Abstract
We previously found that deficiency of the sodium–hydrogen exchanger 1 (NHE1) gene prevented hypoxia-induced pulmonary hypertension and vascular remodeling in mice, which were accompanied by a significantly reduced proliferation of pulmonary artery smooth muscle cells (PASMCs), and which decreased the medial-wall thickness of pulmonary arteries. That finding indicated the involvement of NHE1 in the proliferation and hypertrophy of PASMCs, but the underlying mechanism was not fully understood. To define the mechanism by which the inhibition of NHE1 decreases hypoxic pulmonary hypertension and vascular remodeling, we investigated the role of E2F1, a nuclear transcription factor, in silencing the NHE1 gene–induced inhibition of the proliferation, hypertrophy, and migration of human PASMCs. We found that: (1) silencing of NHE1 by short, interfering RNA (siRNA) significantly inhibited PASMC proliferation and cell cycle progression, decreased hypoxia-induced hypertrophy (in terms of cell size and protein/DNA ratio) and migration (in terms of the wound-healing and migration chamber assays); (2) hypoxia induced the expression of E2F1, which was reversed by NHE1 siRNA; and (3) the overexpression of E2F1 blocked the inhibitory effect of NHE1 siRNA on the proliferation, hypertrophy, and migration of PASMCs. The present study determined that silencing the NHE1 gene significantly inhibited the hypoxia-induced proliferation, hypertrophy, and migration of human PASMCs via repression of the nuclear transcription factor E2F1. This study revealed a novel mechanism underlying the regulation of hypoxic pulmonary hypertension and vascular remodeling via NHE1.
Keywords: sodium–hydrogen exchanger 1, E2F1, PASMC proliferation, hypertrophy, hypoxia
Clinical Relevance
This study determined that silencing the sodium–hydrogen exchanger 1 (NHE1) gene significantly inhibited the hypoxia-induced proliferation, hypertrophy, and migration of human pulmonary artery smooth muscle cells via the repression of nuclear transcription factor E2F1. This study revealed a novel mechanism underlying the regulation by NHE1 of hypoxic pulmonary hypertension and vascular remodeling.
Pulmonary hypertension is caused by many chronic lung diseases associated with prolonged hypoxia, and can result in right ventricular hypertrophy and heart failure. An important pathological feature of pulmonary hypertension involves an increase in medial thickening of pulmonary arteries, resulting from the hyperplasia and hypertrophy of pulmonary artery smooth muscle cells (PASMCs) (1–3). The Na+–H+ exchanger (NHE), a protein localized to plasma and the mitochondrial inner membrane (4), has nine isoforms (5), of which NHE isoform 1 (NHE1) is ubiquitously expressed. We previously reported on the inhibitory effect of reduced NHE activity in the proliferation of PASMCs (6) and on chronic hypoxia–induced pulmonary hypertension and vascular remodeling (7). Increased expression of the NHE1 gene was evident in animals with hypoxia-induced pulmonary hypertension and vascular remodeling (6–10). Moreover, we recently found that deficiency of the NHE1 gene prevented hypoxia-induced pulmonary hypertension and vascular remodeling in mice (11), accompanied by a significantly reduced proliferation of PASMCs and decreased medial wall thickness of the pulmonary arteries. Our findings indicated the involvement of NHE1 in the proliferation and hypertrophy of PASMCs. However, the mechanism by which NHE1 regulated the proliferation and hypertrophy of PASMCs was not fully understood.
The E2F family is essential for cell-cycle progression. E2F1, one member of the family and a downstream factor of cell-cycle modulators and a nuclear transcription factor, plays an important role in governing nuclear transcription factors that regulate the synthesis of DNA (12). Reports indicate that E2F1 is involved in regulating the proliferation of different cells (13–16). We previously determined that p27, a cyclin-dependent kinase inhibitor and an upstream factor of E2F1, plays a critical role in inhibiting the proliferation of PASMCs and the pulmonary hypertension induced by hypoxia (17–19). We also observed a significantly decreased expression of cyclin D1, a downstream factor of p27 and upstream factor of E2F1, in PASMCs isolated from NHE1-deficient mice with decreased hypoxic pulmonary hypertension and vascular remodeling (11). We therefore assumed that the inhibition of NHE1 caused a reduction of PASMC proliferation via the E2F1 regulatory pathway.
Reports indicate that E2F1 is involved in the regulation of hypertrophy (20–22). Studies also showed that the inhibition of NHE1 by inhibitors attenuated the hypertrophy of cardiomyocytes (23–27). Because we also found a significant decrease in the medial wall thickness of pulmonary arteries in NHE1-deficient mice with decreased hypoxic pulmonary hypertension and vascular remodeling (11), we hypothesized that the effects of NHE1 on hypoxia-induced PASMC hypertrophy were also mediated via E2F1. In addition, the migration of PASMCs is reportedly involved in the pathogenesis of pulmonary hypertension (28), and hypoxia and smooth muscle growth-stimulating factors were shown to affect the migration of PASMCs (29–31). Therefore, we investigated the involvement of E2F1 in the regulation by NHE1 of the hypoxia-induced migration of PASMCs.
Materials and Methods
Cells
Human pulmonary artery smooth muscle cells (HPASMCs), human pulmonary artery endothelial cells (HPAECs; Lonza, Inc., Walkersville, MD), and human pulmonary artery fibroblasts (HPAFs; ScienCell Research Laboratories, Carlsbad, CA) were used in this study.
Hypoxia and Hypoxia Chamber
Air gases (1, 2, 3, 5, and 10% O2 in 5% CO2 balance nitrogen; Airgas East, Cambridge, MA) and a hypoxia chamber were used. The hypoxia chamber was placed in a regular CO2 incubator and maintained at 37°C. The concentration of oxygen in the chamber was monitored with an oxygen analyzer, and remained stable, as indicated in the cylinder (19).
NHE1 Short, Interfering RNA and E2F1 cDNA
Two sets of NHE1 short, interfering RNA (siRNA) and scrambled siRNA were obtained from Sigma (St. Louis, MO). The sequences (5′ to 3′) were sense CUGUCUUUGAGGAAAUUCA and antisense UGAAUUUCCUCAAAGACAG for NHE1 siRNA set 1 (NHE1 siRNA1), and sense CCUGUUAAUCAUUCCGUCA and antisense UGACGGAAUGAUUAACAGG for NHE1 siRNA set 2 (NHE1 siRNA2). E2F1 human cDNA (pCMV6-XL6-E2F1) was purchased from Origene (Rockville, MD), and purified according to our previously described methods (17, 19).
siRNA and cDNA Transfection
Transfection agent Lipofectamine-2000 was obtained from Invitrogen (Carlsbad, CA). The procedures for NHE1 siRNA transfection and cotransfection with E2F1 cDNA were performed as described in our previous studies (17, 18).
Exposure to Hypoxia
To implement exposure to hypoxia, PASMCs transfected with siRNA and cDNA were placed in the hypoxia chamber (19). After 24 hours, cells were harvested for the cell proliferation assay and cell-cycle analysis.
Cell Proliferation Assay and Cell-Cycle Analysis
We assayed cell proliferation by using a direct cell count and the bromo-2′deoxyuridine (BrdU) incorporation assay (32). Cell cycles were analyzed using flow cytometry (32).
Measurement of Cell Hypertrophy
Cell hypertrophy was determined by measuring cell size and by calculating the ratio of total protein to DNA content. For the measurement of cell size, PASMCs were plated on chamber slides, and NHE1 siRNA and E2F1 cDNA were transfected. After exposure to hypoxia for 24 hours, cells were washed, fixed, and stained with smooth muscle–specific α-actin (SM α-actin). After hematoxylin counterstaining, SM α-actin–positive cells were chosen from slides, and captured using a digital camera. The size of PASMCs in different groups was measured using an imaging analysis system (IPLab scientific imaging software; Scanalytics, Inc., Fairfax, VA). For the analysis of protein/DNA ratios, PASMCs were plated in six-well plates. After the transfection of NHE1 siRNA and exposure to hypoxia, PASMCs were harvested, and the protein/DNA ratio was determined by measuring total DNA content and total protein content (33, 34).
Cell Migration Assay
A wound-healing assay and migration chamber assay were used to determine the effects of NHE1 on the migration of PASMC, and were performed according to published methods (35, 36).
RT-PCR and Western Blotting
RT-PCR and Western blotting were performed according to previous studies (11, 17–19).
Statistical Analyses
Statistical analyses were performed using Statview (SAS Institute, Inc., Cary, NC), with factorial ANOVA. If the results of ANOVA were significant, multiple comparisons were performed among groups, using the Fisher protected least significant difference test. All values were expressed as mean ± SEM. Significance was set at P < 0.05.
Results
Silencing of NHE1 Significantly Inhibited the Proliferation of Human PASMCs under Normoxia
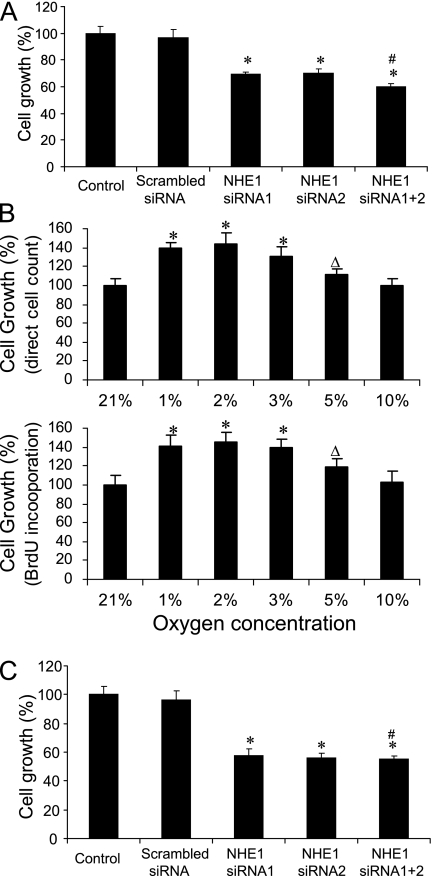
To confirm and compare the inhibitory effects of NHE1 siRNA on the proliferation of PASMCs, we used two sets of NHE1 siRNAs to treat human PASMCs separately. We found that the proliferation of PASMCs was significantly decreased by either set of siRNA, compared with the control group or the scrambled siRNA group, demonstrating an approximately 35% inhibition of growth (Figure 1A). In the meantime, to obtain the maximal inhibition of PASMC proliferation, we simultaneously used two sets of the NHE1 siRNA in one transfection. However, the combination of two sets of siRNA did not significantly change the inhibitory effects of NHE1 in the proliferation of PASMCs, although slightly greater inhibition was evident in the group involving two sets of NHE1 siRNA.
Figure 1.
Effects of silencing the sodium–hydrogen exchanger 1 (NHE1) on the proliferation of human pulmonary artery smooth muscle cells (PASMCs). After transfection with NHE1 short, interfering RNA (siRNA), PASMCs were cultured in normoxia and hypoxia for 24 hours, and harvested for a cell count to assay the proliferation of cells. The effects of NHE1 siRNA on the proliferation of PASMCs were determined under conditions of normoxia (A) and hypoxia (C), setting the control value as 100. *P < 0.05, compared with control and scrambled siRNA groups. #P > 0.05, compared with the NHE1 siRNA1 and siRNA2 groups (n = 9 for each group). (B) Proliferation of PASMCs under different concentrations of hypoxia, setting 21% O2 as 100. Direct cell count (above) and bromo-2′deoxyuridine (BrdU) incorporation assay (below). *P < 0.05 and ΔP > 0.05, compared with each control group (n = 9 for each group).
Silencing of NHE1 Significantly Decreased the Proliferation of Human PASMCs under Hypoxia
After we found that NHE1 siRNA significantly inhibited the proliferation of PASMCs under normoxia, we investigated the effects of NHE1 siRNA on the proliferation of PASMCs under hypoxia. First, we studied the effects of hypoxia on the proliferation of PASMCs by using different concentrations of oxygen. We found a significant increase in cell proliferation in PASMCs exposed to 1, 2, and 3% oxygen, either through direct cell count or the BrdU incorporation assay (Figure 1B). Although a slight stimulation of proliferation was evident in cells exposed to 5 and 10% oxygen, the change was not significant. Because a slightly greater stimulation of PASMC growth was observed in cells exposed to 2% oxygen, we chose 2% oxygen for this study. We found that the silencing of NHE1 siRNA also significantly inhibited the proliferation of PASMCs under hypoxia (Figure 1C), for an inhibition of approximately 45%. No significant difference was evident between the two sets of siRNAs, and the simultaneous use of two sets of siRNAs also did not significantly increase the inhibitory effect under hypoxia.
Silencing of NHE1 Significantly Reduced the Cell-Cycle Progression of Human PASMCs
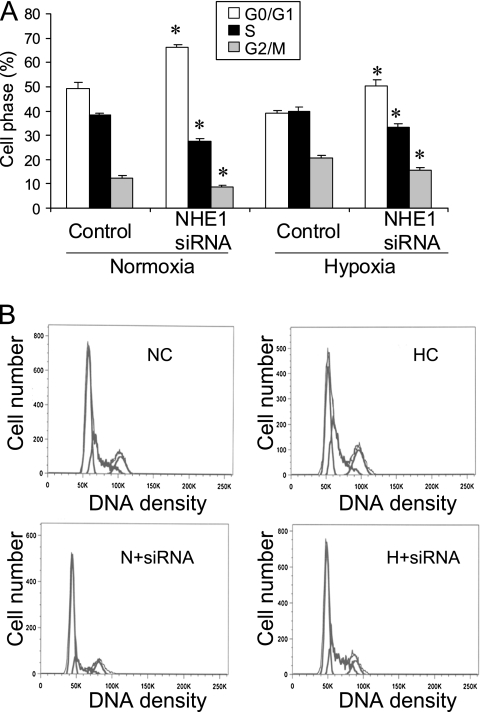
After we observed a significant inhibition in the proliferation of PASMCs with NHE1 siRNA, we investigated whether that inhibition was involved in the change in cell-cycle progression. Flow cytometry was performed to analyze cell phases. We found that silencing the NHE1 gene with siRNA significantly inhibited the cell-cycle progression of PASMCs under normoxia and hypoxia, with a significant decrease in the S phase and G2/M phase, and an increase in the G0/G1 phase in cells treated with NHE1 siRNA (Figure 2).
Figure 2.
Effects of silencing of NHE1 on cell-cycle progression of human PASMCs. After transfection with NHE1 siRNA, PASMCs were cultured in normoxia or hypoxia for 24 hours, and harvested for cell-cycle analysis by flow cytometry. (A) Quantitative data on cell-cycle progression. (B) Representative depictions of flow cytometry. *P < 0.05, compared with the control group (n = 3 for each group). NC, normoxia control; N + siRNA, normoxia + NHE1 siRNA; HC, hypoxia control; H + siRNA, hypoxia + NHE1 siRNA.
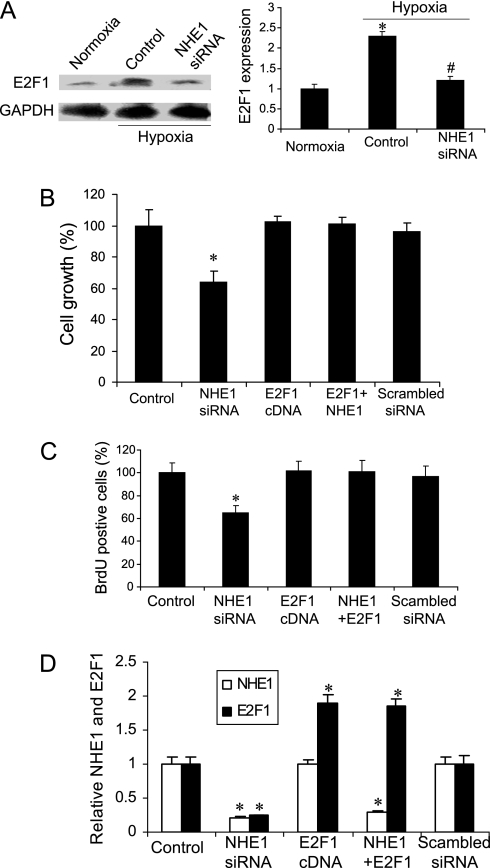
Overexpression of E2F1 Blocked the Inhibition by NHE1 of Hypoxia-Induced Proliferation of Human PASMCs
To determine if E2F1 was involved in the regulation by NHE1 of PASMC proliferation, we analyzed the expression of E2F1 and found that hypoxia significantly increased that expression, and NHE1 siRNA reversed the increase (Figure 3A). We subsequently overexpressed E2F1 via the transfection of E2F1 cDNA into PASMCs, and found that the overexpression of E2F1 completely blocked the inhibitory effects of NHE1 siRNA on the hypoxia-induced proliferation of PASMCs (Figures 3B and 3C). As expected, NHE1 siRNA transfection significantly decreased the hypoxia-induced expression of E2F1, but E2F1 cDNA did not affect the expression of NHE1 (Figure 3D).
Figure 3.
Effects of overexpression of E2F1 on inhibition by NHE1 siRNA of human PASMC proliferation. (A) Expression of E2F1. Protein was isolated from human PASMCs under conditions of normoxia, hypoxia, and hypoxia, and treated with NHE1 siRNA for Western blot analysis. Left, representative imaging. Right, quantitative data. *P < 0.05, compared with other groups. #P > 0.05, compared with normoxia. Cell proliferation data were obtained through direct cell counts (B) and the BrdU incorporation assay (C). After transfection with NHE1 siRNA or E2F1 cDNA, PASMCs were cultured in a hypoxia chamber for 24 hours and harvested for cell counts and a BrdU assay. *P < 0.05, compared with the control group (n = 9 for each group). (D) Quantitative data on the protein expression of NHE1 and E2F1. Proteins isolated from PASMCs treated with NHE1 siRNA and E2F1 cDNA were subjected to Western blot analysis, setting the control value as 1. Data were normalized to Glyceraldehyde-3 phosphate dehydrogenase (GAPDH). *P < 0.05, compared with each control group (n = 3 for each group). NHE1 + E2F1, NHE1 siRNA + E2F1 cDNA.
Silencing of NHE1 Significantly Inhibited the Hypoxia-Induced Hypertrophy of Human PASMCs, and the Overexpression of E2F1 Prevented the Inhibition
Because the hypertrophy of PASMCs is considered an important pathological feature in the thickening of pulmonary artery walls in hypoxia-induced pulmonary hypertension and vascular remodeling, we measured cell size and cell volume to evaluate the effects of NHE1 on the hypoxia-induced hypertrophy of PASMCs. We observed a significant increase in cell size in the PASMCs exposed to hypoxia (Figure 4A). Notably, NHE1 siRNA significantly decreased cell size and protein/DNA ratio, but the overexpression of E2F1 blocked the effects of NHE1 siRNA on the hypoxia-induced hypertrophy of PASMCs (Figures 4B and 4C).
Figure 4.
Effects of NHE1 and E2F1 on hypertrophy of human PASMCs under condition of hypoxia. After transfection with NHE1 siRNA and growing under condition of hypoxia for 24 hours, PASMCs were removed from hypoxia, and a hypertrophy assay was performed. (A) Cell-size assay for cells treated with NHE1 siRNA. (B) NHE1 siRNA plus E2F1 cDNA under condition of hypoxia. (C) Protein/DNA ratio under condition of hypoxia. *P < 0.05, #P > 0.05 as compared with the normoxia control, and $P < 0.05 as compared with hypoxia control in A. *P < 0.05 as compared with the controls in B and C (n = 9 for each group). NHE1 + E2F1, NHE1 siRNA + E2F1 cDNA.
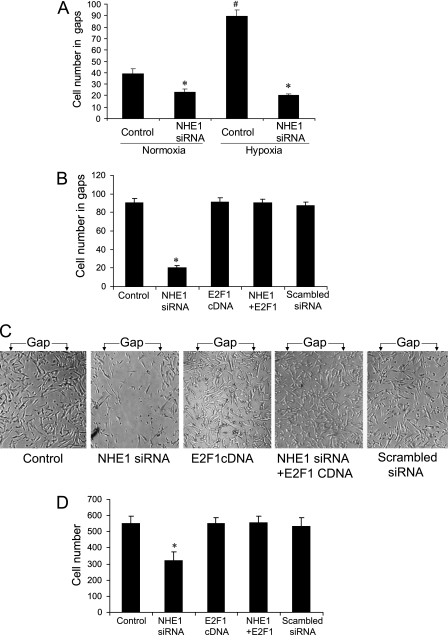
Silencing of NHE1 Significantly Inhibited the Migration of Human PASMCs, and the Overexpression of E2F1 Blocked the Effect
Because NHE1 was reported to be involved in the regulation of cell migration, we investigated whether the migration of PASMCs was mediated by the NHE1 gene. We found that hypoxia significantly increased the migration of PASMCs, and that the silencing of NHE1 siRNA inhibited the migration under normoxia and hypoxia (Figure 5A). In the meantime, we found that the overexpression of E2F1 blocked the inhibition by NHE1 siRNA of hypoxia-induced PASMC migration in the wound-healing assay (Figures 5B and 5C) and in the migration chamber assay (Figure 5D). As shown in Figure 5C, a clearly recognized gap (pipette tip-scratched area) was seen after 24 hours of exposure to hypoxia in the control group, but the gaps were completely filled with cells that resulted in unrecognizable gaps in other groups.
Figure 5.
Effects of NHE1 and E2F1 on migration of human PASMCs under condition of hypoxia. After transfection with NHE1 siRNA and E2F1 cDNA and being cultured in 2% oxygen for 24 hours, a wound-healing assay and migration chamber assay were performed. (A) Wound-healing assay for cells treated with NHE1 siRNA. (B) NHE1 siRNA plus E2F1 cDNA under condition of hypoxia. (C) Representative imaging. (D) Migration chamber assay under condition of hypoxia. *P < 0.05, compared with control group. #P < 0.05, compared with other groups (n = 9 for each group). NHE1 + E2F1 = NHE1 siRNA + E2F1 cDNA. Gap = the scratched area by pipette tip.
Silencing of NHE1 Also Significantly Diminished the Proliferation of Human PAECs and PAFs under Hypoxia
Because the proliferation of PAECs and PAFs is also reportedly involved in the development of pulmonary hypertension, we examined the effects of silencing NHE1 siRNA on the proliferation of PAECs and PAFs, using the same conditions as for PASMCs. We found that NHE1 was expressed in all three types of pulmonary artery cells (Figure 6A), and that the transfection of NHE1 siRNA significantly inhibited the proliferation of the three cell types (Figure 6B).
Figure 6.
Effects of silencing NHE1 on proliferation of human (HPASMCs), human pulmonary artery endothelial cells (HPAECs), and human pulmonary artery fibroblasts (HPAFs) under condition of hypoxia. (A) Representative RT-PCR data show expression of NHE1 mRNA in three cell types. RNA was isolated from HPASMCs, HPAECs, and HPAFs. RT-PCR was performed to measure the expression of NHE1. GAPDH was used as loading control. (B) Cell proliferation data. After transfection with NHE1 siRNA, HPASMCs, HPAECs, and HPAFs were cultured in a 2% oxygen chamber for 24 hours, and harvested for gene expression and cell counts to assay cell proliferation. *P < 0.05, compared with control group (n = 9 for each group).
Discussion
In this study, we found that the silencing of NHE1 by siRNA significantly inhibited the proliferation of human PASMCs (Figure 1) and the cell-cycle progression induced by hypoxia (Figure 2). The inhibitory effect of NHE1 siRNA was blocked by overexpressing E2F1 (Figure 3). We found that NHE1 siRNA attenuated the hypertrophy of human PASMCs (Figure 4) and the migration induced by hypoxia (Figure 5), and that the overexpression of E2F1 prevented these attenuations. NHE1 siRNA also decreased the hypoxia-induced proliferation of human PAECs and PAFs (Figure 6).
The proliferation of PASMCs is considered an important pathological change in the development of the pulmonary hypertension and vascular remodeling induced by hypoxia. In previous studies, we reported that the NHE inhibitors dimethyl amiloride and 5-ethylisopropyl amiloride significantly inhibited the growth of bovine PASMCs (6) in vitro, and inhibited hypoxia-induced pulmonary hypertension in animals (7). However, those NHE inhibitors were not NHE1-specific, but also affected NHE2 and NHE3 (5). Wu and colleagues reported that a new NHE1 inhibitor, sabiporide, significantly inhibited the proliferation of human PASMCs and migration under normoxia (37). However, that inhibitor also affected other NHEs (5). Therefore, the role of NHE1 in the regulation of PASMC proliferation has not been specifically investigated. We recently found that NHE1 gene knockout prevented hypoxia-induced pulmonary hypertension and vascular remodeling in mice, accompanied by a significant decrease in the proliferation of PASMCs (11) and indicating specific effects of the NHE1 gene in regulating the proliferation of PASMCs. In the present study, we used NHE1-specific siRNA to silence NHE1 in human PASMCs under normoxia and hypoxia, and found that silencing NHE1 not only inhibited the proliferation of PASMCs, but also reduced cell-cycle progression. These results demonstrate that NHE1 is a critical regulator of the hypoxia-induced proliferation of PASMCs.
Zhang and colleagues reported a significant increase in the inhibition of NHE1 expression by using two sets of NHE1 siRNA for one transfection (38). To obtain the maximal silencing of the NHE1 gene in PASMCs, we simultaneously transfected two sets of NHE1 siRNA into human PASMCs. However, we did not observe a significantly increased inhibition of PASMC proliferation under normoxia or hypoxia, although a slightly greater inhibition of cell growth was evident in the group with two sets of NHE1 siRNA.
NHE1 has been studied for years, but the mechanism by which NHE1 mediates the proliferation of PASMCs is not well-understood. We previously found that NHE1 gene knockout significantly decreased the expression of Rho-associated, coiled-coil containing protein kinase 1 or Rho-associated protein kinase 1 (ROCK1) and ROCK2, which was accompanied by the significantly increased expression of p27 and decreased expression of cyclin D1 (11). Because E2F1 is a downstream factor of p27 and cyclin D1, we hypothesized that E2F1 plays an important role in the inhibition by NHE1 siRNA of human PASMC proliferation. The E2F family of transcription factors, composed of eight different members, regulates cell-cycle progression (12, 18). E2F1 is one of these members and is essential for cellular proliferation. E2F1 functions to activate nuclear transcription factors and promote cell-cycle progression, after its release from the Rb/E2F1 complex (12, 39). Bindra and colleagues (40) reported that E2Fs may be linked to the transcriptional response to hypoxia. Previous studies demonstrated the involvement of E2F1 in cell proliferation (13–16). Tammali and colleagues (13) found that the inhibition of high glucose–induced and TNF-α–induced rat aorta vascular smooth muscle cell proliferation by aldose reductase was accompanied by the decreased expression of E2F1. Grouwels and colleagues (14) found that the overexpression of E2F1 by an adenovirus E2F1 promoter increased the proliferation of primary β-cells isolated from the pancreas of rats. Amrani and colleaues (15) reported that interferon-γ inhibited the proliferation of airway smooth muscle cells by inhibiting the expression of the E2F1 gene. Goukassian and colleagues (16) reported that the overexpression of E2F1 negated the inhibitory effects of a potent repressor of cellular proliferation, PCA-4230, on the proliferation of human vascular smooth muscle cells. In this study, we found that the expression of E2F1 was induced in PASMCs under hypoxia, and that NHE1 siRNA significantly decreased the induction of EF21 in PASMCs. We also found that the overexpression of E2F1 completely blocked the inhibitory effects of NHE1 siRNA on the hypoxia-induced proliferation of PASMCs. These results indicate that E2F1 signaling is essential for the regulation by NHE1 of PASMC proliferation.
In addition to proliferation, hypertrophy is another pathological characteristic of PASMCs in pulmonary hypertension (41, 42). Studies indicated the relationship between NHE1 and myocardial hypertrophy, showing that the inhibition of NHE1 activity by inhibitors reduced cardiac hypertrophy in animals and cultured cardiomyocytes (23–27). The increased medial wall thickness of pulmonary arteries in pulmonary hypertension and vascular remodeling is involved not only in the proliferation of PASMCs, but also in the hypertrophy of PASMCs. In our previous study, we found a decrease in medial wall thickness of the pulmonary arteries in NHE1-deficient mice. We therefore investigated the effects of NHE1 on the hypertrophy of PASMCs in this study. We found that silencing the NHE1 gene significantly decreased the hypoxia-increased size of human PASMCs. In addition to cell size, cell volume, established by calculating the protein/DNA ratio, was used for the analysis of cell hypertrophy (39), because research indicates that the stimulation of protein synthesis without DNA replication is a feature of hypertrophy (33). We found that silencing NHE1 significantly decreased the protein/DNA ratio in human PASMCs cultured under hypoxia. E2F1 was shown to be involved in the pathogenesis of cardiac hypertrophy. Wohlschlaeger and colleagues (20) reported a significant increase in the expression of E2F1 in myocardial tissues from patients with chronic hear failure–associated cardiomyocyte hypertrophy. Hlaing and colleagues (21) found that E2F1 regulated the hypertrophy of skeletal myoblasts induced by angiotensin II. Vara and colleagues (22) showed that the inhibition of E2F1 function prevented the development of myocyte hypertrophy in rats. In this study, we found that the overexpression of E2F1 also blocked the inhibition by NHE1 siRNA of PASMC hypertrophy, indicating the involvement of E2F1 in the regulation by NHE1 of PASMC hypertrophy.
In addition to proliferation and hypertrophy, the migration of PASMCs is also involved in the vascular remodeling underlying pulmonary hypertension. The extension of PASMCs into nonmuscularized vessels suggests the involvement of smooth muscle cell migration in the pathogenesis of pulmonary hypertension (28). The migration of PASMCs was reportedly induced by sustained hypoxia (29), endothelin-1 (30), serotonin (28), and other factors (31). NHE1 is reportedly involved in regulating the migration of different types of cells (40, 43), and the NHE1 inhibitor sabiporide also inhibited the migration of human PASMCs under normoxia (38). In this study, we found that silencing NHE1 siRNA significantly diminished the migration of PASMCs induced by hypoxia. Interestingly, we also found that the overexpression of the E2F1 gene prevented the inhibition by NHE1 siRNA of human PASMC migration.
The effects of hypoxia on the proliferation of PASMCs were studied in different laboratories, and inconsistent results were reported (44). To determine the best concentration of oxygen that stimulates the proliferation of human PASMCs, we investigated the effects of different levels of oxygen (1, 2, 3, 5, and 10% O2) on the proliferation of PASMCs. We found that 1, 2, and 3% oxygen significantly stimulated the proliferation of PASMCs. Although some investigators obtained controversial results (44), we did not find a significantly increased growth of PASMCs under 5 and 10% oxygen. This result was consistent with the data we obtained in another study (32). The mechanisms underlying the reactions of PASMCs to different oxygen concentrations have not been fully clarified. One report stated that cells in the medial wall of the artery normally exist under conditions of low oxygen (45). Santilli and colleagues (45) measured the concentration of transarterial wall oxygen in rabbit aortas by using an oxygen microelectrode, and found the lowest oxygen concentrations in the medial wall of the artery, compared with the adventitia and lumen. The oxygen concentration was only approximately 4% in the center of the media, but at the same time, oxygen concentrations were around 12% in the lumen and 8% in the adventitia. Because 10% oxygen was widely used to induce pulmonary hypertension and vascular remodeling in rodents, then based on the findings of Santilli and colleagues (45), the oxygen concentration would be lower than 5 or 10% in the media of the pulmonary artery when animals were placed in a hypoxia chamber (10% O2), which resulted in the proliferation and hypertrophy of smooth muscle cells in the medial walls of pulmonary arteries. Thus, a slight reduction of oxygen concentrations to 5 or 10% may not be enough to stimulate the proliferation of PASMCs, because cells in the medial walls of the artery were already under a low oxygen condition. This may explain why investigators generally use 1–3% oxygen for studies related to the proliferation of PASMCs (44, 46, 47).
Moreover, we investigated whether the regulation by E2F1 of NHE is NHE1-specific. We did not detect the expression of NHE2 in human PASMCs. Although the expression of NHE3 was evident in PASMCs, the proliferation of cells was not significantly affected by either the NHE3 inhibitor S-3226 or NHE3 siRNA. Previous reports showed that no expression of NHE2 and NHE3 was evident in human lung tissue (48) and pulmonary endothelial cells (49). Therefore, we did not investigate the effects of E2F1 on NHE2 and NHE3 in this study.
In conclusion, we demonstrated that the specific inhibition of the NHE1 gene by siRNA significantly inhibited the hypoxia-induced proliferation, hypertrophy, and migration of human PASMCs via the repression of nuclear transcription factor E2F1. This observation revealed a novel mechanism underlying the regulation by NHE1 of hypoxic pulmonary hypertension and vascular remodeling.
Footnotes
This work was supported by American Thoracic Society/Pulmonary Hypertension Research Grant PH-08–010 (L.Y.), by National Institutes of Health grant HL39150 (C.A.H.), and by the Susannah Wood Foundation.
Originally Published in Press as DOI: 10.1165/rcmb.2011-0032OC on March 31, 2011
Author Disclosure: None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Stenmark KR, Fagan K, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 2006;99:675–691 [DOI] [PubMed] [Google Scholar]
- 2.Jeffery TK, Wanstall JC. Pulmonary vascular remodeling: a target for therapeutic intervention in pulmonary hypertension. Pharmacol Ther 2001;92:1–20 [DOI] [PubMed] [Google Scholar]
- 3.Rabinovitch M. Pulmonary vascular remodeling in hypoxic pulmonary hypertension. : Yuan J, editor Hypoxic pulmonary vasoconstriction: cellular and molecular mechanisms. Boston: Kluwer Academic Publishers; 2004. pp. 403–418 [Google Scholar]
- 4.Orlowski J, Grinstein S. Na+/H+ exchangers of mammalian cells. J Biol Chem 1997;272:22373–22376 [DOI] [PubMed] [Google Scholar]
- 5.Masereel B, Pochet L, Laeckmann D. An overview of inhibitors of Na(+)/H(+) exchanger. Eur J Med Chem 2003;38:547–554 [DOI] [PubMed] [Google Scholar]
- 6.Quinn DA, Dahlberg CG, Bonventre JV, Scheid CR, Honeyman TW, Joseph PM, Thompson BT, Hales CA. The role of Na+/H+ exchange and growth factors in pulmonary artery smooth muscle cell proliferation. Am J Respir Cell Mol Biol 1996;14:139–145 [DOI] [PubMed] [Google Scholar]
- 7.Quinn DA, Du HK, Thompson BT, Hales CA. Amiloride analogs inhibit chronic hypoxic pulmonary hypertension. Am J Respir Crit Care Med 1998;157:1263–1268 [DOI] [PubMed] [Google Scholar]
- 8.Yao W, Qian G, Yang X. Roles of NHE-1 in the proliferation and apoptosis of pulmonary artery smooth muscle cells in rats. Chin Med J (Engl) 2002;115:107–109 [PubMed] [Google Scholar]
- 9.Rios EJ, Fallon M, Wang J, Shimoda LA. Chronic hypoxia elevates intracellular pH and activates Na+/H+ exchange in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2005;289:L867–L874 [DOI] [PubMed] [Google Scholar]
- 10.Shimoda LA, Fallon M, Pisarcik S, Wang J, Semenza GL. HIF-1 regulates hypoxic induction of NHE1 expression and alkalinzation of intracellular pH in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 2006;291:L941–L949 [DOI] [PubMed] [Google Scholar]
- 11.Yu L, Quinn DA, Garg HG, Hales CA. Deficiency of the NHE1 gene prevents hypoxia-induced pulmonary hypertension and vascular remodeling. Am J Respir Crit Care Med 2008;177:1276–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Israels ED, Israels LG. The cell cycle. Oncologist 2000;5:510–513 [DOI] [PubMed] [Google Scholar]
- 13.Tammali R, Saxena A, Srivastava SK, Ramana KV. Aldose reductase regulates vascular smooth muscle cell proliferation by modulating G1/S phase transition of cell cycle. Endocrinology 2010;151:2140–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grouwels G, Cai Y, Hoebeke I, Leuckx G, Heremans Y, Ziebold U, Stangé G, Chintinne M, Ling Z, Pipeleers D, et al. Ectopic expression of E2F1 stimulates beta-cell proliferation and function. Diabetes 2010;59:1435–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amrani Y, Tliba O, Choubey D, Huang CD, Krymskaya VP, Eszterhas A, Lazaar AL, Panettieri RA., Jr IFN-gamma inhibits human airway smooth muscle cell proliferation by modulating the E2F–1/Rb pathway. Am J Physiol Lung Cell Mol Physiol 2003;284:L1063–L1071 [DOI] [PubMed] [Google Scholar]
- 16.Goukassian D, Sanz-González SM, Pérez-Roger I, Font de Mora J, Ureña J, Andrés V. Inhibition of the cyclin D1/E2F pathway by PCA-4230, a potent repressor of cellular proliferation. Br J Pharmacol 2001;132:1597–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu L, Quinn DA, Garg HG, Hales CA. Cyclin-dependent kinase inhibitor p27Kip1, but not p21WAF1/Cip1, is required for inhibition of hypoxia-induced pulmonary hypertension and remodeling by heparin in mice. Circ Res 2005;97:937–945 [DOI] [PubMed] [Google Scholar]
- 18.Yu L, Quinn DA, Garg HG, Hales CA. Gene expression of cyclin-dependent kinase inhibitors and effect of heparin on their expression in mice with hypoxia-induced pulmonary hypertension. Biochem Biophys Res Commun 2006;345:1565–1572 [DOI] [PubMed] [Google Scholar]
- 19.Yu L, Quinn DA, Garg HG, Hales CA. Heparin inhibits pulmonary artery smooth muscle cell proliferation through GEF-H1/RhoA/ROCK/p27. Am J Respir Cell Mol Biol 2011;44:524–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wohlschlaeger J, Schmitz KJ, Takeda A, Takeda N, Vahlhaus C, Stypmann J, Schmid C, Baba HA, Goukassian D, Sanz-González SM, et al. Inhibition of the cyclin D1/E2F pathway by PCA-4230, a potent repressor of cellular proliferation. Br J Pharmacol 2001;132:1597–1605 J Heart Lung Transplant 2010;29:117–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hlaing M, Spitz P, Padmanabhan K, Cabezas B, Barker CS, Bernstein HS. E2F-1 regulates the expression of a subset of target genes during skeletal myoblast hypertrophy. J Biol Chem 2004;279:43625–43633 [DOI] [PubMed] [Google Scholar]
- 22.Vara D, Bicknell KA, Coxon CH, Brooks G. Inhibition of E2F abrogates the development of cardiac myocyte hypertrophy. J Biol Chem 2003;278:21388–21394 [DOI] [PubMed] [Google Scholar]
- 23.Baartscheer A, Hardziyenka M, Schumacher CA, Belterman CN, van Borren MM, Verkerk AO, Coronel R, Fiolet JW. Chronic inhibition of the Na+/H+-exchanger causes regression of hypertrophy, heart failure, and ionic and electrophysiological remodelling. Br J Pharmacol 2008;154:1266–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dulce RA, Hurtado C, Ennis IL, Garciarena CD, Alvarez MC, Caldiz C, Pierce GN, Portiansky EL, Chiappe de Cingolani GE, Camilión de Hurtado MC. Endothelin-1 induced hypertrophic effect in neonatal rat cardiomyocytes: involvement of Na+/H+ and Na+/Ca2+ exchangers. J Mol Cell Cardiol 2006;41:807–815 [DOI] [PubMed] [Google Scholar]
- 25.Chen L, Gan XT, Haist JV, Feng Q, Lu X, Chakrabarti S, Karmazyn M. Attenuation of compensatory right ventricular hypertrophy and heart failure following monocrotaline-induced pulmonary vascular injury by the Na+–H+ exchange inhibitor cariporide. J Pharmacol Exp Ther 2001;298:469–476 [PubMed] [Google Scholar]
- 26.Xue J, Mraiche F, Zhou D, Karmazyn M, Oka T, Fliegel L, Haddad GG. Elevated myocardial Na+/H+ exchanger isoform 1 activity elicits gene expression that leads to cardiac hypertrophy. Physiol Genomics 2010;42:374–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javadov S, Rajapurohitam V, Kilić A, Zeidan A, Choi A, Karmazyn M. Anti-hypertrophic effect of NHE-1 inhibition involves GSK-3beta dependent attenuation of mitochondrial dysfunction. J Mol Cell Cardiol 2009;46:998–1007 [DOI] [PubMed] [Google Scholar]
- 28.Day RM, Agyeman AS, Segel MJ, Chévere RD, Angelosanto JM, Suzuki YJ, Fanburg BL. Serotonin induces pulmonary artery smooth muscle cell migration. Biochem Pharmacol 2006;71:386–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frid MG, Li M, Gnanasekharan M, Burke DL, Fragoso M, Strassheim D, Sylman JL, Stenmark KR. Sustained hypoxia leads to the emergence of cells with enhanced growth, migratory, and promitogenic potentials within the distal pulmonary artery wall. Am J Physiol Lung Cell Mol Physiol 2009;297:L1059–L1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meoli DF, White RJ. Endothelin-1 induces pulmonary but not aortic smooth muscle cell migration by activating ERK1/2 MAP kinase. Can J Physiol Pharmacol 2010;88:830–839 [DOI] [PubMed] [Google Scholar]
- 31.Zhang R, Zhou L, Li Q, Liu J, Yao W, Wan H. Up-regulation of two actin-associated proteins prompts pulmonary artery smooth muscle cell migration under hypoxia. Am J Respir Cell Mol Biol 2009;41:467–475 [DOI] [PubMed] [Google Scholar]
- 32.Yu L, Hales CA. Hypoxia does neither stimulate pulmonary artery endothelial cell proliferation in mice and rats with pulmonary hypertension and vascular remodeling nor in human pulmonary artery endothelial cells. J Vasc Res 2011;48:465–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen H, Huang XN, Yan W, Chen K, Guo L, Tummalapali L, Dedhar S, St-Arnaud R, Wu C, Sepulveda JL. Role of the integrin-linked kinase/PINCH1/alpha parvin complex in cardiac myocyte hypertrophy. Lab Invest 2005;85:1342–1356 [DOI] [PubMed] [Google Scholar]
- 34.McKay S, de Jongste JC, Saxena PR, Sharma HS. Angiotensin II induces hypertrophy of human airway smooth muscle cells: expression of transcription factors and transforming growth factor–beta1. Am J Respir Cell Mol Biol 1998;18:823–833 [DOI] [PubMed] [Google Scholar]
- 35.James MF, Beauchamp RL, Manchanda N, Kazlauskas A, Ramesh VA. NHERF binding site links the betaPDGFR to the cytoskeleton and regulates cell spreading and migration. J Cell Sci 2004;117:2951–2961 [DOI] [PubMed] [Google Scholar]
- 36.Kwapiszewska G, Wygrecka M, Marsh LM, Schmitt S, Trösser R, Wilhelm J, Helmus K, Eul B, Zakrzewicz A, Ghofrani HA, et al. Fhl-1, a new key protein in pulmonary hypertension. Circulation 2008;118:1183–1194 [DOI] [PubMed] [Google Scholar]
- 37.Wu D, Doods H, Stassen JM. Inhibition of human pulmonary artery smooth muscle cell proliferation and migration by sabiporide, a new specific NHE-1 inhibitor. J Cardiovasc Pharmacol 2006;48:34–40 [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Bobulescu IA, Goyal S, Aronson PS, Baum MG, Moe OW. Characterization of Na+/H+ exchanger NHE8 in cultured renal epithelial cells. Am J Physiol Renal Physiol 2007;293:F761–F766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan YP, Weiss RH. Exogenous attenuation of p21(Waf1/Cip1) decreases mesangial cell hypertrophy as a result of hyperglycemia and IGF-1. J Am Soc Nephrol 2004;15:575–584 [DOI] [PubMed] [Google Scholar]
- 40.Bindra RS, Gibson SL, Meng A, Westermark U, Jasin M, Pierce AJ, Bristow RG, Classon MK, Glazer PM. Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res 2005;65:11597–11604 [DOI] [PubMed] [Google Scholar]
- 41.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med 2007;28:23–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fredenburgh LE, Liang OD, Macias AA, Polte TR, Liu X, Riascos DF, Chung SW, Schissel SL, Ingber DE, Mitsialis SA, et al. Absence of cyclooxygenase-2 exacerbates hypoxia-induced pulmonary hypertension and enhances contractility of vascular smooth muscle cells. Circulation 2008;117:2114–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stock C, Schwab A. Protons make tumor cells move like clockwork. Pflugers Arch 2009;458:981–992 [DOI] [PubMed] [Google Scholar]
- 44.Pak O, Aldashev A, Welsh D, Peacock A. The effects of hypoxia on the cells of the pulmonary vasculature. Eur Respir J 2007;30:364–372 [DOI] [PubMed] [Google Scholar]
- 45.Santilli SM, Tretinyak AS, Lee ES. Transarterial wall oxygen gradients at the deployment site of an intra-arterial stent in the rabbit. Am J Physiol Heart Circ Physiol 2000;279:H1518–H1525 [DOI] [PubMed] [Google Scholar]
- 46.Chen B, Calvert AE, Cui H, Nelin LD. Hypoxia promotes human pulmonary artery smooth muscle cell proliferation through induction of arginase. Am J Physiol Lung Cell Mol Physiol 2009;297:L1151–L1159 [DOI] [PubMed] [Google Scholar]
- 47.Sarkar J, Gou D, Turaka P, Viktorova E, Ramchandran R, Raj JU. MicroRNA-21 plays a role in hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration. Am J Physiol Lung Cell Mol Physiol 2010;299:L861–L871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brant SR, Yun CH, Donowitz M, Tse CM. Cloning, tissue distribution, and functional analysis of the human Na+/N+ exchanger isoform, NHE3. Am J Physiol 1995;269:C198–C206 [DOI] [PubMed] [Google Scholar]
- 49.Cutaia MV, Parks N, Centracchio J, Rounds S, Yip KP, Sun AM. Effect of hypoxic exposure on Na+/H+ antiport activity, isoform expression, and localization in endothelial cells. Am J Physiol 1998;275:L442–L451 [DOI] [PubMed] [Google Scholar]