Abstract
Pulmonary fibrosis is a disease that results in loss of normal lung architecture, but the signaling events that drive tissue destruction are incompletely understood. Wnt/β-catenin signaling is important in normal lung development, but whether abnormal signaling occurs in lung fibrosis due to systemic sclerosis and the consequences of β-catenin signaling toward the fibrogenic phenotype remain poorly defined. In this study, we show nuclear β-catenin accumulation in fibroblastic foci from lungs of patients with systemic sclerosis–associated advanced pulmonary fibrosis. Forced activation of β-catenin signaling in three independently derived sources of normal human lung fibroblasts promotes proliferation and migratory activities but is not sufficient to activate classic markers of fibroblast activation, such as TGF-β, type 1 collagen, α-smooth muscle actin, and connective tissue growth factor. These findings indicate that activation of β-catenin signaling in pulmonary fibroblasts may be a common feature of lung fibrosis, contributing to fibroproliferative and migratory activities associated with the disease.
Keywords: Wnt/β-catenin signaling, scleroderma, fibrosis
Clinical Relevance
Pulmonary fibrosis is a devastating disease with no effective treatment or cure. Understanding the role of Wnt/β-catenin signaling in pulmonary fibrosis will provide the foundation for the development of future therapeutics.
Pulmonary fibrosis is a chronic, progressive, and poorly understood disease that may be idiopathic or associated with systemic sclerosis. In both forms, pulmonary fibrosis is characterized by the proliferation of fibroblasts, resulting in excessive deposition of extracellular matrix and loss of normal alveolar structure. Although pulmonary fibrosis may be attributable to a variety of causes, it is generally thought that the initiating injury activates repair processes that aim to restore the original tissue architecture, but a failure to finely tune the repair process leads to persistent fibroblast activation (1–3). Recent studies using genome-wide expression profiling indicate that signaling pathways required for normal embryonic development are abnormally up-regulated in end-stage lung fibrosis (4). These observations raise the possibility that an inability to limit the activation of core developmental pathways during repair may drive the pathological process. How these core developmental pathways are coordinated to drive the cellular changes associated with fibrosis remains incompletely understood.
The Wnt/β-catenin signaling pathway regulates a wide array of biological processes. These include embryonic axial development, organogenesis, adult stem cell maintenance, and tissue homeostasis (5–7). The diverse effects of β-catenin signaling are ultimately controlled by a transcription complex that contains the DNA-binding factor lymphocyte enhancer factor (LEF)/T-cell factor (TCF) and β-catenin. In this complex, β-catenin serves as an obligate coactivator through its ability to recruit components that promote chromatin remodeling and transcriptional initiation and elongation (reviewed in Ref. 8). Wnts generate the nuclear signaling pool of β-catenin by inhibiting a multiprotein Axin-scaffold complex that continually phosphorylates β-catenin at serines 33 and 37, flagging it for degradation by the ubiquitin/proteosome system. Inhibition of this phosphorylation allows β-catenin to accumulate, enter the nucleus, and complex with TCF proteins. Although β-catenin/TCF-mediated transcription occurs in all tissues, the genes activated by this transcriptional complex are cell-type and -context dependent (reviewed in Ref. 9). Accordingly, the specific targets of β-catenin signaling required for homeostatic repair after injury in the adult lung are still emerging (10, 11).
The contribution of Wnt/β-catenin signaling to fibrogenesis is under active investigation. Microarray analyses of human tissue samples from idiopathic pulmonary fibrosis (IPF) lung and systemic sclerosis (SSc) demonstrate alterations in the expression of a number of β-catenin target genes (matrix metalloproteinase [MMP]-7), osteopontin, cyclin D1, secreted Frizzled-related protein [sFRP]2, peroxisome proliferator-activated receptor δ, and Wnt-inducible signaling protein) and pathway components (Wnt2b, Wnt5b, and Wnt inhibitory factor 1) (12–16). These observations suggest that Wnt/β-catenin signaling activation occurs in these fibrotic conditions. Because the targets of Wnt/β-catenin signaling are tissue-, cell-type–, and context dependent, we sought to address the contribution of Wnt/β-catenin signaling to lung fibrosis by evaluating how forced activation or inhibition of β-catenin/TCF signaling affects the fibrogenic characteristics of three independently derived sources of normal human lung fibroblasts.
Materials and Methods
Cell Culture and Adenoviral Constructs
Normal human lung fibroblasts were obtained from Lonza Walkersville Inc. (Walkersville, MD), human lung fibroblasts (NL-57) from normal lung tissues were obtained from organ donors, and human WI-38 fibroblasts from ATCC (CL-75; Manassas, VA). Culture conditions are described in the online supplement.
The adenovirus (Ad)-S37A-β-cat-HA (HA-hemagglutinin epitope tag) construct was kindly provided by Jan Kitajewski (Columbia University), the myc-ICAT cDNA was provided by Tetsu Akiyama (Japan) and reengineered by Vector Biolabs (Philadelphia, PA) to encode a bicistronic mRNA that translates myc-ICAT and GFP proteins (Ad-ICAT (Myc)-Internal Ribosome Entry Site-GFP), and Ad-Wnt3a-GFP and Ad-GFP viruses were supplied by Tong Chuan He (University of Chicago).
Bromodeoxyuridine Proliferation Assay
Proliferation assay was performed as previously described (17). At least 1,000 cells per cover slip were counted using a Zeiss Axioplan 2 fluorescent microscope (Carl Zeiss, Inc., Thornwood, NJ). Proliferation was determined as the percentage of bromodeoxyuridine-positive cells divided by the number of Hoechst-positive cells.
Ki67 Cell Proliferation Assay
Fibroblasts were infected with the indicated adenovirus, and immunostaining for Ki67 was performed as described in the online supplement. Cells were counted on 10 fields per slip using a Zeiss Axioplan 2 microscope.
Lactate Dehydrogenase Cell Death Assay
Normal human lung fibroblasts (NHLFs) were infected with the indicated adenoviruses, and cell death assay was performed as previously described in (17). Lactate dehydrogenase activity was measured using a Cytotoxicity Detection Kit (Roche, Indianapolis, IN). OD490 was read using a Versamax microplate reader and software (Molecular Devices, Sunnyvale, CA).
Quantification of mRNA
Primer sequences are provided in the online supplement. Real-time quantitative PCR (qPCR) was performed using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) with the MyiQ Single Color Real-Time PCR Detection System and software (Bio-Rad). Glyceraldehyde 3-phosphate dehydrogenase was used as the internal control.
Collagen Contraction Assay
NHLFs were infected with the specified adenovirus and placed in type I collagen gels as described in the online supplement. The diameter of each collagen matrix was measured at the indicated times. The results were expressed as percentage contraction [(initial diameter − end diameter)/initial diameter × 100%].
Cell Migration Assay
Assays were performed as described in the online supplement. In brief, a monolayer of fibroblasts was infected with the indicated adenovirus and Mitomycin C, and wound surface area was quantified using Metamorph analysis software (Molecular Devices). For Transwell migration, adenovirus-infected cells were seeded on the upper chamber of inserts (Becton Dickinson, Redlands, CA), and cells on the reverse side of the membrane from four low magnification fields were counted. Data are expressed as mean ± SD.
Immunohistochemistry
Lung tissue was obtained from three patients with SSc-associated pulmonary fibrosis who were undergoing lung transplant surgery and from three normal donors. Immunohistochemistry was performed using a monoclonal antibody to β-catenin (1:100) (Cat. 610153; BD Biosciences) as described in the online supplement.
Statistics
Statistical analysis was performed by Student's t test or one-way ANOVA followed by Bonferoni's multiple comparison test. A P value of less than 0.05 was considered significant. Statistical calculations were performed using GraphPad Prism software (Graph Pad Software Inc., La Jolla, CA).
Results
Nuclear β-Catenin Is Increased in SSc-Associated Pulmonary Fibrosis
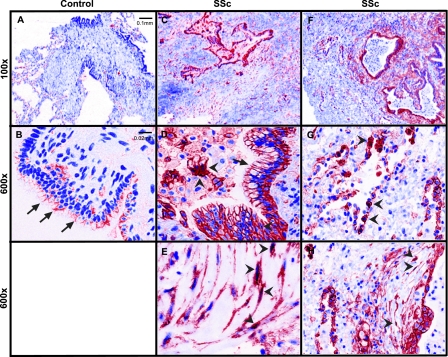
Interstitial lung diseases associated with pulmonary fibrosis can share similar or differing histologic patterns. For example, although IPF presents with the histologic pattern of usual interstitial pneumonia (UIP), lung fibrosis associated with systemic sclerosis may present with the pattern of UIP or nonspecific interstitial pneumonia (18). Whether these distinct histopathologic patterns of fibrosis are driven by activation of common cellular pathways is unclear. Previous work found increased nuclear staining of β-catenin in type II alveolar epithelial cells and fibroblasts from human IPF lung biopsies (19). Because systemic sclerosis-associated pulmonary fibrosis and IPF can display the same histopathologic features of UIP, we sought to clarify whether these two forms of lung fibrosis share a common pathway involving nuclear β-catenin. Lung biopsy samples were collected from three transplant patients with diffuse cutaneous systemic sclerosis and end-stage interstitial lung disease (ILD) (Table 1). Samples from three normal donors whose lungs were not used for transplantation served as controls. As expected, the lungs from control patients without ILD showed β-catenin staining predominantly at cell–cell junctions of airway epithelial cells (Figures 1A and 1B). In marked contrast, junctional as well as nuclear staining of β-catenin was seen in a number of cell types in SSc fibrotic lung (Figures 1C and 1F), including airway and alveolar epithelial cells (Figures 1D and 1G) and cells that appear to be fibroblasts (Figures 1E and 1H). These findings indicate that the signaling pool of β-catenin appears stabilized in systemic sclerosis end-stage fibrotic lungs and suggest that Wnt activation may be a common feature of different etiologies of pulmonary fibrosis.
TABLE 1.
CLINICAL CHARACTERISTICS OF PATIENTS WITH SYSTEMIC SCLEROSIS
| Age (yr) | Sex | Smoking | Pathology | FVC (%) | FEV1 (%) | DlCO (%) | PAP (mm Hg) | |
| SSc 1 | 45 | Male | No | UIP/NSIP | 26 | 28 | 30 | 35/17 |
| SSc 2 | 52 | Female | Yes | UIP | 36 | 44 | 20 | 34/14 |
| SSc 3 | 58 | Male | Yes | UIP | 63 | 77 | 16 | 61/26 |
Definition of abbreviations: NSIP = nonspecific interstitial pneumonia; PAP = pulmonary artery pressure; SSc = systemic sclerosis; UIP = usual interstitial pneumonia.
Figure 1.
Nuclear β-catenin staining is observed in systemic sclerosis (SSc)-associated pulmonary fibrosis. (A) Low (100×) and (B) high (600×) magnification images from a control lung show β-catenin staining at the cell–cell contacts of airway epithelial cells (arrows) but no obvious staining in nuclei of alveolar epithelial or interstitial cells. Control lungs are taken from normal donors whose lungs were not used for transplant. (C–H) Lung section images from patients with SSc-associated pulmonary fibrosis show β-catenin staining at junctions of airway epithelial cells (D, arrows), in nuclei of alveolar epithelial cells (G, arrowheads), and within cytoplasm and nuclei of cells that appear to be fibroblasts (E and H, arrowheads). Images are representative findings from three control patients and three patients with SSc–interstitial lung disease using Zeiss Axioskop with a CRi Nuance multispectral camera.
Wnt/β-Catenin Signaling Is Not Sufficient to Up-Regulate Classic Markers of Fibroblast Activation
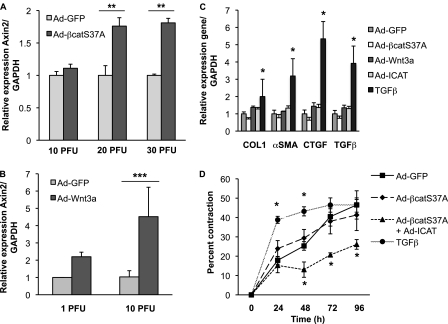
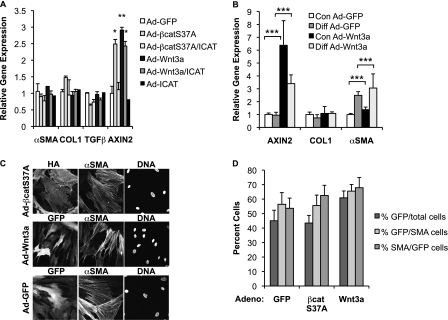
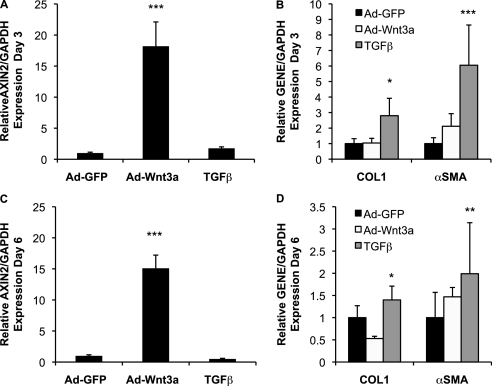
A key effector cell in the development of pulmonary fibrosis is the fibroblast, in which TGF-β signaling is known to up-regulate a number of profibrotic genes, including type 1 collagen (COL1), connective tissue growth factor (CTGF), and α-smooth muscle actin (α-SMA) (20). Given previous reports that Wnt signaling can affect the expression of α-SMA (21–23), we sought to determine whether Wnt/β-catenin signaling was sufficient to drive expression of these same profibrotic genes in NHLFs. Adenoviral expression of Wnt3a and a nondegradable form of β-catenin (Ad-S37A-β-cat) activates β-catenin signaling in NHLFs, as evidenced by increased β-catenin/TCF-mediated transcription using the minimal TCF Optimal Promoter luciferase reporter TOPFLASH and increased protein levels of transcriptionally active and total cytosolic forms of β-catenin (see Figure E1 in the online supplement). We also observed up-regulation of a defined, endogenous, early target of Wnt/β-catenin signaling, AXIN2, by qPCR (Figures 2A and 2B). Activation of β-catenin signaling was not sufficient to promote the expression of classic profibrotic genes typically induced by TGF-β (5 ng/ml) (Figure 2C), a finding that was confirmed in two other primary lung fibroblast lines, NL-57 and WI-38 (Figures 3A and 3B). Even chronic activation of Wnt/β-catenin signaling in NHLF cultures for 6 days failed to significantly up-regulate collagen and α-SMA mRNA or protein (Figure 4; immunoblot data not shown), despite evidence that approximately 60% of infected cells are α-SMA positive (Figures 3C and 3D). Coactivation of β-catenin and TGF-β signaling showed no enhanced expression of these targets (qPCR data not shown), despite evidence for cross-talk between Wnt/β-catenin and TGF-β pathways in other systems (24–27). Moreover, even when using media conditions that up-regulate α-SMA mRNA expression (2-fold) in the human embryonic lung fibroblast line WI-38, additional activation of Wnt/β-catenin signaling fails to significantly enhance α-SMA expression (Figure 3B). Although overexpression of the active, nondegradable form of β-catenin is insufficient to promote fibroblast contraction of a type 1 collagen matrix compared with TGF-β (Figure 2D), β-catenin signaling may be required because cytosolic β-catenin can be detected at baseline in NHLF cultures (Figure E1B) and inhibition by ICAT (inhibitor of β-catenin and TCF) reduces contraction below control levels. Altogether, these findings indicate that CTGF, COL1, α-SMA, and TGF-β are not robust targets of Wnt/β-catenin signaling in lung fibroblast cultures and that the contribution of β-catenin signaling to fibroblast contractility may be independent of α-SMA (28).
Figure 2.
Overexpression of Wnt and nondegradable β-catenin is insufficient to induce expression of TGF-β–responsive genes in normal human lung fibroblasts. Normal human lung fibroblasts (NHLFs) were infected with Ad-GFP (control) or Ad-S37A–β-catenin (A) or Ad-Wnt3a (B) for 24 hours; total RNA was extracted to quantify expression of AXIN2 mRNA by quantitative PCR (qPCR). Ad-S37A–β-catenin and Ad-Wnt3a are sufficient to promote Wnt/β-catenin signaling in NHLFs 24 hours postinfection. Results are expressed as the mean expression ± SD of AXIN2 relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) from three independent experiments (**P < 0.01; ***P < 0.001). (C) NHLFs were treated with Ad-GFP (control), Ad-S37A–β-catenin (20 plaque-forming units (PFU)/cell), Ad-Wnt3a (10 PFU/cell), Ad-ICAT (20 PFU/cell), or TGF-β (5 ng/ml) for 24 hours; total RNA was extracted to quantify expression of type 1 collagen (COL1), α-smooth muscle actin (α-SMA), connective tissue growth factor (CTGF), and TGF-β mRNAs by quantitative PCR. Results are expressed as the mean gene expression ± SD relative to GAPDH from three independent experiments (*P < 0.05). (D) Collagen contraction by NHLFs after treatment with TGF-β (5 ng/ml), Ad-GFP (control), Ad-S37A–β-catenin, or Ad-S37A–β-catenin with Ad-ICAT. Results are expressed as the percentage mean change in matrix diameter size compared with the 0-hour time point ± SD from three independent experiments (*P < 0.05).
Figure 3.
Wnt/β-catenin signaling is not sufficient to induce expression of TGF-β or its established profibrotic targets in NL-57 and WI-38 human fibroblasts. (A) Lung fibroblasts (NL-57) obtained from a patient without lung disease were treated with Ad-GFP (control), Ad-S37A–β-catenin (20 PFU/cell), Ad-Wnt3a (10 PFU/cell), or Ad-ICAT (20 PFU/cell) for 24 hours; total RNA was extracted to quantify expression of α-SMA, COL1, TGF-β, and AXIN2 mRNAs by quantitative PCR. Results are expressed as the mean gene expression ± SD relative to GAPDH from three independent experiments (*P < 0.05; **P < 0.01). (B) Human embryonic fibroblasts (WI-38) were infected with Ad-GFP (control) or Ad-Wnt3a (10 PFU/cell) after undergoing treatment with control or differentiation media for 3 days; total RNA was extracted to quantify expression of AXIN2, COL1, and α-SMA mRNAs by qPCR. Results are expressed as the mean gene expression ± SD relative to GAPDH from three independent experiments (***P < 0.001). (C) Immunofluorescence of NHLFs infected with Ad-GFP, Ad-Wnt3a-GFP, and Ad-S37A–β-catenin–HA for 24 hours. Immunostaining was performed to confirm the presence of hemagglutinin (HA), GFP, and α-SMA; cell nuclei were stained with Hoechst. (D) Infection efficiency with Ad-GFP, Ad-S37A–β-catenin, and Ad-Wnt3a is routinely > 40% and expressed as GFP- or HA-positive cells divided by the total number of cells (%GFP/total cells). More than 50% of α-SMA–expressing cells are infected, expressed as GFP- or HA-positive cells divided by number of α-SMA–expressing cells (%GFP/SMA). Similarly, more than 50% of infected cells are α-SMA positive (%SMA/GFP). Results are expressed as the percent ± SEM from three independent experiments.
Figure 4.
Persistent activation of Wnt/β-catenin signaling does not alter COL1 or α-SMA expression. NHLFs were infected with Ad-GFP (control) or Ad-Wnt3a (10 PFU/cell) or treated with TGF-β (5 ng/ml) every 48 hours until RNA was extracted on Days 3 or 6 postinfection. Although persistent infection with Ad-Wnt3a robustly increases AXIN2 expression on Days 3 (A) and 6 (C), this does not alter COL1 or α-SMA gene expression, in contrast to TGF-β (B and D). Results are expressed as the mean gene expression ± SD relative to GAPDH from three independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001).
Wnt/β-Catenin Signaling Enhances Fibroblast Proliferation but Not Survival
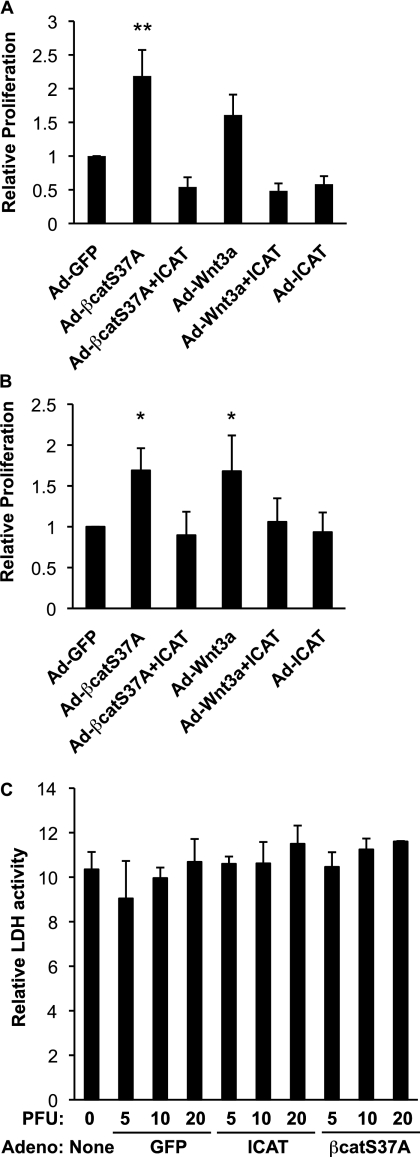
It is well established that Wnt/β-catenin signaling can promote cell proliferation, particularly in epithelial-derived cancers and stem cell populations (29–34), but whether β-catenin signaling drives fibroproliferation through activation of similar gene targets as epithelial cells is unclear. We found that forced activation of β-catenin signaling with adenoviruses expressing Wnt3a or a nondegradable form of β-catenin (Ad-S37A–β-catenin) enhanced bromodeoxyuridine incorporation over Ad-GFP–infected control cells in two independent adult lung fibroblast lines (NL-57 s, Figure 5A; NHLFs, Figure E2A). Wnt/β-catenin signaling also increased the number of fibroblasts expressing the proliferation marker Ki67 (NHLFs; Figure 5B). Although Wnt3a promotes β-catenin stabilization in these fibroblasts (NHLFs, Figure E1B; NL-57 s not shown), it is less potent at driving proliferation than S37A–β-catenin (Figures 5A and E2A). This may be because signaling components further upstream in the Wnt pathway have a greater number of opportunities to be negatively regulated than the more “downstream” components (e.g., β-catenin). The effects of Wnt3a and S37A–β-catenin on proliferation are due to increased β-catenin/TCF transcriptional activity, as opposed to noncanonical effects on adhesion-dependent processes because coinfection with ICAT restores the rate of proliferation to baseline levels (Figures 5A and E2A). The reduced proliferation observed with ICAT is not due to an effect on cell survival because fibroblasts infected with increasing doses of Ad-GFP and Ad-ICAT-GFP failed to promote fibroblast cell death above baseline levels (Figure 5C).
Figure 5.
Wnt/β-catenin signaling promotes proliferation and is not required for the survival of normal human lung fibroblasts. (A) Bromodeoxyuridine (BrdU) incorporation assay. Human lung fibroblasts (NL-57) from nondiseased lungs were infected with Ad-GFP, Ad-S37A–β-catenin, Ad-ICAT, and Ad-Wnt3a for 48 hours and then pulse-labeled with BrdU. Results are expressed as mean ± SD relative to control adenovirus from six independent experiments (**P < 0.01). Similar results were observed with NHLFs (Figure E2). (B) Ki67 assay. Human lung fibroblasts (NHLFs) were infected with Ad-GFP, Ad-S37A–β-catenin, Ad-ICAT, and Ad-Wnt3a for 48 hours and processed for immunofluorescence staining with an antibody to Ki67. Results are expressed as mean ± SD relative to control adenovirus from three to five independent experiments (*P < 0.05). (C) Lactate dehydrogenase (LDH) cell viability assay. NHLFs were infected with Ad-GFP, Ad-S37A–β-catenin, and Ad-ICAT for 72 hours, and media was assessed for LDH activity as described in Materials and Methods. Activation of Wnt/β-catenin signaling neither promotes cell death with increasing PFUs of control adenovirus or adenovirus expressing S37A–β-catenin nor is required for cell survival (Ad-ICAT). Results are expressed as mean levels of LDH activity ± SD from at least three independent experiments.
Wnt/β-Catenin Signaling Enhances Fibroblast Motility
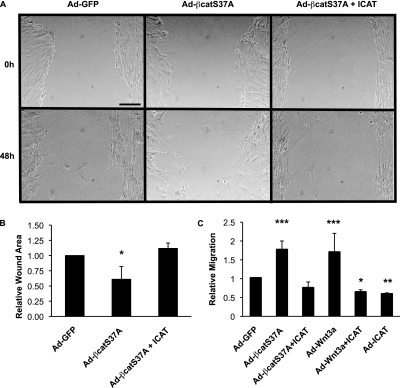
The ability of fibroblasts to migrate to sites of injury to facilitate repair is viewed as a process that may be dysregulated in fibrosis (reviewed in Ref. 2). Therefore, we sought to determine whether β-catenin signaling could affect fibroblast motility. Using a simple “scratch” wound-closure assay on a monolayer of fibroblasts, we found that the stabilized form of β-catenin hastened the rate of wound closure (Figure 6A), revealing reduced wound surface area (Figure 6B). Confirming these findings, we show that increased β-catenin signaling mediated by the nondegradable form of β-catenin or Wnt3a enhances fibroblast migration through a 3-μm-pore Transwell membrane (i.e., Boyden chamber assay; Figure 6C). Similar results were observed in a second adult lung fibroblast line (NL-57; Figure E3A). The effect on motility is specific to β-catenin/TCF signaling activity because coexpression with ICAT returns the level of cell migration to baseline (Figure 6C and Figure E3A).
Figure 6.
Wnt/β-catenin signaling stimulates fibroblast migration. (A) NHLFs were “scratch” wounded, infected with the stated adenoviruses, and photographed at 0-, 24-, and 48-hour time points. Representative images for each infection and 48-hour time point are shown. (B) Quantification of wound size at 48 hours. Results are expressed as mean change ± SD of wound area relative to wound area at 0 time point from four independent experiments (*P < 0.05). (C) Lung fibroblasts (NL-57) obtained from a patient without pulmonary disease were infected with Ad-GFP, Ad-S37A–β-catenin, Ad-ICAT, and Ad-Wnt3a and analyzed in a Boyden chamber assay as described in Materials and Methods. Similar results were observed with NHLFs (Figure E3). Results are expressed as the mean number of cells migrated in each condition relative to control adenovirus ± SD from eight independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001).
It is well established that lung fibroblasts produce proteases that degrade collagens, and recent studies have identified certain collagen proteases as β-catenin/TCF-target genes (35–37). We found that β-catenin signaling mediated by the nondegradable form of β-catenin or Wnt3a is sufficient to enhance NHLF migration through type 1 collagen–coated Transwell membranes but fails to increase invasion through Matrigel (data not shown). These data raised the possibility that Wnt/β-catenin signaling up-regulates proteases that target type 1 collagen. Although lung fibroblasts are known to produce MMP-1 and MMP-2 (38), neither MMP-1 nor MMP-2 mRNA levels are increased by Wnt/β-catenin signaling (Figures E3B and E3C). Moreover, Wnt/β-catenin signaling failed to enhance MMP-2 activities over the already substantial level of baseline collagenase activity assessed by gelatin zymography (data not shown). These data indicate that Wnt/β-catenin signaling activation promotes normal human lung fibroblast migration through type 1 collagen, largely through enhancing the motile characteristics of cells that already have the capacity to degrade matrix.
Discussion
It has long been reasoned that different clinical and histologic presentations of pulmonary fibrosis may share similar molecular mechanisms, but evidence supporting this notion has been lacking. Our detection of nuclear β-catenin staining in fibroblasts and alveolar epithelial cells in end-stage fibrotic lungs from patients with SSc-ILD (Figure 1) demonstrates that β-catenin may be activated in another form of pulmonary fibrosis. These findings corroborate previous observations in samples from patients with IPF (19, 39) and suggest that up-regulation of nuclear β-catenin may be a common feature of end-stage lung fibrosis. The observed increase in β-catenin nuclear staining in human lungs likely reflects bona fide Wnt/β-catenin signaling because microarray data from SSc-affected tissues reveals alterations in multiple Wnts, their regulators, receptors, and targets (16). Although Wnt/β-catenin signaling can be induced in a murine model of lung fibrosis using intratracheal bleomycin (17, 40, 41), the temporal sequence and relative contributions of Wnt activation in epithelial cells, pulmonary interstitial cells, and progenitor cells are not fully established and are under active investigation by our lab. Ongoing studies using whole animal and cell type–specific knockout approaches are required to determine in which cell type β-catenin signaling casually contributes to lung fibrosis.
Since fibroblast proliferation and migration are thought to be critical steps in lung fibrogenesis (42–44), we addressed the contribution of Wnt/β-catenin signaling to these phenotypes using cultures of normal human lung fibroblast and adenoviruses that can force activate (Ad-Wnt3a, Ad-S37A–β-catenin) or inhibit (Ad-ICAT) Wnt/β-catenin signaling. We found that activation of Wnt/β-catenin signaling enhances lung fibroblast proliferation in vitro (Figures 5 and E2), which may contribute to the increase in fibroblast number and activity during fibrogenesis in vivo (2, 3, 45). Although C-MYC and cyclinD1 are established β-catenin/TCF targets in epithelial cell types (46–48), these genes were not obviously up-regulated in NHLFs (Figure E2), suggesting that other targets are responsible for fibroproliferation. Recently, Vugua and colleagues found that Wnt5a can increase fibroblast proliferation through a “noncanonical” or β-catenin/TCF-independent signaling mechanism (49), indicating that canonical and noncanonical Wnts may contribute to fibroproliferation. We also found that Wnt/β-catenin signaling activation can enhance lung fibroblast motility and migration through a type 1 collagen matrix (Figure 6 and data not shown), but the specific targets that promote these activities remain to be determined. For example, although MMP-1, -2, and -9 are known targets of β-catenin/TCF signaling in epithelial and immune cells (35–37) and contribute to cell migrations in these cell types, these genes do not appear to be responsible for the enhanced motility observed our Wnt/β-catenin signaling activated lung fibroblasts (Figure E3). Gene expression analysis from Wnt3a-treated NIH 3T3 embryonic fibroblasts revealed an increase in genes associated with the cell cycle, motility, and adhesion (50), consistent with the Wnt-activated phenotypes characterized in this study. Given that Wnt/β-catenin target genes are cell-type and cell-context dependent, defining the Wnt/β-catenin–regulated target genes in human lung fibroblasts requires similar unbiased microarray approaches.
The transdifferentiation of fibroblasts into myofibroblasts and consequent up-regulation of extracellular matrix components is typically viewed as a hallmark of fibrogenesis (2, 3). Since some of the classic markers of fibroblast “activation” and myofibroblast differentiation contain potential TCF-binding sites in their proximal promoter regions (51, 52), we reasoned that these markers would be co-regulated by β-catenin signaling. However, in striking contrast to the effects of the well established profibrotic factor TGF-β on these fibroblasts (Figures 2C, 2D, and 4B and Figure E4), β-catenin signaling failed to increase mRNA expression of CTGF, COL1, α-SMA, and TGF-β upon either short-term (24 h) (Figure 2) or longer-term (6 d) (Figure 4) Wnt/β-catenin signaling activation. These data indicate that β-catenin signaling in normal human lung fibroblast cultures is not sufficient to activate many of the classic (TGF-β–activated) targets of myofibroblast differentiation. Consistent with these results, Chen and colleagues did not detect increased expression of a fibrotic gene cluster in their analyses of NIH 3T3 cells treated with recombinant Wnt3a (50).
Two recent studies indicate that a systemic elevation of Wnt proteins or injury-induced reductions in a Wnt inhibitor can contribute to fibrosis in other tissues (7, 21). Specifically, age-related up-regulation of canonical Wnts can drive muscle stem cell differentiation along a fibroblastic, as opposed to a myogenic, lineage, resulting in fibrosis at the expense of muscle maintenance (7). Moreover, an injury-induced model of kidney fibrosis was associated with decreased levels of sFRP4, a decoy receptor for Wnt, which correlated with increased levels of the signaling form of β-catenin as well as fibrotic markers such α-SMA (21). Administration of recombinant sFRP4 reduced the levels of active β-catenin and α-SMA expression and the number of myofibroblasts. Since Wnt/β-catenin signaling appears insufficient to up-regulate the expression and number α-SMA–positive lung fibroblasts in culture (Figure 3), β-catenin signaling may contribute to α-SMA expression and myofibroblast abundance in vivo by promoting myofibroblast proliferation or by cell fate decisions that generate the myofibroblastic lineage.
Altogether, the results from our study demonstrate that sustained activation of Wnt/β-catenin signaling in normal adult lung fibroblasts, as might occur in SSc-associated ILD, can enhance activities associated with fibrosis, such as proliferation and motility. Whether these Wnt/β-catenin signaling–dependent fibroblast activities substantially contribute to disease progression needs to be determined using targeted knock-out models. However, given that Wnt/β-catenin signaling controls cell-fate decisions throughout development, often controlling the balance between progenitor cells and their descendants (53), we speculate that cells serving as progenitors to fibroblasts and myofibroblasts may be a key target of Wnt/β-catenin signaling in pulmonary fibrosis. The identification of such fibroblast progenitors in lung and their response to Wnt/β-catenin signaling will be the subject of future investigations.
Supplementary Material
Acknowledgments
The authors thank Drs. J. Iasha Sznajder, Annie Pardo, and Moises Selman for encouragement and insights.
Footnotes
This work was supported by an NRSA from NHLBI (HL78145) (A.P.L.); by funding from the Northwestern Memorial Faculty Foundation (M.J.); by grants HL071643 and ES015024 and an NIEHS ONES Award (G.M.M.); HL071643 and ES013995 (G.R.S.B.); AR050840 (C.A.F.); NIAMS AR42309 (J.V.); and NIH-GM076561 and HL094643 (C.J.G.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0113OC on April 14, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001;134:136–151 [DOI] [PubMed] [Google Scholar]
- 2.Phan SH. The myofibroblast in pulmonary fibrosis. Chest 2002; 122(Suppl)286S–289S [DOI] [PubMed] [Google Scholar]
- 3.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol 2007;170:1807–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med 2008;5:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. Wnt and beta-catenin signalling: diseases and therapies. Nat Rev Genet 2004;5:691–701 [DOI] [PubMed] [Google Scholar]
- 6.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell 2006;127:469–480 [DOI] [PubMed] [Google Scholar]
- 7.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 2007;317:807–810 [DOI] [PubMed] [Google Scholar]
- 8.Logan CY, Nusse R. The wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004;20:781–810 [DOI] [PubMed] [Google Scholar]
- 9.Chien AJ, Conrad WH, Moon RT. A wnt survival guide: from flies to human disease. J Invest Dermatol 2009;129:1614–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrisey EE. Wnt signaling and pulmonary fibrosis. Am J Pathol 2003;162:1393–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. Beta-catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem 2003;278:40231–40238 [DOI] [PubMed] [Google Scholar]
- 12.Selman M, Pardo A, Barrera L, Estrada A, Watson SR, Wilson K, Aziz N, Kaminski N, Zlotnik A. Gene expression profiles distinguish idiopathic pulmonary fibrosis from hypersensitivity pneumonitis. Am J Respir Crit Care Med 2006;173:188–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardo A, Gibson K, Cisneros J, Richards TJ, Yang Y, Becerril C, Yousem S, Herrera I, Ruiz V, Selman M, et al. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med 2005;2:e251– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA 2002;99:6292–6297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selman M, Carrillo G, Estrada A, Mejia M, Becerril C, Cisneros J, Gaxiola M, Perez-Padilla R, Navarro C, Richards T, et al. Accelerated variant of idiopathic pulmonary fibrosis: clinical behavior and gene expression pattern. PLoS ONE 2007;2:e482– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner H, Shearstone JR, Bandaru R, Crowell T, Lynes M, Trojanowska M, Pannu J, Smith E, Jablonska S, Blaszczyk M, et al. Gene profiling of scleroderma skin reveals robust signatures of disease that are imperfectly reflected in the transcript profiles of explanted fibroblasts. Arthritis Rheum 2006;54:1961–1973 [DOI] [PubMed] [Google Scholar]
- 17.Flozak AS, Lam AP, Russell S, Jain M, Peled ON, Sheppard KA, Beri R, Mutlu GM, Budinger GR, Gottardi CJ. Beta-catenin/t-cell factor signaling is activated during lung injury and promotes the survival and migration of alveolar epithelial cells. J Biol Chem 2010;285:3157–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias Am J Respir Crit Care Med 2002; 165:277–304 [DOI] [PubMed] [Google Scholar]
- 19.Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, et al. Aberrant wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol 2003;162:1495–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease. Chest 2004;125:754–765 [DOI] [PubMed] [Google Scholar]
- 21.Surendran K, Schiavi S, Hruska KA. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol 2005;16:2373–2384 [DOI] [PubMed] [Google Scholar]
- 22.Caraci F, Gili E, Calafiore M, Failla M, La Rosa C, Crimi N, Sortino MA, Nicoletti F, Copani A, Vancheri C. Tgf-beta1 targets the gsk-3beta/beta-catenin pathway via erk activation in the transition of human lung fibroblasts into myofibroblasts. Pharmacol Res 2008;57:274–282 [DOI] [PubMed] [Google Scholar]
- 23.DasGupta R, Fuchs E. Multiple roles for activated lef/tcf transcription complexes during hair follicle development and differentiation. Development 1999;126:4557–4568 [DOI] [PubMed] [Google Scholar]
- 24.Dao DY, Yang X, Chen D, Zuscik M, O'Keefe RJ. Axin1 and axin2 are regulated by tgf-beta and mediate cross-talk between tgf-beta and wnt signaling pathways. Ann N Y Acad Sci 2007;1116:82–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W, Rui H, Wang J, Lin S, He Y, Chen M, Li Q, Ye Z, Zhang S, Chan SC, et al. Axin is a scaffold protein in tgf-beta signaling that promotes degradation of smad7 by arkadia. EMBO J 2006;25:1646–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, Cho KR, Fearon ER. Activation of axin2 expression by beta-catenin-t cell factor: a feedback repressor pathway regulating wnt signaling. J Biol Chem 2002;277:21657–21665 [DOI] [PubMed] [Google Scholar]
- 27.Jian H, Shen X, Liu I, Semenov M, He X, Wang XF. Smad3-dependent nuclear translocation of beta-catenin is required for tgf-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev 2006;20:666–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim NG, Xu C, Gumbiner BM. Identification of targets of the wnt pathway destruction complex in addition to beta-catenin. Proc Natl Acad Sci USA 2009;106:5165–5170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Es JH, Jay P, Gregorieff A, van Gijn ME, Jonkheer S, Hatzis P, Thiele A, van den Born M, Begthel H, Brabletz T, et al. Wnt signalling induces maturation of paneth cells in intestinal crypts. Nat Cell Biol 2005;7:381–386 [DOI] [PubMed] [Google Scholar]
- 30.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for wnt signalling in self-renewal of haematopoietic stem cells. Nature 2003;423:409–414 [DOI] [PubMed] [Google Scholar]
- 31.Yu HM, Jerchow B, Sheu TJ, Liu B, Costantini F, Puzas JE, Birchmeier W, Hsu W. The role of axin2 in calvarial morphogenesis and craniosynostosis. Development 2005;132:1995–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, et al. The beta-catenin/tcf-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 2002;111:241–250 [DOI] [PubMed] [Google Scholar]
- 33.Angers S, Moon RT. Proximal events in wnt signal transduction. Nat Rev Mol Cell Biol 2009;10:468–477 [DOI] [PubMed] [Google Scholar]
- 34.Woodhead GJ, Mutch CA, Olson EC, Chenn A. Cell-autonomous beta-catenin signaling regulates cortical precursor proliferation. J Neurosci 2006;26:12620–12630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doyle JL, Haas TL. Differential role of beta-catenin in VEGF and histamine-induced MMP-2 production in microvascular endothelial cells. J Cell Biochem 2009;107:272–283 [DOI] [PubMed] [Google Scholar]
- 36.Denys H, De Wever O, Nusgens B, Kong Y, Sciot R, Le AT, Van Dam K, Jadidizadeh A, Tejpar S, Mareel M, et al. Invasion and mmp expression profile in desmoid tumours. Br J Cancer 2004;90:1443–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jean C, Blanc A, Prade-Houdellier N, Ysebaert L, Hernandez-Pigeon H, Al Saati T, Haure MJ, Coluccia AM, Charveron M, Delabesse E, et al. Epidermal growth factor receptor/beta-catenin/t-cell factor 4/matrix metalloproteinase 1: a new pathway for regulating keratinocyte invasiveness after uva irradiation. Cancer Res 2009;69:3291–3299 [DOI] [PubMed] [Google Scholar]
- 38.Ramos C, Montano M, Becerril C, Cisneros-Lira J, Barrera L, Ruiz V, Pardo A, Selman M. Acidic fibroblast growth factor decreases alpha-smooth muscle actin expression and induces apoptosis in human normal lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 2006;291:L871–L879 [DOI] [PubMed] [Google Scholar]
- 39.Konigshoff M, Balsara N, Pfaff EM, Kramer M, Chrobak I, Seeger W, Eickelberg O. Functional wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS ONE 2008;3:e2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L, Carron B, Yee HT, Yie TA, Hajjou M, Rom W. Wnt pathway in pulmonary fibrosis in the bleomycin mouse model. J Environ Pathol Toxicol Oncol 2009;28:99–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konigshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, Rose F, Fink L, Seeger W, Schaefer L, et al. Wnt1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest 2009;119:772–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katzenstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med 1998;157:1301–1315 [DOI] [PubMed] [Google Scholar]
- 43.Kuhn C, McDonald JA. The roles of the myofibroblast in idiopathic pulmonary fibrosis: ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol 1991;138:1257–1265 [PMC free article] [PubMed] [Google Scholar]
- 44.Kuhn C, III, Boldt J, King TE, Jr, Crouch E, Vartio T, McDonald JA. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis 1989;140:1693–1703 [DOI] [PubMed] [Google Scholar]
- 45.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest 2007;132:1311–1321 [DOI] [PubMed] [Google Scholar]
- 46.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin d1 gene is a target of the beta-catenin/lef-1 pathway. Proc Natl Acad Sci USA 1999;96:5522–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin d1 in colon carcinoma cells. Nature 1999;398:422–426 [DOI] [PubMed] [Google Scholar]
- 48.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-myc as a target of the apc pathway. Science 1998;281:1509–1512 [DOI] [PubMed] [Google Scholar]
- 49.Vuga LJ, Ben-Yehudah A, Kovkarova-Naumovski E, Oriss T, Gibson KF, Feghali-Bostwick C, Kaminski N. Wnt5a is a regulator of fibroblast proliferation and resistance to apoptosis. Am J Respir Cell Mol Biol 2009;41:583–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen S, McLean S, Carter DE, Leask A. The gene expression profile induced by wnt 3a in NIH 3T3 fibroblasts. J Cell Commun Signal 2007;1:175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo Q, Kang Q, Si W, Jiang W, Park JK, Peng Y, Li X, Luu HH, Luo J, Montag AG, et al. Connective tissue growth factor (ctgf) is regulated by wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem 2004;279:55958–55968 [DOI] [PubMed] [Google Scholar]
- 52.Gradl D, Kuhl M, Wedlich D. The wnt/wg signal transducer beta-catenin controls fibronectin expression. Mol Cell Biol 1999;19:5576–5587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature 2005;434:843–850 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.