Abstract
Salt absorption via alveolar epithelial Na+ channels (ENaC) is a critical step for maintaining an airspace free of flooding. Previously, we found that 8-(4-chlorophenylthio)-guanosine-3′,5′-cyclic monophosphate-Na (CPT-cGMP) activated native and heterologous ENaC. To investigate the potential pharmacological relevance, we applied this compound intratracheally to human lungs and found that ex vivo alveolar fluid clearance was increased significantly. Furthermore, this compound eliminated self-inhibition in human lung H441 cells and in oocytes expressing human αβγ but not δβγ channels. To further elucidate this novel mechanism, we constructed mutants abolishing (βΔV348 and γH233R) or augmenting (αY458A and γM432G) self-inhibition. The mutants eliminating self-inhibition lost their responses to CPT-cGMP, whereas those enhancing self-inhibition facilitated the stimulatory effects of this compound. CPT-cGMP was unable to activate a high Po mutant (βS520C) and plasmin proteolytically cleaved channels. Our data suggest that elimination of self-inhibition of αβγ ENaC may be a novel mechanism for CPT-cGMP to stimulate salt reabsorption in human lungs.
Keywords: lung fluid reabsorption, amiloride-sensitive sodium channel, CPT-cGMP, ENaC self-inhibition
Clinical Commentary
This work describes a novel mechanism for a broadly used 8-(4-chlorophenylthio)-guanosine-3′,5′-cyclic monophosphate-Na (CPT-cGMP) to activate lung epithelial sodium channels that govern alveolar fluid clearance. CPT-cGMP may be a potent reagent for removing edema fluid from injured lungs.
Epithelial Na+ channels (ENaC) in polarized absorptive tight epithelium are responsible for the transapical salt influx. Together with basolaterally located Na+/K+–ATPase, the vectorial solute/fluid transport pathway is crucial for maintaining luminal and body fluid volume (1–3). Five subunits have been cloned: α, β, γ, δ, and ε ENaC. Luminal impermeable reagents and hormones have been confirmed to regulate salt reabsorption in lung, kidney, and colon.
ENaC has long been proposed to function as a ligand-gated channel on the basis of observations of other ENaC/DEG family members (1, 4). Acid-sensing Na+ channels, a branch of the ENaC/DEG super gene family, are gated by external protons. Another branch of channels, termed FaNaC, are manipulated by FMRFamide and related tetrapeptides (5). With respect to ENaC channels, a number of luminal reagents, including para-chloromercuribenzoate; benzimidazoly-2-guanidinium; bumetanide; and H+, Zn2+, and Ni2+ ions, have been reported to alter external Na+ self-inhibition, a temperature-sensitive intrinsic phenomenon of ENaC (1, 4, 6–10). Recently, a small molecule (S6939), cpt-cAMP, halothane, glibenclamide, and extracellular chloride ions (Cl−) have been shown to act as ligands to regulate heterologous ENaC (11–16). Among these, halothane and Cl− ions may activate ENaC by eliminating Na+ self-inhibition (17, 18). We recently found that 8-(4-chlorophenylthio)-guanosine-3′,5′-cyclic monophosphate-Na (CPT-cGMP) stimulated human αβγ ENaC in oocytes in a PKG-independent and domain-specific manner (19). The underlying mechanisms are unknown.
Several ectodomains have been reported to modify self-inhibition by unknown mechanisms. Given that self-inhibition is governed by the extracellular Na+ concentrations, the extracellular loop was identified as the critical domain for self-inhibition of Xenopus ENaC by Babini and colleagues (20). By systematic screening mutagenesis, Sheng and coworkers found that external cysteine and histidine residues are essential for self-inhibition of murine ENaC (21, 22). This group recently discovered a number of key amino acid residues in the thumb domains that boosted this intrinsic phenomenon (23). The Cl− binding sites, which affect self-inhibition, are also located in the thumb domains (17). The finger and palm domains have been shown to be critical for external protons and furin to affect self-inhibition (13, 24). It has also been demonstrated that manipulations that result in a maximum increment in opening kinetics were capable of abolishing self-inhibition, for example, serine protease proteolysis and locking all channels in the open state (βS520C, a degenerin mutant). A mutation (αF61L) at the amino terminal of the protein, adjacent to the gating tract in the M1 domain, enhanced self-inhibition (25). Our current understanding of the nature of ENaC gating mechanisms provides no unequivocal answers to the question of how the aforementioned domains affect self-inhibition.
CPT-cGMP, but not parent cGMP, acutely activated human ENaC activity as an external ligand (19, 26). The aim of this study was to examine whether CPT-cGMP could be used as a pharmaceutical strategy to up-regulate alveolar fluid clearance (AFC) via stimulating ENaC and its underlying mechanisms. Our results demonstrate that intratracheally instilled CPT-cGMP significantly enhanced the rate of fluid reabsorption in human lungs. This compound only activated αβγ ENaC externally in a dose-dependent manner via blunting self-inhibition. Mutagenesis of the self-inhibition domains modulated this stimulatory effect.
Methods and Materials
Ex Vivo AFC in Human Lungs
The studies followed the guide principles of the Declaration of the People's Republic of China and were approved by the Ethics Committee of the China Medical University (CMU) at Shenyang, China. All patients were given oral and written informed consent forms. Human lung tissues were obtained during pulmonary resection surgeries of 30 patients with lung cancers (16 patients with lung squamous carcinoma and 14 patients with lung adenocarcinoma) at the First Affiliated Teaching Hospital of CMU (October 2009 to June 2010). There were no fibrous or emphysematous lesions as assessed by preoperative chest CT.
Human lung segments were prepared and AFC measurements were done as described previously (27, 28). Briefly, the segmental bronchus was occluded by a 10-Fr. balloon catheter immediately after removal of the lung, and occluded segments that were located furthest away from the focus of tumor were chosen. A warmed physiologic saline solution (20 ml; 37°C) containing 5% BSA with or without amiloride (1 mM) and/or CPT-GMP (0.5 mM) was instilled into the distal air spaces through the catheter. After instillation, the lungs were inflated with 100% oxygen at an airway pressure of 7 cm H2O. Alveolar fluid was aspirated 60 minutes after instillation. Aspirated alveolar fluid was centrifuged at 3,000 × g for 10 minutes, and the supernatant was obtained for measurement of protein concentrations. AFC values were calculated as follows (29): AFC = [(Vi − Vf)/Vi] × 100, where V is the volume of the instilled albumin solution (i) and the final alveolar fluid (f), and Vf = Vi × Pi/Pf, where P is the concentration of protein in the instilled albumin solution (i) and the final alveolar fluid (f).
Cell cGMP Level Assay
Cell cGMP levels were examined with ELISA kits as described in the online supplement.
Oocyte Expression and Voltage Clamp Studies
Wild-type human α, β, and γ ENaC cDNAs were a gift from Dr. Lingueglia (30). ENaC mutants were constructed as described previously (31). Preparation of oocytes and the two-electrode voltage clamp studies were performed as described in the online supplement (31).
To study the self-inhibition, a bath solution (ND1) with a low concentration of Na+ ions (1 mM) was used (the 95 mM Na+ ions in regular ND96 medium was substituted with equal molar N-methyl-d-glucamine). To elicit self-inhibition, oocytes were held at −60 mV continuously while the bath solutions were switched quickly from ND1 and ND96 solutions, controlled with a SF-77B Perfusion Fast-Step (Warner Ins., Hamden, CT) fluidic exchange system and software pCLAMP as previously described (32, 33). CPT-cGMP (AXXORA, LLC, San Diego, CA) stock solution (50 mM) was prepared in water and stored at −20°C. MTSET (Toronto Research Chemicals, Downsview, ON, Canada) was freshly prepared every 10 minutes.
Patch Clamp Assay
Whole-cell and single-channel patch clamp recordings in H441 cells were performed as described in the online supplement (26).
Statistics
All results are presented as mean ± SE. Dose-response curves were fitted to the Hill equation. One-way ANOVA computations were used to analyze the difference of the means for normally distributed data. P < 0.05 was considered as significant.
Results
CPT-cGMP Stimulates Fluid Resolution in Human Lungs
CPT-cGMP is a cell-permeable compound. Its concentration within human lungs is reduced due to its entrance into alveolar epithelial and nonepithelial cells. We reported that a median effective concentration (EC50) value of 147 μM was required for CPT-cGMP to activate ENaC activity in H441 cells and that the apparent saturated dose was 1 mM (26). In addition, up to 2 mM cGMP analog was applied to primary lung epithelial cells in vitro for ENaC studies (34, 35). As an external ligand, CPT-cGMP was required to maintain an effective dose at least two times above its EC50 value. Therefore, we applied 200 μM of CPT-cGMP to H441 cells and oocytes and 500 μM CPT-cGMP to lungs.
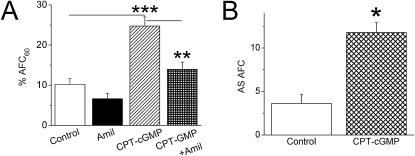
To examine the potential effects of cGMP on fluid resolution in distal lung, we measured ex vivo AFC in isolated human lung lobes. Intratracheally delivered CPT-cGMP markedly increased the AFC value (n = 5–15) (Figure 1A). Moreover, CPT-cGMP stimulated fluid resolution in human lungs ex vivo through augmented ENaC activity, as revealed by amiloride-inhibitable AFC fraction (P < 0.05) (Figure 1B).
Figure 1.
Stimulation of human alveolar fluid clearance (AFC) by 8-(4-chlorophenylthio)-guanosine-3′,5′-cyclic monophosphate-Na (CPT-cGMP). AFC was measured by delivering 0.9% NaCl with 5% BSA into human lung lobes ex vivo. The instillate was collected after 60 minutes, and AFC was measured as described in Materials and Methods. (A) AFC in the presence and absence of amiloride (Amil) and CPT-cGMP. n = 15 for controls; n = 5 for other groups. **P < 0.01; ***P < 0.001. (B) Amiloride-inhibitable AFC fraction associated with ENaC. *P < 0.05.
CPT-cGMP Eliminates Self-Inhibition in Human Lung Epithelial Cells
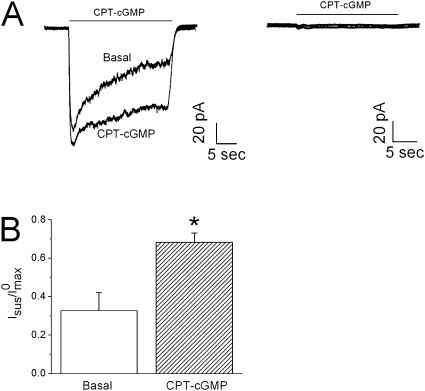
To test the hypothesis that CPT-cGMP activated native ENaC by blunting self-inhibition, we studied the effects of CPT-cGMP on the self-inhibition of native ENaC in H441 cells, a human lung epithelial cell line (Figure 2A). Analogous to the effects on heterologous human α-containing channels, CPT-cGMP significantly elevated the sustained/maximal current ratio (Figure 2B). In sharp contrast, in the presence of amiloride, CPT-cGMP did not evoke currents associated with non-ENaC channels (Figure 2A, right panel).
Figure 2.
CPT-cGMP alters self-inhibition in human lung epithelial cells. (A) Self-inhibition traces recorded at −60 mV in H441 cells in the absence (Basal) and presence of CPT-cGMP and amiloride (right). (B) Sustained (Isus)/maximal (I0max) current ratio. *P < 0.05; n = 5.
CPT-cGMP Stimulates αβγ but Not δβγ ENaC
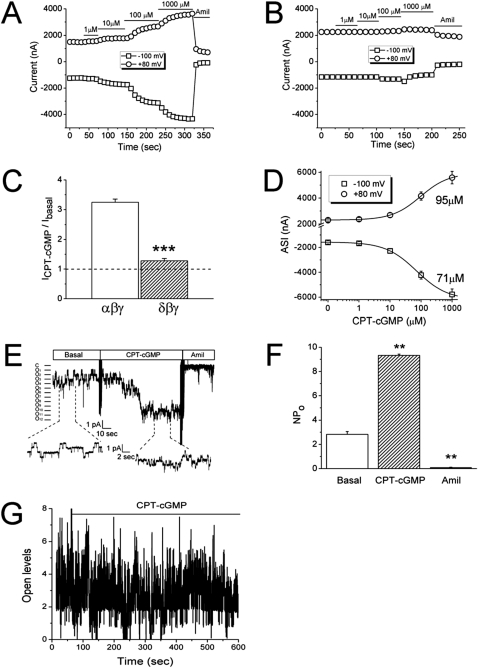
The α and δ subunits are expressed in human lung tissues and primary alveolar type II cells (26, 32). δ ENaC–like channels have been functionally detected in human nasal epithelial cells (36). We previously found that CPT-cGMP activated δαβγ ENaC in oocytes (26). Here we further distinguished the responses of the αβγ and δβγ channels to CPT-cGMP (Figure 3). CPT-cGMP evoked inward and outward currents associated with αβγ but not δβγ channels (Figures 3A and 3B). The increment in αβγ ENaC activity was more than 3-fold (Figure 3C). The EC50 values were 71 ± 2 μM and 95 ± 4 μM, respectively, for currents at −100 and +80 mV (Figure 3D).
Figure 3.
Activation of whole-cell and single-channel epithelial Na+ channel (ENaC) activities by CPT-cGMP. (A and B) Current traces of αβγ (A) and δβγ (B) ENaC channels digitized at the holding potentials of −100 mV and +80 mV. (C) Activation by CPT-cGMP as revealed by increased fold in current levels at −100 mV. ***P < 0.001. (D) Dose–response curves of αβγ ENaC. The median effective concentration values at −100 mV and +80 mV are shown. (E) Single-channel tracings in an outside-out patch. Membrane potential, −60 mV. (F) Single-channel activity before (Basal) and after perfusion of CPT-cGMP and amiloride (Amil). n = 6. **P < 0.01. (G) Single-channel tracings in a cell-attached patch.
Our previous studies found that heterologous ENaC expressed in oocytes may not be regulated by PKG (19). In PKG-deficient cells, the extent of compound stimulation of ENaC was identical to those transfected with scramble siRNA. We substantiated these observations by single-channel recordings. In a cell-free mode that lacks cytosolic PKG signaling molecules, single-channel activity was elevated approximately 3-fold by CPT-cGMP in outside-out patches (Figures 3E and 3F). However, the single-channel activity appeared reduced when CPT-cGMP was applied to cell-attached patches (Figure 3G). Taken together, our results show that heterologous ENaC expressed in oocytes is not regulated by cGMP/PKG pathway. It is reminiscent of the well known fact that ENaC expressed in oocytes is not cAMP/PKA-dependent. We subsequently took the advantage of this expression model to study the mechanisms beyond PKG.
CPT-cGMP Blunts Self-Inhibition of Heterologous ENaC
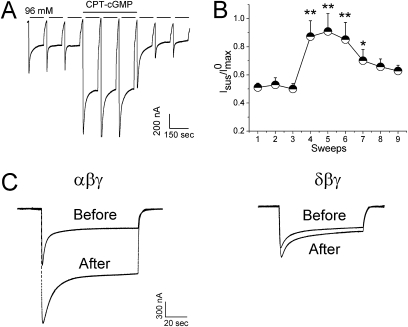
To corroborate the observations in H441 cells, we investigated the effects of CPT-cGMP on self-inhibition associated with human αβγ ENaC in oocytes. The first three sweeps were recorded in the absence of CPT-cGMP as controls (Figure 4A). As anticipated for the self-inhibition, approximately half of the maximal current was the sustained current after fast fluidic exchange (Figure 4A). CPT-cGMP (0.2 mM) stimulated the maximal and the sustained currents reversibly. In the presence of this compound, the sustained/maximal current ratio increased significantly (P < 0.01) (Figure 4B). In contrast to αβγ ENaC, δβγ ENaC showed a slight self-inhibition. Moreover, CPT-cGMP did not alter the kinetics of this process in cells expressing δβγ ENaC (Figure 4C).
Figure 4.
CPT-cGMP modifies self-inhibition of αβγ ENaC in oocytes. Self-inhibition was measured by fast switching the low Na+ bath solution (1 mM) to regular ND96 medium (96 mM). (A) Representative whole-cell current trace digitized at −60 mV. CPT-cGMP (0.2 mM) was added after the first three sweeps and washed out for the last three sweeps. (B) Current ratio. n = 9. *P < 0.05; **P < 0.01 versus the first sweep. (C) Comparison of CPT-cGMP on self-inhibition of αβγ and δβγ ENaC channels. Paired traces were recorded before and after application of CPT-cGMP.
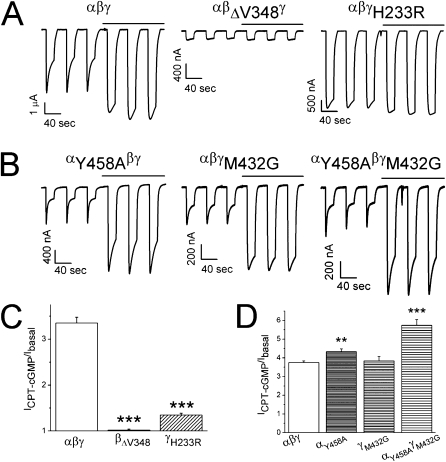
Responses of Self-Inhibition Abolishing Mutants to CPT-cGMP
Because CPT-cGMP regulates self-inhibition, mutation of the self-inhibition domains should modulate its effects. We constructed two self-inhibition–abolishing mutants, βΔV348 and γH233R, as previously reported for murine ENaC (7, 17). The responses of these mutants to CPT-cGMP were almost completely depressed (Figure 5A). In contrast, αβγ ENaC showed a more than 3-fold increment in the maximal currents after CPT-cGMP perfusion (Figure 5C).
Figure 5.
Responses of self-inhibition mutant to CPT-cGMP. (A) Representative current traces of mutants eliminating self-inhibition. Application of CPT-cGMP is indicated by horizontal lines. (B) Current tracings of mutants boosting self-inhibition. (C and D) Fold increased by CPT-cGMP for mutants abolishing (C) and facilitating self-inhibition (D). **P < 0.01 and ***P < 0.001 versus αβγ ENaC. n = 10 to 14.
Responses of Self-Inhibition Boosting Mutants to CPT-cGMP
A series of amino acid residues within the thumb domains, which amplified self-inhibition, have recently been identified (23). Based on the observation that the self-inhibition–abolishing mutant (γH233R) was not sensitive to CPT-cGMP (Figure 5A), we postulated that the self-inhibition boosting mutants (αY458A and γM432G) might be more sensitive to CPT-cGMP and found this to be the case experimentally. The responses to CPT-cGMP (ICPT-cGMP/Ibasal) of αY458A (P < 0.01) and αY458AγM432G (P < 0.001) were increased significantly (Figures 5B and 5D).
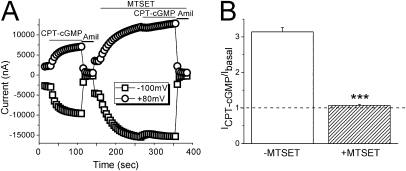
CPT-cGMP Cannot Increase Fully Opened Channel Activity
Elimination of self-inhibition by CPT-cGMP may result in an increment in open probability. We therefore measured the ability of CPT-cGMP to activate DEG mutant channels, which were already fully open (βS520C). CPT-cGMP reversibly activated αβS520Cγ in the absence of MTSET, suggesting that this mutant responds to CPT-cGMP in the same manner as wild-type channels (19). However, reapplication of the same dose of CPT-cGMP, after all channels were fully opened and clamped by MTSET, could not duplicate the stimulation (Figure 6A). The current ratio before and after addition of CPT-GMP was close to 1.0 for fully open channels, significantly less than that in the absence of MTSET for αβS520Cγ (P < 0.001) (Figure 6B).
Figure 6.
Effects of CPT-cGMP on fully opened channels. (A) Representative traces showing the responses of αβS520Cγ to CPT-cGMP (0.2 mM) before and after the addition of MTSET (1 mM). (B) Activation of ENaC by CPT-cGMP. The current ratios are 3.1 ± 0.1 before MTSET and 1.1 ± 0.03 after MTSET, respectively. ***P < 0.001. n = 11.
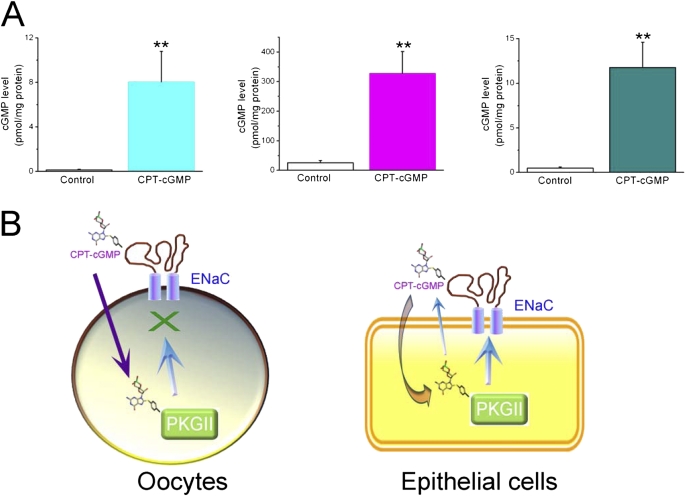
Cell cGMP Levels in Lung Tissues, H441 Cells, and Oocytes
The cGMP level in lung instilled with CPT-cGMP was 8.1 ± 2.7 pmol/mg protein, which was much greater than the level in control lungs (0.2 ± 0.05 pmol/mg protein; n = 6; P < 0.01) (Figure 7A). Perfusion of CPT-cGMP elevated the intracellular cGMP level in H441 cells from 25.5 ± 8.4 to 327.0 ± 74.6 pmol/mg protein (P < 0.01; n = 10). Similarly, an increment in cGMP content in oocytes exposed to CPT-cGMP was seen (from 0.5 ± 0.1 to 11.8 ± 2.9 pmol/mg protein; P < 0.01; n = 100). The compound increased cell cGMP levels 54-, 12-, and 24-fold, respectively, in lungs, H441 cells, and oocytes. The diverse responses may be due to various exposing periods. In addition, the yield of cell protein may vary between these models.
Figure 7.
Analysis of cell cGMP contents by ELISA. (A) Average cell cGMP contents in instilled lungs (left), H441 cells (middle), and oocytes (right). n = 6 lungs, 10 H441 monolayers, and 100 oocytes. **P < 0.01. (B) Divergent regulation of ENaC by CPT-cGMP in oocytes (left) and epithelial cells (right). Heterologous ENaC channels are not regulated by cGMP/PKG and cAMP/PKA pathways. CPT-cGMP therefore does not regulate cloned human ENaC in oocytes via the cGMP/PKG signaling pathway and only serves as an external ligand. This compound stimulates native ENaC function by acting as an external ligand and activating internal PKG in sequence. cGMP is anticipated to accumulate around ENaC as an autocrine.
Discussion
Our results reveal a novel mechanism for CPT-cGMP to stimulate ENaC in human lungs; that is, elimination of external Na+ self-inhibition of native ENaC, an intrinsic feature of the channels. However, mechanistic studies found that this compound cannot up-regulate two self-inhibition abolishing mutants (βΔV348 and γH233R) or a fully opened channel (βS520C). In sharp contrast, mutants boosting self-inhibition displayed a greater response to CPT-cGMP. Although CPT-cGMP does not regulate heterologous δβγ ENaC in oocytes, we cannot exclude the potential regulation of native δ ENaC–containing channels by this compound because ENaC channels expressed in oocytes do not respond to cAMP/PKA and cGMP/PKG pathways.
Two amiloride-sensitive ion transport systems may contribute to the increment in AFC in human lungs instilled with CPT-cGMP. First, apically located ENaC can be regulated by the NO/cGMP/PKG signaling pathway (37, 38). In vitro studies on the regulation of Na+ transport by cGMP across confluent monolayers of cultured rat type II alveolar (ATII) cells have led to contradictory results. cGMP did not alter the short-circuit current (Isc) across rat ATII monolayers (39), supporting a previous study using dome formation as a parameter for active salt and water transport (40). In contrast, cGMP, as well as NO, increased Isc and 22Na influx in tracheal and distal lung epithelia (41, 42). In addition, regulation of Na+ channel activity in A549 and rat ATII cells, as revealed by patch clamp studies, is not consistent. Jain and colleagues reported that cGMP and GSNO significantly decreased single-channel activity in rat ATII cells (34). A stimulatory effect of cGMP has been reported in the same cells (43). Furthermore, different responses of whole-cell Na+ conductance to cGMP and GSNO in A549 cells were published by three independent groups (44–46). Cyclic GMP stimulated amphibian ENaC in urinary bladder epithelium (47, 48). We recently found that CPT-cGMP stimulated human but not murine ENaC (19). The biophysical features of ENaC channels depended on the culture conditions (49, 50). Species- and culture dependence of ENaC properties and the strategies used for elevating cell cGMP content, including NO donors and PKG isoform-specific cGMP analogs, may contribute to these divergent observations. Second, CPT-cGMP may enhance AFC in human lungs through activating cyclic nucleotide-gated Na+ channels. Cyclic GMP increased AFC (30–70%) across rat, rabbit, and sheep adult lungs (51–54) at least partially by activating cyclic nucleotide-gated Na+ channels.
CPT-cGMP also increased amiloride-insensitive AFC in human lungs, possibly due to cGMP-sensitive CFTR and K+ channels. Classical studies clearly demonstrated that CFTR played an essential role in lung liquid reabsorption (55, 56). CFTR was activated by cGMP via PKG isoform II (57, 58). In addition, cGMP may accelerate the K+ recycling across basolateral membrane and may indirectly facilitate transalveolar salt transport (29). We cannot exclude the contribution of these non-ENaC pathways and others at the high dose of CPT-cGMP applied to lungs. When CPT-cGMP is applied luminally (extracellularly), it serves as an external ligand to activate ENaC via releasing self-inhibition of ENaC and then acts as a signal molecule in cytosol to stimulate PKG (Figure 7B). Eventually, the cGMP-sensitive pathways, including CFTR, K channels, and ENaC, are activated to expedite AFC coordinately.
Beyond the cGMP/PKG signal pathway, our data suggest that CPT-cGMP may activate human αβγ ENaC extracellularly by serving as an ENaC ligand, particularly when applied at a concentration above 100 μM. CPT-cGMP activated single-channel activity in outside-out but not cell-attached patches, which separate clamped channels within the pipette from bath CPT-cGMP (19). Even though CPT-cGMP is a cell permeable–specific PKGII activator with an EC50 value in the micromolar range, the time course for activating ENaC in the order of seconds was not supportive of the involvement of this pathway. The EC50 value for CPT-cGMP to rapidly and reversibly activate ENaC is 15-fold greater (71 μM) than that required to activate PKGII (4.6 μM) (59). More specific membrane-permeable analogs of PKGII activators did not stimulate ENaC in a similar manner (19). In addition, specific chlorophenylthio moiety is required to acutely activate ENaC (16, 19).
The following lines of evidence support that CPT-cGMP leads to the relief of the external Na+ self-inhibition of ENaC. First, CPT-cGMP reversibly reduces the declining rate of the maximal current, a hallmark of the release of self-inhibition (6). Second, the sustained/maximal current ratio, another methodology for evaluating self-inhibition, is not altered in peptidase (plasmin)-cleaved channels. Third, and the most direct evidence, is the absence of CPT-cGMP–induced activation in the self-inhibition abolishing mutants βΔV348 and γH233R. Finally, the self-inhibition boosting mutants augment CPT-cGMP–activated current. These results suggest that CPT-cGMP abolishes the Na+ mediated self-inhibition process.
What is the physiological and pharmacological relevance? Under physiological conditions, cytosolic cGMP may be secreted through nucleotide transporters such as CFTR and multidrug resistance proteins (60, 61). In human plasma, the cGMP level is 6 nM (62). In human bronchoalveolar lavage, it is up to 3-fold higher (63). Because cGMP in lung fluid regulates ENaC as autocrine and paracrine, the anticipated concentration at the surface of apical membrane is much greater than the average content in lavage (Figure 7B). As shown by the dose–response curve, this compound abrogates self-inhibition at micromolar level.
Regarding the potential pharmaceutical significance, the concentration of cGMP analogs clinically delivered into patients was up to 1 mg/kg (64, 65). Numerous studies applied cGMP reagents (from 100 μM to 2 mM) to lung tissues and primary alveolar epithelial cells (34, 35, 43). Aspiration of CPT-cGMP may therefore acutely ameliorate the severity of edematous lung injury by two distinct mechanisms for ENaC activation: activation of the cGMP/PKG signal pathways and relief of self-inhibition of ENaC.
Supplementary Material
Acknowledgments
The authors thank Drs. Sadis Matalon, Michael Matthay, and Mark Atkinson for helpful discussions, and Raul Molina for technical assistance.
Footnotes
Supported by NIH grants HL87017 and HL095435 (H-L.J.) and China National Natural Science Foundation grant 30971181 (H.-G.N.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjouranals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0004OC on May 11, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Garty H, Benos DJ. Characteristics and regulatory mechanisms of the amiloride-blockable Na+ channel. Physiol Rev 1988;68:309–373 [DOI] [PubMed] [Google Scholar]
- 2.Garty H. Regulation of the epithelial Na+ channel by aldosterone: open questions and emerging answers. Kidney Int 2000;57:1270–1276 [DOI] [PubMed] [Google Scholar]
- 3.Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu Rev Physiol 2002;64:877–897 [DOI] [PubMed] [Google Scholar]
- 4.Horisberger JD, Chraibi A. Epithelial sodium channel: a ligand-gated channel? Nephron, Physiol 2004;96:37–41 [DOI] [PubMed] [Google Scholar]
- 5.Lingueglia E, Deval E, Lazdunski M. Fmrfamide-gated sodium channel and asic channels: a new class of ionotropic receptors for fmrfamide and related peptides. Peptides 2006;27:1138–1152 [DOI] [PubMed] [Google Scholar]
- 6.Chraibi A, Horisberger JD. Na self inhibition of human epithelial Na channel: temperature dependence and effect of extracellular proteases. J Gen Physiol 2002;120:133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheng S, Perry CJ, Kleyman TR. Extracellular Zn2+; activates epithelial Na+ channels by eliminating Na+ self-inhibition. J Biol Chem 2004;279:31687–31696 [DOI] [PubMed] [Google Scholar]
- 8.Li JH, Kau ST. Bumetanide stimulation of sodium permeability of the apical membrane of toad urinary bladder. J Pharmacol Exp Ther 1988;246:980–985 [PubMed] [Google Scholar]
- 9.Cucu D, Simaels J, Eggermont J, Van Driessche W, Zeiske W. Opposite effects of Ni2+ on Xenopus and rat ENaCs expressed in Xenopus oocytes. Am J Physiol Cell Physiol 2005;289:C946–C958 [DOI] [PubMed] [Google Scholar]
- 10.Cucu D, Simaels J, Van Driessche W, Zeiske W. External Ni2+ and ENaC in A6 cells: Na+ current stimulation by competition at a binding site for amiloride and Na+. J Membr Biol 2003;194:33–45 [DOI] [PubMed] [Google Scholar]
- 11.Chraibi A, Horisberger JD. Stimulation of epithelial sodium channel activity by the sulfonylurea glibenclamide. J Pharmacol Exp Ther 1999;290:341–347 [PubMed] [Google Scholar]
- 12.Chraibi A, Schnizler M, Clauss W, Horisberger JD. Effects of 8-cpt-cAMP on the epithelial sodium channel expressed in Xenopus oocytes. J Membr Biol 2001;183:15–23 [DOI] [PubMed] [Google Scholar]
- 13.Collier DM, Snyder PM. Extracellular protons regulate human ENaC by modulating Na+ self-inhibition. J Biol Chem 2009;284:792–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu M, Echeverri F, Kalabat D, Laita B, Dahan DS, Smith RD, Xu H, Staszewski L, Yamamoto J, Ling J, et al. Small molecule activator of the human epithelial sodium channel. J Biol Chem 2008;283:11981–11994 [DOI] [PubMed] [Google Scholar]
- 15.Ji HL, Benos DJ. Degenerin sites mediate proton activation of deltabetagamma-epithelial sodium channel. J Biol Chem 2004;279:26939–26947 [DOI] [PubMed] [Google Scholar]
- 16.Molina R, Han DY, Su XF, Zhao RZ, Zhao M, Sharp GM, Chang Y, Ji HL. Cpt-cAMP activates human epithelial sodium channels via relieving self-inhibition. Biochim Biophys Acta 2011; 1808:1818–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collier DM, Snyder PM. Extracellular chloride regulates the epithelial sodium channel. J Biol Chem 2009;284:29320–29325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roch A, Shlyonsky V, Goolaerts A, Mies F, Sariban-Sohraby S. Halothane directly modifies Na+ and K+ channel activities in cultured human alveolar epithelial cells. Mol Pharmacol 2006;69:1755–1762 [DOI] [PubMed] [Google Scholar]
- 19.Nie HG, Zhang W, Han DY, Li QN, Li J, Zhao RZ, Su XF, Peng JB, Ji HL. 8-pCPT-cGMP stimulates alphabetagamma-ENaC activity in oocytes as an external ligand requiring specific nucleotide moieties. Am J Physiol Renal Physiol 2010;298:F323–F334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babini E, Geisler HS, Siba M, Grunder S. A new subunit of the epithelial Na+ channel identifies regions involved in Na+ self-inhibition. J Biol Chem 2003;278:28418–28426 [DOI] [PubMed] [Google Scholar]
- 21.Sheng S, Maarouf AB, Bruns JB, Hughey RP, Kleyman TR. Functional role of extracellular loop cysteine residues of the epithelial Na+ channel in Na+ self-inhibition. J Biol Chem 2007;282:20180–20190 [DOI] [PubMed] [Google Scholar]
- 22.Sheng S, Perry CJ, Kleyman TR. External nickel inhibits epithelial sodium channel by binding to histidine residues within the extracellular domains of alpha and gamma subunits and reducing channel open probability. J Biol Chem 2002;277:50098–50111 [DOI] [PubMed] [Google Scholar]
- 23.Maarouf AB, Sheng N, Chen J, Winarski KL, Okumura S, Carattino MD, Boyd CR, Kleyman TR, Sheng S. Novel determinants of epithelial sodium channel gating within extracellular thumb domains. J Biol Chem 2009;284:7756–7765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheng S, Carattino MD, Bruns JB, Hughey RP, Kleyman TR. Furin cleavage activates the epithelial Na+ channel by relieving Na+ self-inhibition. Am J Physiol Renal Physiol 2006;290:F1488–F1496 [DOI] [PubMed] [Google Scholar]
- 25.Huber R, Krueger B, Diakov A, Korbmacher J, Haerteis S, Einsiedel J, Gmeiner P, Azad AK, Cuppens H, Cassiman JJ, et al. Functional characterization of a partial loss-of-function mutation of the epithelial sodium channel (ENaC) associated with atypical cystic fibrosis. Cell Physiol Biochem 2010;25:145–158 [DOI] [PubMed] [Google Scholar]
- 26.Nie HG, Chen L, Han DY, Li J, Song WF, Wei SP, Fang XH, Gu X, Matalon S, Ji HL. Regulation of epithelial sodium channels by cGMP/PKGII. J Physiol 2009;587:2663–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakuma T, Gu X, Wang Z, Maeda S, Sugita M, Sagawa M, Osanai K, Toga H, Ware LB, Folkesson G, et al. Stimulation of alveolar epithelial fluid clearance in human lungs by exogenous epinephrine. Crit Care Med 2006;34:676–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakuma T, Gu X, Sugita M, Sagawa M, Sakuda M, Toga H. Catecholamine clearance from alveolar spaces of rat and human lungs. Respiration 2005;72:189–196 [DOI] [PubMed] [Google Scholar]
- 29.Han DY, Nie HG, Gu X, Nayak RC, Su XF, Fu J, Chang Y, Rao V, Ji HL. K+ channel openers restore verapamil-inhibited lung fluid resolution and transepithelial ion transport. Respir Res 2010;11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lingueglia E, Voilley N, Waldmann R, Lazdunski M, Barbry P. Expression cloning of an epithelial amiloride-sensitive Na+ channel. A new channel type with homologies to Caenorhabditis elegans degenerins. FEBS Lett 1993;318:95–99 [DOI] [PubMed] [Google Scholar]
- 31.Ji HL, Parker S, Langloh AL, Fuller CM, Benos DJ. Point mutations in the post-M2 region of human alpha-ENaC regulate cation selectivity. Am J Physiol Cell Physiol 2001;281:C64–C74 [DOI] [PubMed] [Google Scholar]
- 32.Ji HL, Su XF, Kedar S, Li J, Barbry P, Smith PR, Matalon S, Benos DJ. Delta-subunit confers novel biophysical features to alpha beta gamma-human epithelial sodium channel (ENaC) via a physical interaction. J Biol Chem 2006;281:8233–8241 [DOI] [PubMed] [Google Scholar]
- 33.Su X, Li Q, Shrestha K, Cormet-Boyaka E, Chen L, Smith PR, Sorscher EJ, Benos DJ, Matalon S, Ji HL. Interregulation of proton-gated Na+ channel 3 and cystic fibrosis transmembrane conductance regulator. J Biol Chem 2006;281:36960–36968 [DOI] [PubMed] [Google Scholar]
- 34.Jain L, Chen XJ, Brown LA, Eaton DC. Nitric oxide inhibits lung sodium transport through a cGMP-mediated inhibition of epithelial cation channels. Am J Physiol 1998;274:L475–L484 [DOI] [PubMed] [Google Scholar]
- 35.Chen L, Bosworth CA, Pico T, Collawn JF, Varga K, Gao Z, Clancy JP, Fontenberry JA, Lancaster JR, Jr, Matalon S. Detano and nitrated lipids increase chloride secretion across lung airway cells. Am J Respir Cell Mol Biol 2008;39:50–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bangel-Ruland N, Sobczak K, Christmann T, Kentrup D, Langhorst H, Kusche-Vihrog K, Weber WM. Characterization of the epithelial sodium channel delta-subunit in human nasal epithelium. Am J Respir Cell Mol Biol 2010;42:498–505 [DOI] [PubMed] [Google Scholar]
- 37.Song W, Matalon S. Modulation of alveolar fluid clearance by reactive oxygen-nitrogen intermediates. Am J Physiol Lung Cell Mol Physiol 2007;293:L855–L858 [DOI] [PubMed] [Google Scholar]
- 38.Matalon S, Hardiman KM, Jain L, Eaton DC, Kotlikoff M, Eu JP, Sun J, Meissner G, Stamler JS. Regulation of ion channel structure and function by reactive oxygen-nitrogen species. Am J Physiol Lung Cell Mol Physiol 2003;285:L1184–L1189 [DOI] [PubMed] [Google Scholar]
- 39.Guo Y, DuVall MD, Crow JP, Matalon S. Nitric oxide inhibits Na+ absorption across cultured alveolar type II monolayers. Am J Physiol 1998;274:L369–L377 [DOI] [PubMed] [Google Scholar]
- 40.Goodman BE, Brown SE, Crandall ED. Regulation of transport across pulmonary alveolar epithelial cell monolayers. J Appl Physiol 1984;57:703–710 [DOI] [PubMed] [Google Scholar]
- 41.Rafii B, Gillie DJ, Sulowski C, Hannam V, Cheung T, Otulakowski G, Barker PM, O'Brodovich H. Pulmonary oedema fluid induces non-alpha-ENaC-dependent Na+ transport and fluid absorption in the distal lung. J Physiol 2002;544:537–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwiebert EM, Potter ED, Hwang TH, Woo JS, Ding C, Qiu W, Guggino WB, Levine MA, Guggino SE. cGMP stimulates sodium and chloride currents in rat tracheal airway epithelia. Am J Physiol 1997;272:C911–C922 [DOI] [PubMed] [Google Scholar]
- 43.Kemp PJ, Kim KJ, Borok Z, Crandall ED. Re-evaluating the Na+ conductance of adult rat alveolar type II pneumocytes: evidence for the involvement of cGMP-activated cation channels. J Physiol 2001;536:693–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu W, Leung S, Wright J, Guggino SE. Expression of cyclic nucleotide-gated cation channels in airway epithelial cells. J Membr Biol 1999;171:117–126 [DOI] [PubMed] [Google Scholar]
- 45.Kamosinska B, Radomski A, Man SF, Radomski MW, Duszyk M. Role of inducible nitric-oxide synthase in regulation of whole-cell current in lung epithelial cells. J Pharmacol Exp Ther 2000;295:500–505 [PubMed] [Google Scholar]
- 46.Lazrak A, Samanta A, Matalon S. Biophysical properties and molecular characterization of amiloride-sensitive sodium channels in A549 cells. Am J Physiol Lung Cell Mol Physiol 2000;278:L848–L857 [DOI] [PubMed] [Google Scholar]
- 47.Yamada T, Konno N, Matsuda K, Uchiyama M. Frog atrial natriuretic peptide and cGMP activate amiloride-sensitive Na+ channels in urinary bladder cells of Japanese tree frog, Hyla Japonica. J Comp Physiol B 2007;177:503–508 [DOI] [PubMed] [Google Scholar]
- 48.Yamada T, Matsuda K, Uchiyama M. Frog anp increases the amiloride-sensitive Na(+) channel activity in urinary bladder cells of Japanese tree frog, Hyla Japonica. Gen Comp Endocrinol 2007;152:286–288 [DOI] [PubMed] [Google Scholar]
- 49.Yue G, Hu P, Oh Y, Jilling T, Shoemaker RL, Benos DJ, Cragoe EJ, Jr, Matalon S. Culture-induced alterations in alveolar type II cell Na+ conductance. Am J Physiol 1993;265:C630–C640 [DOI] [PubMed] [Google Scholar]
- 50.Jain L, Chen XJ, Ramosevac S, Brown LA, Eaton DC. Expression of highly selective sodium channels in alveolar type II cells is determined by culture conditions. Am J Physiol Lung Cell Mol Physiol 2001;280:L646–L658 [DOI] [PubMed] [Google Scholar]
- 51.Norlin A, Lu LN, Guggino SE, Matthay MA, Folkesson HG. Contribution of amiloride-insensitive pathways to alveolar fluid clearance in adult rats. J Appl Physiol 2001;90:1489–1496 [DOI] [PubMed] [Google Scholar]
- 52.Junor RW, Benjamin AR, Alexandrou D, Guggino SE, Walters DV. Lack of a role for cyclic nucleotide gated cation channels in lung liquid absorption in fetal sheep. J Physiol 2000;523:493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schutte H, Witzenrath M, Mayer K, Rosseau S, Seeger W, Grimminger F. Short-term “preconditioning” with inhaled nitric oxide protects rabbit lungs against ischemia-reperfusion injury. Transplantation 2001;72:1363–1370 [DOI] [PubMed] [Google Scholar]
- 54.Sakuma T, Zhao Y, Sugita M, Sagawa M, Toga H, Ishibashi T, Nishio M, Matthay MA. Malnutrition impairs alveolar fluid clearance in rat lungs. Am J Physiol Lung Cell Mol Physiol 2004;286:L1268–L1274 [DOI] [PubMed] [Google Scholar]
- 55.Fang X, Song Y, Hirsch J, Galietta LJ, Pedemonte N, Zemans RL, Dolganov G, Verkman AS, Matthay MA. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol 2006;290:L242–L249 [DOI] [PubMed] [Google Scholar]
- 56.Fang X, Fukuda N, Barbry P, Sartori C, Verkman AS, Matthay MA. Novel role for CFTR in fluid absorption from the distal airspaces of the lung. J Gen Physiol 2002;119:199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.French PJ, Bijman J, Edixhoven M, Vaandrager AB, Scholte BJ, Lohmann SM, Nairn AC, de Jonge HR. Isotype-specific activation of cystic fibrosis transmembrane conductance regulator-chloride channels by cGMP-dependent protein kinase II. J Biol Chem 1995;270:26626–26631 [DOI] [PubMed] [Google Scholar]
- 58.Golin-Bisello F, Bradbury N, Ameen N. Sta and cGMP stimulate CFTR translocation to the surface of villus enterocytes in rat jejunum and is regulated by protein kinase G. Am J Physiol Cell Physiol 2005;289:C708–C716 [DOI] [PubMed] [Google Scholar]
- 59.Gamm DM, Francis SH, Angelotti TP, Corbin JD, Uhler MD. The type II isoform of cGMP-dependent protein kinase is dimeric and possesses regulatory and catalytic properties distinct from the type I isoforms. J Biol Chem 1995;270:27380–27388 [DOI] [PubMed] [Google Scholar]
- 60.Sager G, Ravna AW. Cellular efflux of cAMP and cGMP: a question about selectivity. Mini Rev Med Chem 2009;9:1009–1013 [DOI] [PubMed] [Google Scholar]
- 61.Sager G. Cyclic gmp transporters. Neurochem Int 2004;45:865–873 [DOI] [PubMed] [Google Scholar]
- 62.Goette-Di Marco P, Talha S, Enache I, Weiller MA, Charloux A, Massard G, Kessler R, Piquard F, Geny B. Endocrine heart after lung transplantation: increased brain natriuretic peptide is related to right ventricular function. Transpl Int 2010;23:728–735 [DOI] [PubMed] [Google Scholar]
- 63.Arias-Diaz J, Vara E, Torres-Melero J, Garcia C, Baki W, Ramirez-Armengol JA, Balibrea JL. Nitrite/nitrate and cytokine levels in bronchoalveolar lavage fluid of lung cancer patients. Cancer 1994;74:1546–1551 [DOI] [PubMed] [Google Scholar]
- 64.Sandera P, Hillinger S, Stammberger U, Schoedon G, Zalunardo M, Weder W, Schmid RA. 8-Br-cyclic GMP given during reperfusion improves post-transplant lung edema and free radical injury. J Heart Lung Transplant 2000;19:173–178 [DOI] [PubMed] [Google Scholar]
- 65.Hillinger S, Schmid RA, Sandera P, Stammberger U, Schneiter D, Schoedon G, Weder W. 8-Br-cGMP is superior to prostaglandin E1 for lung preservation. Ann Thorac Surg 1999;68:1138–1142, discussion 1143 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.