Abstract
Incomplete combustion produces a pollutant mixture that includes polycyclic aromatic hydrocarbons (PAHs). Previous work by the Columbia Center for Children's Environmental Health (CCCEH) and others linked exposure to PAH with symptoms of asthma and other adverse health effects in young children. Inhaled β2-adrenergic agonists are mainstays in the treatment of reactive airway diseases. These exogenous catecholamines engage membrane-bound β2-adrenergic receptors (β2AR) on airway epithelial and smooth muscle cells to cause airway dilation. We hypothesized that exposure to PAH might similarly interfere with the function of β2AR in airway epithelial or smooth muscle cells, reducing the efficacy of a medication important for the treatment of asthma symptoms. A PAH mixture was devised, based on ambient levels measured prenatally among a cohort of pregnant women participating at the CCCEH. Primary airway epithelial and smooth muscle cells were exposed to varying concentrations of the PAH mixture, and expression, function, and signaling of β2AR were assessed. Murine tracheal epithelial cells and human airway smooth muscle cells, after exposure to a PAH mixture, exhibited reduced expression and function of β2AR. These findings support our hypothesis that environmentally relevant PAHs can impede β2AR-mediated airway relaxation, and suggest a new paradigm where air pollutants not only contribute to the pathogenesis of childhood asthma, but also diminish responsiveness to standard therapy.
Keywords: polycyclic aromatic hydrocarbons, β2-adrenergic receptors
Incomplete combustion produces a pollutant mixture that includes high concentrations of polycyclic aromatic hydrocarbons (PAHs) (1). All of these constituents were associated with adverse respiratory effects (2–4). Although traffic emissions remain the primary source of PAHs in United States cities, PAHs are also emitted readily from industrial sources, cigarette smoke, incense burning, cooking, and space heating (5, 6). Previous work by the Columbia Center for Children's Environmental Health (CCCEH) and others linked exposure to ambient PAHs in particular with asthma-like symptoms in young children and with seroatopy (2, 7, 8). However, the mechanism responsible for these findings has not been elucidated.
Inhaled β2–adrenergic agonists are mainstays in the treatment of asthma and other reactive airway diseases. These exogenous catecholamines engage membrane-bound β2-adrenergic receptors (β2ARs) on airway epithelial and smooth muscle cells to cause airway dilation (9). Data from adipocytes indicate that PAHs impair β-receptor function without reducing membrane-bound receptor numbers (10). We hypothesized that exposure to traffic-related PAHs might interfere with the function of airway β2ARs. This effect would reduce the efficacy of an important asthma medication and worsen asthma symptoms. Our approach involved exposing two different primary lung cell types that play important roles in the pathophysiology of airway obstruction in asthma to a PAH mixture before an assessment of β2AR function. One included murine tracheal epithelial cell (MTEC) monolayers that had been grown on semipermeable supports with an apical air–liquid interface. This method recapitulates the in vivo phenotype of a high-resistance epithelium with functional cilia (11). In addition, human airway smooth muscle (HASM) cells were studied, using similar methods to determine whether PAHs alter the smooth muscle cell function and expression of β2ARs. To mimic urban ambient exposure, the PAH components in these studies were proportionally constituted to resemble New York City concentrations measured during personal monitoring of airborne levels among pregnant women from the CCCEH cohort.
Materials and Methods
Isolation of Primary mTECs
The use of animals for the present experiments was approved by the Animal Care and Use Committee of Columbia University. Mice for these studies were specific-pathogen free C57BL/6 mice obtained from Charles River Laboratories (Wilmington, MA). The mTECs from C57BL/6 mice were isolated and grown on semipermeable supports (Transwell filter inserts; Corning, Inc., Corning, NY), with an apical air–liquid interface as described elsewhere (11). Medium (DMEM–Ham's F-12) was provided initially in the upper and lower chambers. When transmembrane resistance reached 1,000 Ω · cm−2, the medium in the apical chamber was removed to create an air–liquid interface, and the lower compartment medium was changed to 2% Nuserum (BD BioSciences, San Diego, CA) with retinoic acid. This was done on Day 2 or 3 in culture. Cells were cultured for a total of 7–10 days.
Isolation of Primary HASM Cells
HASM cells were isolated from the trachealis muscles of unused human lung alographs, in accordance with guidelines of the Committee on Studies Involving Human Beings at the University of Pennsylvania, as described elsewhere (12–14) and in Methods in the online supplement. HASM cells were plated on tissue culture–treated plastic at a density of 1.0 × 104 cells/cm2 in Ham's F-12 medium, supplemented with 10% FBS and antibiotics. The viability of HASM cells according to Trypan blue exclusion after a 24-hour treatment with 1.86 ng/ml, 2.80 ng/ml, or 5.59 ng/ml PAHs reached 96%, 90%, and 85%, respectively. Cells treated with 10% DMSO reached a viability of 82%.
PAH Mixture
In previous work from the CCCEH, pregnant Dominican and African-American women, aged 18–35 years and living in the Washington Heights, Central Harlem, and South Bronx neighborhoods of New York City, wore continuously active personal air sampling devices for 48 hours during the third trimester of pregnancy. Their exposure to eight carcinogenic PAHs (benz[a]anthracene, chrysene, benzo[b]fluroanthene, benzo[k]fluroanthene, benzo[a]pyrene, indeno[1,2,3-cd]pyrene, disbenz[a,h]anthracene, and benzo[g,h,i]perylene plus pyrene) were determined as described elsewhere (2, 15). A PAH mixture that reproduced the PAH exposure of the CCCEH participants (Table 1) was produced for our cellular experiments. This PAH mixture was suspended in DMSO at 55.9 ng/ml. We then calculated the dose of PAH mixture. The concentration of ambient PAHs ranged from 1–50 ng/m3, with pyrene at the highest concentration of 50 ng/m3. The inhalation dose of pyrene was determined based on a breathing rate of 15 L/minute, exposure for 8 hours per day, a deposition of inhaled pyrene to the lung at 30% of the inhaled dose, and a lung internal surface area of 135 m2. The resulting calculated dose was approximately 1 pg pyrene/cm2 lung.
TABLE 1.
AMBIENT CCCEH PAH CONCENTRATIONS MEASURED DURING 48-HOUR PERSONAL MONITORING (N = 645), AND ASSOCIATED CONTRIBUTIONS TO IN VITRO MIXTURE
| PAH | Proportion (%) | Mean (ng/m3) | In Vitro Stock Mixture (ng/ml) |
| Benzo[a]anthracene | 3.99 | 0.27 | 2.3 |
| Benzo[a]pyrene | 5.13 | 0.42 | 2.9 |
| Benzo[b]fluoranthene | 7.62 | 0.59 | 4.3 |
| Benzo[k]fluoranthene | 1.79 | 0.15 | 1.1 |
| Benzo[g,h,i]perylene | 13.02 | 1.12 | 7.6 |
| Chrysene | 4.82 | 0.35 | 2.8 |
| Dibenzo[a,h]anthracene | 0.89 | 0.06 | 0.5 |
| Indeno[c,d]pyrene | 7.42 | 0.64 | 4.4 |
| Pyrene | 52.59 | 3.69 | 30.0 |
Definition of abbreviations: CCEH, Columbia Center for Children's Environmental Health; PAH, polycyclic aromatic hydrocarbon.
Whole-Cell Membrane Isolation, Western Blot Analysis, and Real-Time PCR
The expression of membrane-bound β2AR was evaluated via Western blot analysis of whole-cell membrane fractions, as described previously (16). For Western blot analysis, 10 μg of whole-cell membrane protein/lane were probed with rabbit anti-mouse or anti-human β2AR antibody (Santa Cruz Scientific, Santa Cruz, CA) and peroxidase-coupled secondary antibodies. To verify equivalent sample loading, blots were stripped and reprobed with murine monoclonal anti-actin antibodies (Chemicon International, Temecula, CA). Quantitative, real-time RT-PCR, using human and murine β2AR primers (Taqman; Applied Biosystems, Foster City, CA) and Applied Biosystem reverse transcriptase reagents, was used to evaluate the steady-state expression of β2AR mRNA. β2AR copy numbers were normalized to copy numbers of glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Measurement of Cellular Cyclic Adenosine Monophosphate Production (β2AR Function)
β2AR function was assessed by measuring concentrations of whole-cell cyclic adenosine monophosphate (cAMP) after treating cells with the β2AR-specific agonist procaterol (10−6 M; 15 minutes at 37°C). In all experiments, cells were pretreated with 10−4 M 3,7-dihydro-1-methyl-3-(2-methylpropyl)-1H-purine-2,6-dione for 15 minutes to inhibit phosphodiesterases. To assess the function of adenylyl cyclase, cells were treated with forskolin (2 × 10−5 M) for 15 minutes at 37°°C. We used an enzyme immunoassay (Cayman Chemical, Ann Arbor, MI), as described previously (17), to quantify cAMP.
Results
Treatment with PAHs Reduces the Function of β2ARs (Procaterol-Induced cAMP Production) and Expression in mTECs
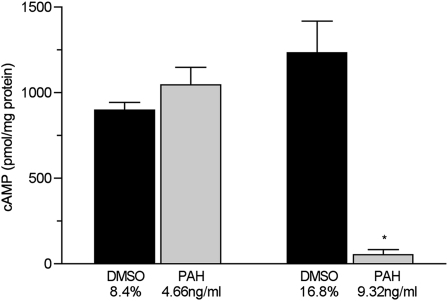
The production of cAMP by cells treated with 9.32 ng/ml of the PAH mixture was reduced by 95% (P = 0.001, versus control cells treated with vehicle, i.e., 16.7% DMSO, n = 6 inserts/condition) (Figure 1). The production of cAMP by cells treated with 4.66 ng/ml of PAHs for 24 hours did not differ from that in vehicle-treated control cells (8.4% DMSO).
Figure 1.
Concentrations of whole-cell, procaterol-induced cyclic adenosine monophosphate (cAMP) in primary murine tracheal epithelial cells exposed to the indicated concentrations of polycyclic aromatic hydrocarbons (PAHs) in DMSO or DMSO-only vehicle in complete medium for 24 hours (n = 6 filters/condition). *P < 0.001, versus all other groups.
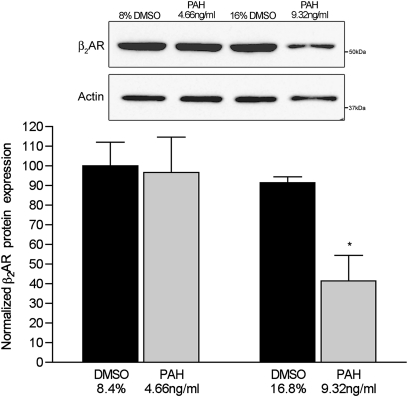
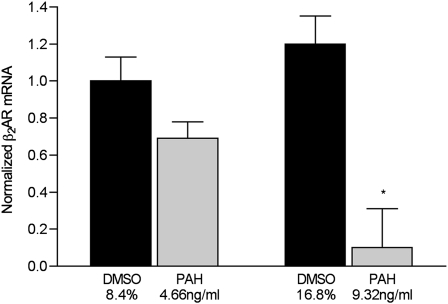
As seen in Figure 2, membrane-bound β2AR protein concentrations in cells exposed to 9.32 ng/ml of PAH were reduced by 61%, compared with vehicle-treated control cells (n = 6 filters/condition, P = 0.001, versus control cells treated with 16.7% DMSO). The expression of membrane-bound receptor was not different from that of control samples in cells treated with 4.66 ng/ml of PAHs for 24 hours (n = 6 filters/condition, P = 0.2 for 4.66 ng/ml PAHs, versus control cells treated with 8.4% DMSO). We also assessed concentrations of β2AR mRNA, normalized to GAPDH, in cells treated with 9.32 and 4.66 ng/ml PAHs for 24 hours. The higher concentration of PAHs reduced the steady-state β2AR message by more than 90% (P < 0.01, versus all other groups; n = 6 filter supports/condition) (Figure 3).
Figure 2.
Concentrations of membrane-bound β2–adrenergic receptors (β2ARs) in murine tracheal epithelial cells exposed to PAHs for 24 hours. Western blot represents whole-cell membrane fractions probed with an anti-human β2AR antibody. Graphical data represent optical density of a 52-kD band normalized to same sample actin concentrations to control for interlane loading variations, and then to control cells exposed to vehicle only (8.4% and 16.8% DMSO in complete medium). Band intensity was arbitrarily set at 100 for control cells treated with 8.4% DMSO, and normalized to actin. *P < 0.01, versus all other groups.
Figure 3.
Expression of normalized β2AR mRNA in murine tracheal epithelial cells exposed to the indicated concentrations of PAHs in DMSO or DMSO-only vehicle in complete medium for 24 hours. Messenger RNA was quantified according to real-time RT-PCR, as described in Materials and Methods. The data are normalized to same-cell glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA. Signal intensity was arbitrarily set at 100 for control cells treated with 8.4% DMSO, to permit comparisons among groups. *P < 0.01 versus all other groups (n = 6 samples/condition).
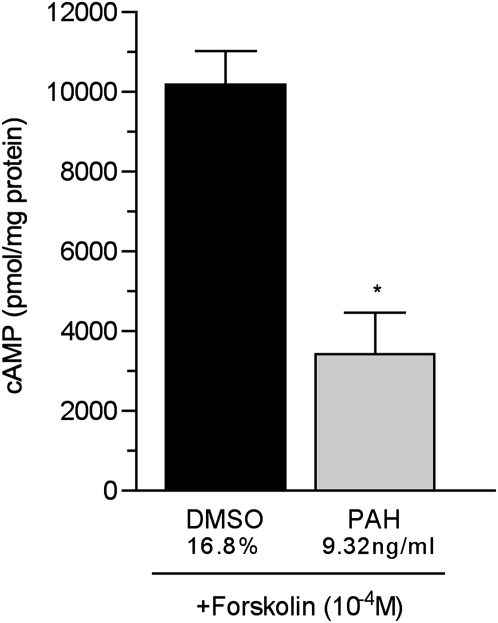
β2AR is a G-protein–coupled receptor that affects concentrations of cAMP through the activation of adenylyl cyclase. The changes in concentrations of whole-cell cAMP shown in Figure 1 could reflect the reduced functional β2AR in the cell membrane, as suggested by the data shown in Figure 2, or the impairment of the β2AR signal transduction pathway. To test for the effects of PAHs on the β2AR signal transduction pathway, cells were treated with an established concentration of the adenylyl cyclase activator forskolin (2 × 10−5 M for 15 minutes) (16). As seen in Figure 4, the forskolin-induced production of cAMP was reduced by 68% in cells treated with 9.32 ng/ml of PAHs for 24 hours, compared with control cells treated with vehicle (16.7% DMSO). These data imply that a proximal component of the β2AR signal transduction pathway is affected by PAHs.
Figure 4.
Concentrations of whole-cell cAMP in murine tracheal epithelial cells exposed to 9.32 ng/ml PAHs for 24 hours. cAMP was measured after treatment with the adenylyl cyclase activator forskolin (2 × 10−5 M) for 15 minutes, to test for adenylyl cyclase function. The data represent cAMP in pmol/mg protein. Control samples were treated with the same concentration of vehicle (16.8% DMSO in complete medium) as the 9.32 ng/ml PAH–treated cells. *P = 0.002, versus vehicle-treated control samples.
Treatment with PAHs Reduces the Function of β2AR (Procaterol-Induced Production of cAMP) and Expression in HASM Cells
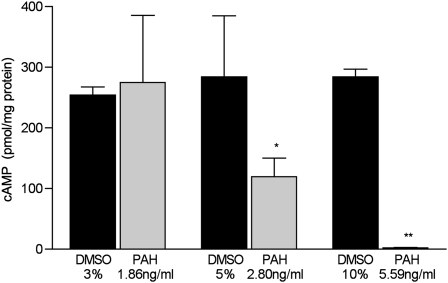
The function of β2AR was assessed in HASM cells by measuring whole-cell concentrations of cAMP after treatment with the β2AR-specific agonist procaterol. As shown in Figure 5, cells treated with 1.86 ng/ml of PAHs showed no reduction in β2AR function, whereas cells treated with 2.80 ng/ml of PAHs demonstrated a β2AR function at 41% of control cells (P = 0.02, versus controls treated with 5% DMSO; n = 6 dishes/condition). HASM cells exposed to 5.59 ng/ml of PAHs demonstrated a β2AR function that was less than 1% of that in control cells treated with 10% DMSO (P = 0.001, versus control cells; n = 6 dishes/condition). The PAH concentrations used in these experiments were 10-fold lower than those used in mTECs, implying that the function of HASM β2AR is more sensitive to PAHs than to mTECs.
Figure 5.
Concentrations of whole-cell cAMP in human airway smooth muscle cells treated with the indicated concentrations of PAH for 24 hours. The data represent pmol of cAMP/mg protein measured in cell lysates after 24-hour treatment with PAHs or vehicle (Control). Control cells were treated with the same concentration of vehicle as PAH cells (3%, 5%, or 10% DMSO in complete medium for 1.86, 2.80, and 5.59 ng/ml PAHs, respectively; n = 6 dishes/condition). *P = 0.05, versus same dilution of vehicle-treated control samples. **P = 0.001, versus same dilution of vehicle-treated control samples.
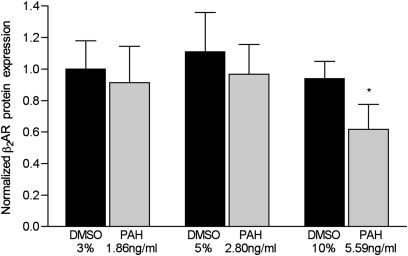
The function of β-receptor in HASM cells treated with the highest concentration of PAHs (5.59 ng/ml) was reduced by 34% (P = 0.04, versus controls treated with 10% DMSO; n = 6 dishes/condition) (Figure 6). Lower concentrations exerted no measurable effect on the function of β2AR in these cells. Despite repeated attempts, β2AR mRNA could not be measured, using a variety of methodologies in HASM cells.
Figure 6.
Concentrations of membrane-bound β2AR in human airway smooth muscle (HASM) cells treated with the indicated concentrations of PAHs for 24 hours. The expression of protein was quantified according to the immunoblot band density, which was then normalized to same-sample actin band density. Control cells were treated with the indicated concentrations of DMSO in complete medium for 24 hours (n = 6 dishes/condition). *P = 0.04, versus control HASM cells treated with 10% DMSO vehicle.
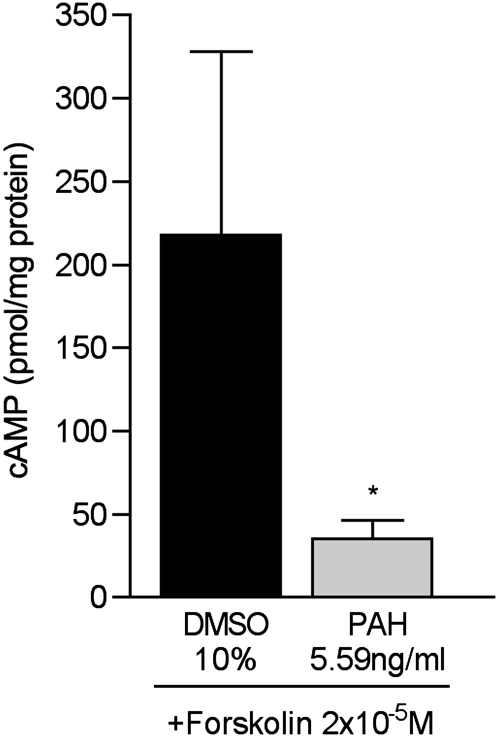
Finally, the forskolin treatment of HASM cells treated with 5.59 ng/ml of PAHs produced whole-cell concentrations of cAMP that were reduced by 84%, compared with those in vehicle-treated control cells (Figure 7) (P = 0.01, versus control cells). The reduced production of cAMP in response to forskolin treatment indicates the reduced function of adenylyl cyclase, a proximal component of the β2AR signal transduction pathway in HASM cells.
Figure 7.
Whole-cell cAMP concentrations in human airway smooth muscle cells treated with 5.59 ng/ml PAHs in 10% DMSO for 24 hours, and then treated with the adenylyl cyclase activator forskolin (2 × 10−5 M) for 15 minutes before the determination of [cAMP]. The data represent cAMP in pmol/mg protein (n = 6 dishes/condition). *P = 0.01, versus control samples treated with 10% DMSO alone.
Discussion
These results indicate that an environmentally relevant mixture of PAHs impairs multiple aspects of the β2AR signal transduction pathway. These findings apply to both primary lung epithelial cells and airway smooth muscle cells. Previously published data from animal and cell-culture studies indicate that airway epithelial cell (18–22) and inflammatory cell (22) β2ARs can be desensitized by β-agonists (23, 24). The regular use of inhaled β-agonists increases bronchial hyperresponsiveness, diminishes β-agonist protection from antigenic stimuli, and causes tolerance to β-agonists, possibly via agonist-induced receptor desensitization (25). The present novel results suggest that the function of β2AR also may be impaired after exposure to traffic-related air pollution, and to PAHs specifically. The loss of receptor function can be attributed to either decreased numbers of receptors in the cell membrane (down-regulation) or a loss of receptor responsiveness to its ligands, because of changes in the receptor or any portion of its signal transduction pathway (desensitization). studying this study, we noted fewer receptors in the cell membrane according to Western blot analysis, and decreased function of adenylyl cyclase, a key member of the β2-receptor signal transduction pathway. Thus both receptor down-regulation and desensitization appear to play a role in the PAH-mediated loss of β2AR function in HASM cells and mTECs. How PAHs affect other pathways of receptor desensitization, such as receptor phosphorylation by β–adrenergic receptor kinases or G-protein expression and function, remains to be established.
The biological mechanisms responsible for the development of asthma symptoms after exposure to air pollution are complex. Several pathways appear important. In one, air pollutants such as diesel, particulate matter, and metals, known triggers of asthma, may induce oxidative stress pathways, causing the formation of excessive reactive oxygen species in the airways and tissue inflammation (3, 4, 26–29). In a second pathway, exposure to diesel may provide a strong adjuvant for allergic sensitization, and such exposure was shown to up-regulate allergic immune mechanisms in the airways (4, 30–33). PAHs, which are components of exhaust from the incomplete combustion of diesel fuel, were linked directly with the development of respiratory disease by our group (2, 7). Mechanistic experiments so far suggest that the inhalation of PAHs can cause acute airway inflammation via the induction of genes associated with the aryl hydrocarbon receptor, oxidative stress, and inflammation (34). In addition, exposure to PAHs such as diesel was associated with a significantly enhanced up-regulation of proallergic cytokine and immunoglobulin production in vivo (8, 35, 36).
This, to the best of our knowledge, is the first study to suggest that another mechanism for the air pollution–related symptoms of asthma may involve the PAH-induced impairment of β2AR signaling. One other similar study indicated that the exposure of adipocytes to PAHs impaired the function of β2AR, without reducing membrane-bound receptor numbers (10).
We acknowledge several limitations to this study. First, like other work in cell systems, the results are not necessarily translatable to what occurs in vivo. The choice of primary cells, alogn with our implementation during the culture of mTECs of the semipermeable supports for an apical air–liquid interface, was intended to improve the translation of these results. In addition, the PAHs studied here comprised a selection of what may be measured in urban air as a consequence of traffic emissions and other sources of pollution. Future studies in animal models and cohort work are needed to validate these findings clinically.
In conclusion, these studies create a new paradigm for asthma morbidity. An environmentally relevant mixture of PAHs may interfere with a key regulatory molecule that is responsible for bronchomotor tone. This new paradigm offers a novel mechanism by which air pollutants may interfere with the treatment for asthma, and contribute to the substantial morbidity associated with this disease. The potential public health impact is large because the prevalence of childhood asthma in urban areas ranges from 8–12% (37, 38).
Supplementary Material
Footnotes
This work was supported by National Institutes of Health grants P50ES015905, P01ES09600, and R01ES008977, and Environmental Protection Agency grant RD-83214101.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0499OC on May 26, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Tewarson A. Ventilation effects on combustion products. Toxicology 1996;115:145–156 [DOI] [PubMed] [Google Scholar]
- 2.Miller RL, Garfinkel R, Horton M, Camann D, Perera F, Whyatt RM, Kinney PL. Polycyclic aromatic hydrocarbons, environmental tobacco smoke, and respiratory symptoms in an inner-city birth cohort. Chest 2004;126:1071–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel MM, Hoepner L, Garfinkel R, Chillrud S, Reyes A, Quinn JW, Perera F, Miller RL. Ambient metals, elemental carbon, and wheeze and cough in New York City children through 24 months of age. Am J Respir Crit Care Med 2009;180:1107–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel MM, Miller RL. Air pollution and childhood asthma: recent advances and future directions. Curr Opin Pediatr 2009;21:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjorseth A, Ramdahl T. Handbook of polycyclic 1 aromatic hydrocarbons: emission 2 sources and recent progress in analytical chemistry. New York: Marcel Dekker; 1985 [Google Scholar]
- 6.Chuang J, Mack G, Kuhlman M, Wilson N. Polycyclic aromatic hydrocarbons and their derivatives in indoor and outdoor air in an eight-home study. Atmos Environ, B Urban Atmos 1991;25:369–380 [Google Scholar]
- 7.Jedrychowski W, Galas A, Pac A, Flak E, Camman D, Rauh V, Perera F. Prenatal ambient air exposure to polycyclic aromatic hydrocarbons and the occurrence of respiratory symptoms over the first year of life. Eur J Epidemiol 2005;20:775–782 [DOI] [PubMed] [Google Scholar]
- 8.Miller RL, Garfinkel R, Lendor C, Hoepner L, Li Z, Romanoff L, Sjodin A, Needham L, Perera FP, Whyatt RM. Polycyclic aromatic hydrocarbon metabolite levels and pediatric asthma–related outcomes in an inner city. Pediatr Allergy Immunol 2010;21:260–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes P. Receptor heterodimerization: a new level of cross-talk. J Clin Invest 2006;116:1210–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irigaray P, Ogier V, Jacquenet S, Notet V, Sibille P, Mejean L, Bihain BE, Yen FT. Benzo[a]pyrene impairs beta-adrenergic stimulation of adipose tissue lipolysis and causes weight gain in mice: a novel molecular mechanism of toxicity for a common food pollutant. FEBS J 2006;273:1362–1372 [DOI] [PubMed] [Google Scholar]
- 11.You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol 2002;283:L1315–L1321 [DOI] [PubMed] [Google Scholar]
- 12.Panettieri RA, Murray RK, DePalo LR, Yadvish PA, Kotlikoff MI. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol 1989;256:C329–C335 [DOI] [PubMed] [Google Scholar]
- 13.Panettieri RA, Jr, Hall IP, Maki CS, Murray RK. Alpha-thrombin increases cytosolic calcium and induces human airway smooth muscle cell proliferation. Am J Respir Cell Mol Biol 1995;13:205–216 [DOI] [PubMed] [Google Scholar]
- 14.Hauck RW, Schulz C, Schomig A, Hoffman RK, Panettieri RA Jr. Alpha-thrombin stimulates contraction of human bronchial rings by activation of protease-activated receptors. Am J Physiol 1999;277:L22–L29 [DOI] [PubMed] [Google Scholar]
- 15.Perera FP, Rauh V, Tsai WY, Kinney P, Camann D, Barr D, Bernert T, Garfinkel R, Tu YH, Diaz D, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect 2003;111:201–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mutlu GM, Adir Y, Jameel M, Akhmedov AT, Welch L, Dumasius V, Meng FJ, Zabner J, Koenig C, Lewis ER, et al. Interdependency of beta–adrenergic receptors and CFTR in regulation of alveolar active Na+ transport. Circ Res 2005;96:999–1005 [DOI] [PubMed] [Google Scholar]
- 17.Factor P, Mutlu GM, Chen L, Mohameed J, Akhmedov AT, Meng FJ, Jilling T, Lewis ER, Johnson MD, Xu A, et al. Adenosine regulation of alveolar fluid clearance. Proc Natl Acad Sci USA 2007;104:4083–4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penn RB, Shaver JR, Zangrilli JG, Pollice M, Fish JE, Peters SP, Benovic JL. Effects of inflammation and acute beta-agonist inhalation on beta 2–AR signaling in human airways. Am J Physiol 1996;271:L601–L608 [DOI] [PubMed] [Google Scholar]
- 19.Penn RB, Panettieri RA, Benovic JL. Mechanisms of acute desensitization of the beta2AR–adenylyl cyclase pathway in human airway smooth muscle. Am J Respir Cell Mol Biol 1998;19:338–348 [DOI] [PubMed] [Google Scholar]
- 20.Turki J, Green SA, Newman KB, Meyers MA, Liggett SB. Human lung cell beta 2–adrenergic receptors desensitize in response to in vivo administered beta-agonist. Am J Physiol 1995;269:L709–L714 [DOI] [PubMed] [Google Scholar]
- 21.Pian MS, Dobbs LG. Evidence for G beta gamma–mediated cross-talk in primary cultures of lung alveolar cells: pertussis toxin–sensitive production of cAMP. J Biol Chem 1995;270:7427–7430 [DOI] [PubMed] [Google Scholar]
- 22.Yukawa T, Ukena D, Kroegel C, Chanez P, Dent G, Chung KF, Barnes PJ. Beta 2–adrenergic receptors on eosinophils: binding and functional studies. Am Rev Respir Dis 1990;141:1446–1452 [DOI] [PubMed] [Google Scholar]
- 23.Nishikawa M, Mak JC, Barnes PJ. Effect of short- and long-acting beta 2–adrenoceptor agonists on pulmonary beta 2–adrenoceptor expression in human lung. Eur J Pharmacol 1996;318:123–129 [DOI] [PubMed] [Google Scholar]
- 24.Nishikawa M, Mak JC, Shirasaki H, Harding SE, Barnes PJ. Long-term exposure to norepinephrine results in down-regulation and reduced mRNA expression of pulmonary beta–adrenergic receptors in guinea pigs. Am J Respir Cell Mol Biol 1994;10:91–99 [DOI] [PubMed] [Google Scholar]
- 25.Beasley R, Pearce N, Crane J, Burgess C. Beta-agonists: what is the evidence that their use increases the risk of asthma morbidity and mortality? J Allergy Clin Immunol 1999;104:S18–S30 [DOI] [PubMed] [Google Scholar]
- 26.Gilliland FD, McConnell R, Peters J, Gong H. A theoretical basis for investigating ambient air pollution and children's respiratory health. Environ Health Perspect 1999;107:403–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L, Poon R, Chen L, Frescura A-M, Montuschi P, Ciabattoni G, Wheeler A, Dales R. Acute effects of air pollution on pulmonary function, airway inflammation, and oxidative stress in asthmatic children. Environ Health Perspect 2009;117:668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romieu I, Barraza-Villarreal A, Escamilla-Nun C, Almstrand A-C, Diaz-Sanchez D, Sly PD, Olin A-C. Exhaled breath malondialdehyde as a marker of effect of exposure to air pollution in children with asthma. J Allergy Clin Immunol 2008;121:903–999 [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Ballaney M, Al-Alem U, Quan C, Jin X, Perera F, Chen L-C, Miller RL. Combined inhaled diesel exhaust particles and allergen exposure alter methylation of T helper genes and IgE production in vivo. Toxicol Sci 2008;102:76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riedel M, Diaz-Sanchez D. Biology of diesel exhaust effects on respiratory function. J Allergy Clin Immunol 2005;115:221–228 [DOI] [PubMed] [Google Scholar]
- 31.Diaz-Sanchez D, Proietti L, Polosa R. Diesel fumes and the rising prevalence of atopy: an urban legend? Curr Allergy Asthma Rep 2003;3:146–152 [DOI] [PubMed] [Google Scholar]
- 32.Batesona TF, Schwartz J. Children's response to air pollutants. J Toxicol Environ Health A 2008;71:238–243 [DOI] [PubMed] [Google Scholar]
- 33.Diaz-Sanchez D, Garcia MP, Wang M, Jyrala M, Saxon A. Nasal challenge with diesel exhaust particles can induce sensitization to a neoallergen in the human mucosa. J Allergy Clin Immunol 1999;104:1183–1188 [DOI] [PubMed] [Google Scholar]
- 34.Rouse RL, Murphy G, Boudreaux MJ, Paulsen DB, Penn AL. Soot nanoparticles promote biotransformation, oxidative stress, and inflammation in murine lungs. Am J Respir Cell Mol Biol 2008;39:198–207 [DOI] [PubMed] [Google Scholar]
- 35.Li N, Wang M, Bramble LA, Schmitz DA, Schauer JJ, Sioutas C, Harkema JR, Nel AE. The adjuvant effect of ambient particulate matter is closely reflected by the particulate oxidant potential. Environ Health Perspect 2009;117:1116–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens T, Cho S-H, Linak WP, Gilmour MI. Differential potentiation of allergic lung disease in mice exposed to chemically distinct diesel samples. Toxicol Sci 2009;107:522–534 [DOI] [PubMed] [Google Scholar]
- 37.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, Scalia MR, Akinbam LJ. National surveillance for asthma: United States, 1980–2004. MMWR Morb Mortal Wkly Rep 2007;56:1–14, 18–54 [PubMed] [Google Scholar]
- 38.Lotz T. Asthma mortality and hospitalizations among children and young adults. MMWR Morb Mortal Wkly Rep 1996;45:350–353 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.