a dose-dependent manner in LECs (Figure 3A), indicating that a direct interaction between A.f. extracts and these cells mediates this response. In addition, we examined the effect of A.f. extracts on the production of IL-6 in the CD45+ cell population isolated from the lungs of unexposed mice. Although a slight increase in IL-6 concentrations occurred in cell supernatants from CD45+ cells after treatment with A.f. (Figure 3A), these concentrations were insignificant compared with the concentrations obtained in A.f.-treated LECs. These results correlate with the constitutive expression of the IL-6 gene in LECs but not in CD45+ cells in the lung, and further demonstrate that LECs, rather than CD45+ cells, are a major source of IL-6 after exposure to A.f.
Figure 3.
Interaction of A.f. extracts with LECs induces the production of IL-6. (A) LECs and CD45+ cells, purified from the lungs of unexposed mice, were treated with indicated doses of A.f. extracts in vitro. After 24 hours, the cell supernatant was analyzed for IL-6 by ELISA. (B) LECs from unexposed mice (n = 3) were treated with A.f. extracts (1 μg/ml) for 24 hours, and both IL-6 and thymic stromal lymphopoietin (TSLP) concentrations were determined in cell supernatants by ELISA. A positive control, provided by the ELISA kit manufacturer, was used to show that the TSLP ELISA assay worked. Data are representative of two or three independent experiments.
Another cytokine that was shown to be produced by LECs and involved in the development of the Th2 immune response is TSLP (22, 23). Thus, we examined whether LECs treated with A.f. extracts in vitro secreted TSLP. In contrast to IL-6, negligible concentrations of TSLP were produced in vitro by A.f.-treated LECs (Figure 3B). Similarly, we did not detect IL-33, also produced by LECs and involved in allergic airway inflammation (24), or TNF-α or eotaxin in supernatants of LECs stimulated by A.f. extracts (data not shown). These data indicate that the direct contact of LECs with A.f. extracts produces IL-6.
β-Glucans in Fungal Extracts Are Responsible for the Induction of IL-6 Production by LECs upon Exposure to Allergens
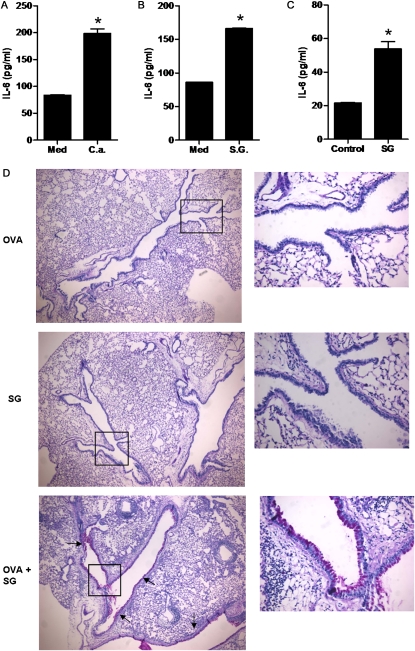
The data thus far indicate that specific components within fungal extracts can directly interact with LECs and activate intracellular signaling pathways that lead to the production of specific cytokines. Because the fungal extracts used were formalin-fixed or heat-inactivated, enzymatic components (e.g., proteases) within the extracts were unlikely to be responsible for the induction of IL-6 in LECs. Bacterial components such as the endotoxin LPS are known to trigger the production of IL-6 through the Toll-like receptor–signaling pathway in macrophages and dendritic cells (21, 25, 26). However, fungi do not intrinsically possess LPS, and the preparations used in these studies were confirmed to be free of LPS contamination. To determine whether the effect of A.f. extracts was attributable to the presence of a common fungal component, we examined whether extracts from other fungi could exert the same effect on LECs. The treatment of LECs in vitro with extracts from C. albicans also induced the production of IL-6 by these cells (Figure 4A). One of the most abundant constituents found in the cell wall of all fungi and plants (representing most allergens), but absent in most bacteria, is the complex polysaccharide β-glucan (27). To determine whether β-glucans can directly induce the production of IL-6 by LECs, we examined the effect of purified β-glucans on these cells. LECs were treated in vitro with purified scleroglucan (from Sclerotium rofsii), a β-(1,3), β-(1,6)-D glucan similar in structure to the β-glucans found in A.f. (28, 29). The treatment of LECs with scleroglucan also triggered the production of IL-6 in these cells (Figure 4B).
Figure 4.
β-glucans induce the production of IL-6 by LECs and promote airway mucous hyperplasia. (A) LECs were treated with medium (Med) or extracts from Candida albicans (C.a.) (1 μg/ml). IL-6 was examined after 24 hours by ELISA. (B) LECs were treated with medium (Med) or purified scleroglucan (SG) (10 μg/ml), and the production of IL-6 was examined as in A. (C) Wild-type mice (n = 3) were oropharyngeally exposed to scleroglucan extracts (SG; 100 μg). Concentrations of IL-6 in bronchoalveolar lavage fluid (BALF) were assessed at 0 and 6 hours after exposure by ELISA. (D) Mice were oropharyngeally exposed to ovalbumin alone (OVA), scleroglucan (SG), or ovalbumin and scleroglucan (OVA+ SG), as described in Materials and Methods. The production of mucus was determined by periodic acid–Schiff (PAS) staining. Magnification, ×40 (left) and ×200 (right; expansions of insets). Arrows indicate PAS-positive (purple) cells in the airways. *Significant difference. Data are representative of two or three independent experiments.
To determine whether β-glucans induce the production of IL-6 in the airways in vivo, mice received oropharyngeal extracts of scleroglucan. After 6 hours, the BALF was harvested, and concentrations of IL-6 were determined by ELISA. Higher concentrations of IL-6 were detected in the BALF of scleroglucan-treated mice (Figure 4C). Thus, the in vivo inhalation of β-glucans alone is sufficient to induce the production of IL-6 in the airways. We previously showed that IL-6 contributes to the development of mucous hyperplasia in response to the oropharyngeal administration of A.f extracts (7). Because β-glucans can enhance the production of IL-6, we tested whether they could function as a local adjuvant for antigens to promote the production of mucus. We therefore administered oropharyngeal ovalbumin as antigen alone or in combination with scleroglucan, to induce allergic airway inflammation previously described with A.f. extracts (7). After three weekly administrations, we examined the production of mucus in the lungs. As expected, no mucus was detected in mice treated with ovalbumin alone as antigen or with scleroglucan alone, but abundant mucus was found in the airways of mice exposed to both ovalbumin and β-glucans (Figure 4D). Thus, fungal β-glucans are sufficient to act as adjuvants of antigens in the lungs to promote mucous hyperplasia.
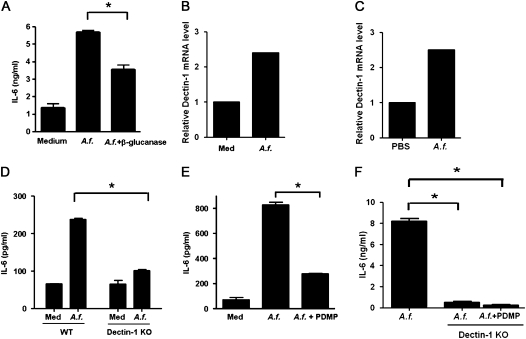
To address the relative contribution of β-glucans present in A.f. extracts to the induction of IL-6 production by LECs, the extracts were first pretreated with β-glucanase. Although a substantial amount of β-glucans remained in the extracts after β-glucanase digestion (25–30% reduction) because of the exaggerated amount of this component in fungi, a significant reduction of IL-6 production by LECs was evident, compared with the production triggered by untreated A.f. extracts (Figure 5A). Dectin-1 is one of the known receptors for β-glucans in a variety of fungal species (30). Dectin-1 was previously reported to be expressed in lung epithelial cells at low concentrations, and it is up-regulated in response to fungal exposure (31). Similarly, we also detected the basal expression of dectin-1 in LECs, and its expression was further up-regulated by exposure to A.f. extracts in vitro (Figure 5B) and in vivo (Figure 5C). To address further the contribution of β-glucans to the A.f.-induced production of IL-6, LECs from wild-type and dectin-1–deficient (Dectin-1 KO) mice were treated with A.f. extracts. Lower concentrations of IL-6 were produced by Dectin-1 KO LECs after treatment with A.f., compared with concentrations from wild-type cells (Figure 5D). Fungal β-glucans were also shown to bind the lactosylceramide present at the cell surface (32, 33). We therefore examined the effects of reducing lactosylceramide in LECs by inhibiting glucosylceramide synthase. Wild-type LECs were treated with A. f. extracts in the presence or absence of PDMP, a known pharmacological inhibitor of glucosylceramide synthase (34). PDMP caused a strong reduction in the concentrations of IL-6 produced by LECs (Figure 5E). In addition, PDMP further reduced the production of IL-6 in LECs from Dectin-1 KO mice to almost undetectable concentrations (Figure 5F). Together, these data demonstrate that β-glucans are the predominant component within the fungal extracts that mediate the induction of IL-6 production by LECs.
Figure 5.
Induction of IL-6 synthesis in LECs by A.f. extracts is mediated by β-glucans present in fungal extracts. (A) LECs were incubated with medium alone (Med) or A.f. extracts (1 μg/ml) previously treated with β-glucanase (A.f. + β-glucanase) or vehicle (A.f.). The production of IL-6 was determined by ELISA after 24 hours. (B) Relative expression of dectin-1 was examined by real-time RT-PCR in LECs after incubation in vitro in the presence of medium alone (Med) or A.f. extracts (1 μg/ml) for 24 hours. (C) Relative expression of dectin-1 was examined by real-time RT-PCR in LECs freshly isolated from wild-type (WT) mice, 6 hours after oropharyngeal exposure to PBS or A.f. extracts (1 μg). (D) LECs isolated from WT or Dectin-1 Knockout (KO) mice were incubated with medium alone or A.f. extracts (1 μg/ml) for 24 hours. Concentrations of IL-6 in cell supernatants were determined by ELISA. (E) LECs from WT mice were incubated with medium alone (Med), A.f. extracts in the presence of L-threo-1-phenyl-2-decanoylamine-3-morpholino-1-propanol (PDMP) (10 μM) (A.f. + PDMP), or vehicle (A.f.). The production of IL-6 was determined by ELISA after 24 hours. (F) LECs isolated from WT mice or Dectin-1 KO mice were incubated with A.f. extracts in the presence or absence of PDMP. The production of IL-6 was determined by ELISA after 24 hours. *Significant difference. Data are representative of two or three independent experiments.
Induction of IL-6 Synthesis in LECs by A.f. Allergen In Vitro and In Vivo Is Mediated by the p38 MAPK/MAP kinase Interacting Kinase Signaling Pathway
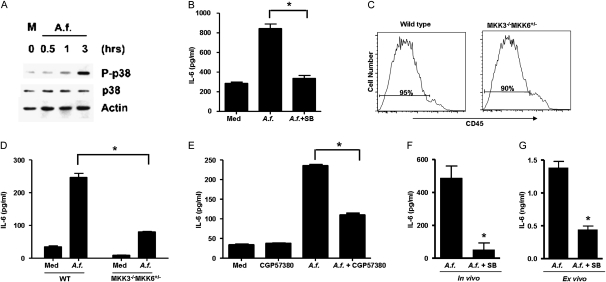
Because the IL-6 gene is constitutively expressed in LECs and A.f. treatment induced the production of IL-6 with little effect on mRNA concentrations, A.f. may regulate IL-6 at the translational level. Although several transcription factors are involved in IL-6 gene transcription, the p38 MAPK pathway is one of the few signaling pathways involved in the translational regulation of IL-6 (35–38). We therefore examine whether A. f. extracts could activate p38 MAPK in LECs via Western blot analysis of phosphorylated p38 MAPK. Treatment with A.f. extract triggered the activation of p38 MAPK (Figure 6A). To examine the contribution of this pathway to the induction of IL-6 production triggered by A.f. extracts, isolated LECs were incubated with A.f. extracts in the presence or absence of the p38 MAPK pharmacological inhibitor SB203580. The presence of SB203580 significantly reduced concentrations of A.f.-induced IL-6, as measured by ELISA (Figure 6B). To demonstrate further the involvement of this pathway in the regulation of IL-6 production induced by A.f. in LECs, we used wild-type and MKK3−/−MKK6+/− mice. MKK3 and MKK6 are the upstream MAPK kinases that phosphorylate and activate p38 MAPK (39, 40). A double deficiency of MKK3 and MKK6 causes embryonic lethality (41). In contrast, MKK3−/−MKK6+/− mice are viable because of the presence of low concentrations of MKK6, but the activity of p38 MAPK is severely impaired (42). LECs were isolated from wild-type and MKK3−/−MKK6+/− cells. A similar enrichment of LECs (CD45-negative cells) was evident in both types of mice (Figure 6C). Wild-type and MKK3−/−MKK6+/− LECs were treated with A.f. extracts, and the production of IL-6 was examined by ELISA. Concentrations of IL-6 were substantially reduced in A.f.-treated MKK3−/−MKK6+/− LECs, compared with A.f.-treated wild-type LECs (Figure 6D).
Figure 6.
The activation of p38 mitogen-activated protein kinase (MAPK) is essential for synthesis of IL-6 by LECs in response to in vitro and in vivo exposure to A.f. (A) LECs from WT mice were treated with medium alone (M) or A.f. extracts (1 μg/ml) for different periods of time. The phosphorylation of p38 MAPK (P-p38), total concentrations of p38 MAPK (p38), and actin (as loading control) were examined by Western blot analysis. (B) LECs from WT mice were treated with A.f. extracts in the presence or absence of SB203580 (5 μM) for 24 hours. Concentrations of IL-6 in cell supernatants were determined by ELISA. (C) FACS analysis of CD45 expression in the LEC population purified from unexposed WT mice and MAPK kinase 3−/− MAPK kinase 6+/− (MKK3−/− MKK6+/−) mice. (D) LECs from wild-type mice (n = 3) and MKK3−/−MKK6+/− mice (n = 3) were treated with A.f. extracts (1 μg/ml). After 24 hours, concentrations of IL-6 in cell supernatants were determined by ELISA. (E) LECs from wild-type mice were treated with A.f. extracts in the presence or absence of the Mnk1 inhibitor CGP57380 (5 μM) for 24 hours. Concentrations of IL-6 in cell supernatants were determined by ELISA. (F and G) Wild-type mice (n = 3) were oropharyngeally exposed to A.f. extracts (1 μg/mouse) and PBS (A.f.) or A.f. extracts and SB203580 (200 μg/mouse) (A.f. + SB). (F) After 6 hours, BALF and total lung tissue were harvested. Concentrations of IL-6 in BALF were determined by ELISA. Lungs from these two groups of mice were homogenated, and LECs were purified and incubated in vitro for 24 hours in the presence of medium alone (defined as ex vivo culture). (G) Concentrations of IL-6 in the supernatant were determined by ELISA. *Significant difference. Data are representative of two or three independent experiments.
The p38 MAPK pathway can regulate protein translation indirectly by phosphorylating and activating the MAP kinase interacting kinases (Mnk1/2) that in turn phosphorylate and activate eukaryotic translation initiation factor 4E (eIF-4E) (43). We therefore examined whether Mnks could also be involved in regulating the production of IL-6 by LECs. Wild-type LECs were treated with A.f. extracts in the presence or absence of a pharmacological Mnk1 inhibitor (CGP57380). The inhibition of Mnk also reduced the production of IL-6 triggered by A.f. extracts (Figure 6E). Together these results show that the p38 MAPK pathway is essential for the production of IL-6 by LECs in response to fungal allergens in vitro. To determine whether p38 MAPK could also regulate the production of IL-6 in the lung in vivo, wild-type mice received A.f. extracts and vehicle (PBS), or A.f. extracts together with the p38 MAPK inhibitor SB203580. The concentrations of IL-6 in BALF were determined 6 hours after exposure. The administration of the p38 MAPK inhibitor caused a pronounced reduction of IL-6 concentrations (Figure 6F). Furthermore, the ex vivo analysis of IL-6 production by LECs purified from these mice showed a marked reduction of IL-6 concentrations in LECs from mice exposed to A.f. and SB203580 (Figure 6G). Thus, activation of the p38 MAPK pathway is important for the synthesis of IL-6 by LECs in response to fungal allergen exposure in vitro and in vivo.
Discussion
Lung epithelium is one of the first physical barriers that inhaled allergens encounter. However, in addition to acting as a structural component of the lung, LECs can contribute to the developing immune response by coordinating the activities of immune cells through the secretion of cytokines and chemokines (22). In this study, we show that the direct interaction between LECs and allergens is sufficient to trigger the production of IL-6, and that airway epithelial cells are the major producers of IL-6 in the lung upon exposure to allergens in vivo. Although a large number of studies examined gene expression in the lung upon the inhalation of allergens, we show that the IL-6 gene is constitutively expressed in vivo specifically in LECs even before exposure. This contrasts with the negligible expression of the IL-6 gene in immune cells present in the lung. The presence of high concentrations of preformed IL-6 mRNA in LECs makes them a prime candidate to secrete this cytokine in the airway, because the translation of preformed IL-6 mRNA could be easily and rapidly achieved by signals triggered by exposure to allergens. This model is supported by our data indicating the presence of significant amounts of IL-6 protein in airway epithelial cells immediately after exposure to allergens, despite marginal changes in IL-6 mRNA concentrations. Because LECs comprise one of the most abundant cellular components in the lung (∼ 30% LECs and ∼ 20% endothelial cells) (44), the rapid production of IL-6 by LECs may explain the high concentrations of this cytokine in the BALF of mice exposed to A.f. extracts (7).
IL-6 gene transcription was shown to be regulated by several transcription factors such as NF-κB, CCAAT/enhancer–binding protein, and activator of protein–1 (45–47). Although less is known about the translational mechanisms that regulate the production of IL-6, the p38 MAPK pathway is one of the signaling pathways involved in the production of IL-6 by promoting the phosphorylation of Mnk1/2 kinases, which activate eIF-4E (36, 37, 43, 48). In our study, we show for the first time, to the best of our knowledge, that the direct contact of fungal allergens with LECs activates p38 MAPK, and that this pathway plays an essential role in regulating the production of IL-6 in the lung in response to allergen exposure. Relative to other signaling pathways, the role of p38 MAPK in the lung remains unexplored, but a recent study indicates the attenuation of lung inflammation and fibrosis in an IL-13–mediated murine model of asthma by a p38 MAPK inhibitor (49). A number of clinical trials tested the efficacy of pharmacological p38 MAPK inhibitors in autoimmune disease, but no clinical studies have been performed in allergic asthma.
Although lung epithelial cells are known to produce other cytokines such as TSLP and IL-33, which are also involved in the allergic airway immune response (22–24), none of these cytokines were detected upon treatment of LECs with A.f. extracts in vitro, unlike IL-6. These results suggest that the direct contact of A.f. extracts with LECs selectively triggers the release of IL-6, probably because of the constitutive expression of the IL-6 gene in these cells, whereas the production of other cytokines assigned to be produced by LECs in vivo may require additional stimuli, such as cytokines derived from immune cells. Supporting this model, high concentrations of IL-6 were found in BALF as soon as 6 hours after in vivo exposure to allergens (Figure 6), but no TSLP or IL-33 was detected (data not shown). We also show in this study for the first time that β-glucans are one of the components of fungal extracts responsible for the induction of IL-6 by primary murine LECs, and that the binding of fungal β-glucans to LECs is sufficient to trigger the production of this cytokine. A recent study showed that β-glucans from Pneumocystis jirovecii, an opportunistic fungus, induce the production of IL-8 by a human epithelial cell line (50). Although no production of IL-6 was reported in that study, those data in conjunction with our findings indicate that β-glucans can directly regulate the production of cytokines/chemokines by lung epithelial cells. In addition to fungi, β-glucans are a major component of the cell wall found in plants, and thus are commonly associated with pollen (51). β-glucans were also found in animal dander and combustion particles (52, 53), which are both environmental triggers of atopic asthma. Although β-glucans are not a structural component of house dust mites, the production of the CCL20 chemokine in lung epithelial cell lines by this common allergen appears to be mediated by β-glucans present in fungi ingested by the mite (54). Thus, β-glucans are a common structural component in a variety of established allergens that can contribute to the development of the typical allergic airway inflammatory response by interacting with airway epithelial cells and triggering the production of regulatory soluble factors. Altering the signaling of β-glucan may therefore constitute a useful therapeutic target for the treatment of allergic asthma.
Acknowledgments
The authors thank Kieren Marr (Division of Infectious Diseases, Oregon Health and Science University, Portland, OR) for providing us with the initial A.f. extracts, Deborah Hogan at Dartmouth Medical School (Dartmouth, NH) for giving us the Candida albicans extracts, and Douglas Johnson (University of Vermont, Burlington, VT) for providing us with the A.f. isolates used in our studies. The authors also thank Douglas Taatjes and Marilyn Wadsworth (Microscopy Imaging Facility, University of Vermont, Burlington, VT) for help with confocal microscopy, Timothy Hunter and Mary Lou Shane (DNA Sequencing Facility, University of Vermont, Burlington, VT) for assistance with real-time RT-PCR analyses, and Colette Charland (Flow Cytometry Facility, University of Vermont, Burlington, VT) for help with flow cytometry analysis. The authors also thank Charlie Irvin, Matthew Poynter, Elizabeth Bonney, and Karen Fortner for helpful discussions.
Footnotes
This work was supported by National Institutes of Health grant RO1 HL69136 and National Center for Research Resources grant P20 RR15557 (M.R.), and by National Institutes of Health/National Heart, Lung and Blood Institute Predoctoral Pulmonary Training Grant T32 HL076122 (W.A.N.).
Originally Published in Press as DOI: 10.1165/rcmb.2011-0054OC on June 3, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Dienz O, Rincon M. The effects of IL-6 on CD4 T cell responses. Clin Immunol 2009;130:27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med 2001;344:350–362 [DOI] [PubMed] [Google Scholar]
- 3.Maddox L, Schwartz DA. The pathophysiology of asthma. Annu Rev Med 2002;53:477–498 [DOI] [PubMed] [Google Scholar]
- 4.Hamelmann E, Gelfand EW. Il-5–induced airway eosinophilia: the key to asthma? Immunol Rev 2001;179:182–191 [DOI] [PubMed] [Google Scholar]
- 5.Ngoc PL, Gold DR, Tzianabos AO, Weiss ST, Celedon JC. Cytokines, allergy, and asthma. Curr Opin Allergy Clin Immunol 2005;5:161–166 [DOI] [PubMed] [Google Scholar]
- 6.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 1998;282:2258–2261 [DOI] [PubMed] [Google Scholar]
- 7.Neveu WA, Allard JB, Dienz O, Wargo MJ, Ciliberto G, Whittaker LA, Rincon M. IL-6 is required for airway mucus production induced by inhaled fungal allergens. J Immunol 2009;183:1732–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virchow JC, Jr, Walker C, Hafner D, Kortsik C, Werner P, Matthys H, Kroegel C. T cells and cytokines in bronchoalveolar lavage fluid after segmental allergen provocation in atopic asthma. Am J Respir Crit Care Med 1995;151:960–968 [DOI] [PubMed] [Google Scholar]
- 9.Bettiol J, Sele J, Henket M, Louis E, Malaise M, Bartsch P, Louis R. Cytokine production from sputum cells after allergenic challenge in IgE-mediated asthma. Allergy 2002;57:1145–1150 [DOI] [PubMed] [Google Scholar]
- 10.Tanaka N, Kamanaka M, Enslen H, Dong C, Wysk M, Davis RJ, Flavell RA. Differential involvement of p38 mitogen-activated protein kinase kinases MKK3 and MKK6 in T-cell apoptosis. EMBO Rep 2002;3:785–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, Sudo K, Akira S, Adachi Y, Ohno N, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol 2007;8:39–46 [DOI] [PubMed] [Google Scholar]
- 12.Allard JB, Poynter ME, Marr KA, Cohn L, Rincon M, Whittaker LA. Aspergillus fumigatus generates an enhanced Th2-biased immune response in mice with defective cystic fibrosis transmembrane conductance regulator. J Immunol 2006;177:5186–5194 [DOI] [PubMed] [Google Scholar]
- 13.Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, Sabio G, Davis RJ, Matthews DE, Doble B, et al. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science 2008;320:667–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rate A, Upham JW, Bosco A, McKenna KL, Holt PG. Airway epithelial cells regulate the functional phenotype of locally differentiating dendritic cells: implications for the pathogenesis of infectious and allergic airway disease. J Immunol 2009;182:72–83 [DOI] [PubMed] [Google Scholar]
- 15.Osterlund C, Gronlund H, Polovic N, Sundstrom S, Gafvelin G, Bucht A. The non-proteolytic house dust mite allergen der p 2 induces NF-kappaB and MAPK dependent activation of bronchial epithelial cells. Clin Exp Allergy 2009;39:1199–1208 [DOI] [PubMed] [Google Scholar]
- 16.Ovrevik J, Lag M, Holme JA, Schwarze PE, Refsnes M. Cytokine and chemokine expression patterns in lung epithelial cells exposed to components characteristic of particulate air pollution. Toxicology 2009;259:46–53 [DOI] [PubMed] [Google Scholar]
- 17.Yoshida Y, Maruyama M, Fujita T, Arai N, Hayashi R, Araya J, Matsui S, Yamashita N, Sugiyama E, Kobayashi M. Reactive oxygen intermediates stimulate interleukin-6 production in human bronchial epithelial cells. Am J Physiol 1999;276:L900–L908 [DOI] [PubMed] [Google Scholar]
- 18.Devlin RB, McKinnon KP, Noah T, Becker S, Koren HS. Ozone-induced release of cytokines and fibronectin by alveolar macrophages and airway epithelial cells. Am J Physiol 1994;266:L612–L619 [DOI] [PubMed] [Google Scholar]
- 19.Cromwell O, Hamid Q, Corrigan CJ, Barkans J, Meng Q, Collins PD, Kay AB. Expression and generation of interleukin-8, IL-6 and granulocyte–macrophage colony–stimulating factor by bronchial epithelial cells and enhancement by IL-1 beta and tumour necrosis factor–alpha. Immunology 1992;77:330–337 [PMC free article] [PubMed] [Google Scholar]
- 20.Losa Garcia JE, Rodriguez FM, Martin de Cabo MR, Garcia Salgado MJ, Losada JP, Villaron LG, Lopez AJ, Arellano JL. Evaluation of inflammatory cytokine secretion by human alveolar macrophages. Mediators Inflamm 1999;8:43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotloff RM, Little J, Elias JA. Human alveolar macrophage and blood monocyte interleukin-6 production. Am J Respir Cell Mol Biol 1990;3:497–505 [DOI] [PubMed] [Google Scholar]
- 22.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol 2007;19:711–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 2002;3:673–680 [DOI] [PubMed] [Google Scholar]
- 24.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. Il-33, an interleukin-1–like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper Type 2–associated cytokines. Immunity 2005;23:479–490 [DOI] [PubMed] [Google Scholar]
- 25.Dodge IL, Carr MW, Cernadas M, Brenner MB. IL-6 production by pulmonary dendritic cells impedes Th1 immune responses. J Immunol 2003;170:4457–4464 [DOI] [PubMed] [Google Scholar]
- 26.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol 2001;1:135–145 [DOI] [PubMed] [Google Scholar]
- 27.Tsoni SV, Brown GD. Beta-glucans and dectin-1. Ann N Y Acad Sci 2008;1143:45–60 [DOI] [PubMed] [Google Scholar]
- 28.Farina JI, Vinarta SC, Cattaneo M, Figueroa LI. Structural stability of sclerotium ROLFSII ATCC 201126 beta-glucan with fermentation time: a chemical, infrared spectroscopic and enzymatic approach. J Appl Microbiol 2009;106:221–232 [DOI] [PubMed] [Google Scholar]
- 29.Fontaine T, Simenel C, Dubreucq G, Adam O, Delepierre M, Lemoine J, Vorgias CE, Diaquin M, Latge JP. Molecular organization of the alkali-insoluble fraction of aspergillus fumigatus cell wall. J Biol Chem 2000;275:41528. [PubMed] [Google Scholar]
- 30.Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol 2006;6:33–43 [DOI] [PubMed] [Google Scholar]
- 31.Cunha C, Di Ianni M, Bozza S, Giovannini G, Zagarella S, Zelante T, D'Angelo C, Pierini A, Pitzurra L, Falzetti F, et al. Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity. Blood 2010;116:5394–5402 [DOI] [PubMed] [Google Scholar]
- 32.Evans SE, Hahn PY, McCann F, Kottom TJ, Pavlovic ZV, Limper AH. Pneumocystis cell wall beta-glucans stimulate alveolar epithelial cell chemokine generation through nuclear factor–kappaB–dependent mechanisms. Am J Respir Cell Mol Biol 2005;32:490–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hahn PY, Evans SE, Kottom TJ, Standing JE, Pagano RE, Limper AH. Pneumocystis carinii cell wall beta-glucan induces release of macrophage inflammatory protein–2 from alveolar epithelial cells via a lactosylceramide-mediated mechanism. J Biol Chem 2003;278:2043–2050 [DOI] [PubMed] [Google Scholar]
- 34.Vunnam RR, Radin NS. Analogs of ceramide that inhibit glucocerebroside synthetase in mouse brain. Chem Phys Lipids 1980;26:265–278 [DOI] [PubMed] [Google Scholar]
- 35.Wang YX, Xu XY, Su WL, Wang Q, Zhu WX, Chen F, Jin G, Liu YJ, Li YD, Sun YP, et al. Activation and clinical significance of p38 MAPK signaling pathway in patients with severe trauma. J Surg Res 2008;161:119–125 [DOI] [PubMed] [Google Scholar]
- 36.Kjellerup RB, Kragballe K, Iversen L, Johansen C. Pro-inflammatory cytokine release in keratinocytes is mediated through the MAPK signal–integrating kinases. Exp Dermatol 2008;17:498–504 [DOI] [PubMed] [Google Scholar]
- 37.Rowlett RM, Chrestensen CA, Nyce M, Harp MG, Pelo JW, Cominelli F, Ernst PB, Pizarro TT, Sturgill TW, Worthington MT. Mnk kinases regulate multiple TLR pathways and innate proinflammatory cytokines in macrophages. Am J Physiol 2008;294:G452–G459 [DOI] [PubMed] [Google Scholar]
- 38.Zhao W, Liu M, Kirkwood KL. P38alpha stabilizes interleukin-6 mRNA via multiple Au-rich elements. J Biol Chem 2008;283:1778–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Derijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science 1995;267:682–685 [DOI] [PubMed] [Google Scholar]
- 40.Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol 1996;16:1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brancho D, Tanaka N, Jaeschke A, Ventura JJ, Kelkar N, Tanaka Y, Kyuuma M, Takeshita T, Flavell RA, Davis RJ. Mechanism of p38 MAP kinase activation in vivo. Genes Dev 2003;17:1969–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wood CD, Thornton TM, Sabio G, Davis RA, Rincon M. Nuclear localization of p38 MAPK in response to DNA damage. Int J Biol Sci 2009;5:428–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J 1997;16:1909–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parent RA. Treatise on pulmonary toxicology, volume I: comparative biology of normal lung. Boca Raton: CRC Press; 1992 [Google Scholar]
- 45.Zhang YH, Lin JX, Vilcek J. Interleukin-6 induction by tumor necrosis factor and interleukin-1 in human fibroblasts involves activation of a nuclear factor binding to a kappa B–like sequence. Mol Cell Biol 1990;10:3818–3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robb BW, Hershko DD, Paxton JH, Luo GJ, Hasselgren PO. Interleukin-10 activates the transcription factor C/EBP and the interleukin-6 gene promoter in human intestinal epithelial cells. Surgery 2002;132:226–231 [DOI] [PubMed] [Google Scholar]
- 47.Hungness ES, Pritts TA, Luo GJ, Sun X, Penner CG, Hasselgren PO. The transcription factor activator protein–1 is activated and interleukin-6 production is increased in interleukin-1beta–stimulated human enterocytes. Shock 2000;14:386–391 [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Flynn A, Waskiewicz AJ, Webb BL, Vries RG, Baines IA, Cooper JA, Proud CG. The phosphorylation of eukaryotic initiation factor EIF4E in response to phorbol esters, cell stresses, and cytokines is mediated by distinct MAP kinase pathways. J Biol Chem 1998;273:9373–9377 [DOI] [PubMed] [Google Scholar]
- 49.Ma JYMS, Kerr I, Mangadu R, Protter AA, Higgins LS. Selective p38A mitogen-activated protein kinase inhibitor attenuates lung inflammation and fibrosis in IL-13 transgenic mouse model of asthma. J Allergy Asthma 2008;1:31–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carmona EM, Lamont JD, Xue A, Wylam M, Limper AH. Pneumocystis cell wall beta-glucan stimulates calcium-dependent signaling of IL-8 secretion by human airway epithelial cells. Respir Res 2010;11:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Novak M, Vetvicka V. Beta-glucans, history, and the present: immunomodulatory aspects and mechanisms of action. J Immunotoxicol 2008;5:47–57 [DOI] [PubMed] [Google Scholar]
- 52.Namork E, Johansen BV, Lovik M. Detection of allergens adsorbed to ambient air particles collected in four European cities. Toxicol Lett 2006;165:71–78 [DOI] [PubMed] [Google Scholar]
- 53.Finkelman MA, Lempitski SJ, Slater JE. Beta-glucans in standardized allergen extracts. J Endotoxin Res 2006;12:241–245 [DOI] [PubMed] [Google Scholar]
- 54.Nathan AT, Peterson EA, Chakir J, Wills-Karp M. Innate immune responses of airway epithelium to house dust mite are mediated through beta-glucan–dependent pathways. J Allergy Clin Immunol 2009;123:612–618 [DOI] [PMC free article] [PubMed] [Google Scholar]