Abstract
The concentration of urokinase plasminogen activator (uPA) is elevated in pathological settings such as acute lung injury, where pulmonary arterial contractility and permeability are disrupted. uPA limits the accretion of fibrin after injury. Here we investigated whether uPA also regulates pulmonary arterial contractility and permeability. Contractility was measured using isolated pulmonary arterial rings. Pulmonary blood flow was measured in vivo by Doppler and pulmonary vascular permeability, according to the extravasation of Evans blue. Our data show that uPA regulates the in vitro pulmonary arterial contractility induced by phenylephrine in a dose-dependent manner through two receptor-dependent pathways, and regulates vascular contractility and permeability in vivo. Physiological concentrations of uPA (≤1 nM) stimulate the contractility of pulmonary arterial rings induced by phenylephrine through the low-density lipoprotein receptor–related protein receptor. The procontractile effect of uPA is independent of its catalytic activity. At pathophysiological concentrations, uPA (20 nM) inhibits contractility and increases vascular permeability. The inhibition of vascular contractility and increase of vascular permeability is mediated through a two-step process that involves docking to N-methyl-d-aspartate receptor–1 (NMDA-R1) on pulmonary vascular smooth muscle cells, and requires catalytic activity. Peptides that specifically inhibit the docking of uPA to NMDA-R, or the uPA variant with a mutated receptor docking site, abolished both the effects of uPA on vascular contractility and permeability, without affecting its catalytic activity. These data show that uPA, at concentrations found under pathological conditions, reduces pulmonary arterial contractility and increases permeability though the activation of NMDA-R1. The selective inhibition of NMDAR-1 activation by uPA can be accomplished without a loss of fibrinolytic activity.
Keywords: urokinase, NMDA-R, lung, permeability
The fibrinolytic system helps limit the deposition of fibrin in the small airways and alveolar compartment of the normal lung (1). Bronchoalveolar lavage (BAL) fluid from healthy individuals contains detectable amounts of urokinase-type plasminogen activator (uPA) (2, 3). The exuberant expression of plasminogen activator inhibitor–1 (PAI-1) and the inhibition of plasmin are characteristic of the fibrinolytic defect in patients with acute respiratory distress syndrome (ARDS) and various forms of lung injury associated with the persistence of alveolar hyaline membranes and disordered lung remodeling (1, 4). Furthermore, intraalveolar fibrin fails to deposit in response to hyperoxia in mice deficient in PAI-1, suggesting that the balance between plasminogen activators and their major inhibitor plays a crucial role in preventing the clearance of alveolar fibrin (5).
However, the involvement of uPA in lung physiology and pathophysiology may extend beyond fibrinolysis. uPA is a multifunctional protein that was implicated in tracheal smooth muscle cell (SMC) contractility (6), the activation of leukocytes (7), and vascular remodeling (8–10), among other activities relevant to lung injury (11–14). Some of these phenotypic changes require the proteolytic activity of uPA, whereas others involve intracellular signaling through cellular receptors, including the uPA receptor (uPAR) (10, 15), the low-density lipoprotein–related receptor (LRP) (16, 17), specific integrins (7), and N-methyl-d-aspartate receptors (NMDA-Rs) (6).
The observation that uPA-deficient mice (uPA−/−) are protected against the pulmonary edema induced by LPS (18) suggests that uPA may also affect vascular tone and permeability within the lung. We previously reported that uPA regulates the vascular contractility of isolated aortic rings (16, 19), and that uPA−/− mice exhibit significantly lower mean arterial blood pressure than do wild-type mice, indicating a predominant procontractile effect of uPA in the systemic arterial circulation under physiological conditions (16). The procontractile effect of uPA is independent of uPAR, and is mediated through LRP (16). LRP antagonists abolish the vasoactivity of uPA in vitro and in vivo, as do ligands that regulate the interaction of uPA with LRP, such as PAI-1 or a PAI-1–derived peptide (EEIIMD) that binds to the “docking site” in uPA for PAI-1 (16). Similar effects of uPA on vascular contractility were observed within the cerebral circulation (17, 20, 21).
The mechanism by which uPA might contribute to the regulation of vascular tone and vascular permeability in the lung is unknown, but could involve signaling through NMDA-Rs. NMDA-Rs are cation channels highly permeable to calcium that were identified in rodent (22–24) and human lungs (6), where they were implicated in the regulation of vascular tone and, more recently, in airway contractility (6). Clues to the function of NMDA-Rs within the pulmonary vasculature may come from their activity within the central nervous system (CNS). Several groups, including our own (21, 25), showed that tissue type plasminogen activator (tPA) and uPA signal through NMDA-Rs. Acting through NMDA-Rs, uPA impairs physiological cerebral vasodilation in response to hypercapnia in the setting of hypoxia and ischemia (26). We also observed that tPA and uPA regulate airway contractility through NMDA-R1 (6).
The molecular basis of the interaction between plasminogen activators (PAs) and NMDA-Rs is incompletely understood. tPA forms a complex with NMDA-R1, as expressed by cortical neurons (25, 27) and tracheal SMC (6). This precedes the cleavage of the NR1 subunit of the receptor by tPA, which is followed by an enhancement of intracellular Ca++ concentration and cell death in the case of neurons (25, 27), and to the stimulation of tracheal contractility induced by acetylcholine in the case of airways (6). It is uncertain whether the cellular consequences result from an occupancy or cleavage of the NR1 subunit, or from events downstream of the receptor binding itself that entail the formation of complexes with cognate serpins and intracellular signaling that also involves LRP (6, 25). The resistance of uPA−/− mice to LPS-induced pulmonary edema, together with the effects of uPA on vascular tone in the CNS and airway contractility, led us to study the effects of uPA on pulmonary arterial contractility and permeability.
Materials and Methods
Materials
The PAI-1–derived peptide EEIIMD was synthesized as described elsewhere (28). MK-801 and glutamate were purchased from Sigma (Rehovot, Israel). Anti-LRP and receptor-associated protein (RAP) were obtained from American Diagnostica (Stamford, CT). Anti–NMDA-R1 antibody was purchased from Biotest (Tel-Aviv, Israel). Polyclonal anti-uPA was provided by UMTEK (Moscow, Russia). Recombinant uPAs were synthesized and characterized as described elsewhere (6).
Human uPA PAI-I Docking-Site Mutant
To generate the uPA PAI-I docking-site (DS) mutant (179–184RHRGGS→AAAAAA), uPA cDNA was amplified by PCR, using the primer pair 5′-GCG GCC ATC TAC CGC GCT GCC GCC GCT GCC GCT GTC ACC TAC GTG TGC GGA GGC AG-3′ and 5′-CTG CCT CCG CAC ACG TAG GTG ACA GCG GCA GCG GCG GCA GCG CGG TAG ATG GCC GC-3′ in pMT/BiP/V5/HisA–uPA plasmid, under the conditions recommended in the QuikChange Site-Directed Mutagenesis Kit manual (Stratagene, La Jolla, CA). The protein was synthesized and characterized as we reported previously (6), and was co-immunoprecipitated as previously described (6, 16, 29) (see the online supplement for additional details).
Contractile Response of Isolated Pulmonary and Aortic Rings
Isometric tension in aortic rings from rats and mice and pulmonic rings from rats were measured as described previously (6, 16) (see the online supplement for additional details).
Pulmonary Arterial Diameter and Flow
Echocardiography was performed before and after an intraperitoneal injection of uPA, using a 13-MHz probe (Vivid 7; GE Medical Systems, Milwaukee, WI). Sprague-Dawley rats (Harlan Laboratories, Jerusalem, Israel) were sedated with intraperitoneal zolazepam (25 mg/kg) and xylazine (50 mg/kg). After shaving the rats’ left hemithoraces, two-dimensional echocardiographic images and pulsed wave Doppler-derived recording were acquired from the short-axis view at the level of the large arteries, at a frame rate of 250–300/second. We measured pulmonary artery diameter (D) and the time velocity integral (TVI) as a surrogate for stroke volume. The cross-sectional area (CSA) of the pulmonary artery and cardiac stroke volume (SV) were calculated according to the formulas CSA = 0.785 × D2, and SV = CSA × TVI. All parameters were evaluated during an average of three consecutive beats. A single echocardiographer, blinded to the specific intervention, performed all acquisitions of data.
Pulmonary Vascular Permeability
Adult, male, 8–10-week-old wild-type (WT) C57BL/6J mice (16) (average weight, 20–25 g), were anesthetized, and uPA (1 mg/kg) was injected into the tail vein. Evans blue dye was infused before measuring extravasation (see the online supplement for additional details).
Co-Immunoprecipitation
Pulmonary arterial rings isolated from uPA−/− mice were incubated with WT uPA, WT uPA together with the PAI-1–derived peptide EEIIMD, or the uPA variant that lacks a functional docking site (ΔDSuPA), and were then precipitated and immunoblotted, as we reported previously (6) (see the online supplement for additional details).
Immunohistochemistry
Formalin-fixed, paraffin-embedded archived normal human lung tissues were used for NMDA-R immunostaining (see the online supplement for additional details).
Statistical Analysis
All data are presented as mean ± SD. Differences were analyzed using the Student t test or one-way ANOVA with the Newman–Keuls post hoc test, as indicated in Results. Statistical significance was set at P < 0.05.
Results
Effects of uPA on Contractility of Pulmonary Arterial Rings
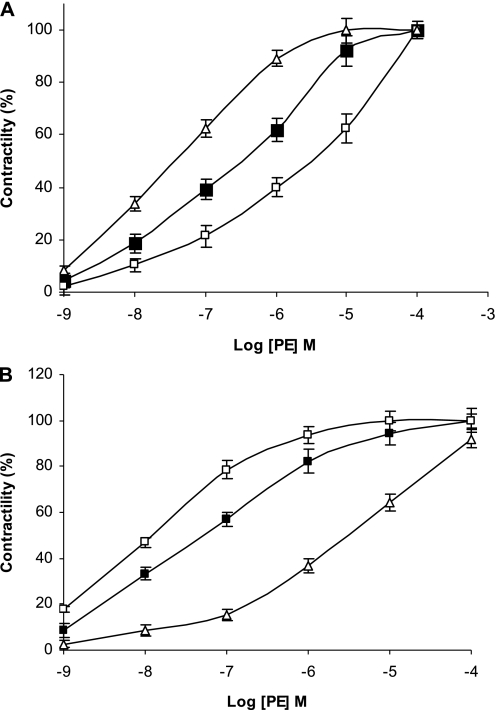
We examined the effects of uPA on pulmonary vascular contractility and permeability. To do so, we first measured the effects of uPA on the contraction of isolated rat pulmonary arterial rings induced by increasing concentrations of phenylephrine (PE). The addition of a physiological concentration of uPA (1 nM) stimulated the contraction of pulmonary arterial rings induced by PE; uPA decreased the 50 percent of effective concentration (EC50) of PE from 28 to 3.5 nM (P < 0.0033, Student t test) (Figure 1A). In contrast, at pathophysiological concentrations (20 nM) measured by us in the plasma of mice 24 hours after acute lung injury induced by bleomycin (20 ± 7 nM versus 1 ± 3 nM in control mice, n = 5; Higazi and colleagues, unpublished observations), uPA impaired the contractility of pulmonary arterial rings, and increased the EC50 of PE approximately sixfold, from 28 to 147 nM (P < 0.0014, Student t test) (Figure 1A).
Figure 1.
Effect of urokinase-type plasminogen activator (uPA) on the contraction of arterial rings. (A) Effect of uPA on the contraction of pulmonary arterial rings. Contraction of isolated pulmonary arterial rings was induced by phenylephrine (PE) at the indicated concentrations in the absence (solid squares) or presence of 1 nM (open squares) or 20 (open triangles) nM uPA. (B) Effect of uPA on the contraction of isolated aortic rings. Contraction of isolated aortic rings was induced by PE at the indicted concentrations in the absence (solid squares) or presence of 1 nM (open squares) or 20 nM (open triangles) uPA. The mean ± SD of four experiments is shown.
We previously observed that 1 nM tPA inhibited the contractility of isolated aortic rings, whereas 20 nM stimulated vasoconstriction (30), a result in seeming contradistinction to the observed effects of uPA on the pulmonary circulation. Therefore, we compared the effects of uPA on the contractility of aortic and pulmonary arterial rings, measured in parallel. Low concentrations of uPA (1 nM) enhanced the contractility of pulmonary arterial rings (Figure 1A), but inhibited the contraction of isolated aortic rings, increasing the EC50 of PE approximately fourfold, from 32 to 127 nM (P < 0.0033) (Figure 1B), whereas 20 nM uPA induced the exact opposite effect, that is, enhanced the contraction of aortic rings, decreasing the EC50 of PE from 36 to 4.1 nM (P < 0.0033) (Figure 1B), and impairing the contraction of pulmonary arterial rings (Figure 1A).
Role of LRP and uPA Catalytic Activity
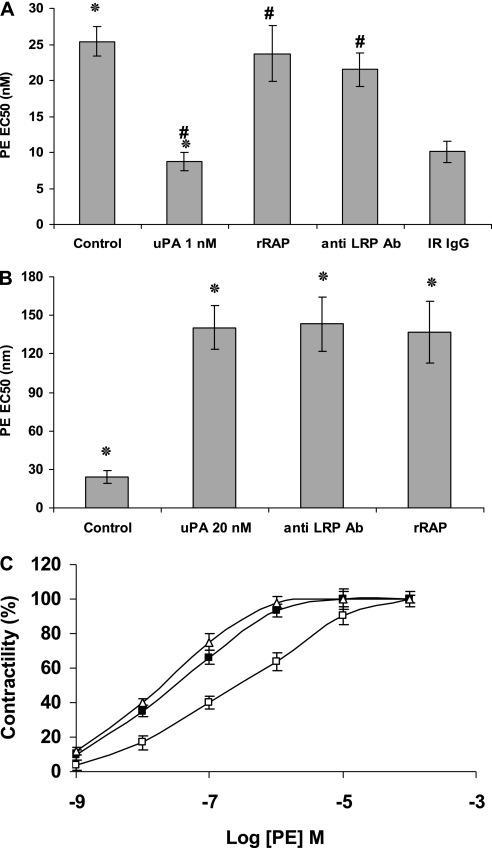
We previously observed that the stimulatory, but not inhibitory, effects of tPA on the contraction of isolated aortic rings were LRP-dependent (30). Therefore, we examined the involvement of this receptor in uPA-induced alterations in pulmonary arterial contractility. Recombinant RAP and the anti–LRP-1 antibody inhibited the procontractile effect of 1 nM uPA (Figure 2A), but did not affect the vasorelaxation induced by 20 nM uPA (Figure 2B). This outcome suggests that the vasorelaxation induced by high concentrations of uPA is mediated through a process that does not require LRP-1 or a related family member. This is similar to our previous finding that the vasoactive effect induced by high concentrations of tPA (20 nM) is independent of LRP (30).
Figure 2.
Involvement of LRP and uPA catalytic activity in uPA-induced alterations of pulmonary arterial contractility. (A) Contraction of isolated pulmonary arterial rings was induced by increasing concentrations of PE from 10−10 M to 10−5 M (for more details, see Methods in the online supplement) in the absence (Control) or presence of 1 nM uPA, alone or together with recombinant receptor-associated protein (rRAP), anti–low-density lipoprotein–related receptor antibody (anti LRP Ab), or irrelevant IgG (IR IgG). (B) Contraction of isolated pulmonary arterial rings was induced by increasing concentrations of PE (as in A) in the absence (Control) or presence of 20 nM uPA, alone or together with rRAP or anti-LRP antibody (anti LRP Ab). (C) Contraction of isolated pulmonary arterial rings was induced by PE at the indicted concentrations in the absence (open squares) or presence of 1 (open triangles) or 20 nM (solid squares) of a catalytically inactive variant of uPa containing a single mutation within the active site (uPA-S356A). The mean ± SD of three experiments is shown. *Statistical significance between columns 1 and 2; #statistical significance between column 1 and the other columns.
In view of these findings, we investigated the role of the catalytic activity of uPA, using a variant containing a single mutation within the active site (uPA-S356A). Catalytically inactive uPA-S356A (1 nM) maintained its procontractile effect on pulmonary arterial rings, but lost its capacity to inhibit contractility when added at a 20-nM concentration (Figure 2C). Thus, the procontractile effect of 1 nM uPA is LRP-dependent and does not require catalytic activity, whereas the inhibition of contraction evident at higher concentrations of uPA requires proteolytic activity and is independent of LRP.
Role of NMDA Receptors in Pulmonary Arterial Contractility
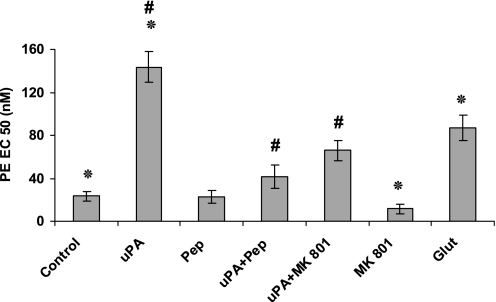
Based on these two sets of results, we turned our attention to NMDA-Rs, which were identified in rat (22–24) and human (6) lungs. Within the brain, the NR1 subunit of NMDA-R1 can bind and undergo cleavage by tPA (25). uPA and tPA share catalytic specificities (e.g., cleaving plasminogen at the same site). Moreover, we reported that uPA and tPA regulate the contractility of isolated tracheal rings by interacting with the NMDA-R1 expressed by tracheal smooth muscle cells (6). To test the possibility that uPA inhibits pulmonary arterial contractility by interacting with NMDA-R1 in the pulmonary vasculature, we examined the effects of the NMDA-R antagonist MK-801. MK-801 blocked the inhibition of vasoconstriction induced by 20 nM uPA, but did not affect the contraction induced by 1 nM uPA (Figure 3). Furthermore, the NMDA-R1 antagonist alone enhanced pulmonary arterial contraction in response to PE (Figure 3), suggesting that the receptor constitutively promotes vasorelaxation. In support of this inference, the NMDA-R1 agonist glutamate inhibited the PE-induced contraction of pulmonary arterial rings (Figure 3).
Figure 3.
Involvement of N-methyl-d-aspartate receptors (NMDA-Rs) in the contraction of pulmonary arterial rings. Contraction of isolated pulmonary arterial rings was induced by PE in the absence (Control) or presence of 20 nM uPA, alone or together with plasminogen activator inhibitor–1 (PAI-1)–derived peptide (uPA + Pep, 1 μM), NMDA-R antagonist MK-801 (100 nM), or the NMDA-R agonist glutamate (Glut, 150 μM). The mean ± SD of three experiments is shown. *Statistical significance between columns 1 and 2; #statistical significance between column 1 and the other columns.
Effects of uPA on Pulmonary Arterial Contractility and Blood Flow In Vivo
No differences were seen among treatment groups at baseline with respect to echocardiographic parameters. The injection of uPA (1 mg/kg) into the tail veins of rats significantly increased the diameter of their pulmonary arteries by approximately 12.5% (P < 0.003) (Table 1). The effect of uPA on arterial diameter was almost totally inhibited by EEIIMD and MK-801 (P < 0.003, versus animals treated with uPA alone) (Table 1). uPA also increased the TVI as a surrogate for SV by approximately 5.9% (P < 0.04). EEIIMD and MK-801 also inhibited the uPA-induced increase in TVI (Table 1). Table 1 also shows that uPA increased the calculated pulmonary arterial cross-sectional area by approximately 25%, and the SV by 35%.
TABLE 1.
PULMONARY ARTERIAL DIAMETER AND FLOW
| Control | P VTI (cm) | SD | PA D (cm) | SD | CSA (cm2) | SV (ml) |
| uPA | 7.84 | 1.4 | 0.32 | 0.076 | 0.0804 | 0.63 |
| uPa + peptide | 8.33 | 1.1 | 0.36 | 0.042 | 0.102 | 0.85 |
| uPA + MK-801 | 7.97 | 1.7 | 0.33 | 0.054 | 0.0855 | 0.681 |
| 8.03 | 1.2 | 0.33 | 0.061 | 0.0855 | 0.686 |
Echocardiography was performed in five different Sprague-Dawley rats (Harlan Laboratories, Jerusalem, Israel) before and after intraperitoneal injections of urokinase-type plasminogen activator (uPA), as described in Materials and Methods. Pulmonary artery diameter (PA D) and the time velocity integral (P TVI), as a surrogate for stroke volume, were measured. The cross-sectional area (CSA) of the pulmonary artery and cardiac stroke volume (SV) were calculated using the formulas CSA = 0.785 × D2, and SV = CSA × TVI. All parameters were evaluated during an average of three consecutive beats. A single echocardiographer, blinded to the specific intervention, performed all data acquisition.
Effects of uPA and NMDARs on Pulmonary Vascular Permeability
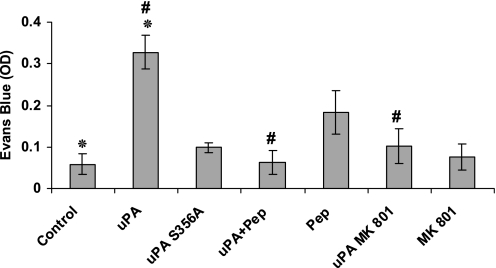
The activation of NMDA-Rs by glutamate in isolated rat lungs was reported to trigger pulmonary edema (22), and uPA−/− mice are protected against LPS-induced pulmonary edema (18). Therefore, we investigated whether the binding of uPA to NMDA-R1 also increases lung permeability. The intravenous injection of uPA (1 mg/kg; estimated plasma concentration, 20 nM) increased lung permeability, as measured by the extravasation of intravenously administered Evans blue into the BAL (Figure 4). Moreover, the induction of vascular permeability by uPA required catalytic activity (Figure 4), and was inhibited by the NMDA-R antagonist MK-801 (Figure 4).
Figure 4.
Effect of uPA and NMDA-Rs on pulmonary vascular permeability. Lung permeability, as measured by the extravasation of intravenously administered Evans blue into the bronchoalveolar lavage, was determined after intravenous injection of saline (Control), wild-type (WT) uPA (uPA, 1 mg/kg), catalytically inactive uPA (uPA S356A), PAI-1 derived peptide (Pep), uPA plus PAI-1–derived peptide (uPA + Pep, 1μM), the NMDA-R antagonist MK-801, (or uPA plus MK-801. The mean ± SD of three experiments is shown. OD = optical density. *Statistical significance between columns 1 and 2; #statistical significance between column 1 and the other columns.
Effects of a PAI-1–Derived Peptide and the uPA DS
We previously reported that the contractile effect of uPA is inhibited by a PAI-1–derived hexapeptide (EEIIMD) that binds to the DS of uPA (16), blocking its interaction with NMDA-Rs (6). EEIIMD affects neither the clearance of uPA nor its catalytic activity (6, 16). Therefore, we examined the effects of EEIIMD on uPA-mediated pulmonary arterial contractility and lung permeability. The intravenous co-administration of EEIIMD blocked the inhibition of pulmonary arterial contractility (Figure 3) and lung permeability induced by uPA (Figure 4).
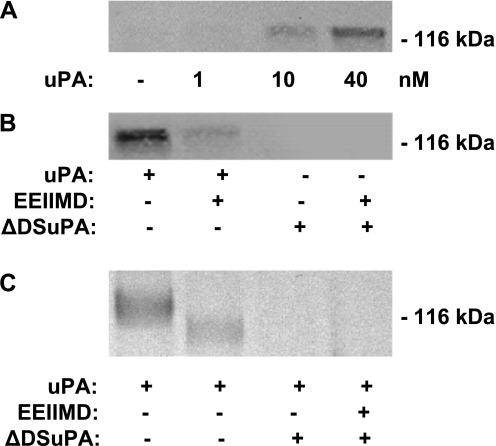
This result posed an apparent paradox, in that EEIIMD blocks the inhibition by uPA of pulmonary arterial contraction and permeability without inhibiting its catalytic activity, which is also required according to the results shown in Figure 2C. This suggested the possibility that uPA inhibits NMDA-R–mediated pulmonary arterial contraction and induces permeability, via a two step-process involving binding through the DS, followed by the expression of catalytic activity, as in the case of tPA (6, 25). To explore this possibility, we preincubated lung homogenates from uPA−/− mice with increasing concentrations of uPA (1–40 nM) at 4°C, followed by precipitation with an antibody against uPA and immunoblotting with an antibody against the NR1 subunit of NMDA-R (6). A single band (molecular weight, approximately 120 kD), corresponding to the estimated size of the NR1 subunit, was evident (Figure 5A). EEIIMD inhibited the formation of uPA/NMDA-R complexes at 4°C (Figure 5B). Furthermore, at 37°C, uPA cleaved the NR1 subunit of NMDA-R, an effect inhibited by EEIIMD (Figure 5C).
Figure 5.
Interaction of uPA and NMDA-R1 in pulmonary arteries. (A) uPA binds to NMDA-R from the pulmonary artery. Homogenates of pulmonary arterial rings isolated from uPA−/− mice were preincubated with the indicated concentrations of uPA at 4°C, and precipitated with an antibody against uPA, followed by immunoblotting with an antibody against the NR1 subunit of NMDA-R1. The results of an experiment representative of three independent analyses are shown. (B) The docking sites in uPA mediate its interactions with NMDA-R. As in A, homogenates of pulmonary arteries rings isolated from uPA−/− mice were preincubated with WT uPA (40 nM) (uPA), WT uPA with PAI-1 derived peptide EEIIMD (EEIIMD, 1 μM), or the uPA variant that lacks a functional docking site (ΔDSuPA) at 4°C, precipitated with an antibody against uPA, followed by immunoblotting with an antibody against the NR1 subunit of NMDA-R1. The results of an experiment representative of three independent analyses are shown. (C) uPA cleaves the NR1 subunit of NMDA-R1. Pulmonary arterial rings isolated from uPA−/− mice were incubated in buffer alone or buffer containing 20 nM WT uPA (uPA), WT uPA with PAI-1–derived peptide EEIIMD (1 μM), or the uPA variant that lacks a functional docking site (ΔDSuPA) for 120 minutes at 37°C. Tissue homogenates were analyzed by SDS-PAGE and Western blotting, using an antibody against the NR1 subunit of NMDA-R1. The results of an experiment representative of three independent analyses are shown.
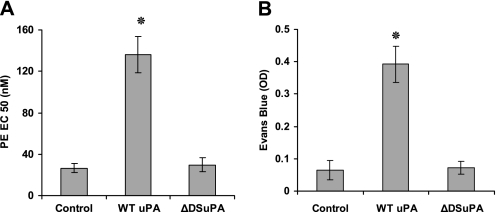
As an independent approach to examining the role of the DS in the interaction between uPA and NMDA-R, we developed a uPA variant in which amino acids 179–184 (RHRGGS), which comprise the DS, were each mutated to alanine (dDSuPA). The uPA DS mutant dDSuPA did not bind to the NMDA receptor (Figure 5B), and did not cleave it (Figure 5C). dDSuPA did not inhibit pulmonary arterial contractility or induce vascular permeability in the lung, although it maintained catalytic activity (Figures 6A and 6B). These data suggest that the binding of uPA to NMDAR-1 through its docking site is required for an inhibition of pulmonary arterial contractility and increased vascular permeability in the lung.
Figure 6.
The uPA variant that lacks a functional docking site (ΔDSuPA) fails to affect the contractility or permeability of the pulmonary vasculature. (A) The contraction of pulmonary arterial rings was induced by PE in the absence (Control) or presence of 20 nM WT uPA or ΔDSuPA. The mean ± SD of three experiments is shown. (B) Lung permeability was measured as in Figure 5 after intravenous injection of saline (Control), WT uPA (uPA, 1 mg/kg), or ΔDSuPA. The mean ± SD of three experiments is shown. EC = effective concentration; OD = optical density.
Finally, to examine the clinical relevance of our findings, we investigated whether NMDA-R1s are expressed in the vasculature of human lungs. We found intense staining for the presence of NMDA-R1 in the vascular smooth muscle of human pulmonary arteries from normal lung tissue (Figure E1 in the online supplement).
Discussion
uPA was implicated in the development of several lung disorders, including acute lung injury (31, 32), ARDS (33–35), pneumonia (36, 37), pulmonary fibrosis (38, 39), and asthma (40, 41). The salutary effect of uPA in these settings is presumed to depend on its ability to lyse the fibrin clots that impair alveolar function (42). However, uPA also activates several intracellular signal-transduction processes through its binding to cell-surface receptors including uPAR, LRP, certain integrins, and NMDA-R1, but their role in regulating critical lung functions (such as vascular contractility and permeability and airway contractility) has received less attention.
The data in this study indicate that uPA regulates pulmonary arterial contractility through two opposing receptor-mediated pathways that sense the ambient concentration of the agonist. At physiological concentrations (1 nM), uPA induces vasoconstriction of isolated pulmonary arterial rings. This process is mediated through LRP, and does not require uPA to exert catalytic activity. In contrast, at pathophysiological concentrations (20 nM), uPA inhibits pulmonary arterial contractility through a process that requires the catalytic site and is mediated via NMDA-R1. Similar elevations of uPA likely occur in human acute lung injury, based on concentrations measured in BAL fluid and the estimated dilution of lung extravascular lining fluid in that compartment (43).
Interestingly, the effects of uPA on pulmonary vessels are opposite those we previously observed in isolated aortic vessels, that is, uPA and tPA at physiological concentrations (1 nM) inhibit the contraction of arterial rings, but promote contraction of pulmonary arterial rings. The explanation for this difference in response is not apparent, but clearly reflects important tissue specificity. Our results are in line with the well-established finding that the pulmonary and systemic circulations respond in an opposite manner to regulators of vascular contractility such as hypoxia and hypercapnia. The effects of high concentrations of uPA are consistent with previous studies showing that the NMDA-R agonist glutamate increases lung permeability (22). Taken together, the acute effects of WT and variant uPAs on receptor-mediated vascular tone and permeability studied here complement recently published data on gene transfection strongly suggesting that the catalytic activity of uPA is required to reach the vessel wall and initiate the more chronic process of aberrant vascular remodeling that may then alter vascular contractility (8). Thus, the nature of the contractile response to uPA and its dependence on, or independence from, catalytic activity appears to depend on its tissue concentration, differences in the tissue-dependent expression of its cognate receptors, and possibly differences in receptor repertoire or signal mechanisms in arterial, venous, and capillary vascular cells within the lung.
Our data also suggest two potentially synergistic means to prevent the deleterious effects of endogenous uPA on vascular permeability under pathological conditions in the lung. Both an antagonist of NMDA-R1 and a peptide that block the DS of uPA and prevent it from binding to NMDA-Rs each help maintain vascular tone and reduce vascular permeability. Moreover, a DS mutant that retains catalytic activity blocks the binding of WT uPA to NMDAR-1, prevents pulmonary arterial dilation, and maintains pulmonary vascular integrity. Our finding that vascular smooth muscle from normal human lung tissue expresses NMDA-R1 (Figure E1) supports the potential clinical relevance of these observations. However, additional experiments will be required to determine the signal transduction pathways involved in the procontractile and inhibitory response, the role of communication between endothelial and vascular smooth muscle cells and lung epithelial cells, and the contribution of uPA and NMDA-R–mediated vascular permeability in pathological conditions associated with elevations of urokinase, including various forms of acute and chronic lung injury.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health grants PO1 HL076406 (S.I., D.B.C., and A.A.-R.H.) and HL82545 (A.A.-R.H.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0302OC on May 26, 2011
Author Disclosure: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Shetty S, Padijnayayveetil J, Tucker T, Stankowska D, Idell S. The fibrinolytic system and the regulation of lung epithelial cell proteolysis, signaling, and cellular viability. Am J Physiol Lung Cell Mol Physiol 2008;295:L967–L975 [DOI] [PubMed] [Google Scholar]
- 2.Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med 2003;31:S213–S220 [DOI] [PubMed] [Google Scholar]
- 3.Idell S, James K, Levin E, Schwartz B, Manchanda N, Maunder R, Martin T, McLarty J, Fair D. Local abnormalities in coagulation and fibrinolytic pathways predispose to alveolar fibrin deposition in the adult respiratory distress syndrome. J Clin Invest 1989;84:695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertozzi P, Astedt B, Zenzius L, Lynch K, LeMaire F, Zapol W, Chapman H. Depressed bronchoalveolar urokinase activity in patients with adult respiratory distress syndrome. N Engl J Med 1990;322:890–897 [DOI] [PubMed] [Google Scholar]
- 5.Barazzone C, Belin D, Piguet F, Vassalli J, Sappino A. Plasminogen activator inhibitor–1 in acute hyperoxic mouse lung injury. J Clin Invest 1996;98:2666–2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nassar T, Yarovoi S, Abu Fanne R, Akkawi S, Jammal M, Allen T, Idell S, Cines D, Higazi A. Regulation of airways contractility by plasminogen activators through NMDA receptor–1. Am J Respir Cell Mol Biol 2010; (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwak S, Mitra S, Bdeir K, Strassheim D, Park J, Kim J, Idell S, Cines D, Abraham E. The kringle domain of urokinase-type plasminogen activator potentiates LPS-induced neutrophil activation through interaction with aVβ3 integrins. J Leukoc Biol 2005;78:937–945 [DOI] [PubMed] [Google Scholar]
- 8.Massey P, Tanaka S, Buckler J, Jiang B, McCourtie A, Qian K, Tom C, Stempien-Otero A, Wen S, Luttrell I, et al. Constriction of carotid arteries by urokinase-type plasminogen activator requires catalytic activity and is independent of NH(2)-terminal domains. Thromb Haemost 2009;102:983–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin-McNulty B, Zhang L, da Cunha V, Vincelette J, Rutledge J, Vergona R, Sullivan M, Wang Y. Urokinase-type plasminogen activator deficiency (uPA-KO) prevented carotid artery ligation–induced vascular remodeling in mice. Transl Res 2007;149:70–75 [DOI] [PubMed] [Google Scholar]
- 10.Kiyan J, Kusch A, Tkachuk S, Krämer J, Haller H, Dietz R, Smith G, Dumler I. Rosuvastatin regulates vascular smooth muscle cell phenotypic modulation in vascular remodeling: role for the urokinase receptor. Atherosclerosis 2007;195:254–261 [DOI] [PubMed] [Google Scholar]
- 11.Bacharach E, Itin A, Keshet E. In vivo patterns of expression of urokinase and its inhibitor PAI-1 suggest a concerted role in regulating physiologic angiogenesis. Proc. Natl. Acad. Sci. USA 1992;89:10686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odekon L, Sato Y, Rifkin D. Urokinase-type plasminogen activator mediates basic fibroblast growth factor–induced bovine endothelial cell migration independent of its proteolytic activity. J Cell Physiol 1992;150:258–263 [DOI] [PubMed] [Google Scholar]
- 13.Pepper M, Belin D, Montesano R, Orci L, Vassalli J. Transforming growth factor–beta 1 modulated basic fibroblast growth factor-induced proteolytic and angiogenic properties of endothelial cells in vitro. J Cell Biol 1990;111:743–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Hinsbergh VWM, Koolwijk P, Hanemaaijer R. Role of fibrin and plasminogen activators in repair-associated angiogenesis: in vitro studies with human endothelial cells. : Goldberg ID, Rosen EM, Regulation of angiogenesis. Basel: Birkhauser Verlag; 1997. pp. 391–411 [DOI] [PubMed] [Google Scholar]
- 15.Higazi AA-R, Upson R, Cohen R, McCrae KR, Manuppello J, Bognacki J, Henkin J, Kounnas M, Strickland D, Preissner KT, et al. Interaction of single chain urokinase with its receptor induces the appearance and disappearance of binding epitopes within the resultant complex for other cell surface proteins. Blood 1996;88:542–551 [PubMed] [Google Scholar]
- 16.Nassar T, Haj-Yeha S, Akkawi S, Kuo A, Bdeir K, Mazar A, Cines DB, Higazi A. Binding of urokinase to low density lipoprotein-related receptor (LRP) regulates vascular smooth muscle cell contraction. J Biol Chem 2002;277:40499–40504 [DOI] [PubMed] [Google Scholar]
- 17.Armstead WM, Cines DB, Bdeir K, Stein SC, Higazi AA. uPA impairs cerebrovasodilation after hypoxia/ischemia through LRP and ERK MAPK. Brain Res 2008;1231:121–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abraham E, Kuhn K, Arcoli J, Strassheim D, Park JS, Shetty S, Idell S. Urokinase-type plasminogen activator potentiates LPS-induced neutrophil activation. J Immunol 2003;I170:5644–5651 [DOI] [PubMed] [Google Scholar]
- 19.Haj-Yejia A, Nassar T, Sachais BS, Kuo A, Bdeir K, Al-Mehdi AB, Mazar A, Cines DB, Higazi AA-R. Urokinase-derived peptides regular vascular smooth muscle cell contraction in vitro and in vivo. FASEB J 2000;14:1411–1422 [DOI] [PubMed] [Google Scholar]
- 20.Armstead WM, Cines BC, Higazi A. Altered NO function contributes to impairment of uPA and tPA cerebrovasodilation after brain injury. J Neurotrauma 2004;21:1204–1211 [DOI] [PubMed] [Google Scholar]
- 21.Armstead W, Cines D, Higazi A. Plasminogen activators contribute to age dependent impairment of NMDA cerebrovasodilation after brain injury. Brain Res Dev Brain Res 2005;156:139–146 [DOI] [PubMed] [Google Scholar]
- 22.Said S, Berisha H, Pakbaz H. Excitotoxicity in the lung: N-methyl-d-aspartate–induced, nitric oxide–dependent, pulmonary edema is attenuated by vasoactive intestinal peptide and by inhibitors of poly(ADP-ribose) polymerase. Proc. Natl. Acad. Sci. USA 1996;93:4688–4692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kathleen G, Dickman J, Youssef G, Mathew M, Said S. Ionotropic glutamate receptors in lungs and airway: molecular basis for glutamate toxicity. Am J Respir Cell Mol Biol 2004;30:139–144 [DOI] [PubMed] [Google Scholar]
- 24.Gill S, Mueller R, Peter P, Mcguire F, Olga O, Pulido M. Potential target sites in peripheral tissues for excitatory neurotransmission and excitotoxicity. Toxicol Pathol 2000;28:277–284 [DOI] [PubMed] [Google Scholar]
- 25.Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, Vivien D, Buisson A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med 2001;7:59–64 [DOI] [PubMed] [Google Scholar]
- 26.Armstead W, Cines D, Higazi A. Plasminogen activators contribute to impairment of hypercapnic and hypotensive cerebrovasodilation after cerebral hypoxia/ischemia in the newborn pig. Stroke 2005;36:2265–2269 [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Atalaya J, Roussel B, Levrat D, Parcq J, Nicole O, Hommet Y, Benchenane K, Castel H, Leprince J, To Van D, et al. Toward safer thrombolytic agents in stroke: molecular requirements for NMDA receptor–mediated neurotoxicity. J Cereb Blood Flow Metab 2008;28:1212–1221 [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Strickland DK, Cines DB, Higazi AA-R. Regulation of single chain urokinase binding, internalization and degradation by a plasminogen activator inhibitor 1–derived peptide. J Biol Cheml 1997;272:27053–27057 [DOI] [PubMed] [Google Scholar]
- 29.Akkawi S, Nassar T, Tarshis M, Cines BC, Higazi A. LRP and avB3 mediate tPA-activation of smooth muscle cells. Am J Physiol Heart Circ Physiol 2006;291:H1351–H1359 [DOI] [PubMed] [Google Scholar]
- 30.Nassar T, Akkawi S, Shina A, Haj-Yehia A, Bdeir K, Tarshis M, Heyman S, Higazi A. The in vitro and in vivo effect of tPA and PAI-1 on blood vessel tone. Blood 2004;103:897–902 [DOI] [PubMed] [Google Scholar]
- 31.Idell S. Extravascular coagulation and fibrin deposition in acute lung injury. New Horiz 1994;2:566–574 [PubMed] [Google Scholar]
- 32.Pinsky D, Liao H, Lawson C, Yan S, Chen J, Carmeliet P, Loskutoff D, Stern D. Coordinated induction of plasminogen activator inhibitor–1 (PAI-1) and inhibition of plasminogen activator gene expression by hypoxia promotes pulmonary vascular deposition. J Clin Invest 1998;102:919–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bone RC, Francis PB, Pierce AR. Intravascular coagulation associated with the adult respiratory distress syndrome. Am J Med 1976;61:585–589 [DOI] [PubMed] [Google Scholar]
- 34.Bachofen M, Weibel E. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med 1982;3:35–56 [PubMed] [Google Scholar]
- 35.Neuhof H, Seeger W, Wolf HRD. Generation of mediators by limited proteolysis during blood coagulation and fibrinolysis: its pathogenetic role in the adult respiratory distress syndrome (ARDS). Resuscitation 1986;14:23–32 [DOI] [PubMed] [Google Scholar]
- 36.Gyetko M, Chen G-H, McDonald RA, Goodman R, Huffnagle GB, Ilkinson CCW, Fuller JA, Toews GB. Urokinase is required for the pulmonary inflammatory response to Cryptococcus neoformans: a murine transgenic model. J Clin Invest 1996;97:1818–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gyetko MR, Sud S, Kendall T, Fuller JA, Newstead MW, Standiford TJ. Urokinase receptor–deficient mice have impaired neutrophil recruitment in response to pulmonary Pseudomonas aeruginosa inefection. J Immunol 2000;165:1513–1519 [DOI] [PubMed] [Google Scholar]
- 38.Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, Simon RH. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor–1 gene. J Clin Invest 1996;97:232–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gunther A, Lubke N, Ruppert C, Weissma N, Grimmminger F, Seeger W. Prevention of lung fibrosis in a rabbit model of bleomycin-induced fibrosis upon aerosol application of heparin or urokinase. Chest 2001;120:4–10 [Google Scholar]
- 40.Wagers S, Norton R, Rinaldi L, Bates J, Sobel B, Irvin C. Extravascular fibrin, plasminogen activator, plasminogen activator inhibitors, and airway hyperresponsiveness. J Clin Invest 2004;114:104–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuramoto E, Nishiuma T, Kobayashi K, Yamamoto M, Kono Y, Funada Y, Kotani T, Sisson R, Simon, Nishimura Y. Inhalation of urokinase-type plasminogen activator (uPA) reduces airway remodeling in a murine asthma model. Am J Physiol Lung Cell Mol Physiol 2008;114:104–111 [DOI] [PubMed] [Google Scholar]
- 42.Hofstra J, Haitsma J, Juffermans N, Levi M, Schultz M. The role of bronchoalveolar hemostasis in the pathogenesis of acute lung injury. Semin Thromb Hemost 2008;34:475–484 [DOI] [PubMed] [Google Scholar]
- 43.Idell S, Koenig K, Fair D, Martin T, McLarty J, Maunder R. Serial abnormalities of fibrin turnover in evolving adult respiratory distress syndrome. Am J Physiol 1991;261:L240–L248 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.