Abstract
It is established that cigarette smoke (CS) causes irreversible oxidations in lung epithelial cells, and can lead to their death. However, its impact on reversible and physiologically relevant redox-dependent protein modifications remains to be investigated. Glutathione is an important antioxidant against inhaled reactive oxygen species as a direct scavenger, but it can also covalently bind protein thiols upon mild oxidative stress to protect them against irreversible oxidation. This posttranslational modification, known as S-glutathionylation, can be reversed under physiological conditions by the enzyme, glutaredoxin 1 (Grx1). The aim of this study was to investigate if CS modifies Grx1, and if this impacts on protein S-glutathionylation and epithelial cell death. Upon exposure of alveolar epithelial cells to CS extract (CSE), a decrease in Grx1 mRNA and protein expression was observed, in conjunction with decreased activity and increased protein S-glutathionylation. Using mass spectrometry, irreversible oxidation of recombinant Grx1 by CSE and acrolein was demonstrated, which was associated with attenuated enzyme activity. Furthermore, carbonylation of Grx1 in epithelial cells after exposure to CSE was shown. Overexpression of Grx1 attenuated CSE-induced increases in protein S-glutathionylation and increased survival. Conversely, primary tracheal epithelial cells of mice lacking Grx1 were more sensitive to CS-induced cell death, with corresponding increases in protein S-glutathionylation. These results show that CS can modulate Grx1, not only at the expression level, but can also directly modify Grx1 itself, decreasing its activity. These findings demonstrate a role for the Grx1/S-glutathionylation redox system in CS-induced lung epithelial cell death.
Keywords: chronic obstructive pulmonary disease, cigarette smoke, cell death, glutaredoxin, protein S-glutathionylation
Clinical Relevance
Cigarette smoke extract (CSE) was found to decrease glutaredoxin 1 (Grx1) expression and increase protein S-glutathionylation. These alterations were shown to be involved in CSE-induced epithelial cell death. Grx1 could therefore help to prevent cell death and potentially the development of emphysema.
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality in the United States (1) that is mainly caused by cigarette smoking. Cigarette smoke (CS) contains 1016 free radicals per cigarette (2), including reactive oxygen species (ROS) and reactive nitrogen species. Inhalation of these oxidants in combination with the production of ROS/reactive nitrogen species by macrophages and neutrophils leads to oxidative stress. Oxidants in CS can cause direct cellular damage by lipid peroxidation (3), DNA damage (4), and irreversible protein oxidations (5, 6).
The pulmonary epithelium is equipped with lining fluid that contains high concentrations of glutathione (GSH) (7), which is an important antioxidant against inhaled ROS. CS is known to acutely deplete GSH, thereby decreasing the lung's antioxidant capacity and making it vulnerable to oxidant-induced injury. As an adaptive response to oxidative stress, such as in smokers, GSH levels increase in the epithelial lining fluid due to up-regulation of the rate-limiting enzyme in GSH synthesis, γ-glutamylcysteine ligase (8). GSH, in concert with its redox cycle partners, serves to maintain the reduced state of protein thiol groups. This can be achieved by direct scavenging of oxidants, or by covalently and reversibly binding protein thiols. The latter formation of mixed disulfides between protein thiols and GSH occurs under physiological conditions, can be induced upon mild oxidative stress, and is known as S-glutathionylation or S-glutathiolation. S-glutathionylation is believed to protect its targeted protein thiols from further irreversible oxidations. In addition, S-glutathionylation can modulate protein function. For instance, our laboratory previously described the inhibition of inhibitory κB kinase β activity, the enzyme responsible for NF-κB activation under proinflammatory conditions, through S-glutathionylation of cysteine 179 after oxidative challenge of lung epithelial cells (9).
Under physiological conditions, S-glutathionylation can be reduced by glutaredoxins (Grxs) (10). Several mammalian Grxs have been identified. Grx1 localizes primarily to the cytosol, whereas Grx2 is present in mitochondria and the nucleus. Recently, Grx3 has gained interest for its altered expression in lung cancer, although this isoform does not exhibit deglutathionylation activity (11).
In the lungs, Grx1 expression is predominant in macrophages and bronchial epithelium, and has been shown to be altered in allergic airway disease (12), COPD (13), and after acute exposure to LPS (14). So far, little is known about the regulation of Grx expression. In the context of COPD, for instance, it is unknown if CS itself influences Grx expression or can modify its activity. Moreover, the effects of CS on protein S-glutathionylation remain to be determined. The aim of this study was, therefore, to investigate the effects of CS on the Grx/protein S-glutathionylation axis in lung epithelial cells, and, furthermore, to investigate their role in CS-induced cell death.
Materials and Methods
Cell Culture
A human transformed alveolar epithelial cell line, A549, was obtained from the American Type Culture Collection (Manassas, VA) and cultured in RPMI 1,640 (Gibco, Grand Island, NY) containing 10% FBS (Biochrome, Berlin, Germany), L-glutamine (2 mM), and penicillin/streptomycin (Invitrogen, Grand Island, NY). At 24 hours before stimulation, cells were cultured in Dulbecco's modified Eagle medium/F12 without phenol red and 0.5% FBS.
Glrx1−/− mice (a kind gift of Dr. Y.-S. Ho, Wayne State University, Detroit, MI), and their control littermates were used to isolate primary tracheal epithelial (MTE) cells, as described previously (15), with minor modifications (16). Cells were cultured in full medium lacking phenol red for 24 hours before stimulation. The University of Vermont Institutional Animal Care and Use Committee granted approval for all procedures.
CS Extract
3R4F Research Cigarettes (University of Kentucky, Lexington, KY) were removed from their filters and CS extract (CSE) was made as previously described (17).
Grx1 Luciferase Reporter Assay
Transient transfections were performed using Fugene (Roche, Indianapolis, IN) according to the manufacturer's instructions using 1.75 μg human Grx1 promotor luciferase plasmid (kindly provided by Dr. J. Park, U.S Department of Agriculture). Cotransfection with 0.25 μg pSV-β-galactosidase was employed to correct for differences in transfection efficiency. Luciferase (Promega, Madison, WI) and β-galactosidase (Tropix, Bedford, MA) activity were measured according to the manufacturer's instructions.
Grx1 Activity Assay
Grx1 activity assay was performed as previously described (18). Data are expressed as micromoles NADPH per minute per milligram protein (19).
Grx1 Catalyzed Cysteine Derivatization for In Situ Detection of S-Glutathionylated Proteins
S-glutathionylated proteins were detected in cells as described previously (16).
Quantitative Determination of Protein S-Glutathionylation Using 5,5′-Dithio-Bis(2-Nitrobenzoic Acid)
Cells were lysed in 137 mM Tris-HCl (pH 8.0), 130 mM NaCl, and 1% NP-40 and cleared by centrifugation. Protein (200 μg) was acetone precipitated for 20 minutes at −20°C and then resuspended and sonicated in extraction buffer. Furthermore, the determination of protein S-glutathionylation was conducted as described previously (20).
Mass Spectrometry
Recombinant Grx1 was incubated for 24 hours at room temperature in the dark in 0.1% trifluoroacetic acid with 2.5 or 5% CSE, or equimolar concentrations of acrolein. For mass spectrometric analysis, 1 μl of recombinant Grx1 (10 pmol/μl) and 1 μl matrix solution (10 mg/ml sinapinic acid in 40% acetonitrile/0.1% trifluoroacetic acid) were spotted on a 384-well target plate of a matrix-assisted laser desorption/ionization (MALDI)–tandem time-of-flight (4,800 MALDI tandem time-of-flight analyzer; Applied Biosystems, Foster City, CA). The instrument was operated in positive linear mode, mid–mass range. Acquisition mass range was 10,000–15,000 Da.
Detection of Grx1 Carbonylation In Vitro
Grx1 was immunoprecipitated from A549 cells and carbonyls were derivatized using an oxyblot kit (Millipore, Billerica, MA). Carbonylation of Grx1 was visualized on an SDS-PAGE gel using the DNP antibody.
Assessment of Cell Viability
Cells were harvested by trypsinization, pelleted, and washed twice with PBS. Next, propidium iodide (1 μg/ml) was added, and cell viability was assessed by flow cytometry.
Results
CSE Down-Regulates Grx1 Expression
To investigate the effect of CS on Grx1 expression, we first exposed A549 cells transiently expressing a human Grx1 promotor luciferase construct to CSE and measured Grx1 promoter activity using a luciferase assay. Results in Figure 1A demonstrate that β-galactosidase–corrected Grx1 luciferase activity was dose-dependently inhibited by treatment with CSE for 48 hours. Significant attenuation of Grx1 promoter activity was also observed after 24 hours of exposure to 2.5% CSE, but not after 4 hours (Figure 1B). Grx1 mRNA (Figure 1C) was negatively affected by 5% CSE after both 24 and 48 hours of exposure. In contrast, no significant alterations in the expression of Grx2 mRNA were observed (data not shown). Protein levels of Grx1 were decreased upon CSE exposure in A549 cells (Figure 1D, left), as well as in MTE cells (Figure 1D, right).
Figure 1.
Cigarette smoke extract (CSE) down-regulates glutaredoxin 1 (Grx1) expression. A549 cells were transiently transfected with a human Grx1 promotor luciferase construct and β-galactosidase and exposed to CSE. (A) Cells were treated for 48 hours with indicated doses of CSE and luciferase activity was measured and corrected for β-galactosidase. (B) Cells were control treated (closed bars) or exposed to 2.5% CSE (shaded bars). After 4, 24, and 48 hours luciferase activity was measured and corrected for β-galactosidase. (C) A549 cells were control treated (closed bars) or exposed to 5% CSE (shaded bars) and expression of Grx1 mRNA was measured by QPCR and corrected for HPRT. Data are expressed as fold-change over control treated cells at 24 hours. (D) Protein expression of Grx1 was determined by Western blotting in A549 cells (left panel) and in primary MTE cells exposed to CSE (right panel). The level of β-actin was measured as a loading control. *P < 0.05 compared with untreated controls, analyzed by ANOVA.
Grx Activity Is Attenuated and Protein S-Glutathionylation Increased by CSE Exposure
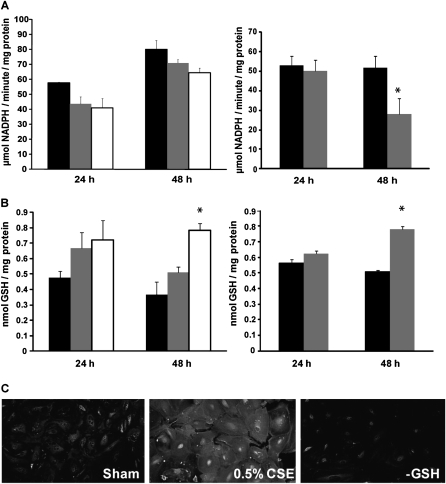
Because of the observed attenuation of Grx1 expression by CSE, we next assessed Grx activity and protein S-glutathionylation in A549 and MTE cells. As expected, Grx activity was attenuated by 2.5 and 5% CSE in A549 cells after 24 and 48 hours, although not significantly (Figure 2A, left), in agreement with results on mRNA and protein expression. In MTE cells, Grx1 activity also decreased after 48 hours, but not 24 hours of exposure to 0.5% CSE (Figure 2A, right). We next assessed the impact of decreased Grx levels and activity on the overall content of cellular protein S-glutathionylation. Results in the left panel of Figure 2B demonstrate that protein S-glutathionylation was augmented after CSE exposure in a dose- and time-dependent fashion in A549 cells. MTE cells showed a significant induction of S-glutathionylated proteins only after 48 hours of CSE exposure (Figure 2B, right), coinciding with the decreased activity at this time point. CSE-induced increases in protein S-glutathionylation were corroborated by in situ detection of S-glutathionylated proteins using Grx1-catalyzed cysteine derivatization in MTE cells stimulated with 0.5% CSE for 48 hours (Figure 2C). Total free GSH in A549 cells was measured using the DTNB recycling method, showed 50% depletion of GSH upon stimulation with 5% CSE for 24 and 48 hours (data not shown). This attenuation of free GSH levels occurs in conjunction with enhanced protein S-glutathionylation.
Figure 2.
Grx activity is attenuated and protein S-glutathionylation increased by CS. A549 cells were control treated (closed bars), exposed to 2.5% (shaded bars) or 5% (open bars) CSE. After 24 and 48 hours Grx activity (A, left panel) and protein S-glutathionylation (B, left panel) were measured. MTE cells were control treated (closed bars) or exposed to 0.5% CSE (shaded bars) for 24 and 48 hours and Grx activity (A, right panel) and protein S-glutathionylation (B, right panel) were measured. (C) Protein S-glutathionylation was visualized using Grx1-catalyzed cysteine derivatization in primary MTE cells treated for 48 hours with 0.5% CSE. −GSH, omission of glutathione (GSH) in the Grx1 reduction mix as a negative control. *P < 0.05 compared with untreated controls, analyzed by ANOVA.
Modulation of Grx1 Protein by CS
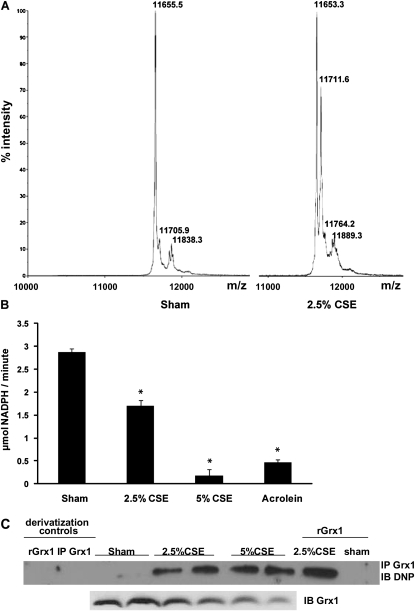
A recent study demonstrated that Grx1 can be oxidized and inhibited by a variety of oxidants (21). To investigate whether CS could directly modify the Grx1 protein, we incubated recombinant human Grx1 with 2.5 and 5% CSE for 24 hours and assessed mass modifications by MALDI-TOF mass spectrometry, as well as Grx activity. Incubation of Grx1 with 2.5% CSE resulted in a mass addition of approximately 58 Da compared with untreated protein (Figure 3A). This mass addition occurred in a concentration-dependent manner, as incubation with 5% CSE further increased the presence of the modified protein to 63.5%, and was nonreversible by DTT (Table 1). Moreover, mass addition did not occur after preincubation of Grx1 with the cysteine alkylating agent, N-ethylmaleimide (data not shown), implying that the mass addition occurs on a cysteine residue. Acrolein is a 58-Da, highly oxidative component of CS, which is reactive toward cysteine residues. We next incubated recombinant Grx1 with an equimolar amount of acrolein to assess whether the mass addition observed after incubation with CSE could be due to acrolein present in CSE. Indeed, here we also detected a DTT-irreversible mass addition of 58 Da (Table 1). Addition of 116 Da, indicative of oxidation of two cysteine residues by acrolein, could also be found in Grx1 using higher concentration of CSE or acrolein (Table 1). Incubation of recombinant Grx1 with CSE dose dependently attenuated its activity in a DTT-irreversible fashion, and similar effects were observed after exposure to acrolein (Figure 3B).
Figure 3.
Modulation of Grx1 protein by CS. (A) Recombinant human Grx1 control (left spectrum), or exposed to 2.5% CSE (right spectrum) for 24 hours at room temperature and analyzed by matrix-assisted laser desorption/ionization (MALDI)–time-of-flight (TOF) mass spectrometry. Numbers in spectra indicate the mass of the product. (B) Assessment of recombinant Grx1 activity after 24 hours of treatments. (C) Carbonylation of immunoprecipitated Grx1 from lysates of A549 cells. Negative derivatization control for recombinant Grx1 (rGrx1) and Grx1 immunoprecipitated from lysates (IP Grx1) exposed to CSE. Derivatization of recombinant Grx1 exposed to CSE and control in the last two lanes. *P < 0.05 compared with untreated control, analyzed by ANOVA.
TABLE 1.
PERCENTAGE OF RECOMBINANT GLUTAREDOXIN 1 THAT IS MODIFIED BY EITHER CIGARETTE SMOKE EXTRACT OR PURIFIED ACROLEIN IN AN EQUIMOLAR CONCENTRATION OF THE RECOMBINANT PROTEIN*
| % Unmodified | % Addition of 58 Da | % Addition of 116 Da | |
| Grx1 untreated | 100 | — | — |
| Grx1 + 2.5% CSE | 61.1 | 33.4 | 5.5 |
| Grx1 + 5% CSE | 36.5 | 53.3 | 10.2 |
| Grx1 + 5% CSE + DTT | 30.2 | 58.1 | 11.7 |
| Grx1 +acrolein equimolar | — | 83.3 | 16.7 |
| Grx1 +acrolein equimolar + DTT | — | 84.7 | 15.3 |
Definition of abbreviations: CSE, cigarette smoke extract; Grx1, glutaredoxin 1.
Both conditions show no changes when preincubated with DTT.
To investigate whether oxidation of Grx1 also occurs in cells, Grx1 was immunoprecipitated form A549 cells stimulated with CSE for 48 hours. Carbonylation of Grx1 was then investigated using an oxyblot kit. Results in Figure 3C indicate that 2.5 and 5% CSE exposure resulted in carbonylation of Grx1, demonstrating that Grx1 is not only oxidized by CSE in a cell-free environment, but that oxidation of Grx1 also takes place in epithelial cells. As a positive control, recombinant Grx1 exposed to CSE was derivatized, showing carbonylation as well.
Effect of Grx1 Modulation on CS-Induced Protein S-Glutathionylation and Epithelial Cell Death
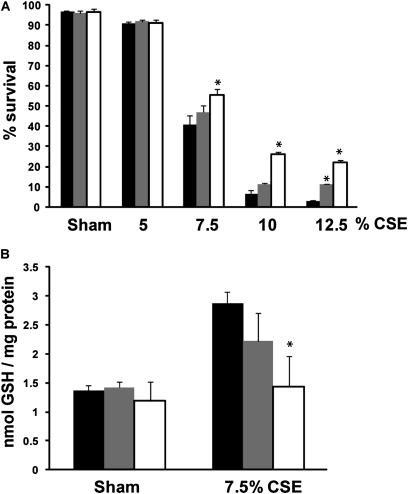
We next sought to investigate whether the observed attenuation of Grx1 by CSE is linked to CSE-induced cell death. We therefore first exposed A549 cells that were transiently transfected with Flag-tagged Grx1 to CSE. In plasmid CMV promotor DNA-transfected cells, CSE dose dependently induced cell death. After 24 hours, cells overexpressing Grx1 demonstrated significant protection against CSE-induced cell death at high doses of CSE. Moreover, transfection with 1 μg Flag-Grx1 offered better protection compared with transfection with 0.5 μg (Figure 4A). In agreement with Figure 2B, CSE increased total protein S-glutathionylation in plasmid CMV promotor DNA-transfected cells. Flag-Grx1 overexpression, however, provided protection against smoke-induced S-glutathionylation in a dose-dependent manner (Figure 4B).
Figure 4.
Overexpression of Grx1 confers partial protection against CS-induced epithelial cell death and smoke-induced increases in protein S-glutathionylation. A549 cells were transiently transfected with plasmid CMV promotor DNA (closed bars), 0.5 μg Flag-Grx1 (shaded bars) or 1 μg Flag-Grx1 (open bars) and treated with CSE for 24 hours. (A) Cell death was assessed by flow cytometry using propidium iodide (PI) uptake. The percentage of analyzed cells that did not take up PI is expressed as percent survival. (B) Protein S-glutathionylation. *P < 0.05 compared with plasmid CMV promotor DNA control exposed to CSE, analyzed by ANOVA.
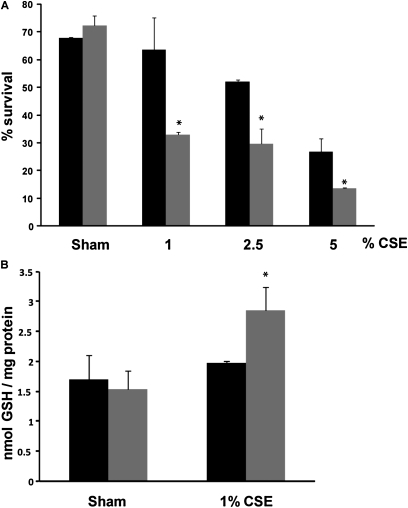
To investigate further the importance of Grx1 in CS-induced cell death, MTE cells isolated from Glrx1−/− mice were used. MTE cells, in general, were more sensitive to CSE-induced cell death compared with A549 cells, as marked death was observed with concentrations as low as 1%. MTE cells lacking Glrx1 demonstrated significantly more cell death compared with cells isolated from control littermates at all doses of CSE tested (Figure 5A). Furthermore, total protein S-glutathionylation was only increased in cells isolated from Glrx1−/− mice using 1% CSE (Figure 5B).
Figure 5.
Increased smoke-induced cell death and protein S-glutathionylation in MTE cells from Glrx1−/− mice. MTE cells isolated from wild-type (closed bars) or Glrx1−/− mice (shaded bars) were treated with CSE for 24 hours. (A) Cell death was assessed by flow cytometry using PI uptake. (B) Protein S-glutathionylation. *P < 0.05 compared with cells from litter mate controls exposed to CSE, analyzed by ANOVA.
Taken together, these data indicate that the decreased content and activity of Grx1 after CSE exposure is indeed responsible for observed increases in total protein S-glutathionylation, and contributes to CSE-induced death of lung epithelial cells.
Discussion
Previous research regarding CS-induced oxidative stress has focused on irreversible oxidations linked to damage, while ignoring the effects of CS on physiologically relevant oxidations that can reversibly modify function. The objective of the present study, therefore, was to investigate whether CS can cause changes in protein S-glutathionylation, an oxidation that can be reversed by Grxs, and if changes in this S-glutathionylation–Grx1 redox system play a role in epithelial cell death provoked by CS.
This is the first report to demonstrate attenuation of Grx1 expression and Grx activity by CSE, in concert with increased protein S-glutathionylation in lung epithelial cells. In patients with COPD, it has been shown that the number of Grx1-positive macrophages was decreased in the lungs, along with decreases in Grx1 protein levels in whole-lung homogenates. In contrast, in sputum supernatants, more Grx1 was detected during acute exacerbations (13). Protein S-glutathionylation was not investigated in the latter study, but elevated levels were reported in blood samples of smokers compared with nonsmokers (22). The present study in cell culture models confirms results of the previous reports regarding the modulation of Grx1 expression and protein S-glutathionylation in patients with COPD and healthy smokers. However, CS probably did not directly affect mRNA expression of Grx1, as attenuated levels of Grx1 mRNA could only be observed after at least 24 hours of exposure. It would appear more likely that CS acts on signaling pathways that modulate transcription factors that, in turn, regulate Grx1 mRNA expression. Interestingly, the decreased expression of Grx1 observed after transforming growth factor (TGF)–β treatment also occurred only after 24 hours (mRNA [12]) or 72 hours (protein [23]). The signaling intermediates and transcription factors involved in the modulation of Grx1 mRNA remain to be investigated. There is only a single study regarding potentially important transcription factor binding sites and regulatory regions in the human Grx1 promoter (24), both of which need to be evaluated in detail in future research. CSE and TGF-β both down-regulate Grx1 mRNA expression, which, for TGF-β, appears to fit into a general repressive effect on antioxidant genes, whereas this is not the case for smoke. No effects of CS on Grx2 mRNA were observed, which is in line with previous studies in which only levels of Grx1, but not of Grx2, were affected (12). It is, however, possible that the activity of Grx2 is altered by smoke exposure, as this isoform is activated when the active site is opened up upon monomerization, which can be accomplished by oxidation (25). Furthermore, the activity assay used here does not distinguish between the different isoforms. Together, these observations could explain why the strong effects observed on Grx1 expression and on recombinant Grx1 activity after CSE exposure do not translate into equally strong effects on total cellular Grx activity.
In addition to the attenuated expression of Grx1 in response to CSE exposure, we observed elevated levels of Grx1 mRNA, protein, and activity in control cells over time in culture. Accordingly, protein S-glutathionylation levels were also decreased over time in culture. Some previous reports have linked Grx1 to cell proliferation. For instance, the enzyme was first discovered as an alternative electron donor for ribonucleotide reductase in Escherichia coli, an enzyme essential to DNA synthesis in proliferating cells (26). In addition, Grx1 has been shown to control actin S-glutathionylation and its polymerization status after growth factor stimulation, which was postulated to play a role in the formation of signal transduction scaffolds and the cellular response to growth factors (27). The increased levels of Grx1 in culture over time could potentially be linked to proliferation, as the experiments were performed at subconfluency, and minor proliferation could still be observed using 0.5% FBS.
In the present study, we show that CS not only attenuated Grx1 expression, but that the Grx1 protein itself was modified by CSE, thereby decreasing its activity (Figures 3A and 3C and Table 1). It was determined that CSE exposure resulted in Grx1 adduct formation through both alkylation by acrolein and carbonylation. Acrolein is the most highly oxidative compound in CS, and is known to irreversibly bind proteins, probing them for rapid proteolytic degradation (6). It is therefore plausible that alkylation of Grx1 leads to proteolytic degradation, a scenario that needs to be formally tested. Nonetheless, results from the present study demonstrate that CS targets Grx1 via multiple mechanisms, which has implications for cell survival.
Cysteines with a low acid dissociation constant are prone to S-glutathionylation upon mild oxidative stress, and when S-glutathionylation occurs at a critical cysteine, this can modify the activity and conformation of the targeted protein. In the present study, we demonstrate that CS exposure enhanced total levels of protein S-glutathionylation (Figures 2B and 2C). Further studies are needed to investigate which particular proteins are targeted by S-glutathionylation. The function of proteins potentially involved in disease pathogenesis, such as inhibitory κB kinase β and NF-κB, activator protein 1, and matrix metalloproteases, have been shown to be affected by S-glutathionylation and, in some instances, by alterations in Grx1 levels (9, 28). Variations in Grx1 and S-glutathionylation of these proteins could, therefore, contribute to the pathophysiology of COPD.
Some of the target proteins of S-glutathionylation are known to modulate cell death (18), a process that has raised interest as a mechanism in the development of COPD (29). Here, we demonstrate that modulation of Grx1 expression, in conjunction with alterations in protein S-glutathionylation, in lung epithelial cells affects their survival in response to CS. So far, Grx1 has been reported to have a cardioprotective role and reduce ROS production after ischemia and reperfusion in Glrx1 transgenic mouse hearts. Conversely, Glrx1−/− mice and Grx1 inhibition by cadmium increased infarct size and ROS production (30). In addition, lens epithelial cells of Glrx1−/− mice exhibited increased sensitivity to oxidative stress, as they had a reduced ability to clear H2O2, and administration of recombinant Grx1 restored antioxidant capacity (31). In the present study, we show that primary MTE cells isolated from Glrx1−/− mice were more sensitive to CS-induced cell death compared with wild-type control animals, in association with enhanced protein S-glutathionylation. Conversely, overexpression of Grx1 in an epithelial cell line was found to protect against CS-induced cell death, while attenuating the induction of S-glutathionylation in response to CSE. Collectively, these data indicate that the decreased expression of Grx1 and attenuation of Grx activity after CSE exposure are indeed responsible for observed increases in total protein S-glutathionylation, and contribute to CSE-induced death of lung epithelial cells. However, additional studies need to be conducted to unravel the target proteins for increased S-glutathionylation that contribute to cell death after CS exposure. Mediators of apoptosis and cell death shown to be modulated by the S-glutathionylation/Grx1 axis include procaspase-3 (32), multiple members of the NF-κB survival pathway (33), ASK1 (34), and Fas (18).
Taken together, the data show increasing evidence for Grx1 as a potential therapeutically relevant candidate for enhancing cell survival upon CS exposure. A previous study showed a similar protective effect using recombinant thioredoxin-1, another member of the thioredoxin family, in the CS exposure model for COPD in mice (35). Restoring Grx1 content in the lungs after exposure to CS may, therefore, have implications in enhancing cell survival, and thus potentially help to prevent the development of emphysema.
Footnotes
This work was supported by Nutrim, an unrestricted grant from GlaxoSmithKline Europe–European COPD Centre of Excellence, NWO/ZonMW VENI grant 016.086.090 (N.L.R.), and National Institutes of Health grant R01HL60014.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0249OC on April 7, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Mannino DM. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest 2002;121 (5 Suppl):121S–126S [DOI] [PubMed] [Google Scholar]
- 2.Pryor WA, Prier DG, Church DF. Electron-spin resonance study of mainstream and sidestream cigarette smoke: nature of the free radicals in gas-phase smoke and in cigarette tar. Environ Health Perspect 1983;47:345–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frei B, Forte TM, Ames BN, Cross CE. Gas phase oxidants of cigarette smoke induce lipid peroxidation and changes in lipoprotein properties in human blood plasma: protective effects of ascorbic acid. Biochem J 1991;277:133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiyosawa H, Suko M, Okudaira H, Murata K, Miyamoto T, Chung MH, Kasai H, Nishimura S. Cigarette smoking induces formation of 8-hydroxydeoxyguanosine, one of the oxidative DNA damages in human peripheral leukocytes. Free Radic Res Commun 1990;11:23–27 [DOI] [PubMed] [Google Scholar]
- 5.Reznick AZ, Cross CE, Hu ML, Suzuki YJ, Khwaja S, Safadi A, Motchnik PA, Packer L, Halliwell B. Modification of plasma proteins by cigarette smoke as measured by protein carbonyl formation. Biochem J 1992;286:607–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panda K, Chattopadhyay R, Chattopadhyay D, Chatterjee IB. Cigarette smoke–induced protein oxidation and proteolysis is exclusively caused by its tar phase: prevention by vitamin C. Toxicol Lett 2001;123:21–32 [DOI] [PubMed] [Google Scholar]
- 7.Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol 1987;63:152–157 [DOI] [PubMed] [Google Scholar]
- 8.Rahman I. Regulation of glutathione in inflammation and chronic lung diseases. Mutat Res 2005;579:58–80 [DOI] [PubMed] [Google Scholar]
- 9.Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS, Matthews DE, et al. Dynamic redox control of nf-kappab through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci USA 2006;103:13086–13091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Jao S, Nanduri S, Starke DW, Mieyal JJ, Qin J. Reactivity of the human thioltransferase (glutaredoxin) C7S, C25S, C78S, C82S mutant and NMR solution structure of its glutathionyl mixed disulfide intermediate reflect catalytic specificity. Biochemistry 1998;37:17145–17156 [DOI] [PubMed] [Google Scholar]
- 11.Cha MK, Kim IH. Preferential overexpression of glutaredoxin3 in human colon and lung carcinoma. Cancer Epidemiol 2009;33:281–287 [DOI] [PubMed] [Google Scholar]
- 12.Reynaert NL, Wouters EF, Janssen-Heininger YM. Modulation of glutaredoxin-1 expression in a mouse model of allergic airway disease. Am J Respir Cell Mol Biol 2007;36:147–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peltoniemi MJ, Rytila PH, Harju TH, Soini YM, Salmenkivi KM, Ruddock LW, Kinnula VL. Modulation of glutaredoxin in the lung and sputum of cigarette smokers and chronic obstructive pulmonary disease. Respir Res 2006;7:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aesif SW, Kuipers I, Anathy V, Guala AS, Reiss JN, Ho YS, Janssen-Heininger YM. Ablation of glutaredoxin-1 attenuates lipopolysaccharide-induced lung inflammation and alveolar macrophage activation. Am J Respir Cell Mol Biol 2011;44:491–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu R, Smith D. Continuous multiplication of rabbit tracheal epithelial cells in a defined, hormone-supplemented medium. In Vitro 1982;18:800–812 [DOI] [PubMed] [Google Scholar]
- 16.Reynaert NL, Ckless K, Guala AS, Wouters EF, van der Vliet A, Janssen-Heininger YM. In situ detection of S-glutathionylated proteins following glutaredoxin-1 catalyzed cysteine derivatization. Biochim Biophys Acta 2006;1760:180–187 [DOI] [PubMed] [Google Scholar]
- 17.Carp H, Janoff A. Possible mechanisms of emphysema in smokers: in vitro suppression of serum elastase–inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis 1978;118:617–621 [DOI] [PubMed] [Google Scholar]
- 18.Anathy V, Aesif SW, Guala AS, Havermans M, Reynaert NL, Ho YS, Budd RC, Janssen-Heininger YM. Redox amplification of apoptosis by caspase-dependent cleavage of glutaredoxin 1 and S-glutathionylation of Fas. J Cell Biol 2009;184:241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gan ZR, Wells WW. Purification and properties of thioltransferase. J Biol Chem 1986;261:996–1001 [PubMed] [Google Scholar]
- 20.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 2006;1:3159–3165 [DOI] [PubMed] [Google Scholar]
- 21.Hashemy SI, Johansson C, Berndt C, Lillig CH, Holmgren A. Oxidation and S-nitrosylation of cysteines in human cytosolic and mitochondrial glutaredoxins: effects on structure and activity. J Biol Chem 2007;282:14428–14436 [DOI] [PubMed] [Google Scholar]
- 22.Muscat JE, Kleinman W, Colosimo S, Muir A, Lazarus P, Park J, Richie JP., Jr Enhanced protein glutathiolation and oxidative stress in cigarette smokers. Free Radic Biol Med 2004;36:464–470 [DOI] [PubMed] [Google Scholar]
- 23.Peltoniemi M, Kaarteenaho-Wiik R, Saily M, Sormunen R, Paakko P, Holmgren A, Soini Y, Kinnula VL. Expression of glutaredoxin is highly cell specific in human lung and is decreased by transforming growth factor–beta in vitro and in interstitial lung diseases in vivo. Hum Pathol 2004;35:1000–1007 [DOI] [PubMed] [Google Scholar]
- 24.Park JB, Levine M. The human glutaredoxin gene: determination of its organization, transcription start point, and promoter analysis. Gene 1997;197:189–193 [DOI] [PubMed] [Google Scholar]
- 25.Lillig CH, Berndt C, Vergnolle O, Lonn ME, Hudemann C, Bill E, Holmgren A. Characterization of human glutaredoxin 2 as iron-sulfur protein: a possible role as redox sensor. Proc Natl Acad Sci USA 2005;102:8168–8173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmgren A. Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc Natl Acad Sci USA 1976;73:2275–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Tekle E, Oubrahim H, Mieyal JJ, Stadtman ER, Chock PB. Stable and controllable RNA interference: investigating the physiological function of glutathionylated actin. Proc Natl Acad Sci USA 2003;100:5103–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krysan K, Lou MF. Regulation of human thioltransferase (httase) gene by AP-1 transcription factor under oxidative stress. Invest Ophthalmol Vis Sci 2002;43:1876–1883 [PubMed] [Google Scholar]
- 29.Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res 2006;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malik G, Nagy N, Ho YS, Maulik N, Das DK. Role of glutaredoxin-1 in cardioprotection: an insight with Glrx1 transgenic and knockout animals. J Mol Cell Cardiol 2008;44:261–269 [DOI] [PubMed] [Google Scholar]
- 31.Lofgren S, Fernando MR, Xing KY, Wang Y, Kuszynski CA, Ho YS, Lou MF. Effect of thioltransferase (glutaredoxin) deletion on cellular sensitivity to oxidative stress and cell proliferation in lens epithelial cells of thioltransferase knockout mouse. Invest Ophthalmol Vis Sci 2008;49:4497–4505 [DOI] [PubMed] [Google Scholar]
- 32.Pan S, Berk BC. Glutathiolation regulates tumor necrosis factor-alpha-induced caspase-3 cleavage and apoptosis: key role for glutaredoxin in the death pathway. Circ Res 2007;100:213–219 [DOI] [PubMed] [Google Scholar]
- 33.Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal 2008;10:1941–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song JJ, Rhee JG, Suntharalingam M, Walsh SA, Spitz DR, Lee YJ. Role of glutaredoxin in metabolic oxidative stress: glutaredoxin as a sensor of oxidative stress mediated by H2O2. J Biol Chem 2002;277:46566–46575 [DOI] [PubMed] [Google Scholar]
- 35.Sato A, Hoshino Y, Hara T, Muro S, Nakamura H, Mishima M, Yodoi J. Thioredoxin-1 ameliorates cigarette smoke–induced lung inflammation and emphysema in mice. J Pharmacol Exp Ther 2008;325:380–388 [DOI] [PubMed] [Google Scholar]