Abstract
Alveolar type II (ATII) cells cultured at an air–liquid (A/L) interface maintain differentiation, but they lose these properties when they are submerged. Others showed that an oxygen tension gradient develops in the culture medium as ATII cells consume oxygen. Therefore, we wondered whether hypoxia inducible factor (HIF) signaling could explain differences in the phenotypes of ATII cells cultured under A/L interface or submerged conditions. ATII cells were isolated from male Sprague-Dawley rats and cultured on inserts coated with a mixture of rat-tail collagen and Matrigel, in medium including 5% rat serum and 10 ng/ml keratinocyte growth factor, with their apical surfaces either exposed to air or submerged. The A/L interface condition maintained the expression of surfactant proteins, whereas that expression was down-regulated under the submerged condition, and the effect was rapid and reversible. Under submerged conditions, there was an increase in HIF1α and HIF2α in nuclear extracts, mRNA levels of HIF inducible genes, vascular endothelial growth factor, glucose transporter–1 (GLUT1), and the protein level of pyruvate dehydrogenase kinase isozyme–1. The expression of surfactant proteins was suppressed and GLUT1 mRNA levels were induced when cells were cultured with 1 mM dimethyloxalyl glycine. The expression of surfactant proteins was restored under submerged conditions with supplemented 60% oxygen. HIF signaling and oxygen tension at the surface of cells appears to be important in regulating the phenotype of rat ATII cells.
Keywords: HIF, ATII cells, surfactant proteins, VEGF, GLUT1
Clinical Relevance
This study provides insight into understanding surfactant expression during focal alveolar hypoxia in human diseases such as acute lung injury, pneumonia, pulmonary edema, or severe small airway obstruction.
The alveolar epithelium is composed of two main cell types, namely, alveolar type I cells and alveolar type II cells (ATII). ATII cells comprise approximately 15% of peripheral lung cells, and cover approximately 5% of the alveolar surface. ATII cells exhibit a distinct morphology, with characteristic lamellar bodies and distinct apical microvilli. These cells produce and secrete surfactant and surfactant proteins (SP-A, SP-B, SP-C, and SP-D), contribute to the regulation of alveolar fluid volume and epithelial repair after lung injury, and play an important role in innate immunity (1).
The importance of using air–liquid (A/L) interface cultures, in which medium is added only to the basolateral surface of cells and the apical surface is exposed to air, was known for many years to be important for the development of cilia in bronchial epithelial cells (2, 3). After the success of this type of culture for airway epithelial cells, the culture of rat and fetal human ATII cells at an A/L interface was also found to promote the maintenance of a differentiated type II phenotype and the expression of surfactant proteins, whereas submersion suppresses the expression of surfactant proteins (4, 5). Although the importance of these A/L interface culture systems is well-accepted, the mechanism for the enhanced differentiation of cultured epithelial cells remains unknown. Epithelial cells with a high density of mitochondria consume a large amount of oxygen. Importantly, Mamchaoui and Saumon showed that rat type II cells consume sufficient oxygen to develop an oxygen tension gradient in culture medium, which is lost when mitochondria are poisoned (6). Oxygen was also reported to be important in the expression of surfactant proteins. Morphologic differentiation and concentrations of SP-A in human fetal lung in vitro are influenced by oxygen in a concentration-dependent manner (7). In murine lung epithelial (MLE)-15 cells, Grek and colleagues reported a significant down-regulation of SP-B and SP-C after exposure to severe hypoxia (8).
Hypoxia inducible factors are central to the regulation of genes involved in hypoxic responses and adaptive processes. The HIFα subunit is constitutively expressed but rapidly degrades under normoxic conditions. Under hypoxic conditions, the α-subunit is stabilized and translocates into the nucleus. After entering the nucleus, HIFα dimerizes with HIFβ, binds to the hypoxia-response element (HRE), and regulates hypoxia inducible genes such as vascular endothelial growth factor (VEGF) and glucose transporter–1 (GLUT1), which are important for minimizing the deleterious effects of hypoxia (9). In alveolar epithelial cells, HIF 1α and HIF2α are expressed, and both are regulated by hypoxia (8, 10–13). HIF is also important at physiologic levels of hypoxia, for example, in tumors (where it is responsible for the Warburg effect [aerobic glycolysis]) (14), in the normal liver (15), in fetal lung (16), and in inflammatory exudates (17), as well as in severe hypoxia (1% oxygen) (12).
VEGF and GLUT1 are commonly used as HIF-responsive genes to indicate increased HIF activity. VEGF, initially identified as a vascular permeability factor, is an important growth factor for endothelial cells. Previously, rat type II cells were shown to express VEGF, particularly in response to hypoxia (18, 19). GLUT1, which is responsible for basal glucose uptake in most tissues, is the predominant glucose transporter in ATII cells (20). Ouiddir and colleagues demonstrated that glucose transport is stimulated by O2 deprivation in rat ATII cells, and that the oxygen-dependent increase in glucose influx is associated with an increase in GLUT1 mRNA and protein levels (21).
Because the mechanism for the enhanced differentiation associated with A/L interface cultures was unknown, we hypothesized that the effect was attributable to oxygen, and that apical culture medium overlying an ATII cell monolayer could pose a significant barrier to the rate of oxygen diffusion. In addition, we wondered whether HIF signaling regulates ATII cell functions at physiologic levels of hypoxia. To test this hypothesis, we isolated primary rat ATII cells, cultured them under A/L interface conditions (cells cultured with no apical medium) or submerged conditions (cells cultured with apical medium), and measured surfactant protein levels and HIF and HIF-inducible gene expression. We then tested whether supplemental oxygen would restore differentiation in submerged cultures.
Materials and Methods
Rat ATII Cell Isolation and Culture
ATII cells were isolated from pathogen-free, adult, male Sprague-Dawley rats (weighing 175–199 g; Harlan, Indianapolis, IN) by dissociation with porcine pancreatic elastase (Roche Diagnostics, Indianapolis, IN) and partial purification on discontinuous density gradients, according to methods described previously (22). This research was approved by the Animal Care Committee at National Jewish Health. More detailed descriptions are available in the online supplement.
Culture of Rat ATII Cells
Culture system for different amounts of apical fluid.
After attachment for 24 hours, cells were rinsed once with Dulbecco's minimal essential medium (DMEM) and then cultured in DMEM containing 5% rat serum (RS), glutamine, antibiotics, and 10 ng/ml keratinocyte growth factor (KGF) for 6 days with 0, 100, 200, or 400 μl of apical media in six-well inserts without rocking, or 400 μL of apical media with rocking. The media were changed every other day.
Culture system for time-course experiment.
After attachment for 24 hours, cells were cultured with RS and KGF for 2, 4, or 6 days, under either A/L interface conditions (basal media, 1.4 ml; apical media, 0 ml) or submerged conditions (basal media, 2.0 ml; apical media, 0.4 ml).
Culture system for switching experiment from A/L interface to submerged condition.
After attachment for 24 hours, cells were cultured with RS and KGF for 6 days under A/L interface conditions. On Day 7, the condition was maintained under A/L interface conditions or switched to the submerged condition for an additional 8, 24, or 48 hours.
Culture system for switching from submerged to A/L interface condition.
After attachment for 24 hours, cells were cultured with RS and KGF for 6 days under submerged conditions. On Day 7, the submerged condition was maintained or switched to the A/L interface condition for an additional 8, 24, or 48 hours.
Extraction of nuclear fraction from rat ATII cells.
After attachment for 24 hours, cells were cultured with RS and KGF for 18 hours under either A/L interface or submerged conditions. Nuclear fractions were isolated from the cells cultured under each condition, using a NE-PER Nuclear and Cytoplasmic Extraction Reagent kit (Thermo Scientific, Rockford, IL). Extracts were stored at −80°C until protein levels of HIF1α and HIF2α were measured by immunoblotting.
Experiments with dimethyloxalyl glycine.
Cells were cultured with the HIFα prolyl hydroxylase inhibitor dimethyloxalyl glycine (DMOG), to stabilize the HIF and increase HIF protein levels. After attachment for 24 hours, cells were cultured with RS and KGF for 6 days under A/L interface, submerged or A/L interface conditions with 1 mM DMOG (Cayman Chemical Co., Ann Arbor, MI).
Culture system with increased oxygen concentrations.
Cells were cultured with supplemental oxygen (60% oxygen, 10% carbon dioxide). After attachment for 24 hours, cells were cultured with RS and KGF for 6 days under either A/L interface or submerged conditions. On Day 7, the conditions were maintained, switched to the alternate condition (A/L interface or submerged condition), or switched to the submerged condition with 60% oxygen for an additional 24 or 48 hours.
Immunoblotting and real-time PCR.
The protein and mRNA expression of corresponding genes was measured by Western blotting and real-time RT-PCR, according to protocols described previously (23, 24). More detailed descriptions are included in the online supplement.
Morphology
Cells were visualized by electron microscopy and immunofluorescence, as described previously (24, 25). A detailed description is included in the online supplement.
Statistical Analysis
All data are presented as means ± SEMs. One-way ANOVA was used to compare differences between two or more groups. Bonferroni or Dunnett post hoc tests were used for multiple comparisons. Statistical significance was set at P < 0.05.
Results
The Amount of Apical Fluid Regulates Surfactant Proteins in Rat Alveolar Type II Cells
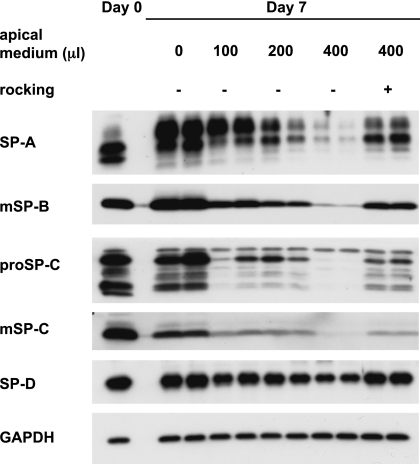
Earlier studies by other investigators indicated that the volume or height of the apical fluid may regulate the expression of surfactant proteins (4, 5), and we had observed better differentiation when cultures of rat type II cells were rocked (23, 26). To test these observations directly, we varied the volume of apical fluid and compared these results to cultures that were rocked. The volume of apical fluid directly altered the level of type II cell differentiation (Figure 1). Increasing the amounts of apical fluid reduced the protein levels of SP-A, mature SP-B, pro–SP-C, mature SP-C, and SP-D. When cells were cultured with 400 μl apical fluid and rocked, the expression of surfactant proteins was partly restored (Figure 1).
Figure 1.
The amount of apical fluid alters surfactant protein (SP) levels. Rat ATII cells were adhered for 1 day and cultured for 6 days in 5% rat serum (RS) with 10 ng/ml keratinocyte growth factor (KGF) and different amounts of apical fluid, with or without rocking. Cells were harvested on Day 7, and protein levels of SPs were analyzed by immunoblotting. Each condition is represented by two lanes from duplicate wells. m, mature.
Submersion of Type II Cells Results in a Time-Dependent Decrease in Expression of Surfactant Proteins
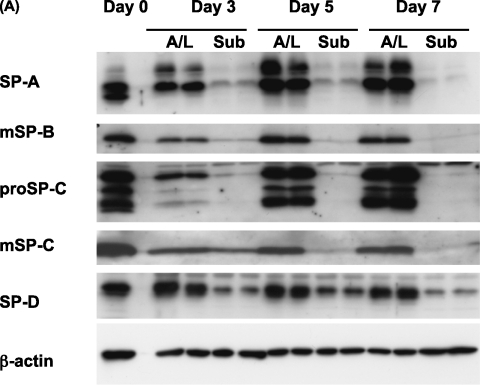
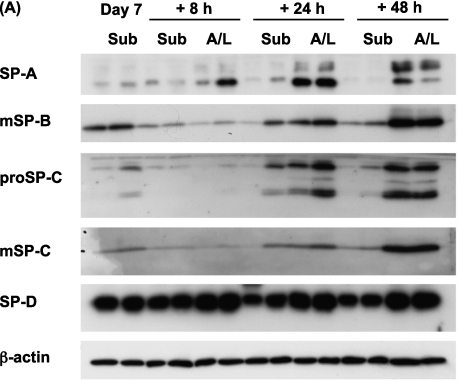
Although submersion decreased the expression of surfactant proteins in type II cells, the time course for this effect was unknown. To address this issue, the expression of surfactant proteins was measured over time under submerged and A/L interface conditions (Figure 2). The levels of surfactant proteins in cell lysates were measured by immunoblotting. By Day 7, the protein levels of SP-A, mature SP-B, and mature SP-C had decreased under the submerged condition. In terms of SP-D, a tendency toward down-regulation under the submerged condition was evident but did not reach statistical significance (Figures 2A and 2B). This effect was also observed by immunocytochemistry. The protein expression of SP-A and pro–SP-C was lower in cells cultured under submerged conditions (Figures E1A and E1B in the online supplement). However, the antibody for mature SP-B, which we used for immunoblotting, did not work for immunocytochemistry. The mRNA levels of SP-A, SP-C, and SP-D were also down-regulated under the submerged condition on Day 7 of culture. However, the mRNA level for SP-B was not statistically decreased (Figure 2C). In addition, according to electron microscopy, cells cultured under submerged conditions appeared flatter and had fewer microvilli and lamellar bodies than those cultured under A/L interface conditions (Figure E1C).
Figure 2.
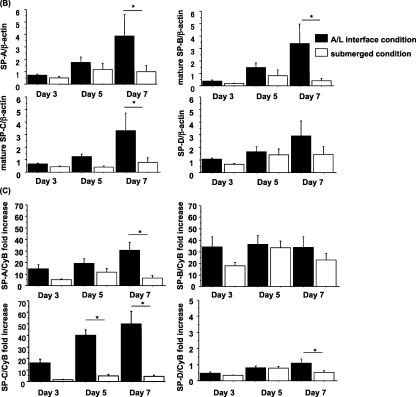
The submerged condition decreases, and the A/L interface condition maintains, the expression of surfactant proteins. (A) Rat ATII cells were adhered for 1 day and cultured for 2, 4, or 6 days in 5% RS and 10 ng/ml KGF. Cells were harvested on Day 3, 5, or 7, and levels of surfactant proteins were analyzed by immunoblotting. Each condition is represented by two lanes from duplicate wells. This blot is representative of five independent experiments. (B) Levels of surfactant proteins from the immunoblotting data were normalized to β-actin and analyzed according to NIH Image (Bethesda, MD) (n = 5). (C) The mRNA concentrations of surfactant proteins were measured by real-time PCR from five independent experiments. These levels were normalized to the constitutive probe cyclophilin B (CyB). Values represent means ± SEMs of five independent experiments. A/L, air–liquid interface condition; Sub, submerged condition. *P < 0.05.
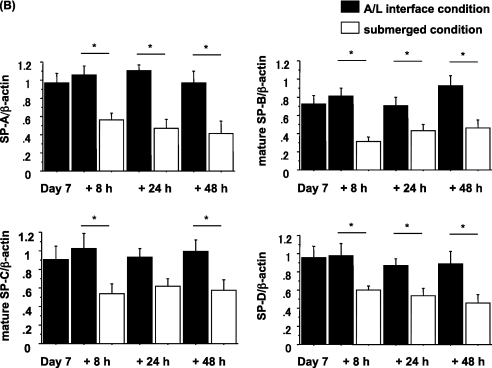
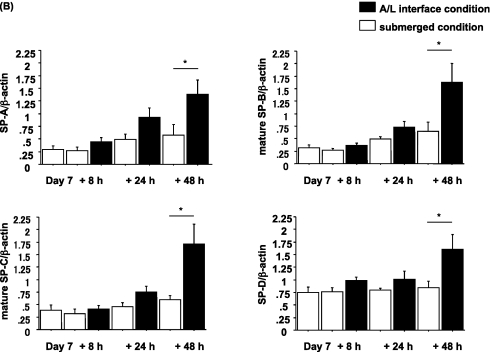
The Changes in Expression of Surfactant Protein Attributable to Culture Conditions Are Rapid and Reversible
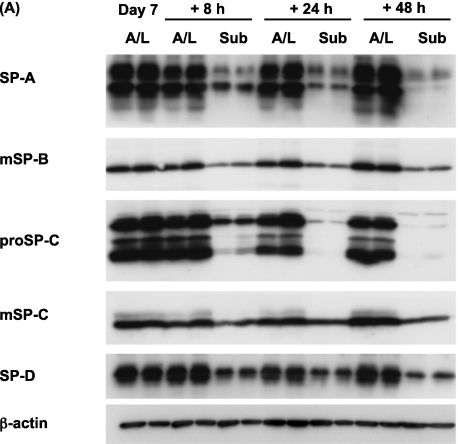
To evaluate whether the changes in expression of surfactant proteins were reversible, we performed two switching experiments, as described in Materials and Methods. The first experiment involved a switch from A/L conditions to submerged conditions. After 6 days of culture under A/L interface conditions, switching to the submerged condition for an additional 8 hours resulted in a dramatic reduction in protein levels (Figures 3A and 3B). The second experiment involved switching from submerged to A/L conditions. After 6 days of culture under the submerged condition, protein levels gradually recovered over 48 hours in the cells switched to the A/L interface condition. (Figures 4A and 4B). Hence, the effect of submersion was rapid and reversible.
Figure 3.
The expression of surfactant proteins is reduced by the addition of apical fluid. (A) Rat ATII cells were adhered for 1 day and cultured for 6 days in 5% RS and 10 ng/ml KGF under the A/L interface condition. They were then cultured for additional 8, 24, or 48 hours, under either the A/L interface or submerged condition, and levels of surfactant proteins were analyzed by immunoblotting. Each condition is represented by two lanes from duplicate wells. This blot is representative of five independent experiments. (B) Levels of surfactant proteins from immunoblotting data were normalized to β-actin and analyzed according to NIH Image (n = 5). Values represent means ± SEMs of five independent experiments. *P < 0.05.
Figure 4.
The A/L interface condition restores surfactant protein expression from the submerged condition. (A) Rat ATII cells were adhered for 1 day and cultured for 6 days in 5% RS and 10 ng/ml KGF under the submerged condition. They were then cultured for an additional 8, 24, or 48 hours in either the submerged condition or the A/L interface condition, and levels of surfactant proteins were analyzed by immunoblotting. Each condition is represented by two lanes from duplicate wells. This blot is representative of five independent experiments. (B) Levels of surfactant proteins from immunoblotting data were normalized to β-actin and analyzed according to NIH Image (n = 5). Values represent means ± SEMs of five independent experiments. *P < 0.05.
HIF Signaling Regulates the Expression of Surfactant Protein In Vitro
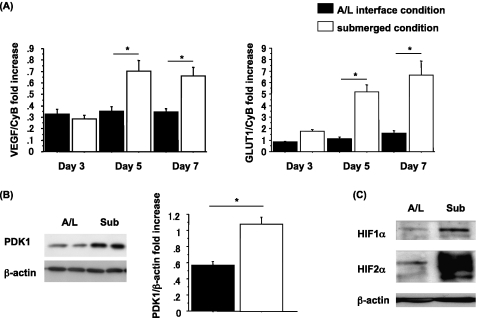
To test our hypothesis, we evaluated the expression of HIF and HIF-inducible genes in submerged and A/L cultures. The mRNA levels of the HIF-inducible genes, VEGF and GLUT1, were up-regulated on Days 5 and 7 under the submerged condition (Figure 5A). The protein level of pyruvate dehydrogenase kinase isozyme–1 (PDK1), which is also an HIF-inducible gene, was increased in the submerged condition on Day 7 (Figure 5B). In addition, we detected an increase in HIF1α and HIF2α in the nuclear extracts of cells cultured under the submerged condition for 18 hours (Figure 5C). In hypoxia, the increase in HIF1α protein was previously shown to be transient, and needs to be measured at an early time point, whereas the expression of HIF2α is more sustained (11).
Figure 5.
Increased expression of hypoxia inducible factor (HIF) and HIF-inducible genes under the submerged condition. (A) Rat ATII cells were adhered for 1 day and cultured for 2, 4, or 6 days in 5% RS and 10 ng/ml KGF. Cells were harvested on Day 3, 5, or 7, and mRNA concentrations of VEGF and GLUT1 were measured by real-time PCR from five independent experiments. These levels were normalized to the constitutive probe cyclophilin B. Values represent means ± SEMs of five independent experiments. (B) Rat ATII cells were harvested on Day 7, and protein levels of pyruvate dehydrogenase kinase isozyme–1 (PDK1) were analyzed by immunoblotting. Each condition is represented by two lanes from duplicate wells. Left, Representative blot of four independent experiments. Right, Protein levels of PDK1 from immunoblotting data analyzed according to NIH Image (n = 4). Protein levels were normalized to β-actin. Values represent means ± SEMs of four independent experiments. (C) Rat ATII cells were adhered for 1 day and cultured for 18 hours in 5% RS and 10 ng/ml KGF, and protein levels of HIF1α and HIF2α from nuclear extract were analyzed by immunoblotting. This blot is representative of three independent experiments. *P < 0.05.
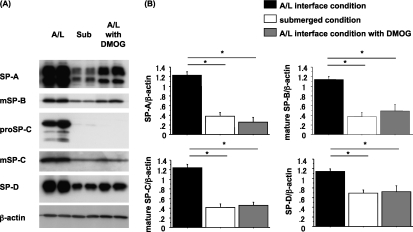
The next series of experiments were designed to determine whether the HIF stabilizer DMOG would replicate the effects of submersion. Therefore, we cultured rat ATII cells under A/L interface conditions with and without DMOG and compared the effects to those in submerged cultures (Figure 6). The expression of surfactant proteins in cells cultured with DMOG was similar to the expression of those cultured under the submerged condition on Day 7 (Figures 6A and 6B). DMOG decreased protein levels of SP-A, mature SP-B, pro–SP-C, mature SP-C, and SP-D, and these decreases mimicked the effects of submersion. In addition, we found no evidence of toxicity in cells cultured in DMOG, based on phase contrast microscopy and the amount of protein in cell lysates, although we cultured cells in 1 mM DMOG for 6 days. The mRNA levels of surfactant proteins were down-regulated, and the mRNA levels of GLUT1 were up-regulated, in cells cultured with DMOG (Figure E2).
Figure 6.
Dimethyloxalyl glycine (DMOG), an HIF stabilizer, decreases the expression of surfactant proteins. (A) Rat ATII cells were adhered for 1 day and cultured for 6 days in 5% RS, 10 ng/ml KGF, 0.58% ethanol, and PBS for 6 days under A/L interface, submerged, or A/L interface conditions with 1 mM DMOG dissolved in 0.58% ethanol and PBS. This blot is representative of four independent experiments. Each condition is represented by two lanes from duplicate wells. Protein levels were normalized by β-actin. (B) Levels of surfactant proteins from immunoblotting data were normalized to β-actin and analyzed according to NIH Image (n = 4). Values represent means ± SEMs of four independent experiments. *P < 0.05.
Supplemental Oxygen Partly Restores the Expression of Surfactant Proteins in Submerged Cultures
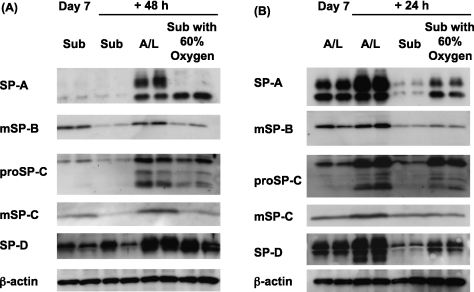
Although the effects appeared to be HIF-dependent and the effects of HIF are known to occur under physiologic oxygen tension (as in the Warburg effect in tumors) (14), we wanted to ensure that the effects were regulated by oxygen. Therefore, we determined whether supplemental oxygen would rescue the expression of surfactant proteins under the submerged condition, and prevent the reduction in surfactant proteins when A/L cultures were submerged (Figure 7). After culturing cells under the submerged condition, they were switched on Day 7 from the submerged condition with 21% oxygen to the submerged condition with 60% oxygen, or switched to the A/L interface condition with 21% oxygen. Cells cultured under the submerged condition with 60% oxygen for 48 hours showed partly restored protein levels of surfactant proteins, compared with those maintained under the submerged condition with 21% oxygen (Figure 7A). We also determined whether supplemental oxygen would prevent the decrease in expression of surfactant proteins, attributable to submersion, in cells cultured under A/L conditions for 6 days (Figure 7B). Cells that had been cultured under A/L conditions were switched to the submerged condition with 21% oxygen, or to the submerged condition with 60% oxygen. Cells cultured under the submerged condition with 60% oxygen for 24 hours expressed higher levels of surfactant proteins than those cultured under the submerged condition with 21% oxygen (Figure 7B).
Figure 7.
Supplemental oxygen restores the expression of surfactant proteins under the submerged condition and prevents the decrease because of submersion. (A) Rat ATII cells were adhered for 1 day and cultured in 5% RS and 10 ng/ml KGF under submerged conditions with 21% oxygen for 6 days. They were then cultured for an additional 48 hours in the submerged condition with 21% oxygen, in the A/L interface condition with 21% oxygen, or in the submerged condition with 60% oxygen. Levels of surfactant proteins were analyzed by immunoblotting. Each condition is represented by two lanes from duplicate wells. This blot is representative of three independent experiments. (B) Rat ATII cells were adhered for 1 day and cultured in 5% RS and 10 ng/ml KGF under A/L interface conditions with 21% oxygen for 6 days. They were then cultured for additional 24 hours under the A/L interface condition with 21% oxygen, under the submerged condition with 21% oxygen, or under the submerged condition with 60% oxygen. Levels of surfactant proteins were analyzed by immunoblotting. Each condition is represented by two lanes from duplicate wells. This blot is representative of three independent experiments.
Discussion
This study shows that apical culture medium overlying an ATII cell monolayer can be a significant barrier to the rate of oxygen diffusion, and that the differentiation of adult rat ATII cells can be regulated by oxygen tension through HIF signaling in vitro.
The evidence to support this conclusion involves the increase in HIF1α and HIF2α from nuclear extracts and the increases in expression of the HIF-inducible genes VEGF, GLUT1, and PDK1, under the submerged condition (Figure 5), indicating that cells cultured under submerged conditions are hypoxic. Many previous studies showed that under hypoxic conditions, HIF translocates from the cytoplasm to the nucleus and induces HIF-inducible genes as an adaptive response to match oxygen supply with metabolic and redox demands (17). Moreover, Mamchaoui and Saumon showed that oxygen tension in the medium above adult rat ATII cells falls under submerged conditions (6). Moreover, rocking, which intermittently exposes cells to air and mixes the medium to prevent the development of a stagnant layer and an oxygen gradient, partly restored the expression of surfactant proteins under the submerged condition (Figure 1). Furthermore, supplemental oxygen partly corrected the expression of surfactant proteins under the submerged condition (Figure 7). Previously, Acarregui and colleagues found that when human fetal lung tissue maintained for 5 days in 1% oxygen was transferred to an environment containing 20% oxygen, rapid morphological development and an induction of SP-A gene expression occurred (7). We also found that the expression of surfactant proteins decreased when cells were cultured with DMOG, a prolyl hydroxylase inhibitor and HIFα stabilizer, under A/L interface conditions (Figure 6). Under normoxic conditions, HIFα subunits are hydroxylated at conserved proline residues by prolyl hydroxylase domain–containing enzymes, whose activities are regulated by oxygen availability. When HIFα is hydroxylated in this manner, it is recognized and marked for proteosomal destruction by ubiquinization and the von Hippel–Lindau protein complex. Prolyl hydroxylase inhibitors (PHIs) are commonly used to mimic hypoxia by stabilizing HIFα. The addition of DMOG mimics the effects of submersion in A/L interface cultures. Grek and colleagues also demonstrated similar effects on the expression of pro–SP-B and pro–SP-C with several PHIs, for example, DMOG, ethyl-3,4-dehydroxybenzoate, and L-mimosine, in MLE-15 cells (8). We also considered the possibility that the effects of A/L interface cultures were attributable to an apical differentiation factor that was concentrated in the absence of added apical fluid. We discounted this hypothesis based on the rocking experiments and the results with supplemental oxygen in which differentiation was maintained but with no reduction of apical fluid. In addition, Dobbs and colleagues used frequent apical washes to test for the presence of a putative apical differentiation factor and were unable to find evidence for its existence (4). Taken together, these findings support the concept that oxygen tension and HIF signaling can regulate the expression of surfactant proteins.
Several observations require additional comment. Differences are evident in the isoforms of SP-A between freshly isolated and cultured rat type II cells, as shown in Figure 1 and as reported previously (27). We attribute this change in electromobility to the amount of glycosylation of SP-A. Rat SP-A contains asparagine-linked oligosaccharide units at the C-terminus that confer a triplet with a molecular mass of 26, 32, and 36 kD under reducing conditions (28). The oligosaccharide moiety of SP-A is involved in the Ca++-dependent aggregation of phospholipid vesicles, in connection with the formation of tubular myelin (29). Freshly isolated rat ATII cells on Day 0 contain mainly the 32-kD glycosylated form of SP-A and nonglycosylated SP-A, whereas cultured type II cells contain both 32-kD and 36-kD glycosylated SP-A. As one possible explanation, the 36-kD isoform is rapidly secreted in vivo, and some problem may occur with the SP-A secretory pathway in cultured type II cells, so that this form accumulates intracellularly. According to another possibility, secreted SP-A is stuck on the surface of the cells in vitro. However, when we performed immunocytochemistry for SP-A, we did not observe excessive SP-A on the surface of cells (Figure E1).
As shown in Figure 5, the increase in HIF1 and HIF2 was evident by 18 hours, whereas known target genes such as VEGF and GLUT1 required more than 3 days to demonstrate increased levels of mRNA. The reason for this delay is unknown. Under severe hypoxia, VEGF mRNA is up-regulated rapidly at 2–18 hours (18, 19). On the other hand, under mild hypoxia (e.g., 10% oxygen), mRNA levels of VEGF do not increase until 18–48 hours (30). Therefore, the timing of up-regulation of target genes is likely related to the severity of hypoxia. HIF DNA-binding activity is also dependent on oxygen concentrations, with low or undetectable activity in normoxia and a maximum increase for very low oxygen concentrations (e.g., 0.5% oxygen) (21, 31). Under the submerged condition, the cells are in mild hypoxia (6). Therefore, we speculate that the lag in response time between observing HIF in the nucleus and the mRNA expression of HIF target genes is primarily caused by HIF DNA-binding activity.
In addition, some observations were not consistent with our hypothesis. The loss of HIF1α early in murine lung development in utero leads to pups that die within hours of birth, with symptoms similar to those of neonatal respiratory distress syndrome (16). The lungs of these pups exhibited impaired alveolar epithelial differentiation and an almost complete loss of surfactant protein expression. Asikainen and colleagues found no difference in expression of surfactant proteins in lungs from preterm baboons treated with or without PHI (32). These observations in fetal and preterm lungs are at variance with our results. This variance may have occurred because those studies used preterm lungs, whereas our study used adult alveolar epithelial cells. Thus the role of HIF may vary in early development compared with the adult state because of the combinatorial nature of the transcription factors necessary for targeted gene expression. Furthermore, the deletion of individual genes may not always provide a complete picture of the physiologic process.
Surprisingly, the expression of surfactant proteins was reversible, and the effect was rapid (Figures 3 and 4). The decrease in expression of surfactant proteins in the switch from the A/L interface condition to the submerged condition occurred within 8 hours. When we switched cultures from the submerged condition to the A/L interface condition, cells were able to restore their expression of surfactant proteins within 48 hours. Hence, their oxygen sensing appears to be quite acute. The observation that the phenotype of type II cells can be dramatically changed by culture conditions is well-established. Previously we and others showed that the ATII cell phenotype and the type I–like cell phenotype is reversible and dependent on soluble factors and the culture substratum or matrix on which cells are plated (24, 33, 34). In this study, the differentiation of rat ATII cells in terms of the expression of surfactant proteins was dependent on the amount of apical fluid and was reversible.
This study contains several limitations. First, the precise mechanisms whereby oxygen improves and submersion inhibits surfactant protein gene expression are not known. However, there are several possible mechanisms. HIFs may bind to HRE on surfactant proteins and repress the expression of surfactant protein genes. HIFs can inhibit the expression of some genes, while enhancing the expression of others (35–37). Potential HRE binding sites in rat surfactant protein regulatory elements were analyzed, using the open-access JASPER database (38). The rat SP-A promoter was sequenced (39). Analysis of the promoter for SP-A revealed three potential HRE sites within that region (positions −936 to −929, −1046 to −1039, and −1475 to −1468). Promoters for rat SP-B and rat SP-C have not been sequenced. However, a 2-kb region upstream from the SP-B and SP-C transcripts also revealed similar HRE sites (SP-B, positions −657 to −650, −898 to −891, −915 to −908, and −1825 to −1818; SP-C, positions −1575 to −1568). In addition, the expression of surfactant proteins may be suppressed through the down-regulation of CCAAT/enhancer binding protein α (C/EBPα) by HIF. C/EBPα is expressed in ATII cells, and the expression of C/EBPα coincides temporally with surfactant production during the terminal differentiation of ATII cells (40). In addition, the expression of SP-A and SP-D increases during the differentiation of type II cells, and their gene promoters contain C/EBP binding sites that comprise important elements regulating their expression (41, 42). The loss of C/EBPα in the respiratory epithelium leads to an arrest in the type II alveolar cell differentiation program (43). Seifeddine and colleagues showed that hypoxia down-regulates C/EBPα in breast cancer cells via several mechanisms, including transcriptional repression mediated by HIF-1α binding to an HRE in the C/EBPα promoter and posttranscriptional effects, such as reduced stability of C/EBPα mRNA and altered cellular distribution of C/EBPα protein (36). Moreover, when HIF1α and C/EBPα interact, they may bring about reciprocal functional changes (44). Interestingly, the protein levels of C/EBPα were down-regulated under the submerged condition in our system (data not shown). As a third possibility, HIF can induce some repressors of gene expression, as shown for certain lipogenic enzymes in the liver (45).
Regarding additional limitations of this study, we did not measure the exact oxygen concentration at the surface of rat ATII cells cultured under the submerged condition because of technical limitations. However, that measurement was performed by Mamchaoui and Saumon (6). We did not show data from cells cultured under severely reduced oxygen tensions. When we cultured rat ATII cells under A/L interface conditions with 1%, 5%, or 10% oxygen, the results were variable, and significant cytotoxicity occurred, limiting our ability to interpret results. In addition to HIF1α, we found a significant increase in HIF2α, a finding consistent with a previous report (46). Most genes regulated by HIF1α are also regulated by HIF2α, and thus the individual contributions of the two HIFs are unclear. Importantly, in liver and hepatic cell lines, some important differences are evident between HIF1α and HIF2α. HIF2α regulates erythropoietin, plasminogen activator inhibitor–1, and some enzymes related to lipid metabolism in the liver (45, 47), whereas HIF1α is more responsible for glucose transport. Hence, additional studies will be required to define the precise role of HIF2α in the regulation of type II cell differentiation. This study was performed with rat type II cells and should be confirmed with human type II cells, because the volume density of type II cell mitochondria is directly related to lung oxygen consumption, and this varies according to species (48). The mitochondria density in type II cells is higher in small rodents. Finally, the pathways whereby oxygen improves and submersion inhibits surfactant protein gene expression will require considerably more work to define, but may lead to opportunities to learn more about the regulation of type II cell function in the adult lung.
In conclusion, we showed that culture of adult rat type II cells at an A/L interface maintained the expression of surfactant proteins. On the other hand, submersion reduced their expression, but this effect was reversible. Under the submerged condition, the HIF-inducible genes GLUT1, VEGF, and PDK1 were up-regulated, and both HIF1α and HIF2α protein levels were increased in nuclear extracts. The HIF stabilizer, DMOG, also down-regulated the expression of surfactant proteins, whereas it up-regulated GLUT1 mRNA levels. Finally, supplemental oxygen partly corrected the impairment of the expression of surfactant proteins in rat ATII cells cultured under the submerged condition. These results indicate that the expression of surfactant proteins and cell differentiation in rat ATII cells in vitro are regulated by oxygen tension through HIF signaling. These findings suggest that focal alveolar hypoxia in a variety of clinical settings, such as acute lung injury, pneumonia, pulmonary edema, or severe small airway obstruction, may impair the production of surfactant.
Supplementary Material
Acknowledgments
The authors thank Karen E. Edeen for assistance with rat ATII isolations, and Dennis Volker, John Shannon, Carl White, and Holger K. Eltzschig for useful consultations and suggestions to improve the experimental design. The authors also thank Teneke M. Warren and Lydia Orth for assistance with preparation of the manuscript.
Footnotes
This work was supported by National Institutes of Health grants HL 029891and AI 083005 (R.J.M.), and by the ExxonMobil Foundation.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0052OC on March 31, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Mason RJ. Biology of alveolar Type II cells. Respirology 2006;11:S12–S15 [DOI] [PubMed] [Google Scholar]
- 2.Whitcutt MJ, Adler KB, Wu R. A biphasic chamber system for maintaining polarity of differentiation of cultured respiratory tract epithelial cells. In Vitro Cell Dev Biol 1988;24:420–428 [DOI] [PubMed] [Google Scholar]
- 3.Yamaya M, Finkbeiner WE, Chun SY, Widdicombe JH. Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol 1992;262:L713–L724 [DOI] [PubMed] [Google Scholar]
- 4.Dobbs LG, Pian MS, Maglio M, Dumars S, Allen L. Maintenance of the differentiated Type II cell phenotype by culture with an apical air surface. Am J Physiol 1997;273:L347–L354 [DOI] [PubMed] [Google Scholar]
- 5.Alcorn JL, Smith ME, Smith JF, Margraf LR, Mendelson CR. Primary cell culture of human Type II pneumonocytes: maintenance of a differentiated phenotype and transfection with recombinant adenoviruses. Am J Respir Cell Mol Biol 1997;17:672–682 [DOI] [PubMed] [Google Scholar]
- 6.Mamchaoui K, Saumon G. A method for measuring the oxygen consumption of intact cell monolayers. Am J Physiol Lung Cell Mol Physiol 2000;278:L858–L863 [DOI] [PubMed] [Google Scholar]
- 7.Acarregui MJ, Snyder JM, Mendelson CR. Oxygen modulates the differentiation of human fetal lung in vitro and its responsiveness to cAMP. Am J Physiol 1993;264:L465–L474 [DOI] [PubMed] [Google Scholar]
- 8.Grek CL, Newton DA, Spyropoulos DD, Baatz JE. Hypoxia upregulates expression of hemoglobin in alveolar epithelial cells. Am J Respir Cell Mol Biol 2010;44:439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clerici C, Planes C. Gene regulation in the adaptive process to hypoxia in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 2009;296:L267–L274 [DOI] [PubMed] [Google Scholar]
- 10.Li QF, Wang XR, Yang YW, Lin H. Hypoxia upregulates hypoxia inducible factor (HIF) 3alpha expression in lung epithelial cells: characterization and comparison with HIF-1alpha. Cell Res 2006;16:548–558 [DOI] [PubMed] [Google Scholar]
- 11.Uchida T, Rossignol F, Matthay MA, Mounier R, Couette S, Clottes E, Clerici C. Prolonged hypoxia differentially regulates hypoxia-inducible factor (HIF) 1alpha and HIF-2alpha expression in lung epithelial cells: implication of natural antisense HIF-1alpha. J Biol Chem 2004;279:14871–14878 [DOI] [PubMed] [Google Scholar]
- 12.Yu AY, Frid MG, Shimoda LA, Wiener CM, Stenmark K, Semenza GL. Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor 1 in the lung. Am J Physiol 1998;275:L818–L826 [DOI] [PubMed] [Google Scholar]
- 13.Jain M, Sznajder JI. Effects of hypoxia on the alveolar epithelium. Proc Am Thorac Soc 2005;2:202–205 [DOI] [PubMed] [Google Scholar]
- 14.Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer 2008;8:705–713 [DOI] [PubMed] [Google Scholar]
- 15.Jungermann K, Kietzmann T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology 2000;31:255–260 [DOI] [PubMed] [Google Scholar]
- 16.Saini Y, Harkema JR, LaPres JJ. HIF1alpha is essential for normal intrauterine differentiation of alveolar epithelium and surfactant production in the newborn lung of mice. J Biol Chem 2008;283:33650–33657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell 2010;40:294–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuder RM, Flook BE, Voelkel NF. Increased gene expression for VEGF and the VEGF receptors Kdr/Flk and Flt in lungs exposed to acute or to chronic hypoxia: modulation of gene expression by nitric oxide. J Clin Invest 1995;95:1798–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pham I, Uchida T, Planes C, Ware LB, Kaner R, Matthay MA, Clerici C. Hypoxia upregulates VEGF expression in alveolar epithelial cells in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol 2002;283:L1133–L1142 [DOI] [PubMed] [Google Scholar]
- 20.Clerici C, Matthay MA. Hypoxia regulates gene expression of alveolar epithelial transport proteins. J Appl Physiol 2000;88:1890–1896 [DOI] [PubMed] [Google Scholar]
- 21.Ouiddir A, Planes C, Fernandes I, VanHesse A, Clerici C. Hypoxia upregulates activity and expression of the glucose transporter GLUT1 in alveolar epithelial cells. Am J Respir Cell Mol Biol 1999;21:710–718 [DOI] [PubMed] [Google Scholar]
- 22.Dobbs LG, Mason RJ. Pulmonary alveolar Type II cells isolated from rats: release of phosphatidylcholine in response to beta-adrenergic stimulation. J Clin Invest 1979;63:378–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mason RJ, Pan T, Edeen KE, Nielsen LD, Zhang F, Longphre M, Eckart MR, Neben S. Keratinocyte growth factor and the transcription factors C/EBP alpha, C/EBP delta, and SREBP-1c regulate fatty acid synthesis in alveolar Type II cells. J Clin Invest 2003;112:244–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Edeen K, Manzer R, Chang Y, Wang S, Chen X, Funk CJ, Cosgrove GP, Fang X, Mason RJ. Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro. Am J Respir Cell Mol Biol 2007;36:661–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mason RJ, Lewis MC, Edeen KE, McCormick-Shannon K, Nielsen LD, Shannon JM. Maintenance of surfactant protein A and D secretion by rat alveolar Type II cells in vitro. Am J Physiol Lung Cell Mol Physiol 2002;282:L249–L258 [DOI] [PubMed] [Google Scholar]
- 26.Xu X, McCormick-Shannon K, Voelker DR, Mason RJ. KGF increases SP-A and SP-D mRNA levels and secretion in cultured rat alveolar Type II cells. Am J Respir Cell Mol Biol 1998;18:168–178 [DOI] [PubMed] [Google Scholar]
- 27.Ito Y, Mason RJ. The effect of interleukin-13 (IL-13) and interferon-gamma (IFN-gamma) on expression of surfactant proteins in adult human alveolar Type II cells in vitro. Respir Res 2010;11:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawgood S, Benson BJ, Hamilton RL., Jr Effects of a surfactant-associated protein and calcium ions on the structure and surface activity of lung surfactant lipids. Biochemistry 1985;24:184–190 [DOI] [PubMed] [Google Scholar]
- 29.Haagsman HP, Elfring RH, van Buel BL, Voorhout WF. The lung lectin surfactant protein A aggregates phospholipid vesicles via a novel mechanism. Biochem J 1991;275:273–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christou H, Yoshida A, Arthur V, Morita T, Kourembanas S. Increased vascular endothelial growth factor production in the lungs of rats with hypoxia-induced pulmonary hypertension. Am J Respir Cell Mol Biol 1998;18:768–776 [DOI] [PubMed] [Google Scholar]
- 31.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol 1996;271:C1172–C1180 [DOI] [PubMed] [Google Scholar]
- 32.Asikainen TM, Chang LY, Coalson JJ, Schneider BK, Waleh NS, Ikegami M, Shannon JM, Winter VT, Grubb P, Clyman RI, et al. Improved lung growth and function through hypoxia-inducible factor in primate chronic lung disease of prematurity. FASEB J 2006;20:1698–1700 [DOI] [PubMed] [Google Scholar]
- 33.Olsen CO, Isakson BE, Seedorf GJ, Lubman RL, Boitano S. Extracellular matrix driven alveolar epithelial cell differentiation in vitro. Exp Lung Res 2005;31:461–482 [DOI] [PubMed] [Google Scholar]
- 34.Danto SI, Shannon JM, Borok Z, Zabski SM, Crandall ED. Reversible transdifferentiation of alveolar epithelial cells. Am J Respir Cell Mol Biol 1995;12:497–502 [DOI] [PubMed] [Google Scholar]
- 35.Ahmad A, Ahmad S, Glover L, Miller SM, Shannon JM, Guo X, Franklin WA, Bridges JP, Schaack JB, Colgan SP, et al. Adenosine A2A receptor is a unique angiogenic target of HIF-2alpha in pulmonary endothelial cells. Proc Natl Acad Sci USA 2009;106:10684–10689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seifeddine R, Dreiem A, Blanc E, Fulchignoni-Lataud MC, Le Frere Belda MA, Lecuru F, Mayi TH, Mazure N, Favaudon V, Massaad C, et al. Hypoxia down-regulates CCAAT/enhancer binding protein–alpha expression in breast cancer cells. Cancer Res 2008;68:2158–2165 [DOI] [PubMed] [Google Scholar]
- 37.Choi SM, Cho HJ, Cho H, Kim KH, Kim JB, Park H. STRA13/DEC1 and DEC2 inhibit sterol regulatory element binding protein–1c in a hypoxia-inducible factor–dependent mechanism. Nucleic Acids Res 2008;36:6372–6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bryne JC, Valen E, Tang MH, Marstrand T, Winther O, da Piedade I, Krogh A, Lenhard B, Sandelin A. JASPAR, the open access database of transcription factor binding profiles: new content and tools in the 2008 update. Nucleic Acids Res 2008;36:D102–D106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith CI, Rosenberg E, Reisher SR, Li F, Kefalides P, Fisher AB, Feinstein SI. Sequence of rat surfactant protein a gene and functional mapping of its upstream region. Am J Physiol 1995;269:L603–L612 [DOI] [PubMed] [Google Scholar]
- 40.Li F, Rosenberg E, Smith CI, Notarfrancesco K, Reisher SR, Shuman H, Feinstein SI. Correlation of expression of transcription factor C/EBP alpha and surfactant protein genes in lung cells. Am J Physiol 1995;269:L241–L247 [DOI] [PubMed] [Google Scholar]
- 41.He Y, Crouch E. Surfactant protein D gene regulation: interactions among the conserved CCAAT/enhancer-binding protein elements. J Biol Chem 2002;277:19530–19537 [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg E, Li F, Reisher SR, Wang M, Gonzales LW, Ewing JR, Malek S, Ballard PL, Notarfrancesco K, Shuman H, et al. Members of the C/EBP transcription factor family stimulate expression of the human and rat surfactant protein A (SP-A) genes. Biochim Biophys Acta 2002;1575:82–90 [DOI] [PubMed] [Google Scholar]
- 43.Basseres DS, Levantini E, Ji H, Monti S, Elf S, Dayaram T, Fenyus M, Kocher O, Golub T, Wong KK, et al. Respiratory failure due to differentiation arrest and expansion of alveolar cells following lung-specific loss of the transcription factor C/EBPalpha in mice. Mol Cell Biol 2006;26:1109–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janardhan HP. The HIF-1 alpha-C/EBP alpha axis. Sci Signal 2008;1:jc2. [DOI] [PubMed] [Google Scholar]
- 45.Rankin EB, Rha J, Selak MA, Unger TL, Keith B, Liu Q, Haase VH. Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol Cell Biol 2009;29:4527–4538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiesener MS, Jurgensen JS, Rosenberger C, Scholze CK, Horstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei UA, Pugh CW, et al. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J 2003;17:271–273 [DOI] [PubMed] [Google Scholar]
- 47.Hu CJ, Sataur A, Wang L, Chen H, Simon MC. The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1alpha and HIF-2alpha. Mol Biol Cell 2007;18:4528–4542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Massaro GD, Gail DB, Massaro D. Lung oxygen consumption and mitochondria of alveolar epithelial and endothelial cells. J Appl Physiol 1975;38:588–592 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.