Abstract
Lung macrophages use the scavenger receptor MARCO to bind and ingest bacteria, particulate matter, and post cellular debris. We investigated the role of MARCO in influenza A virus (IAV) pneumonia. In contrast to higher susceptibility to bacterial infection, MARCO−/− mice had lower morbidity and mortality from influenza pneumonia than wild-type (WT) mice. The early course of influenza in MARCO−/− lungs was marked by an enhanced but transient neutrophilic inflammatory response and significantly lower viral replication compared with the WT mice. At later time points, no significant differences in lung histopathology or absolute numbers of T lymphocyte influx were evident. Uptake of IAV by WT and MARCO−/− bronchoalveolar lavage macrophages in vitro was similar. By LPS coadministration, we demonstrated that rapid neutrophil and monocyte influx during the onset of influenza suppressed viral replication, indicating a protective role of early inflammation. We hypothesized that the presence of increased basal proinflammatory post cellular debris in the absence of scavenging function lowered the inflammatory response threshold to IAV in MARCO−/− mice. Indeed, MARCO−/− mice showed increased accumulation of proinflammatory oxidized lipoproteins in the bronchoalveolar lavage early in the infection process, which are the potential mediators of the observed enhanced inflammation. These results indicate that MARCO suppresses a protective early inflammatory response to influenza, which modulates viral clearance and delays recovery.
Keywords: inflammation, scavenger receptors, leukocytes, chemokines, pathology, oxidized lipoproteins
Clinical Relevance
This research presents the beneficial effect of a well controlled burst of inflammatory reaction within the first couple days of influenza. The scavenger receptor MARCO suppresses this early inflammatory response. Therefore, polymorphisms in human MARCO may be related to a differential innate immune response to influenza.
Influenza is a significant public health concern, being responsible for large numbers of deaths and hospitalizations each year in the United States (1) and worldwide.
A successful immune response to influenza balances antiviral mechanisms and the regulation of the inflammation to prevent excessive tissue damage. The early events in the innate immune response often hold the key to such processes (2). After activation by virus, alveolar macrophages (AMs) become highly phagocytic, produce high amounts of inflammatory cytokines such as IL-6 and TNF-α (3), and initiate a cascade of immune responses. AM depletion studies have shown that AMs are indispensable in controlling influenza virus in mice (4) and pigs (5) and in preventing uncontrolled expansion of virus-specific cytotoxic lymphocytes in mouse lungs (6). Activation of macrophage inflammasome pathways by viral RNA is one mechanism linked to decreased morbidity in mouse models (7) and operates in part through recruitment of neutrophils. More generally, neutrophils, which rapidly infiltrate the lung after influenza A virus (IAV) infection, release microbicidal products, such as reactive oxygen species, cationic peptides, eicosanoids, proteolytic enzymes, and elastase (8). Depletion of lung neutrophils in mice, followed by IAV infection, resulted in higher viral titers and increased mortality (9, 10). Mice deficient in mouse chemoattractant protein (MCP)1 (11) showed diminished recruitment of AMs and granulocytes and impaired influenza recovery.
Conversely, marked increases in neutrophils and AMs in the lungs and exaggerated cytokine and chemokine production characterize severe pneumonia and mortality due to IAV, H1N1, H5N1 (12), or experimental recombinant influenza (1918HA/NA:Tx/91) (4). Thus, an optimum inflammatory response and neutrophil influx that is neither insufficient nor excessive is a key feature in the successful resolution of influenza pneumonia.
One factor that could modulate the innate immune response and the degree of neutrophil activation is the functional state of resident macrophages. Alveolar macrophages can play an immunomodulatory role (13) and help in the restoration of immune homeostasis during pulmonary infections (14).
We further explored the function of AMs in the host response to influenza, focusing on whether scavenger receptors play a role. These pattern recognition molecules mediate recognition and uptake of foreign material and modified host molecules (e.g., oxidized proteins and lipids) and exhibit a large array of ligand specificity. Lung macrophages use scavenger receptor A (SRA)-1 and macrophage receptor with collagenous structure (MARCO) to mediate uptake and clearance of lung pathogens, Streptococcus, unopsonized micro- and nanoparticulate matter, and ozone-generated oxidized lipid intermediates (15–19). In all these studies, MARCO−/− mice showed increased morbidity or mortality because of decreased macrophage-mediated uptake and clearance, leading to exacerbated neutrophil response and enhanced lung pathology. In systemic viral infections, SRA contributes to the uptake and clearance of adenovirus by Kupffer cells (20, 21), resistance to herpes virus (22), and human cytomegalovirus recognition by monocytic cells (23). Based on these findings, we initially postulated that the scavenger receptor MARCO would improve host resistance and influenza resolution. In contrast, our findings suggest that MARCO suppresses an early inflammatory response in innate immunity against IAV through clearance of proinflammatory oxidized lipids in post cellular debris. In MARCO-deficient mice, an enhanced early burst of inflammation featuring a moderately increased neutrophil influx is linked to improved survival.
Materials and Methods
Materials, Animals, Virus, and Infection
CD11c, MHCII, DX5, CD3, CD4, and CD8 antibodies were obtained from Miltenyi Biotec (Auburn, CA). CD16/34, F4/80, and CD11b antibodies and the IL1b ELISA kit were obtained from eBiosystems (San Diego, CA). IL-17 antibody was obtained from R&D Systems (Minneapolis, MN). 1-palmitoyl 2-(oxovaleroyl)-sn-glycero-3-phosphocholine (POVPC) was obtained from Avanti Polar Lipids (Alabaster, AL). EO6 antibody was a gift from Professor Joseph Witztum. Poly I:C was obtained from Dr. Farhad Imani. MARCO−/− mice were provided by K. Tryggvason (University of Oulu, Finland). BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, ME). All animal protocols were approved by the Ethical Care and Use of Animal Committee, Center for Animal Resources and Comparative Medicine, Harvard University. Influenza strain A/PR/8/34 (H1N1) was obtained from Charles River Laboratories (Wilmington, MA).
Adult male wild-type (WT) or MARCO−/− mice were anesthetized with 100 mg ketamine (Vedco, St. Joseph, MO) and 20 mg xylazine solution per kg per mouse and infected intranasally with 10 HAU APR8 in 50 μl PBS. Mice were killed with intraperitoneal sodium pentobarbital injection. Mice were monitored twice daily for 16 days after infection for survival. A 10-point scoring system was devised to measure influenza-related morbidity in mice (see Figure E1 in the online supplement).
Lung Fixation and Histology
Formalin-fixed, paraffin-embedded, 8-μm sections were stained with H&E. Features of viral pneumonitis (inflammatory cells, edema, cell fragmentation, hemorrhage, and interstitial expansion) were evaluated semiquantitatively based on an index generated by multiplying a severity score (0–3) by the extent of involvement in the section (0–3 score).
Flow Cytometry
A minimum of 5 × 105 cells were used per immunoreaction. Cells were incubated in Fc-Block (CD16/34), resuspended in the required antibody or isotype control, and incubated at 4°C for 30 minutes. Cells were washed and analyzed by a BD Canto II flow cytometer (BD Biosciences, Sparks, MD).
Quantitative PCR Analysis of Gene Expression
RT2 Profile PCR array for mouse inflammatory cytokines and receptors (SA Biosciences, Frederick, MD) was performed with 1 μg total RNA from lung or total bronchoalveolar lavage (BAL) cells from five mice per group. Individual real-time PCR assays were done with predesigned assays from Applied Biosystems (Foster City, CA).
Virus Labeling, In Vitro Infection, Fluorescent Imaging, and Quantitation
Sucrose-gradient purified influenza A/PR/8/34 (1 mg) labeled with a FITC antibody labeling kit (Pierce Thermo Scientific, Rockford, IL) was used to infect naive AMs (2.5 HAU FITC-labeled virus to 104 cells). Scanning cytometry was performed as described elsewhere (24). Alternatively, 1 mg virus was labeled for 2 hours with 2.5 μl of 25 mM lipophilic dye and DiD (1,1'-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt) (Invitrogen, Carlsbad, CA).
Oxidized Phospholids ELISA Assay
Cell-free BAL supernatant (50 μl) or surfactant-associated material fraction, obtained by centrifugation of the supernatant at 60,000 × g and dissolving the pellet in 100 μl PBS, was used for ELISA. Additional experiments were performed with crude organic extraction of surfactant-associated material following the Bligh and Dyer method and the method of Hοrkko and colleagues (25). Results were normalized against standard using POVPC as an EO6 ligand (26).
Statistics
Student's t test (unpaired, two tailed) was used to calculate statistical significance. P values less than 0.05 were considered significant.
Results
MARCO Deficiency Allows Greater Survival in Influenza
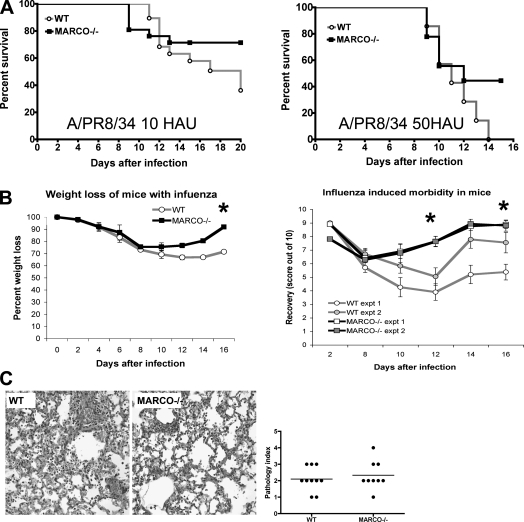
We compared survival in WT and MARCO−/− BALB/c mice with moderate (10 HAU, equivalent to 200 PFU or 1 TCID) (Figure 1A, left) and high (50 HAU) doses (Figure 1A, right) of IAV (A/PR8/34). MARCO deficiency was associated with increased survival at both doses. WT mice had significantly greater weight loss (Figure 1B, left). MARCO−/− mice exhibited more efficient recovery from influenza-induced morbidity (Figure 1B, right; Figure E1). Mice killed 8 days after infection (10 HAU) exhibited similar degrees of histopathology in both groups (Figure 1C). Similarly, no clear differences were observed in survivors from both the groups 16 days after infection to account for the reduced mortality in the MARCO−/− group (data not shown). No other remarkable groupwise differences were found in the heart, liver, spleen, or gut histopathology examined at this time (data not shown), ruling out the possibilities of preexisting infections or other health conditions.
Figure 1.
MARCO-deficient mice have increased survival and enhanced recovery from influenza. (A) Male BALB/c wild-type (WT) and MARCO−/− mice (8–10 per group) were infected with 10 HAU (left) and 50 HAU (right) influenza A virus and monitored for the indicated period. Data on 10 HAU are representative of two independent experiments. (B) Left: MARCO−/− mice have lower weight loss than WT mice. Data are representative of three independent experiments. *P < 0.03. Right: MARCO−/− mice show enhanced recovery compared with WT mice (*P < 0.05) (for additional information, see Figure E1). Data are representative of two independent experiments. (C) Histopathology of WT (left) and MARCO−/− (right) lungs on Day 8 after infection showing similar degrees of pathology (n = 9–10 mice per group). Slides were scored for severity and extent of pathology, and the differences are shown in the graph.
MARCO−/− Mice Exhibit Enhanced Acute Early Inflammation with IAV
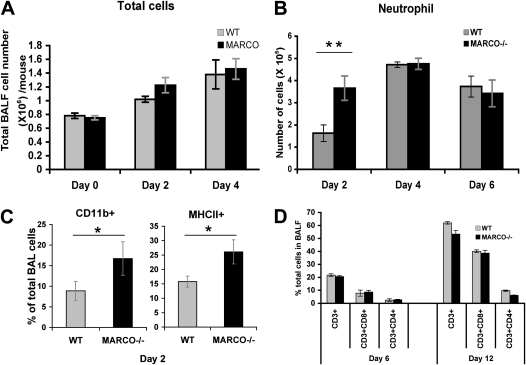
Investigation of differential cellular influx in the lungs over the course of influenza showed an acute early neutrophil response in the MARCO−/− group within 2 days (Figure 2; Figure E2), with decreases in macrophages accounting for similar total cell yields. By Day 4 after infection, the neutrophil levels were comparable in the two groups (Figure 2B). Flow cytometric evaluation of BAL cells showed increased CD11b+ cells in the MARCO−/− group relative to WT, consistent with the observation of increased neutrophil influx (Figure 2C, left). A small yet significant increase in MHCII+ cells (further characterized as CD11chiCD11blo; data not shown) was found in the BAL fluid (BALF) of the knockout animals at Day 2 after infection, suggesting increased activation of resident phagocytic cells (Figure 2C, right). No significant differences were observed in the natural killer cell population and resident macrophage or dendritic cells in the BALF (Figure E2). The WT and MARCO−/− mice showed similar amounts of total CD3+ cellular influx in the lung, as well as CD4+ and CD8+ subpopulations, on 6 and 12 days after infection (Figure 2D).
Figure 2.
Differential cellular composition in bronchoalveolar lavage (BAL) of infected lungs. Control and infected WT and MARCO−/− mice (at least six mice per group per time point) were analyzed by BAL at indicated intervals after infection. (A) Total number of cells in BAL fluid. (B) Total neutrophil counts per mouse by microscopic examination of cytological spins in BAL samples. (C). Flow cytometric determination of CD11b and MHC II–positive cells in BAL at Day 2 after infection. *P < 0.015; **P < 0.001. (D) Flow cytometric evaluation of total lymphocytes in BAL, percent CD3-expressing cells, and CD3+CD4+ versus CD3+CD8+ subpopulations in each group at Days 6 and 12 after infection. Each figure is representative of two or three independent experiments.
Enhanced Inflammatory Gene Induction in MARCO−/− Lungs
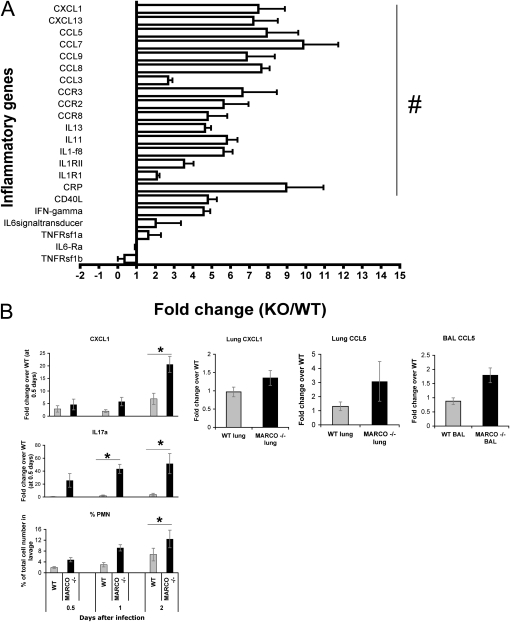
The observation of enhanced early inflammatory response in the MARCO−/− lungs was supported by the gene expression data. Probing for differential expression of 84 mouse inflammatory genes (RT2 Profiler Array) indicated enhanced induction of the chemokines CXCL1 (KC), CCL5 (RANTES) CCL7 (MCP3), and CCL8 (MCP2) in the MARCO−/− mice at Day 2 (Figure 3A). Prominent chemokine receptors CCR2, CCR3 (receptor for eotaxin, CCL5, and CCL7), CCR4 (receptor for MIP1, RANTES, and MCP1), and CCR7showed a similar trend. The relative CXCL1 and CCL5 mRNA levels between the infected WT and MARCO−/− lungs were further assayed by individual real-time PCR assays (Figure 3B), which verified the array results. Because IL-17 is related to neutrophil influx via C-X-C chemokine induction (27), we investigated IL-17 mRNA induction. Over the course of 0.5 to 2 days after infection, induction of IL-17 and CXCL1 mRNA correlated with increased neutrophils in the BALF (Figure 3B, left). However, our trials of IL-17 neutralization did not suppress influenza-induced neutrophil influx in either group, indicating the lack of an effective role of IL-17 in this process (Figure E3).
Figure 3.
MARCO−/− lungs have higher inflammatory gene expression after infection. (A) RT2 Profiler PCR array for mouse inflammatory cytokine and receptors was performed with lung RNA extracts at Day 2 after infection, comparing five each of WT and MARCO−/− samples. Results are expressed as expression fold changes of MARCO−/− over WT (mean ± SD). #P < 0.05. (B) Quantitative real-time PCR showing enhanced chemokine expression. Left: Temporal expression pattern of IL-17a and CXCL1 in BAL cells with respect to lung neutrophil influx (n = 4–7 mice per group). Right: CXCL1 and CCL5 expression in MARCO−/− versus WT BAL cells and post-BAL lungs at Day 2 after infection (n = 3 mice per group). Data are representative of duplicate experiments.
MARCO Is Not Necessary for IAV Entry but Regulates Downstream Inflammatory Gene Induction in Macrophages
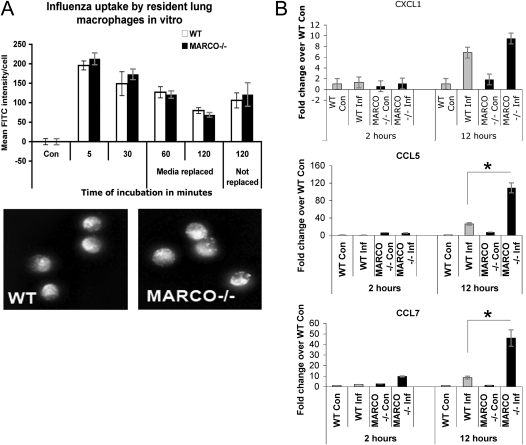
We sought to determine if differences in the outcome after infection reflect impaired uptake or removal of virions by MARCO−/− phagocytes. Resident AMs from naive WT and MARCO−/− infected with IAV ex vivo showed no significant differences in IAV uptake (Figure 4A). To rule out a possible effect of the anionic FITC-tag on scavenger receptor–mediated virus uptake, we performed similar experiments with IAV labeled with DiD and measured relative uptake of the labeled virus by microscopy and flow cytometry. The results were similar to those seen for FITC-labeled virus uptake (data not shown). In addition, blocking of class A scavenger receptors with polyinosinic acid (poly-I) before infection led to no significant differences in viral uptake by macrophages (data not shown). Taken together, these results indicate that the absence of MARCO does not change the quantitative uptake of IAV by lung macrophages.
Figure 4.
MARCO deficiency does not affect virus entry into lung macrophages but induces higher inflammatory gene expression than WT macrophages. (A) Alveolar macrophages from WT and MARCO−/− mice (n = 6 mice per group) and were infected ex vivo with fluorescence–labeled influenza A virus (IAV). Upper panel: FITC fluorescence intensity plotted over time per cell in each group. Mean (± SE) of quadruplicate wells. Lower panel: Collapsed-stack confocal images of representative fields at 5 minutes after inoculation. (B) BAL cells were infected in vitro with unlabeled IAV , and excess unbound virus was washed off after 30 minutes of incubation at 37°C. Cells were incubated for additional periods as indicated, and RNA was extracted for quantitative real time RT-PCR analysis of inflammatory genes, CXCL1, CCL5 and CCL7. Data are representative of two experiments. *P < 0.05; **P < 0.001.
Although uptake of the virus was not impaired in MARCO−/− macrophages, in vitro infection was accompanied by higher expression of CCL5 and CCL7 expression after 12 hours of infection (Figure 4B). No statistically significant difference in expression of the neutrophil chemoattractant CXCL1 was observed (CXCL1, P = 0.09; CCL5, P = 2.13; 9E05, CCL7, P = 0.00126). The supernatants of the cells in culture were tested for LDH activity and were comparable between the groups (data not shown), indicating that the results were not due to differential cell viability.
Suppression of Viral Outgrowth in MARCO−/− Mice: Early Inflammation versus Viral RNA Replication
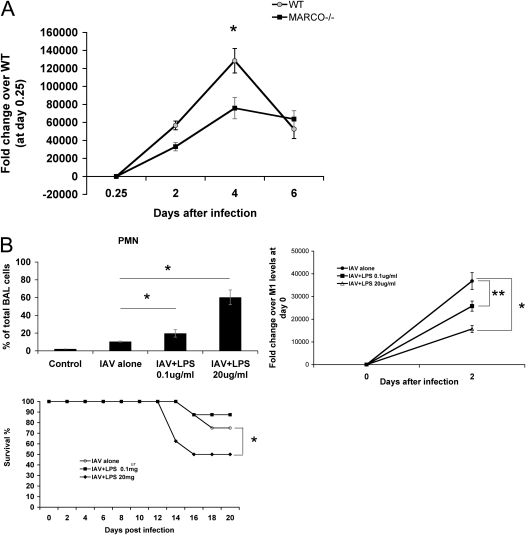
To determine whether the enhanced inflammatory response early in influenza altered viral replication in MARCO−/− mice, we performed real-time PCR quantitation of viral M1 RNA in lungs periodically after infection. The viral RNA levels peaked at Day 4 (Figure 5A). MARCO−/− mice had lower viral M1 RNA expression on Days 2 and 4 after infection compared with WT mice. At 4 days after infection, WT lungs had twice the level of viral RNA seen in MARCO−/− (Figure 5A). The viral replication decreased after Day 4 of infection, and by Day 6 there was no significant difference between the two groups.
Figure 5.
(A) Suppression of viral replication in MARCO−/− mice. Quantitative real-time RT-PCR was performed for the IAV M1 gene using total lung RNA at the indicated intervals and normalized with mouse GAPDH. Representative of duplicate experiments, with at least four mice per time point per group. *P < 0.003. (B) LPS aerosol treatment after infection of WT mice induces higher inflammation and suppresses viral outgrowth but does not render survival advantage. WT mice were infected with IAV and left untreated or treated with 0.1 or 20 μg/ml LPS aerosolized for 20 minutes, 12 hours after infection. Upper left panel: Cytological spins from BAL at Day 2 after infection. Upper right panel: RNA extracted from total lung homogenate was used to detect viral M1gene by quantitative real time RT-PCR. Representative of two independent experiments (n = 5 mice per group). *P < 0.05; **P < 0.03. Lower panel: Mice (8–10 per group) were infected with IAV and left untreated or were treated with indicated doses of LPS aerosol as above, and their mortality was monitored. Mortality of mice treated with LPS 20 μg/ml aerosol was significantly different than the untreated infected group. *P < 0.01.
We hypothesized that the enhanced early inflammation might be responsible for reducing viral proliferation in the MARCO−/− group. To test this, we enhanced the acute inflammation in WT mice after influenza infection by LPS treatment. Exposure to LPS aerosols (0.1 or 20 μg/ml) for 20 minutes at 12 hours after infection led to a substantial influx of inflammatory cells in the lungs seen in lung lavage samples 36 hours later (i.e., 48 hours after infection) (Figure 5B, left). A lower concentration of LPS (0.1 μg/ml) induced a moderate increase in BAL neutrophils (19.7% at 48 hours compared with 10.4% in the no-LPS infected group). This level of enhanced neutrophil response was accompanied by a suppression of viral M1 RNA by 30% (Figure 5B, upper right). Exposure to 20 μg/ml LPS led to a much greater neutrophil influx, 60.2% of BAL cells at 48 hours, and viral replication suppression by 63 ± 11% within 24 hours and by 54 ± 7.7% at 48 hours compared with no LPS challenge after infection (Figure 5B, upper panel). Despite the potentially beneficial effect of LPS-induced enhanced early inflammation on limiting viral replication, LPS led to increased mortality compared with IAV alone (Figure 5B, lower panel), indicating that a decrease in virus load does not necessarily correlate with a survival advantage. With 20 μg/ml LPS, excessive neutrophil influx and inflammatory tissue damage resulted in high mortality, consistent with the cytokine storm hypothesis of influenza mortality (7, 28). These results indicate that an early but regulated (i.e., not excessive) inflammatory response is beneficial for resolving IAV infection and increasing survival, analogous to observations in the MARCO−/− mice after infection.
MARCO-Mediated Suppression of Beneficial Early Inflammation Relates to Its Scavenging Function
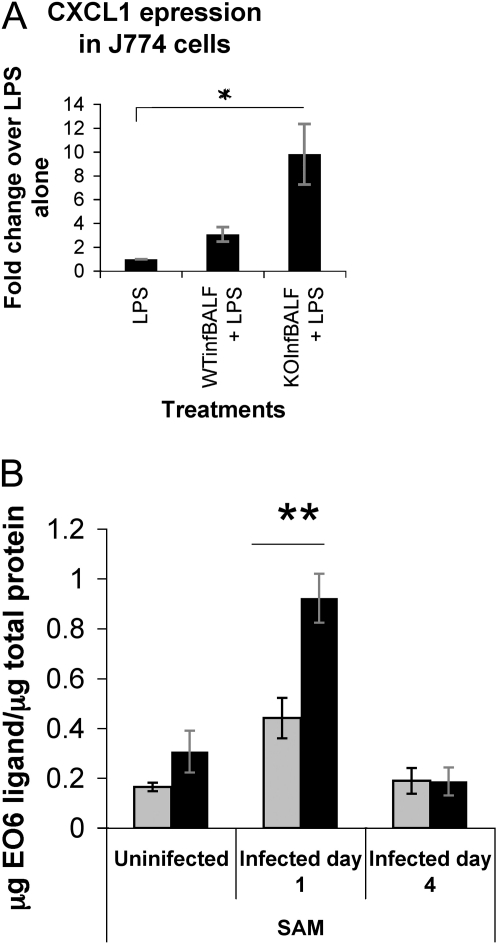
Based on prior findings (18), we postulated that MARCO deficiency might impair the scavenging activity of macrophages, leading to accumulation of proinflammatory mediators in the alveolar milieu, which is likely to lower the inflammatory threshold during an infection. In keeping with this hypothesis, we found that MARCO−/− surfactant fluid caused higher induction of CXCL1 compared with WT (Figure 6A). In addition, the MARCO−/− fluid led to greater TNF release compared with WT in macrophages stimulated with LPS (data not shown) in the absence of detectable NO generation in the BAL (Figure E4).
Figure 6.
(A) Inflammatory potential of BALF of mice infected for 8 hours. Mice were instilled with influenza, their BALF fluid collected 8 hours after infection and used for pretreatment of J774 macrophages (see Figure E1). CXCL1 expression in J774 cells with PBS vehicle or WT (WTinfBALF) and MARCO−/− BALF (KOInfBALF) pretreatment for 30 minutes, followed by LPS stimulation (200 ng/ml overnight). CXCL1 expression was undetermined in unstimulated cells. Average of samples from three mice per group. (B) PosT cellular oxidized lipoprotein accumulation in MARCO−/− lungs. BAL fluids were analyzed for oxidized lipoproteins by EO6 antibody ELISA 1 and 4 days after infection. Using dose response curve of POVPC as standard for EO6 binding (not shown), ligands for EO6 antibody in BAL were quantitated. EO6 reactive lipoproteins in SAM fraction in BAL of the infected WT and MARCO−/− mice at indicated periods after infection. Data are representative of three independent experiments. All results were normalized against protein content of the samples. *P < 0.05; **P < 0.03.
Infection-related cell damage leads to oxidation of phospholipids as breakdown products (29–31), which are proinflammatory (32, 33) and readily cleared by scavenger receptors (18). We measured oxidized phospholipids (ox-PL) in BALF of infected WT and knockout mice using the EO6 monoclonal antibody. Murine monoclonal antibody EO6 specifically binds to the phosphorylcholine head group of oxidized, but not native, phospholipids (25, 34). In ELISA assays using the EO6 antibody, we compared oxidized phospholipid content in phospholipid-enriched extracts (60,000 × g pellet) from BALF of infected mice (Figure 6B). There was a 2-fold greater amount of ox-PL reacting with EO6 antibody in samples form MARCO−/− mice (adjusted for protein content) compared with the WT mice at Day 1 (Figure 6B). By Day 4 after infection, the oxidized phospholipid content of BAL samples from the two groups were comparable and lower than seen on Day 1, which coincides with our data of transient higher inflammation in the MARCO−/− lungs. We next determined the effect of ox-PL pretreatment and IAV infection on cytokine expression of MARCO−/− AMs. Pretreatment of the alveolar macrophages with 10 μM POVPC, an important ox-PL constituent of oxidized low-density lipoprotein (LDL) in surfactant fluids, for 30 minutes before IAV infection ex vivo augmented CXCL1 and CCL5 expression by 15.8 and 16.4%, respectively (Table 1). This indicates that the presence of oxidized phospholipids enhances the inflammatory response of MARCO−/− AMs to IAV.
TABLE 1.
PRETREATMENT OF ALVEOLAR MACROPHAGES WITH OXIDIZED PHOSPHOLIPIDS AUGMENT INFLUENZA INDUCED CHEMOKINE GENERATION*
| Treatments | Fold Change over Vehicle Control (mean ± SE) | Mean Increase with Pretreatment | |
| CXCL1 | Vehicle | 1.00 ± 0.06 | |
| Infection | 10.49 ± 0.202 | ||
| POVPC + infection | 12.15 ± 0.837 | 15.8% | |
| CCL5 | Vehicle | 1.00 ± 0.079 | |
| Infection | 3.80 ± 0.075 | ||
| POVPC + infection | 4.43 ± 0.315 | 16.4% |
Definition of abbreviations: POVPC = 1-palmitoyl 2-(oxovaleroyl)-sn-glycero-3-phosphocholine.
* Alveolar macrophages isolated from naive MARCO−/− mice were cultured in complete RPMI for 4 h and then pretreated with 10 μM POVPC for 30 min in PBS before infection with influenza A virus. The cells were incubated in complete RPMI for 4 h, after which RNA was extracted from the cells. CXCL1 and CCL5 message levels were determined by Taqman quantitative real-time PCR.
Discussion
Studies of streptococcal infections (16), adenoviral infections, and viral hepatitis (20, 21) indicate that scavenger receptors participate in the uptake and clearance of these pathogens from the lung and other sites. In contrast, IAV infection in MARCO-deficient mice resulted in better survival and earlier recovery from weight loss and morbidity symptoms than the WT control mice in the three trials reported in this manuscript. Similar findings of increased survival with influenza in MARCO−/− mice have been observed by another laboratory (Z. Chroneos, personal communication).
The course of infection was marked by a transient increase in inflammation early in the innate defense phase in the absence of obvious differences in the adaptive response measured by the lymphocyte counts (see Figure 2). The molecular mechanism that links the transient inflammatory up-regulation to an enhanced recovery later is unclear, although it is evident that inflammatory cells suppress viral replication and may result in effectively lower viral levels during the peak period (Figure 5). The total lymphocyte influx in the lungs remained comparable between the groups. Kai and colleagues reported that IL-10–deficient mice showed increased survival via increased TH17 response in the absence of alteration in the magnitude of cellular response (35). In addition, lower virus load does not necessarily correlate with survival advantages (35). Our results also show that LPS administration 12 hours after influenza infection could effectively lower the viral mRNA levels, but the excessive inflammation that LPS caused resulted in increased death rates of mice, as expected (Figure 5). Our findings support the interpretation that a critical window for a beneficial inflammatory response exists for influenza immunity, where there is a mild but early inflammatory response within the first 2 to 3 days followed by effective down-regulation and prevention of further inflammatory amplification sequelae. MARCO−/− mice exhibited this pattern.
In an earlier study, therapeutic blockage of MIP-2 blocked PMN influx in the lungs and reduced the severity of lung pathology to IAV (36). Fujisawa (9) showed that depletion of neutrophils in rats by the use of monoclonal antibody RB6 was associated with significant elevations in the pulmonary viral titers on Days 3 through 5 and that it took longer for the viral titer to decrease than in control rats. Although these reports appear to contradict regarding the role of inflammatory cellular influx in influenza, they illustrate the importance of timing and of the level of neutrophil response in influenza in leading to a beneficial versus a deleterious role. Recently, Tate and colleagues (10) reported the use of anti-Ly6G (1A8) antibody with increased specificity to neutrophils to block their influx before influenza. They reported that viral titer was greatly increased in the experimentally neutropenic mice and was associated with a worse pathological outcome and edema. We show here in our MARCO-deficient model that a more subtle differential neutrophil response, without exaggerated peak levels or duration of neutrophil influx, resulted in lower peak RNA levels of the virus and was associated with early recovery and better survival.
We inquired if MARCO mediated the uptake of IAV. MARCO deficiency did not result in quantitative differences in IAV entry in macrophages. However, MARCO−/− macrophages showed higher levels of IL1b (data not shown), CCL5, and CCL7 mRNA upon infection in vitro (Figure 4). This suggested that differential intracellular responses exist as a consequence of MARCO, probably in the mode of viral entry and intracellular processing, and we are currently investigating this. The role of IL-17 during the initial stages before 2 days after infection is not clear. Our initial attempts to neutralize IL-17 in vivo using antibody did not result in abrogation of early neutrophil influx in the WT or the MARCO−/− lungs at Day 2 after infection (data not shown), but additional studies are needed to fully address this question. It is possible that the release of IL-17 was delayed further from our messenger RNA studies or that neutrophils expressed higher IL-17, which may have implications later in the disease process.
We considered the most likely initial cause of enhanced acute inflammation in MARCO−/− mice to be the compromised scavenging of oxidized phospholipid and postcellular debris, which trigger proinflammatory gene expression. As initially shown in studies related to atherosclerosis, oxidized phospholipids and LDLs are scavenged by SRA and CD36 (37–39). Macrophage class A scavenger receptors readily take up oxidized liposomes (40, 41). In prior studies of acute inflammation in ozone-damaged lungs, we found that the absence of MARCO leads to diminished clearance of proinflammatory oxidized phospholipids, with a resultant robust acute inflammatory effect. We have shown here (Figure 6A) that an inflammatory component existed in the BALF of MARCO−/− mice as early as 8 hours after infection, when neutrophil influx or reactive oxygen species were not detectable. We also noted that even in the uninfected state, BALF of MARCO−/− mice contained greater oxidized phospholipid than the WT mice but did not have detectable increased neutrophil levels or proinflammatory gene messenger levels at basal state. We believe that a threshold value is reached with additional release and accumulation of ox-PL upon infection and initiation of cell death, which then triggers increased inflammation. Fitting with our observation of an early yet transient rise in inflammation in the MARCO−/− mice's response to influenza, the enhanced levels of ox-PL were not evident at 4 days after infection (Figure 6), although cell death must have increased at this time. One possibility is that the function of MARCO in this case is significant in the early stages, mostly in the resident macrophages encountering the earliest wave of oxidized lipids and cell debris from the first cellular victims of infection. Due to functional redundancy of these scavenger receptors, it is possible that activated neutrophils and macrophages recruited into the lungs have compensatory scavenger receptors, which may nullify the effect of the SR deficiency at a later period. Further study is needed to evaluate a time-dependent role of MARCO versus other scavenger receptors during lung infection. In summary, this study identifies an unexpected harmful role for the macrophage scavenger receptor MARCO in early innate immune responses to influenza. We speculate that possible variations in expression or functional efficacy of this receptor in the human population could contribute to individual variation in influenza disease severity.
Acknowledgments
The authors thank Dr. Glen Deloid for help in the microscopic evaluation of phagocytosis.
Footnotes
Supported by NIH grant ES011008–08 ES 00002 (L.Z.).
Originally Published in Press as DOI: 10.1165/rcmb.2010-0349OC on May 11, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Bridges CB, Harper SA, Fukuda K, Uyeki TM, Cox NJ, Singleton JA. Prevention and control of influenza: recommendations of the advisory committee on immunization practices (acip). MMWR Recomm Rep 2003;52(RR-8):1–34; quiz CE31–34 [PubMed] [Google Scholar]
- 2.McGill J, Heusel JW, Legge KL. Innate immune control and regulation of influenza virus infections. J Leukoc Biol 2009;86:803–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker S, Quay J, Soukup J. Cytokine (tumor necrosis factor, il-6, and il-8) production by respiratory syncytial virus-infected human alveolar macrophages. J Immunol 1991;147:4307–4312 [PubMed] [Google Scholar]
- 4.Tumpey TM, Garcia-Sastre A, Taubenberger JK, Palese P, Swayne DE, Pantin-Jackwood MJ, Schultz-Cherry S, Solorzano A, Van Rooijen N, Katz JM, et al. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol 2005;79:14933–14944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HM, Lee YW, Lee KJ, Kim HS, Cho SW, van Rooijen N, Guan Y, Seo SH. Alveolar macrophages are indispensable for controlling influenza viruses in lungs of pigs. J Virol 2008;82:4265–4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wijburg OL, DiNatale S, Vadolas J, van Rooijen N, Strugnell RA. Alveolar macrophages regulate the induction of primary cytotoxic t-lymphocyte responses during influenza virus infection. J Virol 1997;71:9450–9457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The nlrp3 inflammasome mediates in vivo innate immunity to influenza a virus through recognition of viral rna. Immunity 2009;30:556–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee WL, Downey GP. Leukocyte elastase: physiological functions and role in acute lung injury. Am J Respir Crit Care Med 2001;164:896–904 [DOI] [PubMed] [Google Scholar]
- 9.Fujisawa H. Neutrophils play an essential role in cooperation with antibody in both protection against and recovery from pulmonary infection with influenza virus in mice. J Virol 2008;82:2772–2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tate MD, Deng YM, Jones JE, Anderson GP, Brooks AG, Reading PC. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J Immunol 2009;183:7441–7450 [DOI] [PubMed] [Google Scholar]
- 11.Dessing MC, van der Sluijs KF, Florquin S, van der Poll T. Monocyte chemoattractant protein 1 contributes to an adequate immune response in influenza pneumonia. Clinical Immunol 2007;125:328–336 [DOI] [PubMed] [Google Scholar]
- 12.Perrone LA, Plowden JK, Garcia-Sastre A, Katz JM, Tumpey TM. H5n1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog 2008;4:e1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med 1989;170:499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taut K, Winter C, Briles DE, Paton JC, Christman JW, Maus R, Baumann R, Welte T, Maus UA. Macrophage turnover kinetics in the lungs of mice infected with streptococcus pneumoniae. Am J Respir Cell Mol Biol 2008;38:105–113 [DOI] [PubMed] [Google Scholar]
- 15.Arredouani M, Yang Z, Ning Y, Qin G, Soininen R, Tryggvason K, Kobzik L. The scavenger receptor marco is required for lung defense against pneumococcal pneumonia and inhaled particles. J Exp Med 2004;200:267–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arredouani MS, Palecanda A, Koziel H, Huang YC, Imrich A, Sulahian TH, Ning YY, Yang Z, Pikkarainen T, Sankala M, et al. Marco is the major binding receptor for unopsonized particles and bacteria on human alveolar macrophages. J Immunol 2005;175:6058–6064 [DOI] [PubMed] [Google Scholar]
- 17.Arredouani MS, Yang Z, Imrich A, Ning Y, Qin G, Kobzik L. The macrophage scavenger receptor sr-ai/ii and lung defense against pneumococci and particles. Am J Respir Cell Mol Biol 2006;35:474–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahl M, Bauer AK, Arredouani M, Soininen R, Tryggvason K, Kleeberger SR, Kobzik L. Protection against inhaled oxidants through scavenging of oxidized lipids by macrophage receptors marco and sr-ai/ii. J Clin Invest 2007;117:757–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanno S, Furuyama A, Hirano S. A murine scavenger receptor marco recognizes polystyrene nanoparticles. Toxicol Sci 2007;97:398–406 [DOI] [PubMed] [Google Scholar]
- 20.Haisma HJ, Boesjes M, Beerens AM, van der Strate BW, Curiel DT, Pluddemann A, Gordon S, Bellu AR. Scavenger receptor a: a new route for adenovirus 5. Mol Pharm 2009;6:366–374 [DOI] [PubMed] [Google Scholar]
- 21.Xu Z, Tian J, Smith JS, Byrnes AP. Clearance of adenovirus by kupffer cells is mediated by scavenger receptors, natural antibodies, and complement. J Virol 2008;82:11705–11713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 1997;386:292–296 [DOI] [PubMed] [Google Scholar]
- 23.Yew KH, Carsten B, Harrison C. Scavenger receptor a1 is required for sensing hcmv by endosomal tlr-3/-9 in monocytic thp-1 cells. Mol Immunol 2010;47:883–893 [DOI] [PubMed] [Google Scholar]
- 24.DeLoid GM, Sulahian TH, Imrich A, Kobzik L. Heterogeneity in macrophage phagocytosis of staphylococcus aureus strains: high-throughput scanning cytometry-based analysis. PLoS ONE 2009;4:e6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horkko S, Bird DA, Miller E, Itabe H, Leitinger N, Subbanagounder G, Berliner JA, Friedman P, Dennis EA, Curtiss LK, et al. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest 1999;103:117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw PX, Horkko S, Chang MK, Curtiss LK, Palinski W, Silverman GJ, Witztum JL. Natural antibodies with the t15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest 2000;105:1731–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A. Neutrophil recruitment by human il-17 via c–x-c chemokine release in the airways. J Immunol 1999;162:2347–2352 [PubMed] [Google Scholar]
- 28.Lee NL. Role of cytokines and chemokines in severe and complicated influenza infections. Hong Kong Med J 2009;15:38–41 [PubMed] [Google Scholar]
- 29.Huber J, Vales A, Mitulovic G, Blumer M, Schmid R, Witztum JL, Binder BR, Leitinger N. Oxidized membrane vesicles and blebs from apoptotic cells contain biologically active oxidized phospholipids that induce monocyte-endothelial interactions. Arterioscler Thromb Vasc Biol 2002;22:101–107 [DOI] [PubMed] [Google Scholar]
- 30.Matsura T, Serinkan BF, Jiang J, Kagan VE. Phosphatidylserine peroxidation/externalization during staurosporine-induced apoptosis in hl-60 cells. FEBS Lett 2002;524:25–30 [DOI] [PubMed] [Google Scholar]
- 31.Lusis AJ. Atherosclerosis. Nature 2000;407:233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh M, Leitinger N, de Martin R, Onai N, Matsushima K, Vora DK, Berliner JA, Reddy ST. Increased transcription of il-8 in endothelial cells is differentially regulated by tnf-alpha and oxidized phospholipids. Arterioscler Thromb Vasc Biol 2001;21:1585–1591 [DOI] [PubMed] [Google Scholar]
- 33.Lee H, Shi W, Tontonoz P, Wang S, Subbanagounder G, Hedrick CC, Hama S, Borromeo C, Evans RM, Berliner JA, et al. Role for peroxisome proliferator-activated receptor alpha in oxidized phospholipid-induced synthesis of monocyte chemotactic protein-1 and interleukin-8 by endothelial cells. Circ Res 2000;87:516–521 [DOI] [PubMed] [Google Scholar]
- 34.Friedman P, Horkko S, Steinberg D, Witztum JL, Dennis EA. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids: importance of schiff base formation and aldol condensation. J Biol Chem 2002;277:7010–7020 [DOI] [PubMed] [Google Scholar]
- 35.McKinstry KK, Strutt TM, Buck A, Curtis JD, Dibble JP, Huston G, Tighe M, Hamada H, Sell S, Dutton RW, et al. Il-10 deficiency unleashes an influenza-specific th17 response and enhances survival against high-dose challenge. J Immunol 2009;182:7353–7363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakai S, Kawamata H, Mantani N, Kogure T, Shimada Y, Terasawa K, Sakai T, Imanishi N, Ochiai H. Therapeutic effect of anti-macrophage inflammatory protein 2 antibody on influenza virus-induced pneumonia in mice. J Virol 2000;74:2472–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park YM, Febbraio M, Silverstein RL. Cd36 modulates migration of mouse and human macrophages in response to oxidized ldl and may contribute to macrophage trapping in the arterial intima. J Clin Invest 2009;119:136–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boullier A, Gillotte KL, Horkko S, Green SR, Friedman P, Dennis EA, Witztum JL, Steinberg D, Quehenberger O. The binding of oxidized low density lipoprotein to mouse cd36 is mediated in part by oxidized phospholipids that are associated with both the lipid and protein moieties of the lipoprotein. J Biol Chem 2000;275:9163–9169 [DOI] [PubMed] [Google Scholar]
- 39.Han J, Hajjar DP, Febbraio M, Nicholson AC. Native and modified low density lipoproteins increase the functional expression of the macrophage class b scavenger receptor, cd36. J Biol Chem 1997;272:21654–21659 [DOI] [PubMed] [Google Scholar]
- 40.Kamps JA, Scherphof GL. Receptor versus non-receptor mediated clearance of liposomes. Adv Drug Deliv Rev 1998;32:81–97 [DOI] [PubMed] [Google Scholar]
- 41.Kamps JA, Morselt HW, Scherphof GL. Uptake of liposomes containing phosphatidylserine by liver cells in vivo and by sinusoidal liver cells in primary culture: in vivo-in vitro differences. Biochem Biophys Res Commun 1999;256:57–62 [DOI] [PubMed] [Google Scholar]