Abstract
We identified a previously unrecognized component of airborne particulate matter (PM) formed in combustion and thermal processes, namely, environmentally persistent free radicals (EPFRs). The pulmonary health effects of EPFRs are currently unknown. In the present study, we used a model EPFR-containing pollutant-particle system referred to as MCP230. We evaluated the effects of MCP230 on the phenotype and function of bone marrow–derived dendritic cells (BMDCs) in vitro and lung dendritic cells (DCs) in vivo, and the subsequent T-cell response. We also investigated the adjuvant role of MCP230 on airway inflammation in a mouse model of asthma. MCP230 decreased intracellular reduced glutathione (GSH) and the GSH/oxidized glutathione ratio in BMDCs, and up-regulated the expression of costimulatory molecules CD80 and CD86 on DCs. The maturation of DCs was blocked by inhibiting oxidative stress or the uptake of MCP230. BMDCs exposed to MCP230 increased their antigen-specific T-cell proliferation in vitro. In a model of asthma, exposure to MCP230 exacerbated pulmonary inflammation, which was attributed to the increase of neutrophils and macrophages but not eosinophils. This result correlated with an increase in Th17 cells and cytokines, compared with non–MCP230-treated but ovalbumin (OVA)–challenged mice. The percentage of Th2 cells was comparable between OVA and OVA + MCP230 mice. Our data demonstrate that combustion-generated, EPFR-containing PM directly induced the maturation of DCs in an uptake-dependent and oxidative stress–dependent manner. Furthermore, EPFR-containing PM induced a Th17-biased phenotype in lung, accompanied by significant pulmonary neutrophilia. Exposure to EPFR-containing PM may constitute an important and unrecognized risk factor in the exacerbation and development of a severe asthma phenotype in humans.
Keywords: EPFR, dendritic cell, asthma, Th17, neutrophil
Clinical Relevance
We identified a previously unrecognized component of airborne particulate matter (PM) formed in combustion and thermal processes, namely, environmentally persistent free radicals (EPFRs). Exposure to EPFR-containing PM results in Th17-specific pulmonary immune responses. These data suggest that exposure to EPFR-containing PM may constitute an important and unrecognized risk factor in the exacerbation and development of a severe asthma phenotype in humans.
Epidemiological studies demonstrated that increased asthma prevalence and exacerbation are closely associated with increased ambient air pollution (1–3). In particular, severe asthma, which is usually not sensitive to glucocorticoid treatment, is mostly associated with exposure to air pollution (4). Ambient air pollution is a complex mixture of particulates and volatiles arising from various sources, with 40–70% of airborne particulate matter (PM) attributed to emissions from various types of combustion or thermal processes (5). A tremendous amount of attention has been paid to the contributions of vehicular exhaust (such as diesel exhaust particulates; DEPs) to the health effects associated with exposures to PM. In contrast, comparatively little is known about the health effects associated with exposure to particle-associated pollutant by-products of combustion and the thermal treatment of hazardous wastes and hazardous materials. Unfortunately, the complexity and resulting nonuniformity of the composition of combustion-generated particles, and particularly those generated from treating hazardous substances, often limits attempts to determine the mechanism of their toxicity.
In the present study, we used a common by-product of waste combustion, 2-monochlorophenol (2-MCP), associated with a transition metal–containing fly ash. This pollutant-particle system is a known source for the formation of polychlorinated dibenzo-p-dioxins and dibenzofurans. When chlorinated phenols, such as 2-MCP, react with transition metal–laden particles at slightly elevated temperatures, such as those occurring in low-temperature thermal treatment units or the post-flame, cool zone of high-temperature combustors, they form environmentally persistent free radicals (EPFRs). We are studying the specific EPFR of 2-MCP that is formed by reaction with fly ash containing Cu(II)O at 230°C (referred to as MCP230). Our previous studies demonstrated that EPFRs such as MCP230 induced cellular oxidative stress and cytotoxicity (6). However, the impact of exposure to MCP230 on the development and exacerbation of asthma remains unknown.
Dendritic cells (DCs) are recognized as professional antigen-presenting cells (APCs), exhibiting a potent antigen-presenting ability (7). DCs are crucial in regulating immune responses, and play an important role in the induction of asthma. Several studies showed that oxidative stress induces the maturation of DCs. Mature DCs present antigens to naive T cells, and direct the T-cell response. The role of EPFRs in inducing the maturation and function of DCs is unknown. This study sought to evaluate the effects of the EPFR-containing pollutant-particle system, MCP230, on the phenotype and function of DCs and the subsequent T-cell response. In addition, we investigated the adjuvant role of EPFRs on airway inflammation in a mouse model of asthma.
Materials and Methods
Mice
BALB/c mice (aged 6–8 weeks) and ovalbumin specific, T-cell receptor (OVA-TCR) transgenic (Balb/C-Tg[DO11.1010]Loh/J) or DO11.10 mice were purchased from Harlan (Indianapolis, Indiana) and the Jackson Laboratory (Bar Harbor, ME), respectively. Animal protocols were prepared in accordance with the Guide for the Care and Use of Laboratory Animals (8), and were approved by the Institutional Animal Care and Use Committee at the Louisiana State University Health Sciences Center.
Particles
EPFR-containing particles were produced as previously described (6). The particle size was confirmed as 0.2 μm in diameter, according to transmission electron microscopy and flow cytometry.
Generation and Treatment of Bone Marrow–Derived DCs
Our methods are described in the online supplement.
Quantification of Intracellular Reduced Glutathione and Oxidized Glutathione of Bone Marrow–Derived DCs
Intracellular reduced glutathione (GSH) and oxidized glutathione (GSSG) were quantified in bone marrow–derived DC (BMDC) lysates according to the method of Rahman and colleagues (9), as described in the online supplement.
Proliferation and Activation of T Cells by MCP230-Pretreated BMDCs
Naive CD4+ T cells were purified from the spleens of DO11.10 mice by negative selection (Stemcell, Vancouver, British Columbia, Canada). BMDCs were treated with 200 ng/ml ovalbumin peptide (OVA323–339; Anaspec, Fremont, CA), along with MCP230 or vehicle (sham group), in medium for 24 hours. DCs exposed to MCP230 or vehicle in the presence or absence of alpha-phenyl N-tertiary-butyl nitrone (PBN) or an uptake blocker were cocultured with purified CD4+ T cells for 72 hours at a ratio of 1:5. CD4+ T cells were labeled with 0.5 μM carboxyfluorescein diacetate, succinimidyl ester (CFSE) (Invitrogen, Carlsbad, CA). The dilution of CFSE and induction of CD69 were used to determine the proliferation and activation, respectively, of CD4+ T cells.
Asthma Model and Exposure to MCP230
Mice were divided into three groups: saline-exposed controls (sham), ovalbumin-exposed (OVA), and OVA + MCP230. The OVA and OVA + MCP230 mice were immunized intraperitoneally on Days 0 and 14 with 20 μg of OVA (Sigma-Aldrich, St. Louis, MO), emulsified in 100 μl of Imject Alum (Pierce, Rockford, IL). On Days 24, 25, and 26, mice were challenged with aerosolized OVA (1% in saline for 20 minutes). Age-matched and sex-matched mice that had been sensitized and challenged with saline at each time point were used as negative controls (sham). OVA + MCP230 mice received 50 μg of MCP230 suspended in sterile saline containing 0.02% Tween-80 by oropharyngeal aspiration. Sham and OVA mice received the same volume of vehicle.
Bronchoalveolar Lavage Fluid Cellularity and Measurement of Cytokines
Our methods used are described in the online supplement.
Lung Histopathology
After bronchoalveolar lavage fluid (BALF) was acquired, lungs were infused with HistoChoice (Amresco, Solon, OH) and isolated. Each lung section was stained with hematoxylin and eosin.
Assessment of Pulmonary T-Cell Populations and Maturation of DCs
A single-cell suspension of lung cells was prepared, using a standardized protocol (10). Pulmonary T-cell populations and the maturation of DCs were examined by flow cytometry (as detailed in the online supplement).
Expression of IL-17 in the Lung
The expression of IL-17A and IL-17F genes in lungs was analyzed by real-time PCR (as detailed in the online supplement).
Statistical Analysis
Data were analyzed using InStat (GraphPad Software, version 3.0). ANOVA was used to determine levels of differences between all groups. Comparisons of all pairs were performed according to Tukey-Kramer tests. Data are presented as means ± SEM. P < 0.05 was considered statistically significant.
Results
MCP230 Induces an Innate Immune Response, Recruitment of Neutrophils, and Maturation of DCs in Mouse Lungs
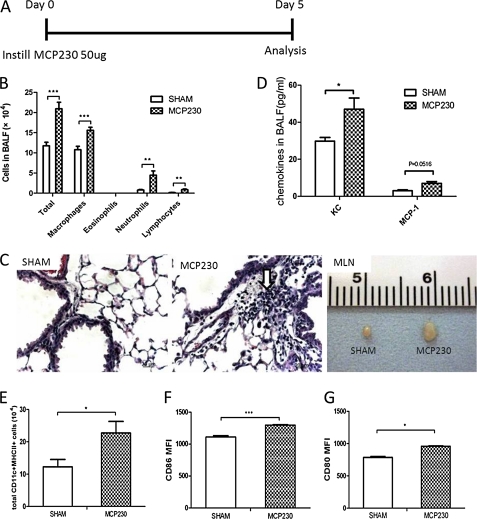
To investigate whether exposure to MCP230 induced airway inflammation, MCP230 was administered to mice via oropharyngeal aspiration (Figure 1A). Exposure to MCP230 increased the total number of BALF leukocytes. This increase was attributable to the recruitment of neutrophils, monocytes, and lymphocytes (Figure 1B). Histopathology revealed inflammatory cell infiltration (Figure 1C) around vessels and bronchia. This MCP230-induced cellular response correlated with increased concentrations of chemokine (C-X-C motif) ligand 1 (KC) and monocyte chemoattractant protein 1 (MCP-1) in the BALF (Figure 1D).
Figure 1.
Exposure to the environmentally persistent free radical (EPFR)–containing pollutant-particle system MCP230 induced an innate immune response in lungs and the maturation of pulmonary dendritic cells (DCs). (A) Mice received MCP230 via oropharyngeal aspiration on Day 0. Bronchoalveolar lavage fluid (BALF) and lungs were isolated for analyses of cellularity and chemokines on Day 5. (B) Exposure to MCP230 increased the total numbers of leukocytes, neutrophils, monocytes, and lymphocytes in BALF. (C) Representative micrographs demonstrate inflammatory cell infiltrates in the perivascular and peribronchiolar regions (arrow). The mediastinal lymph node (MLN) was enlarged upon instillation of MCP230. (D) Exposure to MCP230 increased concentrations of chemokine (C-X-C motif) ligand 1 (KC) and MCP–1 in BALF. (E) Exposure to MCP230 increased the total number of lung DCs (CD11c+MHCII+ cells). (F and G) The mean fluorescence intensity (MFI) of CD86 (F) and CD80 (G) in lung DCs was determined by flow cytometry. *P < 0.05. **P < 0.01. ***P < 0.001.
The total number of DCs in the lungs of mice exposed to MCP230 was significantly increased over that of sham-treated animals (Figure 1E). The increase in number of DCs was accompanied by an increase in the maturation status of pulmonary DCs, as evidenced by the increased expression of costimulatory molecules CD86 and CD80 (Figures 1F and 1G). Upon necropsy, we also found that the mediastinal lymph nodes (MLNs) were enlarged in mice treated with MCP230 (Figure 1C).
MCP230-Induced Maturation of DCs Is Inhibited by Treatment with Antioxidants or Uptake Blockers
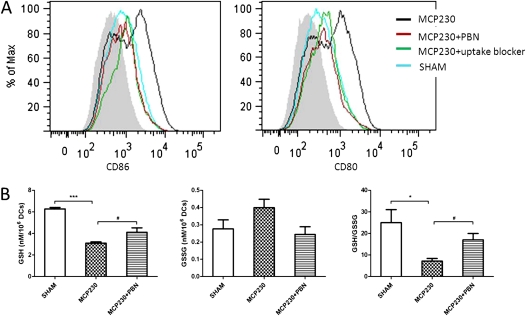
To test whether exposure to MCP230 directly induced the maturation of DCs, BMDCs were exposed to MCP230 particles. We found that exposure to MCP230 significantly up-regulated CD86 and CD80 on CD11c+MHCII+ BMDCs (Figure 2A). This increase was blocked after the addition of the antioxidant PBN and the uptake blocker cocktail (Figure 2A). The uptake blocker cocktail reduced the uptake of FITC–dextran, but failed to inhibit the maturation of BMDCs in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF), as evidenced by increased concentrations of CD80 and CD86 (data not shown). These data indicate that the uptake blocker cocktail inhibits the uptake of MCP230, and blocks the maturation of BMDCs, by virtue of its ability to inhibit macropinocytosis.
Figure 2.
Maturation of DCs and oxidative stress after exposure to MCP230 in vitro. (A) Expression of costimulatory molecules in CD11c+ MHCII+ bone marrow–derived dendritic cells (BMDCs). BMDCs were exposed to vehicle (SHAM) or MCP230, or pretreated with PBN or uptake blocker. Twenty-four hours later, the expression of CD86 and CD80 in CD11c+ MHCII+ BMDCs was determined by flow cytometry. Gray area in histogram represents isotype control. (B) MCP230 regulates BMDC glutathione homeostasis. After exposure to MCP230 for 24 hours, DCs were collected and lysed. Intracellular reduced glutathione (GSH) and oxidized glutathione (GSSG) were measured in cell lysates. *P < 0.05. #P < 0.05. ***P < 0.001.
The ratio of cellular GSH/GSSG is a hallmark of oxidative stress. To examine whether exposure to MCP230 induced oxidative stress in BMDCs, the GSH/GSSG ratio of BMDCs after stimulation with particles was tested. As shown in Figure 2B, a decrease in the GSH/GSSG ratio was evident in cells exposed to MCP230. This decrease was reduced in cells pretreated with 10 mM PBN for 1 hour. These data suggest that the maturation of DCs after exposure to MCP230 is dependent on particle uptake and the ability to induce oxidative stress.
MCP230-Pretreated DCs Induce CD4+ T-Cell Proliferation and Activation
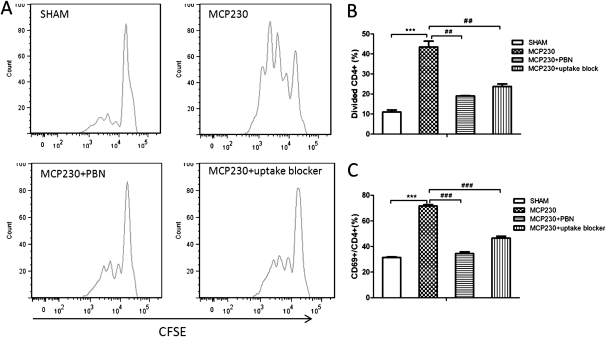
Because MCP230 may directly induce the maturation of BMDCs, we further examined the ability of MCP230-pretreated BMDCs to induce the proliferation and activation of CD4+ T cells. In this experiment, OVA323–339 peptide and OVA-TCR transgenic mice (DO11.10) were used. As shown in Figure 3, BMDCs co-exposed to MCP230 and OVA323–339 induced significant proliferation (Figures 3A and 3B) and activation (Figure 3C) of CD4+ T cells from DO11.10 mice. The ability of MCP230 to induce the activation and proliferation of T cells was inhibited by PBN or the uptake blocker cocktail (Figure 3).
Figure 3.
MCP230 alters the capacity of DCs to prime T cells. BMDCs were exposed to MCP230 or vehicle (SHAM) and ovalbumin peptide (OVA323–339), with or without pretreatment with PBN or uptake blocker, for 24 hours. DCs were then cocultured with carboxyfluorescein diacetate, succinimidyl ester (CFSE)-labeled naive DO11.10 TCR+CD4+ T cells for 72 hours. Fluorescence intensities were measured by flow cytometry. (A) Representative histogram of CFSE division profiles of CD4+ T cells in each group. Data indicate percentages of dividing CD4+ T cells (B) and CD69+ activated CD4+ T cells (C). ##P < 0.01. ###P < 0.001. ***P < 0.001.
MCP230 Enhances Pulmonary Inflammation in a Mouse Model of Asthma by Inducing Maturation of DCs
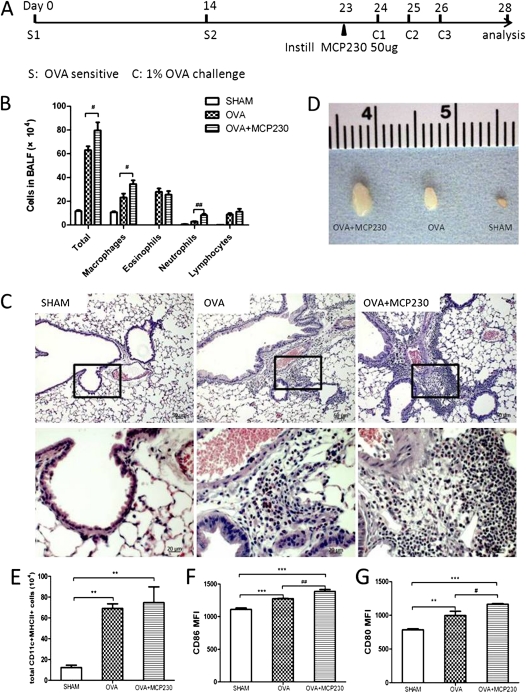
Because our in vivo and in vitro studies demonstrated that exposure to MCP230 was capable of inducing the maturation of DCs and enhancing the capacity of DCs to stimulate the proliferation and activation of T cells, we were interested in the role of MCP230 exposure in exacerbating asthma. A mouse model of asthma was established by OVA sensitization and challenge. MCP230 was instilled into the bronchi of mice 24 hours before their first OVA challenge (Figure 4A). Mice treated with MCP230 recruited more inflammatory cells to the lung compared with sham mice exposed only to vehicle or OVA mice, as evidenced by the total number of cells recruited to the BALF (Figure 4B) and peribronchial regions (Figure 4C). Differential cell counts of the BALF demonstrated significant elevations in the numbers of neutrophils and macrophages, but not eosinophils (Figure 4B). Upon necropsy, we observed a tremendous increase in the size of MLNs from mice of the OVA + MCP230 group, compared with that of OVA mice (Figure 4D).
Figure 4.
MCP230 enhanced pulmonary inflammation in a mouse model of asthma by inducing the maturation of DCs. (A) Schematic of the protocol used to induce allergic inflammation (i.e., mouse model of asthma). OVA complexed to Imject Alum was administered intraperitoneally on Protocol Days 0 and 14. Mice were then challenged with aerosolized OVA on Protocol Days 24, 25, and 26. The OVA + MCP230 group was exposed to MCP230 on Protocol Day 23. Sham control mice were exposed to saline and vehicle. (B) BALF was acquired, and cells were differentiated and counted. (C) Representative micrographs of the lungs of mice exposed to sham, OVA, or OVA + MCP230 (hematoxylin and eosin stain). (D) MLNs from OVA + MCP230 mice were larger than those of OVA mice. (E–G) Lung cells were isolated and stained with fluorescently labeled antibodies to determine the numbers and maturation status of DCs. Numbers of pulmonary DCs (E) and the MFIs of CD86 (F) and CD80 (G) in lung DCs were determined by flow cytometry. #P < 0.05. ##P < 0.01. **P < 0.01. ***P < 0.001.
The status of CD11c+MHCII+ DC maturation was also examined. Compared with sham mice, the number of DCs in the lungs of OVA-sensitized and OVA-challenged mice was significantly higher (Figure 4E). Total numbers of DCs did not differ between OVA + MCP230 and OVA mice. The expression of the costimulatory molecules CD86 and CD80 had increased (Figures 4F and 4G) in both OVA and OVA + MCP230 mice. Treatment with MCP230 induced a significantly greater expression of both CD86 and CD80, compared with OVA alone (Figures 4F and 4G).
MCP230 Increases IL-17–Producing T Cells in Lung Tissues, but Not IL-4–Producing T Cells
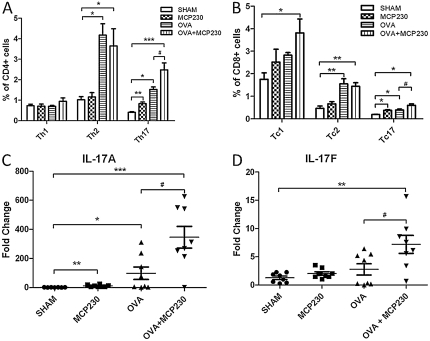
To examine the role of MCP230 in the differentiation of pulmonary T cells, pulmonary T-cell subpopulations were measured using intracellular cytokine staining. The results were gated on CD4+ or CD8+ populations. Sensitization or challenge with OVA initiated strong Th2 and Tc2 immune responses and weak Th17 and Tc17 responses, whereas the IFN-γ response was not statistically different from that in sham-treated mice (Figures 5A and 5B). The exposure of “asthmatic mice” to MCP230 resulted in a significant increase in the number of T cells producing IL-17, compared with non–MCP230-treated but OVA-challenged mice (Figures 5A and 5B). The elevated concentration of IL-17A and IL-17F mRNA in the lungs of the OVA + MCP230 mice confirmed this phenomenon (Figures 5C and 5D). The percentage of T cells producing IL-4 was comparable between the OVA and OVA + MCP230 mice.
Figure 5.
MCP230 influences pulmonary T-cell responses in a mouse model of asthma. (A and B) Lung cells were isolated, stimulated with phorbol myristate acetate and ionomycin, and stained with surface antibodies (CD4 and CD8) and intracellular antibodies (IFN-γ, IL-4, and IL-17A). Subsets of T cells were then quantified using flow cytometry. (C and D) The expression of IL-17A and IL-17F genes in whole-lung homogenates was tested by real-time PCR. #P < 0.05. *P < 0.05. **P < 0.01. ***P < 0.001.
The cytokines in BALF were also analyzed. In total, 13 cytokines and chemokines were measured, and 11 (IL-4, IL-5, IL-1β, IL-10, IL-13, IFN-γ, IL-12p40, TNF-α, KC, MCP-1, and macrophage inflammatory protein 2 [MIP-2]) were detectable in BALF. Higher levels of IL-4, IL-5, IL-13, and KC (Table 1) were detected in the BALF of OVA-sensitized/challenged mice, compared with sham-treated mice. The exposure of asthmatic mice to MCP230 resulted in a further increase in the concentrations of IL-13, KC, and MCP-1 over those of OVA mice. The concentrations of IL-10 and IFN-γ in BALF were comparable in all three groups.
TABLE 1.
CYTOKINES AND CHEMOKINES IN BRONCOALVEOLAR LAVAGE FLUID (pg/ml)
| Sham Group | OVA Group | OVA + MCP230 Group | |
| IFN-γ | 6.50 ± 1.38 | 6.66 ± 3.28 | 9.82 ± 0.97 |
| IL-4 | 3.13 ± 1.07 | 13.64 ± 1.62† | 18.53 ± 4.16† |
| IL-5 | 7.90 ± 0.42 | 57.43 ± 11.31* | 63.24 ± 9.32† |
| IL-1β | 1.47 ± 0.42 | 4.02 ± 1.36 | 6.07 ± 0.86 |
| IL-13 | 4.52 ± 0.83 | 27.13 ± 4.51† | 46.90 ± 7.59‡§ |
| IL-10 | 6.38 ± 1.07 | 8.40 ± 1.99 | 12.17 ± 2.87 |
| IL-12p40 | 5.56 ± 1.18 | 7.90 ± 2.23 | 14.62 ± 3.45 |
| TNF-α | 4.26 ± 0.18 | 4.25 ± 0.28 | 5.20 ± 0.54 |
| KC | 29.78 ± 1.99 | 193.30 ± 17.84‡ | 270.45 ± 29.67‡§ |
| MCP-1 | 3.04 ± 0.43 | 5.34 ± 1.49 | 9.65 ± 1.52* |
| MIP-2 | 23.19 ± 5.22 | 18.35 ± 3.50 | 31.59 ± 6.33 |
Definition of abbreviations: OVA, ovalbumin; MCP230, EPFR of 2-MCP that is formed by reaction with Cu(II)O containing fly-ash at 230°C; KC = chemokine (C-X-C motif) ligand 1; MCP-1 = monocyte chemoattractant protein 1; MIP-2 = macrophage inflammatory protein 2.
Cytokines and chemokines in BALF were examined using a multiplex mouse cytokine/chemokine assay kit. Results are expressed as means ± SEM (n = 7–8 mice/group).
P < 0.05.
P < 0.01.
P < 0.001 versus sham group.
P < 0.05 versus OVA group.
Discussion
This study explored the effects of a recently identified component of airborne PM formed during processes of combustion, namely, the EPFRs. The EPFR-containing ultrafine particle used in this study was created by combusting 1,2-monochlorophenol in the presence of a metal catalyst and silica substrate (i.e., MCP230). In particular, this study explored the role of MCP230 in altering pulmonary immunologic homeostasis in normal mice or mice with allergic airway inflammation (i.e., mice with asthma). Our data demonstrated that MCP230 directly induced the maturation of pulmonary DCs and enhanced OVA-induced allergic airway inflammation. This did not correlate with eosinophilic inflammation, as expected of a traditional model of OVA-induced asthma, but rather with pulmonary neutrophilia. The observed neutrophilia appeared to be directly related to MCP230 exposure. In addition, MCP230 enhanced the Th17 immune response observed after OVA sensitization and challenge. Neutrophilic inflammation and Th17 immune responses are predominantly associated with persistent and difficult-to-control asthma (i.e., with poor responses to corticosteroids) (11–16).
Exposure to MCP230 induced the maturation of lung DCs in vivo, and stimulated the expression of the costimulatory molecules CD80 and CD86 in BMDCs in vitro, suggesting that MCP230 directly stimulates the maturation of DCs. Comparable effects on the maturation of DCs were observed after exposure of human blood-derived monocytes or BMDCs to freshly collected air pollution particles and ultrafine carbon black particles (17–19).
The mechanism by which ambient PM induces the maturation of DCs remains unclear. However, it seems logical to assume that particle uptake, by either specialized phagocytic or endocytic mechanisms, is necessary to promote the maturation of DCs. Indeed, our data demonstrate that particle uptake is necessary for increasing the expression of the costimulatory molecules CD86 and CD80.
Oxidative stress is also thought to play an important role in inducing the maturation of DCs (19–21). GSH is an important tripeptidethiol antioxidant, and its intracellular concentration and a decreased GSH/GSSG ratio are indicators of oxidative stress (9). In this study, exposure to MCP230 induced oxidative stress in BMDCs, as evidenced by decreased GSH and GSH/GSSG ratio. The antioxidant PBN was able to decrease the MCP230-induced oxidative stress of BMDCs, and also inhibited their maturation (i.e., decreased the expression of CD86 and CD80). Because amorphous silica particles, which do not contain an EPFR, failed to induce the maturation of BMDCs, these data cumulatively suggest that the pro-oxidant property of MCP230 is responsible for inducing the maturation of DCs.
We further examined the effects of pulmonary exposure to MCP230 on pulmonary DCs in the presence of an antigen (OVA). As expected, MCP230 enhanced the expression of costimulatory molecules on DCs in the presence of the antigen. Furthermore, these more mature DCs were capable of inducing stronger CD4+ T-cell proliferation and activation responses. These data are compatible with the enhanced inflammation in the lung and enlarged MLNs in the MCP230-exposed and OVA-challenged mice. These findings suggest that MCP230 amplifies the functional activation of DCs by inducing the elevated expression of costimulatory molecules, thus exerting a durable effect on the antigen-presenting ability of DCs. In mice with asthma, MCP230 appeared to synergize with allergen, to exacerbate pulmonary pathophysiology.
In this study, exposure to MCP230 elicited an enhanced Th17 immune response in a mouse model of asthma. This result is contrary to previous reports of Th responses after many other exposures, including PM and DEPs (22–24). Many studies with DEPs, for example, demonstrated that the generation of oxidative stress by DEPs favors a Th2 skewing of the immune response, while suppressing Th1 differentiation (24–27). This Th2 skew correlated with significant eosinophilic inflammation in the lung and an OVA–IgG1 response in already sensitized animals (28). In this study, the Th2 immune responses of OVA + MCP230–exposed animals were not statistically different from those of OVA–sensitized or OVA–challenged animals, and the Th17 responses were significantly greater. This result is compatible with the demonstration in our model of increased IL-17A gene expression in the lung and increased neutrophil concentrations in the BALF of mice exposed to MCP230.
These differences in immune responses are most likely related to the differences in chemical composition of various PMs and DEPs and MCP230. For example, carbonaceous particles and a quinone in DEPs were shown to promote Th1 immune responses, whereas chemical components extracted with benzene–ethanol initiated Th2 responses (22, 23). Th17 and associated cytokines were implicated in the development and promotion of steroid-resistant asthma and airway inflammation and hyperresponsiveness in humans and mice (12, 16). The mechanism by which MCP230 induces Th17 but not Th2 immune responses is not clear. But in total, our data suggest that exposure to MCP230 may be a significant and unrealized risk factor in the development of steroid-resistant asthma.
In conclusion, our data demonstrate that EPFR-containing PM formed during processes of combustion directly induced the maturation of DCs in an uptake-dependent and oxidative stress–dependent manner. Alterations in the maturation of DCs were accompanied by alterations in Th cell subsets in the lung. The local Th17-biased phenotype (increased Th17 cells and cytokines) was accompanied by significant pulmonary neutrophilia. Pulmonary neutrophilic inflammation, the presence of Th17 cytokines, and elevated numbers of Th17 cells in the lung are reminiscent of severe, steroid-insensitive asthma in humans. Thus, exposure to EPFR-containing PM may constitute an important and unrecognized risk factor in the exacerbation and development of a severe and steroid-resistant asthma phenotype in humans.
Supplementary Material
Acknowledgments
Special thanks go to Drs. Jay Kolls and Kong Chen of Louisiana State University Health Sciences Center Genetics for their advice and assistance.
Footnotes
This work was supported by grants from the National Institute of Environmental Health Sciences (5R01ES015050 and P42ES013648) to S.A.C. The contents of this manuscript are solely the responsibility of the authors, and do not necessarily represent the official views of the National Institutes of Health.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-00010C on April 14, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Holguin F. Traffic, outdoor air pollution, and asthma. Immunol Allergy Clin North Am 2008;28:577–588 [DOI] [PubMed] [Google Scholar]
- 2.Patel MM, Miller RL. Air pollution and childhood asthma: recent advances and future directions. Curr Opin Pediatr 2009;21:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salam MT, Islam T, Gilliland FD. Recent evidence for adverse effects of residential proximity to traffic sources on asthma. Curr Opin Pulm Med 2008;14:3–8 [DOI] [PubMed] [Google Scholar]
- 4.Mamessier E, Nieves A, Vervloet D, Magnan A. Diesel exhaust particles enhance T-cell activation in severe asthmatics. Allergy 2006;61:581–588 [DOI] [PubMed] [Google Scholar]
- 5.Cass GR. Comments on sources, atmospheric levels, and characterization of airborne particulate matter. Inhal Toxicol 1995;7:765–768 [Google Scholar]
- 6.Balakrishna S, Lomnicki S, McAvey KM, Cole RB, Dellinger B, Cormier SA. Environmentally persistent free radicals amplify ultrafine particle mediated cellular oxidative stress and cytotoxicity. Part Fibre Toxicol 2009;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossi M, Young JW. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J Immunol 2005;175:1373–1381 [DOI] [PubMed] [Google Scholar]
- 8.National Research Council Guide for the care and use of laboratory animals. Washington, DC: National Academic Press; 1996 [Google Scholar]
- 9.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 2006;1:3159–3165 [DOI] [PubMed] [Google Scholar]
- 10.Ormerod MG. Preparing suspensions of single cells. : Ormerod MG, editor Flow cytometry: a practical approach. Oxford: Oxford University Press; 2000: pp. 35–46 [Google Scholar]
- 11.Berry M, Morgan A, Shaw DE, Parker D, Green R, Brightling C, Bradding P, Wardlaw AJ, Pavord ID. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax 2007;62:1043–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, et al. Th17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol 2008;181:4089–4097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma: evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest 2001;119:1329–1336 [DOI] [PubMed] [Google Scholar]
- 14.Alcorn JF, Crowe CR, Kolls JK. Th17 cells in asthma and COPD. Annu Rev Physiol 2010;72:495–516 [DOI] [PubMed] [Google Scholar]
- 15.Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax 2002;57:875–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Ramli W, Prefontaine D, Chouiali F, Martin JG, Olivenstein R, Lemiere C, Hamid QT. (h)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol 2009;123:1185–1187 [DOI] [PubMed] [Google Scholar]
- 17.Becker S, Soukup J, van Zijverden M, van der Pijl A, Bol M, van Pinxteren FA, de Haar C, Penninks AH, van Loveren H, Pieters R. Coarse (pm(2.5–10)), fine (pm(2.5)), and ultrafine air pollution particles induce/increase immune costimulatory receptors on human blood-derived monocytes but not on alveolar macrophages. J Toxicol Environ Health A 2003;66:847–859 [DOI] [PubMed] [Google Scholar]
- 18.de Haar C, Kool M, Hassing I, Bol M, Lambrecht BN, Pieters R. Lung dendritic cells are stimulated by ultrafine particles and play a key role in particle adjuvant activity. J Allergy Clin Immunol 2008;121:1246–1254 [DOI] [PubMed] [Google Scholar]
- 19.Williams MA, Rangasamy T, Bauer SM, Killedar S, Karp M, Kensler TW, Yamamoto M, Breysse P, Biswal S, Georas SN. Disruption of the transcription factor NRF2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter. J Immunol 2008;181:4545–4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Csillag A, Boldogh I, Pazmandi K, Magyarics Z, Gogolak P, Sur S, Rajnavolgyi E, Bacsi A. Pollen-induced oxidative stress influences both innate and adaptive immune responses via altering dendritic cell functions. J Immunol 2010;184:2377–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue K, Yanagisawa R, Koike E, Nishikawa M, Takano H. Repeated pulmonary exposure to single-walled carbon nanotubes exacerbates allergic inflammation of the airway: possible role of oxidative stress. Free Radic Biol Med 2010;48:924–934 [DOI] [PubMed] [Google Scholar]
- 22.Yanagisawa R, Takano H, Inoue KI, Ichinose T, Sadakane K, Yoshino S, Yamaki K, Yoshikawa T, Hayakawa K. Components of diesel exhaust particles differentially affect Th1/Th2 response in a murine model of allergic airway inflammation. Clin Exp Allergy 2006;36:386–395 [DOI] [PubMed] [Google Scholar]
- 23.Inoue K, Takano H, Hiyoshi K, Ichinose T, Sadakane K, Yanagisawa R, Tomura S, Kumagai Y. Naphthoquinone enhances antigen-related airway inflammation in mice. Eur Respir J 2007;29:259–267 [DOI] [PubMed] [Google Scholar]
- 24.Ohtani T, Nakagawa S, Kurosawa M, Mizuashi M, Ozawa M, Aiba S. Cellular basis of the role of diesel exhaust particles in inducing Th2-dominant response. J Immunol 2005;174:2412–2419 [DOI] [PubMed] [Google Scholar]
- 25.Bleck B, Tse DB, Curotto de Lafaille MA, Zhang F, Reibman J. Diesel exhaust particle–exposed human bronchial epithelial cells induce dendritic cell maturation and polarization via thymic stromal lymphopoietin. J Clin Immunol 2008;28:147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan RC, Wang M, Li N, Yanagawa Y, Onoe K, Lee JJ, Nel AE. Pro-oxidative diesel exhaust particle chemicals inhibit LPS-induced dendritic cell responses involved in T-helper differentiation. J Allergy Clin Immunol 2006;118:455–465 [DOI] [PubMed] [Google Scholar]
- 27.Porter M, Karp M, Killedar S, Bauer SM, Guo J, Williams D, Breysse P, Georas SN, Williams MA. Diesel-enriched particulate matter functionally activates human dendritic cells. Am J Respir Cell Mol Biol 2007;37:706–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li N, Harkema JR, Lewandowski RP, Wang M, Bramble LA, Gookin GR, Ning Z, Kleinman MT, Sioutas C, Nel AE. Ambient ultrafine particles provide a strong adjuvant effect in the secondary immune response: implication for traffic-related asthma flares. Am J Physiol Lung Cell Mol Physiol 2010;299:L374–L383 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.