Abstract
Allergic asthma is characterized by acute influxes of proinflammatory leukocytes in response to allergen stimulation, followed by quiescent (chronic) periods between allergen challenges, during which sustained, low-level inflammation is evident. These chronic phases of disease are thought to be mediated by populations of leukocytes persisting within airways and tissues. The lack of any in situ proliferation by these cells, along with their limited lifespan, suggests that a continual recruitment of leukocytes from the circulation is needed to maintain disease chronicity. The mechanisms regulating this persistent recruitment of leukocytes are unknown. Although classic leukocyte-attracting chemokines are highly elevated after acute allergen challenge, they return to baseline levels within 24 hours, and remain close to undetectable during the chronic phase. In the present study, we investigated whether an alternative family of chemoattractants, namely, extracellular cyclophilins, might instead play a role in regulating the recruitment and persistence of leukocytes during chronic asthma, because their production is known to be more sustained during inflammatory responses. Using a new murine model of chronic allergic asthma, elevated concentrations of extracellular cyclophilin A, but not classic chemokines, were indeed detected during the chronic phase of asthma. Furthermore, blocking the activity of cyclophilins during this phase reduced the number of persisting leukocytes by up to 80%. This reduction was also associated with a significant inhibition of acute disease reactivation upon subsequent allergen challenge. These findings suggest that blocking the function of cyclophilins during the chronic phase of asthma may provide a novel therapeutic strategy for regulating disease chronicity and severity.
Keywords: chronic asthma, cyclophilins, cyclosporine A, NIM811
Clinical Relevance
This study focused on reducing the chronic rather than acute phase of allergic asthma. We show that targeting an alternative family of chemoattractants, namely, extracellular cyclophilins, can lead to a reduced persistence of leukocytes during the chronic phase, and also reduces the severity of subsequent acute asthma reactivation. These findings provide a potential novel target for reducing chronic inflammation in allergic asthma.
Allergic asthma is a complex disease of airway inflammation that results from a variety of genetic and environmental factors, and is characterized by acute airway constriction, the hypersecretion of mucus, and lung inflammation (1). Although their exact immunopathophysiology is not clearly understood, acute asthmatic responses are known to be driven by specific subsets of leukocytes, namely, antigen-specific T cells and IgE-switched B-cells, mast cells, and eosinophils, as well as neutrophils in some cases (2). Samples from the airways of patients with asthma show an increased presence of CD4+ T cells expressing the activation markers CD25 and major histocompatibility complex Class II (3, 4). These activated CD4+ T cells create a cytokine environment that favors the initiation and perpetuation of the asthmatic response, making them important regulators of disease pathogenesis (2, 5, 6). The Th2-associated cytokines IL-4, IL-5, and IL-13 are found at increased concentrations in asthmatic airway cells and fluid, and they colocalize with T-cell markers in biopsies (7, 8). These Th2 cytokines promote the generation of IgE-secreting B-cells, and the mobilization and activation of eosinophils (9). After they are recruited and activated, these leukocytes exacerbate inflammation by secreting a myriad of cytokines, chemokines, and enzymes, resulting in the additional recruitment of proinflammatory leukocytes, tissue damage, and airway constriction.
Allergic asthma is a chronic disease insofar as airway inflammation is never completely resolved, even in the absence of acute allergen challenge. One of the downstream effects of this unresolved inflammation involves the increased deposition of collagen, resulting in airway remodeling that can drastically reduce airway diameter (10). Another hallmark of chronic asthma involves the sustained presence of proinflammatory leukocytes within airways and submucosal tissue during the quiescent (or chronic) phases of the disease, despite an absence of allergen. Indeed, elevated numbers of eosinophils, activated lymphocytes, and mast cells, as well as increased concentrations of IL-5, were found in biopsies of patients with asthma in clinical remission (11). Along with promoting tissue pathology, the sustained presence of activated, antigen-specific T cells and other effector leukocytes within asthmatic airways and tissues creates an environment that is primed for rapid re-initiation upon allergen challenge. The factors regulating the observed persistence of leukocytes in the absence of allergen stimulation are unknown. However, insofar as most effector leukocytes have a limited lifespan (12–14) and do not proliferate in situ (15), the persistent airway inflammation seen during chronic asthma must involve recruitment stimuli to maintain an elevated numbers of leukocytes.
Obvious candidates that could regulate this recruitment comprise the chemokines known to be associated with asthma, including eotaxins 1–3, regulated upon activation, normal T-cell expressed and presumably secreted (RANTES), macrophage inflammatory protein (MIP)-1a, and monocyte chemotactic protein (MCP)-1, all of which were shown to increase after exposure to allergens. Although an acute burst of production of these classic chemokines occurs within 2–4 hours of exposure, they return to baseline concentrations within 24 hours (16, 17). In addition, studies in which patients with asthma were sampled during remission phases of their disease showed concentrations of chemokines similar to those in healthy control subjects, despite elevated numbers of eosinophils and T cells in their lung airways (11). Similar findings were reported for eotaxin in a guinea pig model of asthma (18), and for eotaxin, RANTES, MIP-1α, and MCP-1 in a murine model (19). These observations demonstrate a timeline whereby the majority of chemokines associated with the recruitment of asthma-associated leukocytes, including T cells and eosinophils, are produced acutely after allergen challenge, but return to low, or even baseline, concentrations within 24 hours. This finding begs the question of how the recruitment of leukocytes may be regulated during the chronic phases of asthma, when acute allergen challenge is absent. Although low, residual concentrations of chemokines may be sufficient to mediate this recruitment, alternative types of chemoattractants may take over as regulatory factors.
Cyclophilins are present in high abundance in all eukaryotic cells (20). Although cyclophilins exhibit many different functions (20), they are probably best known as receptors for the immunosuppressive drug cyclosporine A (CsA) (21). However, cyclophilins can also be secreted in response to inflammatory stimuli (22, 23), and high concentrations of extracellular cyclophilins were reported in several inflammatory diseases (24–26). Interestingly, extracellular cyclophilins demonstrate potent chemoattractant properties both in vitro (27–30) and in vivo (23), suggesting a capacity to contribute to the recruitment of leukocytes during inflammatory responses. In support of this idea, we previously showed that blocking cyclophilin function in vivo, using various nonimmunosuppressive analogues of CsA, reduced the recruitment of leukocytes into inflamed tissues by 40–80% in two different murine models of acute lung inflammation (27, 31). In one of these, an ovalbumin (OVA)–induced model of acute allergic asthma, the inhibition of cyclophilins resulted not only in a reduced influx of leukocytes into the airways and lung tissue, but also led to reductions in Th2-associated cytokines, mucus hypersecretion, and airway resistance (31).
Although these studies demonstrated a significant role for cyclophilins in mediating the recruitment of leukocytes during the acute phase of an asthmatic response, their potential contribution to the chronic recruitment of leukocytes was not examined. Thus, in the present study, we investigated whether inhibiting the function of cyclophilins during the chronic phase of asthma would lead to a reduction in the persistence of pulmonary leukocytes, and whether this reduction would in turn exert an impact on the severity of subsequent acute responses upon allergen challenge (i.e., acute reactivation).
Several murine models of chronic allergic lung inflammation that mimic most of the hallmark features of chronic human asthma, including the persistence of leukocytes, were previously described (32). Using a modified version of one of these models, we show here that: (1) extracellular cyclophilins (but not classic chemokines) are present during the chronic phase of disease; (2) inhibiting the function of cyclophilins during the chronic phase leads to a 45–80% reduction in the persistence of leukocytes; and (3) inhibiting the function of cyclophilins during the chronic phase also results in a significant decrease in the severity of acute disease reactivation upon allergen challenge, including reduced influx of leukocytes, tissue pathology, and airway hyperreactivity.
Materials and Methods
Murine Model of Chronic Asthma
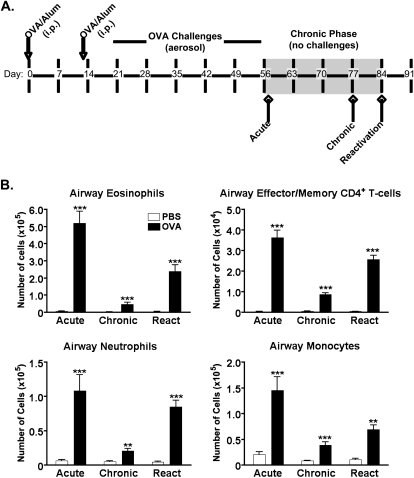
Female BALB/c mice were purchased from commercial vendors. A previously published model of chronic asthma (33) was adapted for the present study. Mice were sensitized by an intraperitoneal injection of 50 μg ovalbumin (OVA) in alum on Days 0 and 13 (Figure 1A, arrows), and were then challenged seven times between Days 18–28 for 20 minutes per day with aerosolized 5% OVA (or PBS), followed by three times per week for 4 weeks. To mimic the chronic (quiescent) phase of asthma, mice were housed for 4 weeks without challenge (Figure 1A, shaded area). Acute responses were then reactivated by two challenges of aerosolized 5% OVA (or PBS). For kinetics experiments, groups of animals were killed 24 hours after the final OVA challenge (Acute), during the chronic phase (Chronic), or 24 hours after the final reactivation challenge (Reactivation).
Figure 1.
Kinetics of leukocytic infiltration in a murine model of chronic allergic asthma. (A) Female BALB/c mice were sensitized by intraperitoneal injection of 100 μg ovalbumin (OVA) in alum on Days 0 and 13. Mice were then challenged with an aerosolized solution of 5% OVA in PBS (or PBS alone) seven times between Days 19–28, and then three times per week for 4 weeks. Groups of mice were either killed 1 day after the final aerosol challenge (Acute), or housed for an additional 4 weeks without OVA challenge (Chronic Phase). An acute response was then reactivated by an additional aerosolized OVA challenge (Reactivation). (B) Leukocytic infiltration was assessed in the airways by flow cytometric analyses of bronchoalveolar lavage (BAL) fluid collected at three time points: 24 hours after the final repetitive challenge (Acute), 3 weeks into the chronic phase (Chronic), and 24 hours after the final reactivation challenge (Reactivation). Data show the mean ± SEM for numbers of leukocytes at the Acute, Chronic, and Reactivation time points. A Student t test was used to establish significant differences between the OVA and PBS groups (n = 6–12 mice per group). **P < 0.005. ***P < 0.0005.
N–Methyl-4–Isoleucine–Cyclosporin Intervention
The nonimmunosuppressive CsA analogue, N–methyl-4–isoleucine–cyclosporin (NIM811) (34, 35), was generously provided by the Novartis Institute for Biomedical Research (Cambridge, MA). Based on previous studies (31), 200-μg doses of NIM811 were delivered in 15% Cremophor-EL (Sigma-Aldrich, St. Louis, MO) by intraperitoneal injections given twice per week during the 4-week chronic phase.
Analysis of Airway Cells and Tissues
Airway fluid and cells were collected by bronchoalveolar lavage (BAL). The supernatant (BALF) was separated, and cell pellets were treated with red blood cell lysis buffer and counted. Cells were stained for flow cytometric analysis, using the antibodies PE–Cy5–anti-CD4 plus FITC–anti-CD62L (effector/memory CD4+ T cells), APC–anti-Gr1 (neutrophils), and PE–anti-CD11c (airway monocytes). Eosinophils were identified according to their forward scatter/side scatter distribution, as previously described (31). Whole lungs were processed for histologic analysis, as previously described (31). Six-micrometer sections were stained with Masson's Trichrome and periodic acid–Schiff (PAS; Histoserv, Inc., Germantown, MD). Changes in the deposition of mucus and collagen were scored blindly, using a previously described semiquantitative scale (36) ranging from 0 (normal) to 4 (pronounced mucus or collagen along the airways).
Measuring Extracellular Cyclophilins and Chemokines
Extracellular cyclophilins A (CypA) and B (CypB) were quantified by Western blot analysis, as previously described (31). Concentrations of MCP-1, MCP-3, RANTES, MIP-1α, MIP-1β, and granulocyte-macrophage colony-stimulating factor (GM-CSF) were assessed using a Bender MedSystems FlowCytomix bead array (eBioscience, San Diego, CA). Concentrations of eotaxin and IL-16 were measured by ELISA (R&D Systems, Minneapolis, MN).
Measuring Airway Hyperresponsiveness
Airway resistance in response to increasing doses of acetyl-β–methylcholine (Sigma-Aldrich) was measured using the FinePointeRC system (Buxco Research Systems, Wilmington, NC). Measurements were performed 24 hours after the final reactivation challenge, as previously described (31).
Chemotaxis Assays
Chemotaxis assays were performed as previously described for activated CD4+ T cells (31) and for monocytes and neutrophils (37). Purified leukocytes (104) were added to upper wells, and 100 ng/ml (neutrophils and CD4+ T cells) or 200 ng/ml (monocytes) CypA was added to the lower wells, as previously optimized (27–29, 31). The activity of CypA was blocked with 2 μM NIM811 added to the upper and lower wells.
Statistical Analyses
Two-tailed unpaired t tests were used to compare the two experimental groups, and two-way ANOVA (with the Bonferroni post hoc test) was used for comparisons of airway hyperresponsiveness.
Results
Murine Model of Chronic Allergic Asthma Demonstrates Persistence of Leukocytes during the Chronic Phase
To determine the contribution of cyclophilins to disease severity during chronic allergic asthma, we first had to establish and characterize a suitable murine model that would provide us with the persistence of leukocytes and acute reactivation responses observed in human disease. For this, we adapted a model of chronic asthma described by McMillan and Lloyd (33). Figure 1A shows the optimized regimen used in all our present experiments. For the initial kinetics experiments, we examined changes in leukocyte numbers at various time points during the regimen: 24 hours after an acute challenge (Acute), 3 weeks into the chronic phase (Chronic), and 24 hours after the acute reactivation challenge (Reactivation). As shown in Figure 1B, a robust influx of eosinophils and CD4+ effector/memory T cells (CD4+/CD62Llo), as well as increases in neutrophils and monocytes, were evident in the airways of OVA-challenged mice at the Acute time point. Importantly, this inflammation never completely resolved, even after 3 weeks without OVA challenges, as demonstrated by the persisting numbers of the four cell subsets in the OVA group, relative to the PBS group, at the Chronic time point. The reactivation of an acute response by re-exposure to OVA challenges resulted in a marked influx of all subsets of leukocytes.
Extracellular CypA but Not Classic Chemokines Remain Elevated during the Chronic Phase of Allergic Asthma
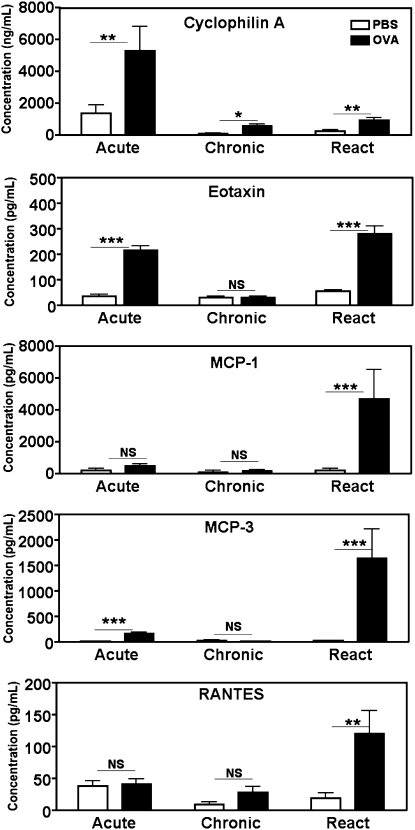
We next investigated whether extracellular cyclophilins (CypA and CypB) or classic chemokines might be present during the chronic phase of our asthma model. Elevated concentrations of several chemoattractants (CypA, eotaxin, RANTES, MCP-1, and MCP-3) were evident at the Acute or Reactivation time points (Figure 2). Another chemoattractant reported to be associated with the recruitment of T cells in asthma, IL-16 (38), was also elevated during Reactivation (data not shown). However, only CypA remained significantly above baseline concentrations (relative to PBS control mice) at the Chronic time point. In terms of relative differences, the concentrations of CypA in the OVA groups were 3.9-fold and 3.8-fold above those of their PBS cohorts at the Acute and Reactivation time points, respectively, and 5.6-fold above that of the PBS group at the Chronic time point. CypB remained at baseline concentration throughout the regimen, and concentrations of MIP-1α, MIP-1β, and GM-CSF were below the sensitivity threshold of the cytometric bead array (data not shown). Taken together, these findings suggest that, unlike classic chemokines, extracellular CypA persists during the chronic phase of allergic asthma.
Figure 2.
Elevated concentrations of cyclophilin A (CypA) are present during the chronic phase of asthma. Concentrations of CypA, eotaxin, regulated upon activation, normal T-cell expressed and presumably secreted (RANTES), monocyte chemotactic protein (MCP)-1, and MCP-3 were measured in the BAL fluid of mice killed at the time points specified in Figure 1. Measurements were determined via Western blot analysis (CypA), ELISA (eotaxin), or cytometric bead array (RANTES, MCP-1, and MCP-3). Data show the mean ± SEM for concentrations of each chemoattractant in OVA-challenged versus PBS-challenged groups. A Student t test was used to establish statistically significant differences between OVA and PBS data at each time point (n = 4–12 mice per group). **P < 0.005. ***P < 0.0005.
Inhibiting the Activity of Cyclophilins Reduces the Persistence of Leukocytes during Chronic Asthma
To test whether the extracellular cyclophilins detected at the chronic time point of our model might play a role in regulating the persistence of leukocytes, we investigated the impact of blocking their activity during the chronic phase of disease. Groups of mice were treated with 200 μg of a nonimmunosuppressive analogue of CsA, NIM811, twice per week during the 4-week chronic time period. Numbers of leukocytes were assessed 3 weeks into the chronic phase, on Day 77. Strikingly, the numbers of persisting eosinophils, effector/memory CD4+ T cells, neutrophils, and monocytes were all highly reduced (45–80%) in mice treated with NIM811, compared with mice treated with diluent alone (Table 1).
TABLE 1.
INHIBITING THE FUNCTION OF CYCLOPHILINS DURING THE CHRONIC PHASE OF ASTHMA REDUCES THE PERSISTENCE OF LEUKOCYTES
| PBS | DIL | NIM811 | Percentage of Reduction | P | |
| Eosinophils | 1,582 ± 546.1 | 6,070 ± 675.5 | 1,865 ± 325.5 | 69.3 | 0.0006 |
| Effector/memory CD4+ T cells | 16.98 ± 5.281 | 412.5 ± 74.15 | 45.09 ± 6.242 | 82.1 | 0.0005 |
| Neutrophils | 680.6 ± 212.4 | 3,370 ± 688.7 | 929.7 ± 195.3 | 70.0 | 0.01 |
| Monocytes | 4,191 ± 1,103.9 | 12,022.5 ± 2,123.2 | 6,600 ± 1,026.7 | 45.1 | 0.02 |
Chronic allergic asthma was induced as described in Figure 1A. Mice were then treated with 200 μg N-methyl-4-isoleucine-cyclosporin (NIM811) or diluent (DIL) alone, via intraperitoneal injection twice per week during the 4-week chronic phase of the regimen. Groups of mice were killed on Day 77 (3 weeks into the chronic phase), and BAL leukocytes were analyzed by flow cytometry. Data show the mean ± SEM for numbers of leukocytes, with n = 4–5 mice per treatment group. A Student t test was used to establish statistically significant differences between the NIM811-treated and diluent-treated groups.
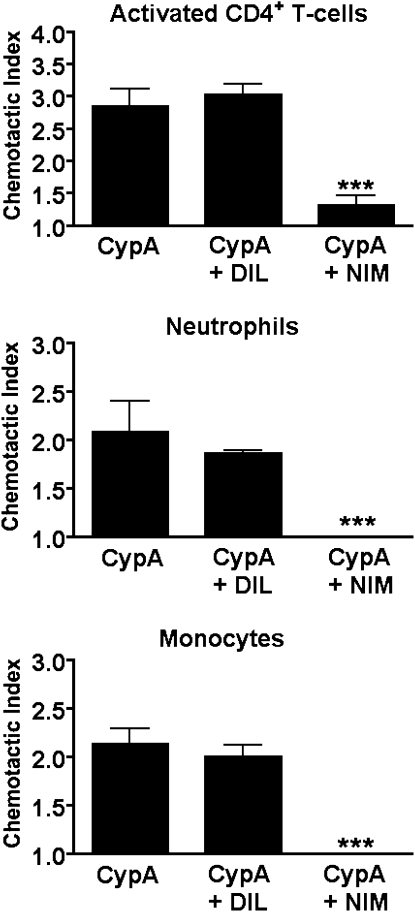
To provide supporting evidence that NIM811 has the biochemical capacity to inhibit the CypA-mediated recruitment of leukocytes, in vitro chemotaxis studies were performed using purified populations of activated CD4+ T cells, neutrophils, and monocytes. The migration of all three subsets of leukocytes was inhibited by more than 90% in the presence of NIM811 (Figure 3). Eosinophils were not tested in this experiment, because we (29) and others (39) previously showed that eosinophils do not migrate directly in response to extracellular CypA in vitro. Importantly, previous experiments in our laboratory showed that NIM811 is highly specific for the cyclophilin-mediated migration of leukocytes, and exerts no impact on the leukocyte chemotaxis induced by noncyclophilin chemoattractants (27). Taken together, these findings demonstrate that blocking the function of cyclophilins during the chronic phase of asthma results in a marked reduction of leukocytic persistence, and that the intervention drug used for these studies has a potent inhibitory impact on the CypA-mediated migration of leukocytes.
Figure 3.
N-methyl-4-isoleucine-cyclosporin (NIM811) blocks the CypA-induced migration of leukocytes in vitro. Purified populations of activated CD4+ T cells, neutrophils, and monocytes were set up in Boyden chemotaxis chambers in the presence of recombinant CypA ± 2 μM NIM811 (NIM) or diluent alone (DIL). Data show the mean chemotactic indices ± SEM for each group (n = 6 wells/group), as calculated by comparing the number of cells migrating in each test group relative to medium alone. A Student t test was used to establish statistically significant differences between NIM811-treated and DIL-treated groups. ***P < 0.0001.
Inhibiting the Activity of Cyclophilins during the Chronic Phase of Asthma Reduces the Disease Severity Induced upon Acute Reactivation
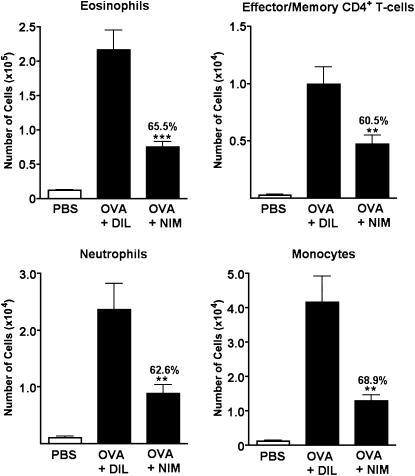
After establishing that the inhibition of cyclophilin activity can reduce the persistence of leukocytes, we examined whether this would result in reduced acute inflammatory responses upon re-exposure to allergens. Our working hypothesis stated that reducing the chronic persistence of leukocytes would also lead to less severe inflammation during the reactivation of acute asthma, because fewer resident effector cells would be present to initiate an acute response. Thus, in the present experiments, we tested the impact of the cyclophilin-blocking regimen already described (NIM811 delivered twice per week during the 4-week chronic phase) on the pathology of acute asthma reactivation. As shown in Figure 4, a significant decrease in acute leukocyte influx was indeed evident. Total numbers of eosinophils, effector/memory CD4+ T cells, neutrophils, and monocytes were reduced by more than 60%.
Figure 4.
Inhibiting the function of cyclophilins during the chronic phase of asthma reduces the numbers of leukocytes recruited upon acute allergen challenge. Chronic allergic asthma was induced as already described, and 200 μg of NIM811, or diluent alone, were administered by intraperitoneal injection twice per week during the 4-week chronic phase. A separate group of mice received PBS aerosol challenges throughout the regimen, without intervention, to provide baseline data. Five days after the final intervention injection, all mice received two aerosol challenges of either PBS or OVA to induce an acute asthmatic response (Reactivation). Numbers of leukocytes were assessed in BAL fluid by flow cytometry 24 hours after the second acute reactivation challenge. Data show the mean ± SEM for numbers of leukocytes (n = 14–15 mice per group). The percent reduction between NIM811-treated and diluent-treated groups is also shown. A Student t test was used to establish statistically significant differences between the NIM and DIL groups. **P < 0.005. ***P < 0.0001.
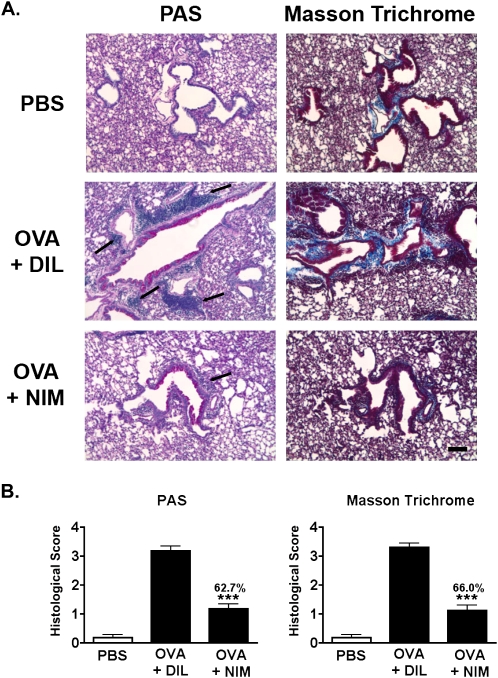
In addition to airway inflammation, the pathology of asthma was examined by histology. Whole lungs from the Reactivation time point were sectioned and stained for the presence of mucus (PAS) and the deposition of collagen (Masson's Trichrome). The lungs of mice with asthma treated with NIM811 during the chronic phase showed a marked reduction in the number of inflammatory foci around the small airways and a decreased secretion of airway mucus (Figure 5A, left). Furthermore, lungs from NIM811-treated mice demonstrated reduced airway remodeling, as evidenced by a reduction in collagen staining (Figure 5A, right). The reductions in mucus and collagen mediated by treatment with NIM811 were both greater than 60%, based on a semiquantitative analysis (Figure 5B).
Figure 5.
Inhibiting the function of cyclophilins during the chronic phase of asthma reduces tissue pathology after an acute reactivation of asthma. Lung pathology was analyzed in mice killed 24 hours after their second acute reactivation challenge, using the same timeline described in Figure 4. (A) Whole lungs from PBS-treated, diluent-treated, and NIM811-treated mice were fixed and embedded in paraffin, and 6-μm sections were cut and stained with periodic acid-Schiff (PAS) (left) or Masson's Trichrome (right). Black arrows denote areas of inflammatory foci. Images are shown at ×10 magnification. Scale bar = 10 μm. (B) Changes in the deposition of mucus and collagen were scored, using a semiquantitative scale ranging from 0 (normal) to 4 (pronounced deposition of mucus or collagen). A Student t test was used to identify significant differences between NIM-treated and DIL-treated groups (n = 6–8 mice per group). ***P < 0.0001.
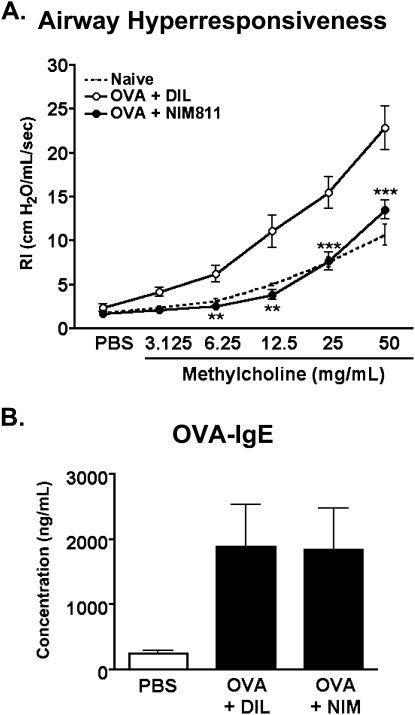
To address whether the observed reductions in airway leukocytic infiltration and remodeling would translate to improved lung function, airway hyperresponsiveness, as measured by alterations in bronchial resistance to methylcholine challenge, was analyzed at the Reactivation time point. As shown in Figure 6A, mice treated with NIM811 showed a significant reduction in the airway resistance associated with asthma. Indeed, NIM811-treated animals demonstrated responses similar to those observed in naive mice.
Figure 6.
Inhibiting the function of cyclophilins during the chronic phase of asthma significantly reduces airway hyperresponsiveness in mice with asthma. (A) Airway resistance was measured in NIM811-treated versus diluent-treated mice, 24 hours after their second reactivation challenge, using the same timeline described in Figure 4. A separate group of naive mice was analyzed to provide baseline data for nonasthmatic lung function. Individual mice from each group were anesthetized, and a tracheostomy tube was inserted for ventilation via the Buxco FinePointe RC system. The animals were then challenged with increasing doses of methylcholine, and measurements of maximum airway resistance (RI) were determined using Buxco FinePointe software. Data show the mean ± SEM at individual doses of methylcholine for each group (n = 7–10 mice per group). Two-way ANOVA was used to establish statistically significant differences between the NIM811 and DIL groups. **P < 0.001. ***P < 0.0001. (B) Peripheral blood was collected by cardiac puncture from mice killed at the Reactivation time point. Concentrations of the OVA-specific IgE present in individual serum samples were measured according to ELISA (MD Biosciences Inc., St. Paul, MN). Data show the mean ± SEM for concentrations of OVA-specific IgE (n = 7 mice per group).
As a whole, these findings indicate that inhibiting the function of cyclophilins during the chronic phase of asthma not only affects the persistence of leukocytes during this phase, but leads to a significant reduction in the subsequent acute responses reactivated by allergen exposure. Importantly, total cell numbers in spleens and peripheral lymph nodes remained comparable between NIM811-treated and diluent-treated animals (see Figure E1 in the online supplement), providing evidence that the observed reduction in inflammatory responses was not attributable to NIM811 inducing peripheral leukopenia. This result is supported by previously studies showing no cytotoxicity by NIM811 in a wide range of leukocytic subsets (35), minimal impact on the capacity of T cells to be activated and to proliferate in vitro (see Figure E2A in the online supplement), and no impact on the production of cytokines by T cells isolated from OVA-primed mice treated with NIM811 (see Figure E2B in online supplement). Further evidence that NIM811 does not affect the priming and activation of T and B cells is provided by the detection of similar concentrations of serum OVA–specific IgE in NIM-treated and diluent-treated groups in the present study (Figure 6B). Thus, the major inflammatory parameter affected by treatment with NIM811 during the chronic phase of asthma is likely the recruitment of leukocytes, with a reduction in this recruitment exerting a downstream impact on acute reactivation responses.
Discussion
One of the hallmark features of chronic allergic asthma involves persistent low-level inflammation in the absence of allergen stimulation. This chronic inflammation is, in turn, responsible for the permanent remodeling of airways that drastically reduces airway diameter. Although acute or short-term animal models of asthma reproduce the robust transient inflammation mediated in response to allergen exposure, such models fail to exhibit the persistent inflammation and lung changes characteristic of chronic disease. The murine model described here not only demonstrates acute inflammatory responses after allergen stimulation, but also reproduces the chronic (quiescent) phase, characterized by the sustained presence of proinflammatory leukocytes. This model enabled us to investigate potential factors contributing to the persistence of leukocytes in chronic asthma and the impact of reducing the persistence of leukocytes on subsequent acute responses.
Cyclophilins, widely known for their role in protein folding and as the target of CsA, are also known as potent leukocyte chemoattractants (40). In previous studies, we showed that extracellular cyclophilins contribute significantly to the recruitment of leukocytes during acute lung inflammation (27, 29, 31). In the present study, we investigated whether cyclophilins may also play a role in the recruitment of leukocytes during chronic phases of asthma, with a focus on their contribution to the chronic persistence of leukocytes. Strikingly, we found that out of all the chemoattractants measured during the chronic phase of disease, only extracellular CypA remained elevated above PBS control levels. This finding fits with observations in human patients with asthma, in whom bronchial biopsies performed during disease remission showed an absence of classic leukocyte chemokines, despite the presence of persisting proinflammatory leukocytes (11). Although our experiments were focused on measuring the chemokines most commonly associated with allergic asthma, we acknowledge the important caveat that other less obvious chemoattractants may have been present and were not measured. Nevertheless, strong supporting evidence that the presence of CypA during chronic disease plays a role in leukocytic persistence was provided by blocking studies in which mice were treated during the chronic phase of disease with the nonimmunosuppressive CsA analogue, NIM811. Treatment reduced the persistence of CD4+ effector/memory T cells, eosinophils, neutrophils, and monocytes by up to 80%. Importantly, previous studies demonstrated the capacity of NIM811 to bind to cyclophilins with high affinity, higher even than that of unmodified CsA (34, 41, 42). Moreover, both CsA and various analogues of CsA were shown to inhibit all functions mediated by cyclophilins (42–44), including chemotaxis (31, 45).
We believe the present study to be of importance because we focused on the inhibiting events that occur during the chronic phase of asthma. Previous studies designed to block the function of chemoattractants in animal models of asthma typically provided treatment throughout the course of disease, using either intervention regimens or knockout mice (46, 47), thereby spanning both the chronic and acute phases. We propose that reducing the persistence of leukocytes during the chronic phase will result in fewer proinflammatory leukocytes available for reactivation upon exposure to allergens, which in turn will result in a reduced severity of acute inflammatory responses. In support of this concept, mice treated with NIM811 during the chronic phase of disease demonstrated a markedly reduced acute influx of leukocytes and tissue pathology after acute reactivation by allergen challenge. The numbers of all leukocyte subsets were reduced by at least 60%, and the hypersecretion of mucus (as measured by PAS staining) and airway remodeling (based on the abundance of collagen deposition) were both reduced by more than 60%. These reductions in inflammation and remodeling also translated to profound improvements in lung function, and specifically a reduction in airway resistance in response to methylcholine challenge.
Collectively, our findings provide supporting evidence that inhibiting the activity of cyclophilins during the chronic phase of asthma exerts a downstream impact on subsequent acute responses. Of note, treatment with the inhibitory drug was discontinued at least 4 days before the allergen challenge. Based on previously published pharmacokinetics data, the half-life of NIM811 is less than 48 hours (35, 48), and thus the observed reductions in acute inflammatory responses are unlikely attributable to the direct effect of any residual drug. This concept was further confirmed by performing supplemental experiments in which the final dose of NIM811 was omitted, so that acute reactivation was not induced until 1 week after the final injection of NIM811. With this altered intervention regimen, we also observed a reduced acute infiltration of leukocytes into the airways (up to 82%), as well as highly significant reductions in airway resistance in response to methycholine challenges (data not shown). This result strongly suggests that the improvements seen with NIM811 treatment are attributable to the cumulative effects of reducing the persistence of leukocytes by blocking cyclophilin activity during the chronic phase, and do not simply reflect the timing of the final dose of drug in relation to the reactivation of acute asthma.
In conclusion, our findings demonstrate for the first time, to the best of our knowledge, the potential role of cyclophilins in contributing to leukocytic persistence and disease severity in chronic asthma. The effectiveness of NIM811 at reducing not only the persistence of leukocytes, but also airway remodeling and inflammation upon the reactivation of acute asthma, suggests that cyclophilins may be an important therapeutic target for managing chronic asthma. In contrast to unmodified CsA, where the primary cellular effect is the inhibition of T-cell activation, nonimmunosuppressive NIM811 does not affect calcium-dependent signaling, providing an attractive alternative method of reducing inflammation by targeting leukocytic recruitment without causing immunosuppression.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health grant AI067254 (S.L.C.) and American Heart Association predoctoral award 0815226E (E.J.S.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0007OC on April 14, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Cookson W. The alliance of genes and environment in asthma and allergy. Nature 1999;402(Suppl):B5–B11 [DOI] [PubMed] [Google Scholar]
- 2.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol 2004;22:789–815 [DOI] [PubMed] [Google Scholar]
- 3.Corrigan CJ, Hartnell A, Kay AB. T lymphocyte activation in acute severe asthma. Lancet 1988;1:1129–1132 [DOI] [PubMed] [Google Scholar]
- 4.Walker C, Kaegi MK, Braun P, Blaser K. Activated T cells and eosinophilia in bronchoalveolar lavages from subjects with asthma correlated with disease severity. J Allergy Clin Immunol 1991;88:935–942 [DOI] [PubMed] [Google Scholar]
- 5.Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol 2003;111:450–463, quiz 464 [DOI] [PubMed] [Google Scholar]
- 6.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol 1999;17:255–281 [DOI] [PubMed] [Google Scholar]
- 7.Huang SK, Xiao HQ, Kleine-Tebbe J, Paciotti G, Marsh DG, Lichtenstein LM, Liu MC. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol 1995;155:2688–2694 [PubMed] [Google Scholar]
- 8.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant Th2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 1992;326:298–304 [DOI] [PubMed] [Google Scholar]
- 9.Romagnani S. Cytokines and chemoattractants in allergic inflammation. Mol Immunol 2002;38:881–885 [DOI] [PubMed] [Google Scholar]
- 10.Homer RJ, Elias JA. Consequences of long-term inflammation: airway remodeling. Clin Chest Med 2000;21:331–343 (ix.) [DOI] [PubMed] [Google Scholar]
- 11.van den Toorn LM, Overbeek SE, de Jongste JC, Leman K, Hoogsteden HC, Prins JB. Airway inflammation is present during clinical remission of atopic asthma. Am J Respir Crit Care Med 2001;164:2107–2113 [DOI] [PubMed] [Google Scholar]
- 12.Medoff BD, Thomas SY, Luster AD. T cell trafficking in allergic asthma: the ins and outs. Annu Rev Immunol 2008;26:205–232 [DOI] [PubMed] [Google Scholar]
- 13.Robertson JM, MacLeod M, Marsden VS, Kappler JW, Marrack P. Not all CD4+ memory T cells are long lived. Immunol Rev 2006;211:49–57 [DOI] [PubMed] [Google Scholar]
- 14.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol 2006;24:147–174 [DOI] [PubMed] [Google Scholar]
- 15.Harris NL, Watt V, Ronchese F, Le Gros G. Differential T cell function and fate in lymph node and nonlymphoid tissues. J Exp Med 2002;195:317–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown JR, Kleimberg J, Marini M, Sun G, Bellini A, Mattoli S. Kinetics of eotaxin expression and its relationship to eosinophil accumulation and activation in bronchial biopsies and bronchoalveolar lavage (BAL) of asthmatic patients after allergen inhalation. Clin Exp Immunol 1998;114:137–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holgate ST, Bodey KS, Janezic A, Frew AJ, Kaplan AP, Teran LM. Release of RANTES, MIP-1 alpha, and MCP-1 into asthmatic airways following endobronchial allergen challenge. Am J Respir Crit Care Med 1997;156:1377–1383 [DOI] [PubMed] [Google Scholar]
- 18.Humbles AA, Conroy DM, Marleau S, Rankin SM, Palframan RT, Proudfoot AE, Wells TN, Li D, Jeffery PK, Griffiths-Johnson DA, et al. Kinetics of eotaxin generation and its relationship to eosinophil accumulation in allergic airways disease: analysis in a guinea pig model in vivo. J Exp Med 1997;186:601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalo JA, Lloyd CM, Kremer L, Finger E, Martinez AC, Siegelman MH, Cybulsky M, Gutierrez-Ramos JC. Eosinophil recruitment to the lung in a murine model of allergic inflammation: the role of T cells, chemokines, and adhesion receptors. J Clin Invest 1996;98:2332–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P, Heitman J. The cyclophilins. Genome Biol 2005;6:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science 1984;226:544–547 [DOI] [PubMed] [Google Scholar]
- 22.Jin ZG, Melaragno MG, Liao DF, Yan C, Haendeler J, Suh YA, Lambeth JD, Berk BC. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res 2000;87:789–796 [DOI] [PubMed] [Google Scholar]
- 23.Sherry B, Yarlett N, Strupp A, Cerami A. Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages. Proc Natl Acad Sci USA 1992;89:3511–3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Billich A, Winkler G, Aschauer H, Rot A, Peichl P. Presence of cyclophilin A in synovial fluids of patients with rheumatoid arthritis. J Exp Med 1997;185:975–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki J, Jin ZG, Meoli DF, Matoba T, Berk BC. Cyclophilin A is secreted by a vesicular pathway in vascular smooth muscle cells. Circ Res 2006;98:811–817 [DOI] [PubMed] [Google Scholar]
- 26.Tegeder I, Schumacher A, John S, Geiger H, Geisslinger G, Bang H, Brune K. Elevated serum cyclophilin levels in patients with severe sepsis. J Clin Immunol 1997;17:380–386 [DOI] [PubMed] [Google Scholar]
- 27.Arora K, Gwinn WM, Bower MA, Watson A, Okwumabua I, MacDonald HR, Bukrinsky MI, Constant SL. Extracellular cyclophilins contribute to the regulation of inflammatory responses. J Immunol 2005;175:517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damsker JM, Bukrinsky MI, Constant SL. Preferential chemotaxis of activated human CD4+ T cells by extracellular cyclophilin A. J Leukoc Biol 2007;82:613–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gwinn WM, Damsker JM, Falahati R, Okwumabua I, Kelly-Welch A, Keegan AD, Vanpouille C, Lee JJ, Dent LA, Leitenberg D, et al. Novel approach to inhibit asthma-mediated lung inflammation using anti-CD147 intervention. J Immunol 2006;177:4870–4879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Q, Leiva MC, Fischkoff SA, Handschumacher RE, Lyttle CR. Leukocyte chemotactic activity of cyclophilin. J Biol Chem 1992;267:11968–11971 [PubMed] [Google Scholar]
- 31.Balsley MA, Malesevic M, Stemmy EJ, Gigley J, Jurjus RA, Herzog D, Bukrinsky MI, Fischer G, Constant SL. A cell-impermeable cyclosporine a derivative reduces pathology in a mouse model of allergic lung inflammation. J Immunol 2010;185:7663–7670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fulkerson PC, Rothenberg ME, Hogan SP. Building a better mouse model: experimental models of chronic asthma. Clin Exp Allergy 2005;35:1251–1253 [DOI] [PubMed] [Google Scholar]
- 33.McMillan SJ, Lloyd CM. Prolonged allergen challenge in mice leads to persistent airway remodelling. Clin Exp Allergy 2004;34:497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Billich A, Hammerschmid F, Peichl P, Wenger R, Zenke G, Quesniaux V, Rosenwirth B. Mode of action of SDZ NIM 811, a nonimmunosuppressive cyclosporin A analog with activity against human immunodeficiency virus (HIV) Type 1: interference with HIV protein–cyclophilin A interactions. J Virol 1995;69:2451–2461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenwirth B, Billich A, Steinkasserer A, Peichl P, Donatsch P, Lawen A, Wenger R, Traber P. SDZ NIM 811: anti-HIV nonimmunosuppressive natural cyclosporine. Drugs Future 1995;20:579–583 [Google Scholar]
- 36.Elekes K, Helyes Z, Kereskai L, Sandor K, Pinter E, Pozsgai G, Tekus V, Banvolgyi A, Nemeth J, Szuts T, et al. Inhibitory effects of synthetic somatostatin receptor Subtype 4 agonists on acute and chronic airway inflammation and hyperreactivity in the mouse. Eur J Pharmacol 2008;578:313–322 [DOI] [PubMed] [Google Scholar]
- 37.Damsker JM, Okwumabua I, Pushkarsky T, Arora K, Bukrinsky MI, Constant SL. Targeting the chemotactic function of CD147 reduces collagen-induced arthritis. Immunology 2009;126:55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lynch EL, Little FF, Wilson KC, Center DM, Cruikshank WW. Immunomodulatory cytokines in asthmatic inflammation. Cytokine Growth Factor Rev 2003;14:489–502 [DOI] [PubMed] [Google Scholar]
- 39.Cormier SA, Taranova AG, Bedient C, Nguyen T, Protheroe C, Pero R, Dimina D, Ochkur SI, O'Neill K, Colbert D, et al. Pivotal advance: eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J Leukoc Biol 2006;79:1131–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bukrinsky MI. Cyclophilins: unexpected messengers in intercellular communications. Trends Immunol 2002;23:323–325 [DOI] [PubMed] [Google Scholar]
- 41.Quesniaux VF, Schreier MH, Wenger RM, Hiestand PC, Harding MW, Van Regenmortel MH. Cyclophilin binds to the region of cyclosporine involved in its immunosuppressive activity. Eur J Immunol 1987;17:1359–1365 [DOI] [PubMed] [Google Scholar]
- 42.Gallay PA. Cyclophilin inhibitors. Clin Liver Dis 2009;13:403–417 [DOI] [PubMed] [Google Scholar]
- 43.Daum S, Schumann M, Mathea S, Aumuller T, Balsley MA, Constant SL, de Lacroix BF, Kruska F, Braun M, Schiene-Fischer C. Isoform-specific inhibition of cyclophilins. Biochemistry 2009;48:6268–6277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mikol V, Kallen J, Walkinshaw MD. X-ray structure of a cyclophilin B/cyclosporin complex: comparison with cyclophilin A and delineation of its calcineurin-binding domain. Proc Natl Acad Sci USA 1994;91:5183–5186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malesevic M, Kuhling J, Erdmann F, Balsley MA, Bukrinsky MI, Constant SL, Fischer G. A cyclosporin derivative discriminates between extracellular and intracellular cyclophilins. Angew Chem Int Ed Engl 2009;49:213–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bisset LR, Schmid-Grendelmeier P. Chemokines and their receptors in the pathogenesis of allergic asthma: progress and perspective. Curr Opin Pulm Med 2005;11:35–42 [DOI] [PubMed] [Google Scholar]
- 47.Lukacs NW. Role of chemokines in the pathogenesis of asthma. Nat Rev Immunol 2001;1:108–116 [DOI] [PubMed] [Google Scholar]
- 48.Li W, Luo S, Hayes M, He H, Tse FL. Determination of N-methyl-4-isoleucine-cyclosporin (NIM811) in human whole blood by high performance liquid chromatography-tandem mass spectrometry. Biomed Chromatogr 2007;21:249–256 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.